Abstract
Objective: Recently, ribosome binding protein 1 (RRBP1) is reported to be involved in tumorigenesis. However, the expression and clinical significance of RRBP1 in prostate cancer (PCa) remains unknown. This study is aimed to investigate the expression and clinical significance of RRBP1 in PCa.
Materials and methods: RRBP1 expression was firstly detected in 6 cases of PCa and matched adjacent non-cancerous prostate tissues by reverse transcription-quantitative PCR (RT-qPCR) and Western blot. Then, RRBP1 expression was further detected in 127 cases of PCa and 40 cases of non-cancerous prostate tissues by immunohistochemistry (IHC). The relationship of RRBP1 with clinical-pathological characters and patients’ prognosis was analyzed in PCa.
Results: RT-qPCR and Western blot analysis showed that RRBP1 expression levels in PCa tissues were significantly higher compared with those in matched adjacent non-cancerous prostate tissues. IHC results shown that the high-expression rate of RRBP1 in PCa was 69.3%, which was significantly greater than those in non-cancerous prostate tissues (15.0%, P<0.001). RRBP1 expression was significantly associated with T stage, lymph node metastasis, PSA and Gleason score in PCa. Survival analysis indicated that patients with RRBP1 low-expression presented longer survival time compared with those with RRBP1 high-expression. Moreover, RRBP1 as well as T stage, lymph node metastasis and Gleason score could serve as independent prognostic factors in PCa.
Conclusion: RRBP1 is highly expressed in PCa and correlates with prognosis, which may serve as a potential biomarker in PCa.
Keywords: prostate cancer, RRBP1, prognosis, PSA, Gleason score
Introduction
Prostate cancer (PCa) is considered as one of the most common urinary tumors among men in the world, which accounts for the second leading cause of cancer-related mortality.1–3 It is reported that the 5-year survival rate of locoregional PCa is nearly 99%, while about one-third of those patients may progress to metastatic PCa.4–6 Metastatic PCa is the major cause of cancer death. Locoregional PCa can be cured by radical prostatectomy (RP) or radiation therapy, but only palliative treatments can be used to metastatic PCa.7,8 Therefore, early detection of PCa is important. However, available diagnostic and prognostic tools for PCa are still insufficient.9,10 Identifying novel diagnostic and prognostic biomarker of PCa is required.
Ribosome binding protein 1 (RRBP1), an important endoplasmic reticulum membrane protein, is composed of 1,410 amino acid including a transmembrane domain, an acidic coiled-coil COOH-terminal domain and a ribosome-binding domain.11 RRBP1 is crucial for the transportation and secretion of nascent proteins.11,12 Moreover, RRBP1 plays a key role in ribosome binding and the terminal differentiation of secretory cells and tissues.13–15 RRBP1 is mainly localized on the endoplasmic reticulum and can be detected in nucleus and cytoplasm.16 Recent studies reported that RRBP1 was highly expressed in esophageal carcinoma, breast cancer, lung cancer and colorectal cancer.15,17–20 Furthermore, RRBP1 is confirmed to be a potential prognostic biomarker in esophageal carcinoma, breast cancer and colorectal cancer.15,17,21 Thus, current data indicates that RRBP1 is an oncogene and may be involved in tumor formation and progression. However, association between RRBP1 and PCa has never been reported.
In this study, RRBP1 expression was detected in PCa and matched adjacent non-cancerous prostate tissues. Meanwhile, the association between RRBP1 expression and clinical-pathological characters and prognosis was evaluated in PCa.
Material and methods
Patients and samples
All tissue samples were collected from Hunan Provincial People’s Hospital, the first affiliated hospital of Hunan Normal University, between January 2012 and December 2014. A total of 127 PCa patients were enrolled in this study. Before puncture biopsy, no patient received radiotherapy, chemotherapy or immunotherapy. Pathological diagnosis was performed by the pathologists in Hunan Provincial People’s Hospital. Based on the pathological diagnosis, patients with stage T1–T2 received radical surgery resection. Patients with stage T3–T4 received radiotherapy, chemotherapy, endocrine therapy or immunotherapy. Clinicopathological characters including age, smoke history, T stage, lymph node metastasis, PSA level and Gleason score were obtained from hospital records. A total of 127 cases of PCa and 40 cases of matched adjacent non-cancerous prostate tissues were collected and stored as paraffin embedding tissues. In 127 cases of PCa patients, only 6 cases of fresh PCa tissues and matched adjacent non-cancerous prostate tissues were collected and stored at liquid nitrogen. All patients signed informed consent and completed follow-up. The mean survival time was 49.1 months (range 10–76 months). This study was supported by the Medical Ethics Committee of Hunan Provincial People’s Hospital, first affiliated hospital of Hunan Normal University and all patients.
Reverse transcription-quantitative PCR (RT-qPCR)
All the manipulation was performed as previously described.22 Total RNA was extracted from fresh tissues using Trizol reagents (Invitrogen, Waltham, MA, USA) according to manufacturer’s protocol (Invitrogen). Then, RNA was reversely transcribed to cDNA by using a reverse transcription kit (Takara Biotechnology, Japan). Finally, the levels of mRNA were tested according to the manufacturer’s instructions. The sequences of the primers of RRBP1 were 5′-TTCAAC-GAGGGCGAGGCCCAG-3′ (F) and 5′-CGTGCCTGCACA-GCCGTGATCT-3′ (R). The primer sequences of GAPDH were 5′-GCATCCTGCACCACCAACTG-3′ and 5′-GCCTG-CTTCACCACCTTCTT-3′. Reaction conditions were recorded as follow: 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 30 s. The levels of mRNA were compared by the 2−ΔΔCt method. The expression of GAPDH was used as an internal control gene for standardizing the expression of target genes. Experiments were repeated in triplicate.
Western blot
All the manipulation was performed as previously described.22 Total proteins were extracted from fresh tissues by RIPA lysis buffer (KeyGEN, China) and measured by BCA Protein Assay Kit (KeyGEN). A total of 50 μg of protein was separated on 10% SDS-polyacrylamide gels (SDS-PAGE) and then transferred onto polyvinylidene fluoride (PVDF) membranes. After blocked with 5% nonfat milk PBST solution for 1 hr at room temperature, membranes were incubated with polyclonal RRBP1 antibody (1:1,000) (Proteintech, China) and β-actin antibody (1:2,000) (Abcam, USA) at 4 °C overnight, respectively. Then, membranes were washed with PBST and incubated with secondary antibodies (ZhongshanJingqiao, China) for 1 hr at room temperature. Protein band was visualized by ECL Plus Kit (Beyotime, China). Relative protein expression levels were statistically analyzed by measuring the gray density of each protein band. The expression of β-actin was used as an internal control protein for standardizing the expression of target protein. Experiments were repeated in triplicate.
Immunohistochemistry (IHC)
All manipulation was performed as previously described.23 Tissue sections were dewaxed with xylene and rehydrated with alcohol. Antigen retrieval was performed by 0.01 mol/L citrate buffer (pH 6.0). Then, sections were blocked with hydrogen peroxide and fetal bovine serum, and incubated with polyclonal RRBP1 antibody (1:200) (Proteintech). After incubated with biotinylated secondary antibody, sections were developed by 3,3ʹ-diaminobenzidine (DAB) and stained with hematoxylin. According to the staining intensity and percentage of positive cells, total scores of each sample were calculated by multiplying the two scores. The scores of staining intensity and positive cells percentage were recorded as follow: blank (0), weak (1), moderate (2) and strong (3): <5% (0), ≥5% to <25% (1), 25–50% (2), >50% (3). Total scores of each sample ranged from 0 to 9. RRBP1 expression was divided into high-expression (≥4) and low-expression (<4).
Statistical analysis
SPSS 19.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. All data were presented as mean ± SD. The difference between two groups was compared by independent sample t-test. When the data was not normally distributed, the difference was compared by a non-parametric test. Association between RRBP1 expression and clinicopathological characters was analyzed by Pearson chi-square test (χ2). Kaplan–Meier method and Cox’s regression model were used for survival analysis. P<0.05 was considered as a statistical difference.
Results
RRBP1 is highly expressed in PCa
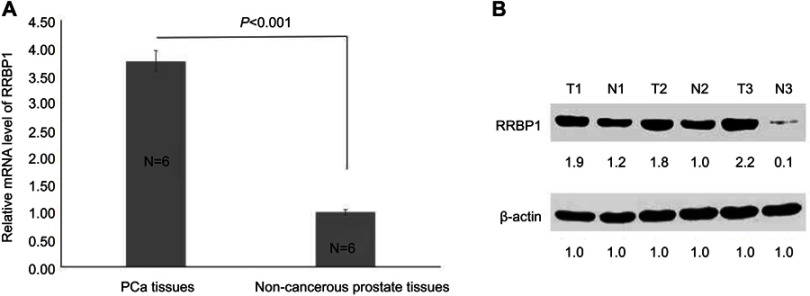
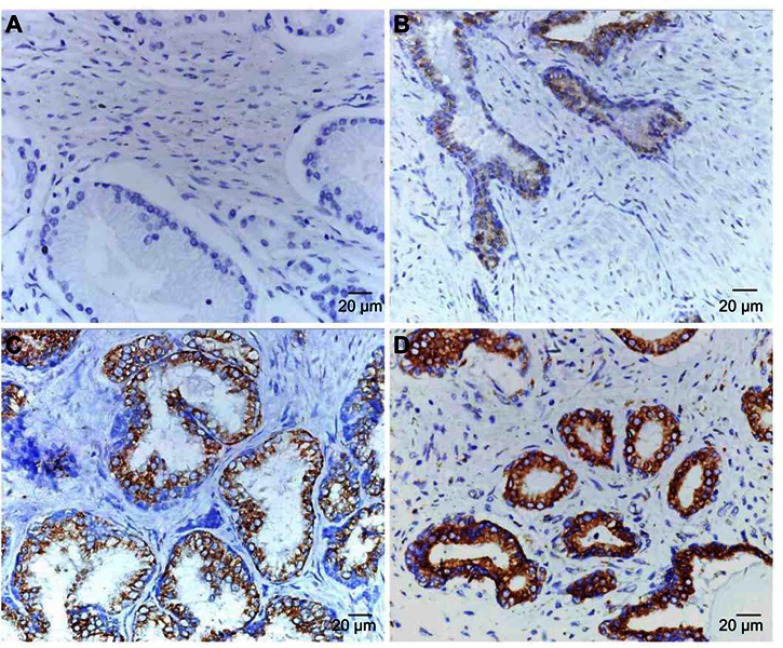
To investigate the correlation between RRBP1 and PCa, RRBP1 expression was firstly detected in 6 cases of fresh PCa tissues and matched adjacent non-cancer prostate tissues by RT-qPCR and Western blot assays. Results showed RRBP1 mRNA levels were significantly higher in PCa tissues compared with those in matched adjacent non-cancer prostate tissues (Figure 1A, P<0.001). Meanwhile, significantly higher levels of RRBP1 protein were observed in PCa tissues (Figure 1B, P<0.05). Subsequently, RRBP1 expression was further detected in 127 cases of PCa and 40 cases of matched adjacent non-cancer prostate tissues by IHC. As shown in Figure 2, positive expression of RRBP1 was mainly observed in cell cytoplasm as brown. Statistical analysis has shown that the high-expression rate of RRBP1 in PCa tissues was 69.3%, which was significantly higher than those in non-cancer prostate tissues (15.0%, Table 1, P<0.001). These data indicated that RRBP1 was highly expressed in PCa.
Figure 1.
RRBP1 was highly expressed in PCa. (A) Relative mRNA level of RRBP1 was detected in PCa and non-cancerous prostate tissues by RT-qPCR, and mean ± SD represented three independent experiments. (B) RRBP1 protein expression level was detected in PCa and non-cancerous prostate tissues by Western blot, and the gray density of each protein brand was recorded.
Abbreviations: T, tumor; N, non-cancerous prostate tissues; RRBP1, ribosome binding protein 1; PCa, prostate cancer; RT-qPCR, reverse transcription-quantitative PCR.
Figure 2.
RRBP1 expression was detected in PCa by IHC. (A) No positive staining of RRBP1 in non-cancerous prostate tissues (Score = 0, ×400). (B) Weak staining of RRBP1 in PCa (Score = 1, ×400). (C) Moderate staining of RRBP1 in PCa (Score = 4, ×400). (D) Strong staining of RRBP1 in PCa (Score = 9, ×400).
Abbreviations: RRBP1, ribosome binding protein 1; IHC, immunohistochemistry; PCa, prostate cancer.
Table 1.
RRBP1 expression was detected in PCa tissues by IHC
| Types | N | RRBP1 | P-value | |
|---|---|---|---|---|
| Low-expression | High-expression | |||
| PCa tissues | 127 | 39 (30.7%) | 88 (69.3%) | <0.001 |
| Non-cancerous prostate tissues | 40 | 34 (85.0%) | 6 (15.0%) | |
Abbreviations: RRBP1, ribosome binding protein 1; IHC, immunohistochemistry; PCa, prostate cancer.
RRBP1 expression correlates with clinicopathological characters and patients’ prognosis
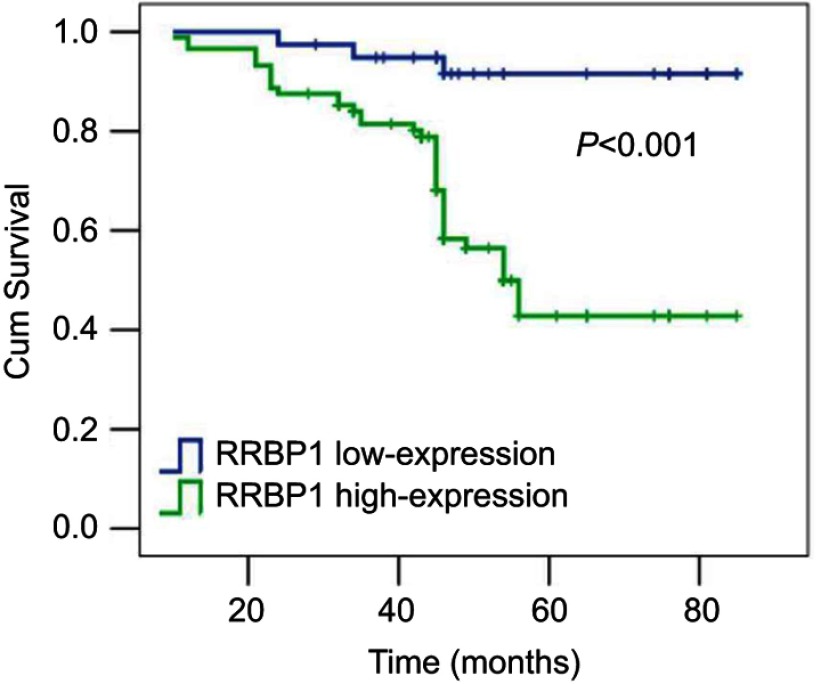
Then, the correlation between RRBP1 expression and clinicopathological characters and patients’ prognosis was further analyzed in 127 cases of PCa. Results have shown that RRBP1 expression significantly correlated with T stage, lymph node metastasis, PSA level and Gleason score (Table 2, P<0.05), but was not significantly connected with age and smoke history (Table 2, P>0.05). RRBP1 expression levels were significantly higher in patients with T3–T4 stage or lymph node metastasis. Patients with more higher PSA level (>20 ng/ml) or Gleason score (≥8) presented more higher levels of RRBP1. Kaplan–Meier survival analysis has shown that patients with RRBP1 high-expression presented poorer prognosis. The mean survival time of patients with RRBP1 high-expression was 56 months, which was significantly reduced than those with RRBP1 low-expression (73 months) (Table 3, Figure 3, P<0.001). Meanwhile, T stage, lymph node metastasis, PSA and Gleason score were associated with patients’ prognosis (Table 3, P<0.05). Cox regression analysis showed RRBP1 expression, T stage, lymph node metastasis and Gleason score were independent prognostic factors in PCa (Table 4, P<0.05).
Table 2.
The association between RRBP1 expression and clinical-pathological characters was analyzed in PCa
| Clinical-pathological characters | N | RRBP1 | P-value | |
|---|---|---|---|---|
| Low-expression | High-expression | |||
| Age (years) | ||||
| ≤64 | 67 | 16 | 51 | 0.086 |
| >64 | 60 | 23 | 37 | |
| Smoke history | ||||
| Negative | 36 | 9 | 27 | 0.522 |
| Positive | 91 | 30 | 61 | |
| T stage | ||||
| T1–T2 | 75 | 34 | 41 | <0.001 |
| T3–T4 | 52 | 5 | 47 | |
| Lymph node metastasis | ||||
| Negative | 106 | 37 | 69 | 0.021 |
| Positive | 21 | 2 | 19 | |
| PSA (ng/ml) | <0.001 | |||
| ≤20 | 67 | 30 | 37 | |
| >20 | 60 | 9 | 51 | |
| Gleason score | ||||
| <8 | 65 | 31 | 34 | <0.001 |
| ≥8 | 62 | 8 | 54 | |
Abbreviations: RRBP1, ribosome binding protein 1; PCa, prostate cancer.
Table 3.
Patients’ survival was evaluated by Kaplan–Meier analysis
| Variables | N | Mean survival time (month, 95% CI) |
P-value |
|---|---|---|---|
| RRBP1 | |||
| Low-expression | 39 | 73 (69–76) | <0.001 |
| High-expression | 88 | 56 (51–60) | |
| Age (years) | |||
| ≤64 | 67 | 64 (58–69) | 0.251 |
| >64 | 60 | 60 (55–65) | |
| Smoke history | |||
| Negative | 36 | 59 (53–66) | 0.403 |
| Positive | 91 | 63 (58–67) | |
| T stage | |||
| T1–T2 | 75 | 65 (61–70) | 0.011 |
| T3–T4 | 52 | 56 (50–62) | |
| Lymph node metastasis | |||
| Negative | 106 | 64 (60–68) | <0.001 |
| Positive | 21 | 48 (37–58) | |
| PSA (ng/ml) | |||
| ≤20 | 67 | 67 (63–71) | 0.002 |
| >20 | 60 | 55 (49–61) | |
| Gleason score | |||
| <8 | 65 | 69 (65–72) | <0.001 |
| ≥8 | 62 | 54 (48–60) |
Figure 3.
Kaplan–Meier survival analysis showing RRBP1 high-expression that presented poor prognosis in PCa.
Abbreviations: RRBP1, ribosome binding protein 1; PCa, prostate cancer.
Table 4.
Patients’ survival was evaluated by Cox regression analysis
| Variables | Hazard rate | 95% CI | P-value |
|---|---|---|---|
| RRBP1 expression (high-expression vs Low-expression) | 6.924 | 1.995–24.035 | 0.002 |
| Age (>64 years vs ≤64 years) | 1.375 | 0.692–2.740 | 0.363 |
| Smoke history (positive vs negative) | 1.648 | 0.738–3.681 | 0.223 |
| T stage (T3–T4 vs T1–T2) | 6.410 | 1.842–22.222 | 0.004 |
| Lymph node metastasis (positive vs negative) | 4.027 | 1.691–9.590 | 0.002 |
| PSA (>20 ng/ml vs ≤20 ng/ml) | 1.017 | 0.419–2.475 | 0.969 |
| Gleason score (≥8 vs ≥8) | 6.534 | 1.964–21.737 | 0.002 |
Discussion
In this study, to investigate the correlation between RRBP1 expression and PCa, we firstly detected RRBP1 expression in PCa and matched adjacent non-cancer prostate tissues by RT-qPCR and Western blot assays. Results showed that RRBP1 mRNA and protein levels in PCa tissues were significantly greater than those in non-cancer prostate tissues, indicating RRBP1 high-expression was associated with the formation of PCa. Meanwhile, IHC assay received the same results that RRBP1 was highly expressed in PCa, which further supported that RRBP1 high-expression was connected with the formation of PCa. Moreover, IHC results shown that RRBP1 was hard to detect in non-cancer prostate tissues, suggesting that RRBP1 detection might be helpful for the diagnosis of PCa.
It was well known that T stage, lymph node metastasis, PSA level and Gleason score correlated with PCa progression and affected patients’ prognosis.24–28 Thus, the correlation between RRBP1 expression and clinicopathological characters and patients’ prognosis was further analyzed in PCa. Results showed RRBP1 expression significantly correlated with T stage, lymph node metastasis, PSA level and Gleason score, suggesting that RRBP1 high-expression was implicated in the progression of PCa. Kaplan–Meier survival analysis showed that RRBP1 expression as well as T stage, lymph node metastasis, PSA level and Gleason score were connected with patients’ prognosis. Meanwhile, Cox regression analysis showed RRBP1 expression as well as T stage, lymph node metastasis and Gleason score were independent prognostic factors in PCa. These data indicated that RRBP1 could serve as a novel prognostic biomarker in PCa, which was consistent with the findings in esophageal carcinoma, breast cancer and colorectal cancer.15,17,21
In addition, there are a few limitations in this study. On the one hand, it is inevitable to exclude the selection bias because of the potential differences in specimens collection. On the other hand, it is hard to eliminate the histological differences in adjacent non-cancer prostate tissues. Therefore, further investigations are required to validate our findings.
Conclusion
Our data indicate that RRBP1 is highly expressed in PCa, which may be helpful for the diagnosis of PCa. RRBP1 high-expression predicts unfavorable prognosis, which may be beneficial for the prediction of prognosis in PCa.
Acknowlegment
Thanks to all patients who participated in this study. This study was carried out in accordance with the principles of the Declaration of Helsinki. This work was supported by the Renshu Research Fund of Hunan Provincial People’s Hospital (no. 201708 to Tieqiu Li), the Natural Science Foundation of Hunan Province of China (no. 2018JJ6103 to Tieqiu Li) and Scientific Research Foundation of Administration of Traditional Chinese Medicine of Hunan Province of China (no. 201765 to Tieqiu Li). This study was also supported by the Key Research and Development Project of Science and Technology Bureau of Changsha (no. kq1801095 to Tieqiu Li).
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kumar C, Rasool RU, Iqra Z, et al. Alkyne-azide cycloaddition analogues of dehydrozingerone as potential anti-prostate cancer inhibitors via the PI3K/Akt/NF-kB pathway. Medchemcomm. 2017;8(11):2115–2124. doi: 10.1039/c7md00267j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hassanipour S, Fathalipour M, Salehiniya H. The incidence of prostate cancer in Iran: a systematic review and meta-analysis. Prostate Int. 2018;6(2):41–45. doi: 10.1016/j.prnil.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou X, Li Q, He J, et al. HnRNP-L promotes prostate cancer progression by enhancing cell cycling and inhibiting apoptosis. Oncotarget. 2017;8(12):19342–19353. doi: 10.18632/oncotarget.14258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han Y, Xu J, Kim J, Wu X, Gu J. LINE-1 methylation in peripheral blood leukocytes and clinical characteristics and prognosis of prostate cancer patients. Oncotarget. 2017;8(55):94020–94027. doi: 10.18632/oncotarget.21511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng P, Li H, Xu P, et al. High lncRNA HULC expression is associated with poor prognosis and promotes tumor progression by regulating epithelial–mesenchymal transition in prostate cancer. Arch Med Sci. 2018;14(3):679–686. doi: 10.5114/aoms.2017.69147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thurtle DR, Gordon EM, Brierly RD, Conway CJ, McLoughlin J. General practitioner perception of prostate-specific antigen testing has improved, but more awareness of prostate cancer risk in younger patients is still awaited. Prostate Int. 2018;6(2):61–65. doi: 10.1016/j.prnil.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mavrogenis AF, Angelini A, Vottis C, et al. Modern palliative treatments for metastatic bone disease: awareness of advantages, disadvantages, and guidance. Clin J Pain. 2016;32(4):337–350. doi: 10.1097/AJP.0000000000000255 [DOI] [PubMed] [Google Scholar]
- 8.Ren L, Chen J, Zhang X. Increased expression of tumor protein D54 is associated with clinical progression and poor prognosis in patients with prostate cancer. Oncol Lett. 2017;14(6):7739–7744. doi: 10.3892/ol.2017.7214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loeb S, Bjurlin MA, Nicholson J, et al Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65(6):1046–1055. doi: 10.1016/j.eururo.2013.12.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izumi K, Shigehara K, Nohara T, Narimoto K, Kadono Y, Mizokami A. Both high and low serum total testosterone levels indicate poor prognosis in patients with prostate cancer. Anticancer Res. 2017;37(10):5559–5564. doi: 10.21873/anticanres.11988 [DOI] [PubMed] [Google Scholar]
- 11.Wanker EE, Sun Y, Savitz AJ, et al. Functional characterization of the 180-kD ribosome receptor in vivo. J. Cell Biol. 1995;130(1):29e39. doi: 10.1083/jcb.130.1.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benyamini P, Webster P, Meyer DI. Knockdown of p180 eliminates the terminal differentiation of a secretory cell line. Mol Biol Cell. 2009;20(2):732–744. doi: 10.1091/mbc.e08-07-0682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savitz AJ, Meyer DI. 180-kD ribosome receptor is essential for both ribosome binding and protein translocation. J Cell Biol. 1993;120(4):853–863. doi: 10.1083/jcb.120.4.853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu GH, Mao CZ, Wu HY, et al. Expression profile of rrbp1 genes during embryonic development and in adult tissues of Xenopus laevis. Gene Expr Patterns. 2017;23–24:1–6. doi: 10.1016/j.gep.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Wang M, Zhang M, et al. Expression and significance of RRBP1 in esophageal carcinoma. Cancer Manag Res. 2018;10:1243–1249. doi: 10.2147/CMAR.S158013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsen JV, Blagoev B, Gnad F, et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127(3):635–648. doi: 10.1016/j.cell.2006.09.026 [DOI] [PubMed] [Google Scholar]
- 17.Liang X, Sun S, Zhang X, et al. Expression of ribosome-binding protein 1 correlates with shorter survival in Her-2 positive breast cancer. Cancer Sci. 2015;106(6):740–746. doi: 10.1111/cas.12666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Telikicherla D, Marimuthu A, Kashyap MK, et al. Overexpression of ribosome binding protein 1 (RRBP1) in breast cancer. Clin Proteomics. 2012;9(1):7. doi: 10.1186/1559-0275-9-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai HY, Yang YF, Wu AT, et al. Endoplasmic reticulum ribosome-binding protein 1 (RRBP1) overexpression is frequently found in lung cancer patients and alleviates intracellular stress-induced apoptosis through the enhancement of GRP78. Oncogene. 2013;32(41):4921–4931. doi: 10.1038/onc.2012.514 [DOI] [PubMed] [Google Scholar]
- 20.Krasnov GS, NIu O, Khankin SL, et al. Colorectal cancer 2D-proteomics: identification of altered protein expression. Mol Biol (Mosk). 2009;43(2):348–356. [DOI] [PubMed] [Google Scholar]
- 21.Pan Y, Cao F, Guo A, et al. Endoplasmic reticulum ribosome-binding protein 1, RRBP1, promotes progression of colorectal cancer and predicts an unfavourable prognosis. Br J Cancer. 2015;113(5):763–772. doi: 10.1038/bjc.2015.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei B, Zhou X, Lv D, et al. Apoptotic and nonapoptotic function of caspase 7 in spermatogenesis. Asian J Androl. 2017;19(1):47–51. doi: 10.4103/1008-682X.169563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T, Hong X, Zhao J, et al. Gelsolin-like actin-capping protein is associated with patient prognosis, cellular apoptosis and proliferation in prostate cancer. Biomark Med. 2016;10(12):1251–1260. doi: 10.2217/bmm-2016-0186 [DOI] [PubMed] [Google Scholar]
- 24.Carlsson SV, Tafe LJ, Chade DC, et al. Pathological features of lymph node metastasis for predicting biochemical recurrence after radical prostatectomy for prostate cancer. J Urol. 2013;189(4):1314–1318. doi: 10.1016/j.juro.2012.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drobkova H, Jurecekova J, Grendar M, et al. Testosterone as a prospective predictor of pathological Gleason score and pathological stage in prostate cancer. Gen Physiol Biophys. 2017;36(5):549–556. doi: 10.4149/gpb_2017044 [DOI] [PubMed] [Google Scholar]
- 26.Freiberger C, Berneking V, Vogeli TA, et al. Long-term prognostic significance of rising PSA levels following radiotherapy for localized prostate cancer – focus on overall survival. Radiat Oncol. 2017;12(1):98. doi: 10.1186/s13014-017-0837-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.May M, Gunia S, Helke C, Braun KP, Pickenhain S, Hoschke B. Is it possible to provide a prognosis after radical prostatectomy for prostate cancer by means of a PSA regression model? Int J Biol Markers. 2005;20(2):112–118. [DOI] [PubMed] [Google Scholar]
- 28.Helpap B, Ringli D, Tonhauser J, et al. The significance of accurate determination of Gleason score for therapeutic options and prognosis of prostate cancer. Pathol Oncol Res. 2016;22(2):349–356. doi: 10.1007/s12253-015-0013-x [DOI] [PubMed] [Google Scholar]