Abstract
Candida parapsilosis (C. parapsilosis) and Rhodotorula mucilaginosa (R. mucilaginosa) have emerged as a potential pathogen in immunosuppressed hosts; they rarely induce onychomycosis in immunocompetent hosts without assistance from other pathogens. Here we present onychomycosis induced by two strains on different toenails in an immunocompetent young adult. The patient presented with onychomycosis on left and right first toenails due to R. mucilaginosa and C. parapsilosis, respectively. Based on the diagnosis, he had been orally treated with itraconazole 200 twice daily for one week every four weeks that repeated 7 times; however, the toenails did not respond satisfactorily to the treatment. After two months of drug cessation, we confirmed that the two toenails were infected with different fungi. R. mucilaginosa was isolated from the left first toenail, and C. parapsilosis was isolated from the right first toenail. Identifications were confirmed by morphological and cultural characteristics as well as by DNA molecular analysis. After determining in vitro drug susceptibility, the patient was successfully treated with a topical application of ketoconazole cream on the left toenail and oral itraconazole. It is the rare known case of different nails being infected by R. mucilaginosa and C. parapsilosis respectively.
Keywords: Onychomycosis, Rhodotorula mucilaginosa, Candida parapsilosis, Yeast
1. Introduction
Onychomycosis is an infection of the nail plate, which is caused by three groups of fungal pathogens: dermatophyte molds (DM), non-DM (NDM) and yeasts [1,2]. Candida species are the most common pathogenic yeast [3], with Candida albicans being the most frequent isolated species. Candida parapsilosis (C. parapsilosis) was known to be occasionally responsible for pathological lesions of the nails, but has now emerging as one of the important etiological agents of onychomycosis.
Rhodotorula mucilaginosa (R. mucilaginosa), also a yeast species, is commonly reported as an etiological agent of opportunistic infections in humans, particularly in immunocompromised hosts [4]. It rarely induces onychomycosis in immunocompetent patients.
Nail infection with environmental yeasts is a rather rare event, but they are difficult to treat due to their high resistance to antifungals. Recently, we have confirmed an ongoing nail infection with an unusual yeast fungus. A young adult with 13-year-old onychomycosis was resistant to oral application of itraconazole. The mycological diagnosis for onychomycosis is C. parapsilosis and R. Mucilaginosa, which are isolated respectively from right toenail and left toe nail. The isolated agent, R. mucilaginosa, is resistant to most azoles, but is susceptible to ketoconazole. The onychomycosis was successfully treated by following the drug susceptibilities of the two strains in vitro. The patient has no onychomycosis recurrence at the one-year follow up point.
2. Case
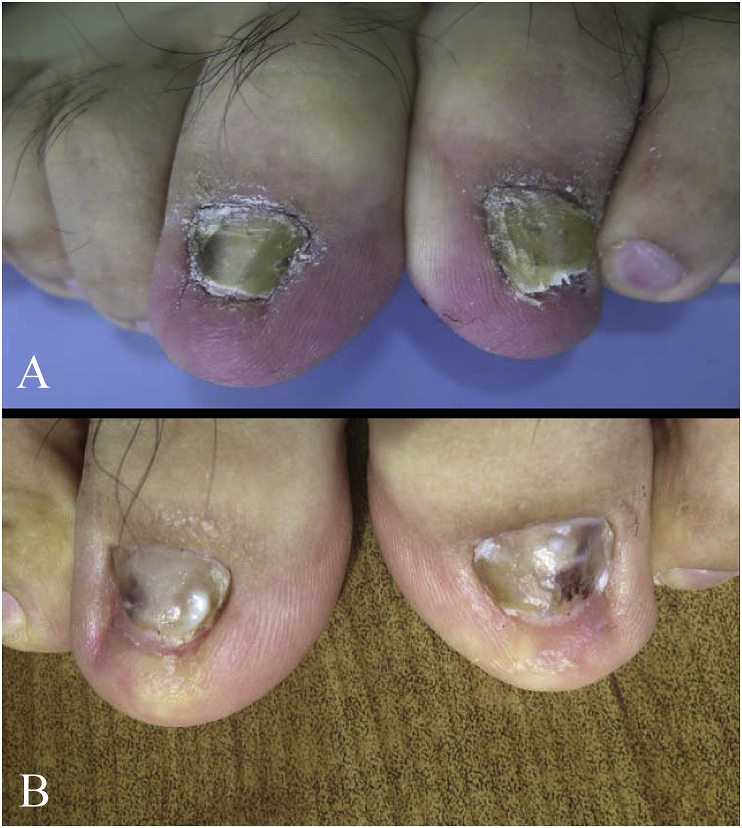
A 26-year-old Chinese male visited our department in October 2015 (day 0) with yellow color and hypertrophy on both the left and right first toenails, with a somewhat periungual erythema (Fig. 1) at both sites. Sixteen years ago (-16 years), the patient had the periungual erythema of the first left toe with slight pain, he did not receive any treatment; and fifteen years ago (-15 years) he had a slight painful periungual erythema on the right first toenail. Twelve years ago (-12 years), the patient first noticed that both the right and left first toenails showed yellow color and abnormal thickness. Physical examination showed that infection had destroyed the entire nail plate, and the affected nails had become yellow, with subungual hyperkeratosis. The patient has a history of playing football. Thirteen months ago (at -13 months), the patient was treated with oral itraconazole 200 mg twice daily for one week out of every month for four months, but did not respond well to the treatment. The treatment regimen was then changed to oral itraconazole 200mg twice per day for one week, every two months. Three cycles of this new treatment were given, but little improvement was seen. The patient was then prescribed for topical amorolfine cream twice per day for two months. Once more, no good response to the treatment was seen on either nail. The patient did not report any other disease during this time.
Fig. 1.
(A) Toenails affected by onychomycosis; (B) two months after initial treatment.
At day 0, the patient was given a full examination. Laboratory findings were all within normal limits. Serological tests for hepatitis B virus, HIV antibody and anti-nuclear antibodies were all negative. Chest radiograph showed unremarkable findings. We did not find any immunosuppression.
At day 0, after nail asepsis with 70% ethanol, the distal and lateral fragments of both toenails plates with subungual debris were removed with two different sterile nail clippers. Direct microscopic examination of debris from both toenails in 20% KOH was negative for fungal infection. The nail clippings and subungual debris were inoculated onto the slopes of PDA containing chloramphenicol with and without cycloheximide (0.5 mg/ml), and OA, respectively, at 26 °C. There was no fungal colony growth on the plate at day +30 days. We did not give the patient any other treatment by then. At day +30 days, toenail clippings and debris were given the same tests. Again, direct microscopic examination and inoculation did not reveal any fungal infection or fungal colony growth.
At + 60 days, direct microscopic examination of both the toenail crisp did not reveal fungal infection; however, at + 67 days, colony growth was found on the agar slopes (Fig. 2).
Fig. 2.
(A) C. parapsilosis colony on Sabouraud Dextrose Agar (SDA); (B) R. mucilaginosa control colony on SDA.
The identity of the isolated agent was reconfirmed by sequencing of the ITS1/ITS4 region of rRNA and compared with reference sequences deposited in GenBank at day +83 days. The isolated strains showed not only the typical morphological features of C. parapsilosis and R. mucilaginosa (Fig. 2), but also perfect homology to the D1/D2 sequence of C. parapsilosis and R. mucilaginosa in GenBank. The isolated agents have been added to the collection of Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College in Nanjing, Jiangsu. C. parapsilosis and R. mucilaginosa are under accession number CMCCF 2160013 and CMCCF2160014, respectively.
The in vitro susceptibility of the two strains was determined by using the microdilution method according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) M38A at + 83 days. The minimal inhibitory concentrations (MICs) were defined as the lowest concentration at which no growth occurred which led to results as show in Table 1 and Table 2.
Table 1.
The in vitro drug susceptibility of Rhodotorula mucilaginosa.
| Drug | MIC (μg/ml) | Drug | MIC (μg/ml) |
|---|---|---|---|
| Itraconazole | 0.125 | Nystatin | 1 |
| Fluconazole | 8 | Bifonazole | 0.125 |
| Terbinafine | 0.25 | Econazole | 1 |
| Nafitifine | >4 | ketoconazole | 0.06 |
Table 2.
The in vitro drug susceptibility of Candida parapsilosis.
| Drug | MIC (μg/ml) | Drug | MIC (μg/ml) |
|---|---|---|---|
| Itraconazole | >4 | Nystatin | 1 |
| Fluconazole | 32 | Bifonazole | >4 |
| Terbinafine | >4 | Econazole | 1 |
| Nafitifine | >4 | Ketoconazole | 0.25 |
After mycological confirmation and MIC test, the treatment followed. According to the results of antifungal susceptibility testing in vitro, both the strains were susceptive to ketoconazole. However, since no oral ketoconazole tablets were available in China, the patient was treated with oral itraconazole 200 mg once daily, along with topical terbinafine cream twice per day on the right toenail and topical ketoconazole cream on the left toenail. The condition then improved satisfactorily. After 2 month of treatment of oral therapy with itraconazole, the periungual lesion healed completely (Fig. 1). Subsequently itraconazole therapy was maintained at a dose of 200 mg per day for an additional two months until +5 months. The patient has remained available for follow up. There was no onychomycosis recurrence at the one-year follow-up.
3. Discussion
Onychomycosis is a fungal infection of the nail. Candida species [5] and Rhodotorula species can be the causative agents of onychomycosis in earlier studies. Hay et al. noticed three patterns of nail diseases due to Candida: total dystrophic onychomycosis (mostly seen in chronic mucocutaneous candidiasis); proximal and lateral nail dystrophy (secondary to chronic paronychia); and distal and lateral nail dystrophy, which usually associated with onycholysis, sloughing of the nail with peripheral vascular disease, and finger- and toenail abnormalities [6].
C. parapsilosis, having long been known as a common contaminant in clinical nail specimens, is now emerging as one of the important etiological agents of onychomycosis [7]. Although C. parapsilosis nail infections are commonly reported in association with other fungal pathogens [8,9], there have been some reported cases of onychomycosis due to C. parapsilosis alone, along with an assortment of unusual predisposing conditions, such as minor trauma, or heart disease, or receiving antibiotic drugs over long periods either as an adult or as a child [7,10]. Earlier reports even suggested that C. parapsilosis could be the most prevalent Candida species in both finger- and toenail infections [9].
R. mucilaginosa is a basidiomycetous yeast. Rhodotorula is a pigmented yeast, a normal environmental inhabitant but it can cause opportunistic infections associated with the blood stream, endocarditis, meningitis, peritonitis, and endophthalmitis [[11], [12], [13], [14], [15]]. Rhodotorula infection in immunocompetent persons is extremely rare and its role as causative agent in onychomycosis is long held in doubt, as R. mucilaginosa (rubra) has been reported only once in the literature as causative agent of onychomycosis. The first case of onychomycosis caused by R. mucilaginosa was described by Cunha et al. The patient was immunocompetent, and the onychomycosis affected only the nail of the hallux [16]. Authors suggested that this yeast could be a primary agent of onychomycosis. Another case report showed that nails subject to psoriasis were secondarily infected by R. mucilaginous [17]. Both cases caused by R. mucilaginous appeared in fingernails or toenails of immunocompetent individuals.
The clinical presentation of our case was not typical of those described in the literature [7,17]. Also, onychomycosis due to C. parapsilosis and R. mucilaginous on different toenails in the same patient. In our case, the patient is immunocompetent, but has the habit of playing football and a history of ungula whitlow. In this case, we speculate that unnoticed minor trauma and paronychia may be related to each toenail's infection.
Both isolate agents presented in this case were found to be highly resistant to azoles. Our present isolated strain, R. mucilaginous, also was resistant to several kinds of azole, such as itraconazole and fluconazole; however, it is interesting that the strain is susceptible to ketoconazole. Because no oral preparation of ketoconazole in the domestic market in China, this could be explained fewer strains resistant to this drug. In the present case, although the onychomycosis did not respond well to oral itraconazole alone, we still failed to isolate fungi after stopping all anti-fungal treatment for two months.
In conclusion, we report an onychomycosis case in the an immunocompetent patient, in which C. parapsilosis and R. mucilaginous caused the infection of different toe nails. We speculate that this young adult was affected by two distinct opportunistic agents owing to the patient's outdoor activities and paronychia. One of possibilities to have negative fungal detection until +67 days may be due to the patient who had received antifungal drugs within a year even through two or more months stopping antifungals is recommended. The other possibility is that the yeast R. mucilaginous and C. parapsilosis are naturally resistant to azoles that survived through the abuse of azoles.
Conflict of interest
There are none.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (NM. 81773337 and 81401653) and funded by the Shandong Traditional Chinese Medicine Science and Technology Development Plans, China (NM 2017-415), the Medical and Health Science Technology Project of Shandong Province, China (NM 2017WS345), and the Natural Science Foundation of Shandong Province, China (NM. ZR2015HL127).
References
- 1.Shimoyama H., Satoh K., Makimura K., Sei Y. Epidemiological survey of onychomycosis pathogens in Japan by real-time PCR. Med. Mycol. 2018;0:1–6. doi: 10.1093/mmy/myy096. [Epub ahead of print] PubMed PMID: 30380094. [DOI] [PubMed] [Google Scholar]
- 2.Welsh O., Vera-Cabrera L., Welsh E. Onychomycosis. Clin. Dermatol. 2010;28(2):151–159. doi: 10.1016/j.clindermatol.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Chadeganipour M., Mohammadi R. Causative agents of onychomycosis: a 7-year study. J. Clin. Lab. Anal. 2016;30(6):1013–1020. doi: 10.1002/jcla.21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wirth F., Goldani L.Z. Epidemiology of Rhodotorula: an emerging pathogen. Interdiscip. Perspect. Infect. Dis. 2012;2012:465717. doi: 10.1155/2012/465717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fich F., Abarzúa-Araya A., Pérez M., Nauhm Y., León E. Candida parapsilosis and Candida guillermondii: emerging pathogens in nail candidiasis. Indian J. Dermatol. 2014;59(1):24–29. doi: 10.4103/0019-5154.123485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hay R.J., Baran R., Moore M.K., Wilkinson J.D. Candida onychomycosis–an evaluation of the role of Candida species in nail disease. Br. J. Dermatol. 1988;118(1):47–58. doi: 10.1111/j.1365-2133.1988.tb01749.x. [DOI] [PubMed] [Google Scholar]
- 7.Hosuru S.S., Hamal D., Nayak N., Gokhale S. Onychomycosis due to Candida parapsilosis in a child with ventricular septal defect: an unusual predisposition. Case Rep. Pediatr. 2016;2016 doi: 10.1155/2016/7026068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautret P., Rodier M.H., Kauffmann-Lacroix C., Jacquemin J.L. Case report and review. Onychomycosis due to Candida parapsilosis. Mycoses. 2000;43(11–12):433–435. [PubMed] [Google Scholar]
- 9.Segal R., Kimchi A., Kritzman A., Inbar R., Segal Z. The frequency of Candida parapsilosis in onychomycosis. An epidemiological survey in Israel. Mycoses. 2000;43(9–10):349–353. doi: 10.1046/j.1439-0507.2000.00582.x. [DOI] [PubMed] [Google Scholar]
- 10.Koklu E., Gunes T., Kurtoglu S., Gokoglu S., Koklu S. Onychomycosis in a premature infant caused by Candida parapsilosis. Pediatr. Dermatol. 2007;24(2):155–156. doi: 10.1111/j.1525-1470.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- 11.Duggal S., Jain H., Tyagi A., Sharma A., Chugh T.D. Rhodotorula fungemia: two cases and a brief review. Med. Mycol. 2011;49(8):879–882. doi: 10.3109/13693786.2011.583694. [DOI] [PubMed] [Google Scholar]
- 12.Mohd N.F., Tan L.H., Na S.L., Ng K.P. Meningitis caused by Rhodotorula mucilaginosa in HIV-infected patient: a case report and review of the literature. Mycopathologia. 2015;180(1–2):95–98. doi: 10.1007/s11046-015-9879-0. [DOI] [PubMed] [Google Scholar]
- 13.Kitazawa T., Ishigaki S., Seo K., Yoshino Y., Ota Y. Catheter-related bloodstream infection due to Rhodotorula mucilaginosa with normal serum (1→3)-β-D-glucan level. J. Mycol. Med. 2018;28(2):393–395. doi: 10.1016/j.mycmed.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 14.García-Suárez J., Gómez-Herruz P., Cuadros J.A., Guillén H., Burgaleta C. Rhodotorula mucilaginosa catheter-related fungaemia in a patient with multiple myeloma. Mycoses. 2011;54(4):e214–216. doi: 10.1111/j.1439-0507.2009.01816.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim H.A., Hyun M., Ryu S.Y. Catheter-associated Rhodotorula mucilaginosa fungemia in an immunocompetent host. Infect. Chemother. 2013;45(3):339–342. doi: 10.3947/ic.2013.45.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Cunha C.M.M., dos Santos S.L.P., Dornelas-Ribeiro M., Vermelho A.B., Rozental S. Identification, antifungal susceptibility and scanning electron microscopy of a keratinolytic strain of Rhodotorula mucilaginosa: a primary causative agent of onychomycosis. FEMS Immunol. Med. Microbiol. 2009;55(3):396–403. doi: 10.1111/j.1574-695X.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- 17.Martini K., Müller H., Huemer H.P., Höpfl R. Nail psoriasis masqueraded by secondary infection with Rhodotorula mucilaginosa. Mycoses. 2013;56(6):690–692. doi: 10.1111/myc.12091. [DOI] [PubMed] [Google Scholar]