Abstract
Objective
Chronic inflammation and expression of the TP53 gene are two biomarkers that have been identified as particularly important in the etiology and progression of cancer. While much is known about the determinants of inflammation, there is currently little information regarding the causes of variation in the functioning of TP53, even though it has been recognized for 40 years as the most potent of the cancer suppressor genes. The current paper explores the interrelationship between these two biomarkers and investigates the extent to which they are influenced by the social environment.
Methods
Using structural equation modeling (SEM) and longitudinal observational data from a sample of several hundred African Americans, we tested the hypothesis that adversity – operationalized as racial discrimination- and coping resources – operationalized as religiosity and black friends - influence expression of TP53, for better or worse, through their impact on inflammation.
Results
Correlational analysis showed inflammation and TP53 to be inversely related. Further, discrimination was positively related to inflammation and negatively related to TP53 expression, whereas religiosity and black friends were both negatively related to inflammation and positively related to TP53 expression. Finally, SEM indicated that the effect of the social environmental variables on TP53 expression was indirect through level of inflammation.
Conclusions
In addition to its established contribution to cancer through DNA damage and cell proliferation, inflammation likely increases cancer risk indirectly by inhibiting expression of the TP53 cancer suppressor gene. Hence environmental and stress management interventions may do more than reduce inflammation's cell damaging effects; they may also lessen the chances of cancer by increasing expression of TP53.
Highlights
-
•
Discrimination, religiosity, and black friends showed significant associations with inflammation and TP53.
-
•
The effect of discrimination, religiosity, and black friends on TP53 was indirect through level of inflammation.
-
•
Inflammation likely increases cancer risk indirectly by suppressing the TP53 cancer suppressor gene.
-
•
Environmental and stress management interventions may lessen the chances of cancer by increasing expression of TP53.
1. Introduction
Cancer is one of the leading causes of death worldwide. In the U.S., 32% of persons 75 and older have been diagnosed with cancer and roughly 40% of individuals develop cancer at some point during their lifetime, a probability that is even higher for Black Americans (National Cancer Institute, 2017). Chronic inflammation and expression of the TP53 gene are two biomarkers that have been identified as particularly important in the etiology and progression of cancer. While much is known about the determinants of inflammation, there is currently little information regarding the causes of variation in the functioning of TP53, even though it has been recognized for 40 years as the most potent of the cancer suppressor genes (Cooks, Harris, & Oren, 2014). The current paper explores the impact of social adversity and coping resources on these two biomarkers. Using longitudinal data from a sample of several hundred African Americans, we test the hypothesis that the social environment influences expression of TP53, for better or worse, through their impact on inflammation.
1.1. Chronic inflammation
A profusion of studies over several decades has documented the role of chronic inflammation in a variety of cellular processes (e.g., DNA damage, cell proliferation) that effect the onset and progression of cancer (Korniluk, Koper, Kemona, and Dyumicka-Piekarska, 2017; Shalapour & Karin, 2015). Modern medicine's explanation for inflammation emphasizes the role of exercise, diet, and unhealthy habits such as smoking. While these are all important health risk factors, together they still leave most of the variance in inflammation unexplained. In recent years, several studies have reported a link between exposure to social adversity and inflammation. Findings suggest that social conditions such as loneliness, financial distress, and racial discrimination are associated with increased inflammation and that this relation holds even after controlling for health risk behaviors such as smoking, excess drinking, lack of exercise, BMI, etc. (Fagundes & Way, 2014; Simons et al., 2018; Slavich & Cole, 2013). There is strong evidence that psychosocial stress amplifies risk for cancer and it seems likely that inflammation is an important mediator of this effect (Powell, Tarr, & Sheridan, 2013).
Perhaps the most compelling explanation for this association between social adversity and inflammation has been provided by Steven Cole and his colleagues (Cole, 2014; Slavich & Cole, 2013). The inflammatory program of the innate immune system is comprised of proinflammatory genes that combat injury and tissue damage by fighting infection and promoting new cell growth. Cole argues that a psychological orientation of threat and guardedness leads to increased expression of the inflammatory program as the organism is preparing for possible attack and injury. Presumably, this pattern of gene expression evolved to help adapt molecular physiology to the types of sporadic and transient physical threats that characterized our ancestral environments (Cole, 2014).
If our immune system is wired to produce a heightened inflammatory response to anticipated threat, perceiving the social environment as friendly and supportive should be associated with low inflammation. Consonant with this expectation, recent studies have reported that social support, religious involvement, and eudaimonic happiness are negatively related to inflammation (Ferraro & Kim, 2014; Fredrickson et al., 2015).
1.2. TP53 gene and P53 protein
The TP53 gene is the most powerful and well-known of the cancer suppressor genes. It produces a protein labeled p53 which regulates cell division. When DNA in a cell becomes damaged or mutated, P53 activates various genes to repair the damage (Levine & Oren, 2009). If repair is not possible, it prevents the cell from dividing and signals it to undergo apoptosis (cell death). These activities stop mutated cells from dividing, thereby preventing the development of cancer cells (Levine & Oren, 2009; Ozaki & Nakagawara, 2011). These functions have lead TP53 to be labeled “the guardian of the genome.” Mice deficient in p53 develop spontaneous cancers, and the TP53 gene is dysfunctional in most human cancers (Levine, 2011; Ozaki & Nakagawara, 2011). Indeed, once established, tumors grow unimpeded, at least in part, by fostering PT53 mutations within the cancerous cells (Oliver, Hollstein, & Hainaut, 2010; Rivlin, Brosh, Oren, and Rotter, 2011). Given the importance of this gene in the prevention of cancer, a crucial question concerns the factors that reduce its expression.
Interestingly enough, a potent predictor of TP53 silencing is inflammation. Evidence from laboratory and animal research strongly suggests that expression of inflammatory genes (e.g., NFkb, IL-6) and TP53 expression are inversely related (Brighenti et al., 2014; Gudkov, Gurova, & Komarova, 2011). The current study attempts to replicate this inverse association using observational data with humans. In addition to inflammation, there is some evidence from animal models that social stress (restraint, social defeat) predicts a reduction in P53 (Feng, Liu, Zhang, Wenwei, & Hu, 2012). To our knowledge no one has examined the effect of the social environment on TP53 using humans. While Cole and colleagues argue that the inflammatory response has evolved as a functional reaction to threat and adversity, it is not clear why such perceptions would result in reduced expression of TP53. One possibility, however, is that inflammation, in its attempt to heal injury and grow new tissue, requires the shutting down of genes such as TP53 that arrest growth and induce death (apoptosis) of damaged cells. This suggests a model where social stress fosters inflammation which, in turn, down regulates expression of TP53, whereas the availability of coping resources decreases inflammation and, in turn, up-regulates expression of TP53. This mediational model is tested in the present study.
1.3. The current study
Past research indicates that traditional measures of stress, such as low SES, are weak predictors of health and well-being among African Americans (Walsemann, Goosby& Farr, 2016; Turner, Brown, & Hale, 2017). Presumably this is because upward mobility for African Americans does not necessarily provide relief from race related stressors (Geronimus et al., 2016). Advanced education, for example, often leads to increased discrimination and race-related challenges (Pearson, 2008). And, affluence offers no protection from the burdens of segregation and neighborhood disadvantage (Massey, 2017). Such findings indicate the importance of focusing on race-related stressors and coping resources that are central to the lives of African Americans (Geronimus et al., 2016; Simons et al., 2018; Walsemann, Goosby, & Farr, 2016). Toward this aim, the present study employs discrimination as an indicator of stress and both religiosity and having black friends as indicators of coping resources.
A large body of research has established that exposure to racism is associated with poor health and well-being (Williams, 2012; J.C. Phelan, Link, & Tehranifar, 2010). Importantly for the present study, numerous investigations have also linked experiences of racial discrimination to increased inflammation (Brody, Yu, Miller, & Chen, 2015; Lewis, Aiello, Leurgans, Kelly, & Barnes, 2010; Simons et al., 2018). Turning to coping resources, there is strong evidence regarding the importance of the Black church as an institution in the African American community. African Americans are significantly more religious than the rest of America as more than half attend religious services at least once a week, more than three in four pray daily, and nearly nine-in-ten state that they are “absolutely certain” that God exists (Pew Research Center, 2009). In addition to being a center for worship, however, the black church is a safe haven where African Americans can congregate, socialize and speak freely without fear of persecution. Significantly for the present study, research has linked religiosity to reduced inflammation, decreased risk for cancer, and enhanced health (Ferraro & Kim, 2014; Ironson, Lucette, Hylton, Pargament, & Krause, 2018; Kinney et al., 2003).
Finally, studies suggest that having black friends, especially close black friends, is an important source of social support for African Americans. This research has examined the role of same- and cross-race friends in social support exchanges, and the benefits of these two types of friendship for African Americans' emotional wellbeing. Findings from these studies indicate that African Americans perceive greater intimacy and emotional closeness to black compared to white friends (Aboud, Mendelson, & Purdy, 2003; Kao & Joyner, 2004), and report receiving more of several types of support from their same race friends (Davis & High, 2019). Further, those with intraracial best friends report greater emotional well-being than those with interracial best friends (McGill, Way, & Hughes, 2012). Together, these studies suggest that intraracial friendships are an important source of protection, support, and solidarity in a racially charged society.
We expect that discrimination correlates positively with inflammation and negatively with expression of TP53. Conversely, religious participation and having a social network of black friends is expected to be negatively related to inflammation and positively related to TP53 expression. Finally, it is hypothesized that inflammation will mediate the effect of the three social environmental variables - discrimination, religiosity, and black friends - on expression of TP53. In other words, the effect of social context on the viability of the cancer suppressor gene TP53 is expected to be indirect through level of inflammation.
Changes in the epigenetic regulation (and in turn expression) of genes tend to follow a rather slow, gradual progression. Thus to the extent that the social environment influences these processes, it likely to be chronic, persistent exposure to an adverse or supportive set of conditions, rather than short-term acute events, that exert an effect (Horvath and Raj, 2018). Consonant with this observation, we use longitudinal assessments (from age 18 to 29) of the three social environmental variables to predict inflammatory and TP53 gene expression at age 29.
2. Method
2.1. Sample
Our research utilizes the seven waves of data that have been collected for the Family and Community Health Study (FACHS), an investigation of neighborhood and family processes that contribute to the health and development of African American children (see Gibbons, Gerrard, Cleveland, Wills, & Brody, 2004; Simons et al., 2011 for details). The FACHS sample consists of several hundred African American families living in Georgia and Iowa at the initiation of the study. Each family included a child who was in 5th grade at wave 1 (1997–98). In 2014–2015 when the targets were roughly 29 years of age, a 7th Wave of data was collected that included blood draws. Given the logistics of scheduling visits by phlebotomists, only members of the sample residing in Georgia, Iowa, or a contiguous state were identified as eligible. After also excluding persons who were deceased, incarcerated, or otherwise unreachable, we were left with a pool of 479 individuals, 413 (86%) of whom agreed to be interviewed and to provide blood. Average education for these individuals was 13.1 years (9% < HS, 38% HS/GED, 18% vocational school, 24% 2–3 years of college, 14% college graduate, 2% graduate school). Median income was roughly $25,000 (30% < $13,000, 20% > $36,000, 9% > $52,000). Analyses indicated that those individuals who did not participate in waves 6 and 7 did not differ significantly from those who participated with regard to wave 1 scores on a wide variety of child and caretaker characteristics.
2.2. Procedures
The protocol and all study procedures were approved by the University Institutional Review Board of the University of Georgia (Title: FACHS IV; Protocol # Study00000172). African American university students and community members were trained as field interviewers. Questions were administered in the respondent's home using computer assisted interviewing (see Gibbons et al., 2004). At Wave 7 participants were also asked to provide a blood sample. A certified phlebotomist drew five tubes of blood at each participant's home. Two of the tubes were spun immediately to separate serum into 3 cryo-vials that were then frozen and stored in a −80° freezer until used for the analyses described in the Measures section.
2.3. Measures
2.3.1. TP53 gene expression
All available blood samples were sent to the Rutgers repository for genome-wide transcriptomic analysis. After excluding samples with poor quality (n = 81) and samples with no amplification (n = 3), we were left with a total sample of 379 individuals. Details regarding cleaning, coding, and tests for batch effects are provided in the Online Supplement. Following protocol, TP53 expression scores consist of normalized data that was log2 transformed after quantile normalization.
2.3.2. Elevated inflammation
Using the mRNA data assayed by the Rutgers repository, an inflammatory score was constructed for each respondent by summing the normalized transcriptional data for each of the 19 circulating leukocytes utilized by Cole (2014) and others (Fredrickson et al., 2015) in their CTRA measure of inflammatory response (see Online Supplement).
2.3.3. Racial discrimination
At each wave of data collection (W4-W7), respondents completed 13 items from the Schedule of Racist Events (Landrine & Klonoff, 1996). This instrument has strong psychometric properties and has been used extensively in studies of African Americans (Simons et al., 2018). The items assess the frequency (1 = never, 4 = several times) with which various discriminatory events (racial slurs, hassled by police, disrespectful sales clerks, false accusations by authority figures) have been experienced in the last year because of being African American. Coefficient alpha for the scale was above 0.75 at every wave and scores were summed across waves to form a cumulative measure of perceived discrimination.
2.3.4. Religiosity
At waves 4–7, respondents completed a five-item scale regarding the importance of spiritual beliefs in their daily life (1 = unimportant; 5 = very important) and frequency (1 = never; 5 = daily) of participation in various religious services and personal activities (prayer, meditation). Coefficient alpha was roughly 0.80 at each wave. Scores were averaged across waves to form a composite measure of religiosity across the past several years.
2.3.5. Black friends
At waves 4–7, respondents were asked, “What proportion of your casual friends is African American?” and “What proportion of your close friends is African American?” The response format for each of the items ranged from 1 (10% or less) to 5 (greater than 80%). Finally, a composite measure of the proportion of black friends was created by averaging the scores for the two items across waves.
2.3.6. Control variables: social class and health risk factors
Several variables that have been linked to health were included as statistical covariates: Gender, education (8th grade thru post graduate study), age, weekly income (in dollars), work status (1 = employed), relationship status (married or cohabiting (no, yes)), and health insurance (no, yes). We also controlled for various health risk behaviors assessed at waves 4–7 and summed across waves to form measures of cumulative risk: smoked cigarettes in the past year (0 = no, 1 = yes), alcohol consumption in the past year (0 = never, 5 = every day), diet (two items asked about frequency of fruit and vegetable consumption during the previous 7 days), exercise (two items asked how often in the past 7 days the respondent exercised or participated in physical activity for at least 30 min.).
Finally, Self-reported illness was assessed at wave 7. Respondents were asked (0 = not experienced, 3 = severe symptoms), “In the past 3 months, have you experienced any of the following symptoms?” The list consisted of 18 symptoms, including cough, runny nose, swollen glands, sore throat, fever, asthma or allergies, urinary problems, nausea, diarrhea, dizziness, breathlessness, racing heartbeat, palpitations, or chest pain, and numbness or tingling. Items were summed to form an index of self-reported illness. Cronbach α for the scale was 0.886.
3. Results
At each wave (4–7), the independent variables correlated with inflammation and TP53 in the expected direction. That is, at each wave discrimination showed a positive association with inflammation and a negative association TP53, whereas both religiosity and black friends were negatively related to inflammation and positively related to TP53. While all of these association were in the expected direction, in most cases they only approached statistical significance (p ≤ ,15). When the variables were summed across waves, however, the summary measures achieved statistical significant. This pattern of findings is consistent with the idea that the impact of the social environment on inflammation and TP53 is a cumulative process.
Table 1 presents the zero-order correlation matrix for the study variables. As expected, there is a negative association (−0.213, p ≤ .01) between inflammation and TP53 expression. Also as predicted, inflammation shows a positive association with racial discrimination (0.106, p ≤ .05) and a negative relation with both religiosity (−0.106, p ≤ .05) and intraracial friends (−0.130, p ≤ .05). Further, as anticipated TP53 expression shows a negative association with racial discrimination (−118, p ≤ .05) and a positive relation with both religiosity (0.107, p ≤ .05) and black friends (0.087, p ≤ .10). Two of the control variables – cigarette use and illness – are related to TP53 whereas only one of the controls, illness, shows a significant association with inflammation.
Table 1.
Correlations, means, and standard deviations among the study variables (n = 385).
| Variable or statistic | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. TP53 (Age 29) | ── | ||||||||||||||
| 2. Inflammation (Age 29) | -.213** | ── | |||||||||||||
| 3. Racial discrimination (Age 10–29) | -.118* | .106* | ── | ||||||||||||
| 4. Religiosity (Age 18–29) | .107* | -.106* | -.117* | ── | |||||||||||
| 5. Black friends (Age 18–29) | .087† | -.130* | -.004 | .038 | ── | ||||||||||
| 6. Males | .083 | -.094† | .105* | -.154** | .002 | ── | |||||||||
| 7. Education (Age 29) | -.001 | .024 | .101* | .211** | -.064 | -.074 | ── | ||||||||
| 8. Income (Age 29) | .030 | -.054 | .050 | .060 | -.008 | -.026 | .032 | ── | |||||||
| 9. Married or cohabiting (Age 29) | .038 | .004 | .040 | .059 | -.110* | -.003 | .091† | .101* | ── | ||||||
| 10. Health insurance (Age 29) | -.059 | -.033 | .030 | .027 | -.065 | -.139** | .220** | .050 | .065 | ── | |||||
| 11. Health diet (Ages 21–29) | .048 | .005 | .098† | .169** | -.001 | -.133** | .188** | .011 | .048 | .099† | ── | ||||
| 12. Exercise (Ages 21–29) | -.068 | .034 | .243** | .039 | -.146** | .265** | .162** | .047 | .032 | .069 | .271** | ── | |||
| 13. Alcohol use (Ages 21–29) | -.003 | .008 | .248** | -.232** | -.109* | .178** | .083 | .025 | -.022 | .028 | -.091 | .067 | ── | ||
| 14. Cigarette use (Ages 21–29) | -.105* | -.002 | .128* | -.183** | -.142** | .070 | -.277** | -.035 | -.030 | -.044 | -.090† | .036 | .269** | ── | |
| 15. Illness (Age 29) | -.149** | .167** | .315** | -.066 | -.144** | -.272** | .075 | .034 | .045 | .174** | .024 | .030 | .216** | .100† | ── |
| Mean | 5.657 | .001 | 21.085 | 3.926 | 4.082 | .370 | 13.081 | 6.160 | .283 | .813 | 6.121 | 4.917 | 2.239 | 2.025 | 5.761 |
| SD | .369 | .079 | 5.309 | 1.537 | 1.024 | .485 | 1.723 | 28.648 | .451 | .390 | 1.815 | 1.521 | 1.144 | 1.459 | 6.477 |
†p ≤ .10, *p ≤ .05, **p ≤ .01 (two-tailed test).
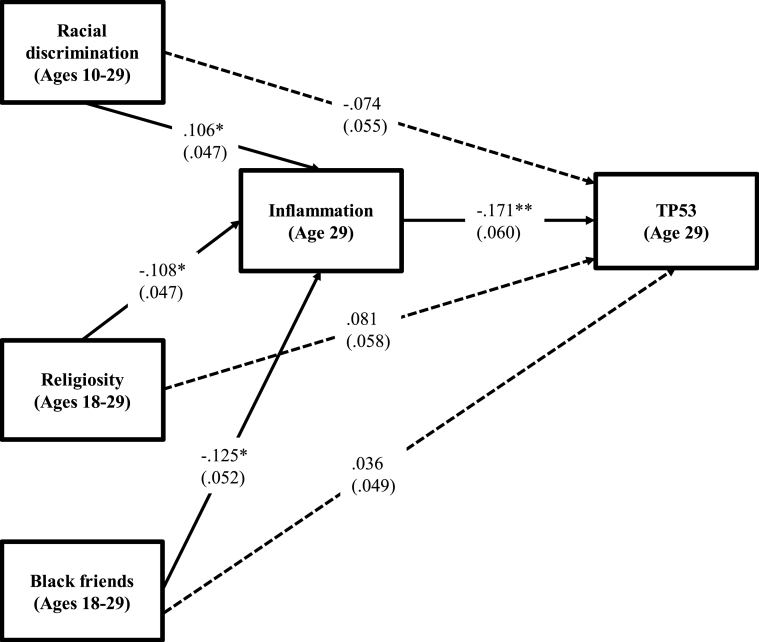
Fig. 1 presents an SEM model designed to test the study hypotheses. The goodness of fit indices all indicate a good fit to the data. The SEM shows rather consistent support for the hypothesized mediational model. Racial discrimination shows a positive association (β = 0.106, p ≤ .05) with inflammation. In contrast, both religiosity (β = −0.112, p ≤ .05) and black friends (β = −0.125, p ≤ .05) are negatively related to inflammation. Inflammation, in turn, is negatively related to TP53 expression (β = −0.189, p ≤ .05). Importantly given the study hypotheses, none of the socioenvironmental variables is significantly related to TP53 once the effect of inflammation is taken into account. The bootstrapping technique in MPlus with 1,000 replications indicated that the mediated effects of racial discrimination (indirect effect = −0.018, 95% CI [-0.048, −0.002]), religiosity (indirect effect = 0.019, 95% CI [0.004, 0.045]), and black friends (indirect effect = 0.021, 95% CI [0.003, 0.054]) on TP53 through inflammation are significant and account for about 19% of the total variance for racial discrimination, about 20% of the total variance for religiosity, and about 36% of the total variance for black friends. Thus the SEM findings provide support for the hypothesis that adversity and coping resources may indirectly reduce expression of TP53 through their impact on inflammation.
Fig. 1.
Effects of racial discrimination, religiosity, and black friends on TP53 gene expression levels through pro-inflammatory response, Note. Chi-square = 9.081, df = 9, p = .436; CFI = 1.000; RMSEA = 0.000. Values are standardized parameter estimates and standard errors are in parentheses. Males, education, married/cohabited, health insurance, healthy diet, exercise, alcohol use, cigarette use, and illness are controlled in these analyses. **p ≤ .01; *p ≤ .05; †p ≤ .10 (two-tailed tests), n = 385.
4. Discussion
Prior laboratory and animal research has documented a negative relation between inflammation and expression of the critical TP53 cancer suppressor gene. Findings from the present study corroborated this inverse association using observational data. Further, past research has established that for African Americans adverse social experiences such as racial discrimination increase inflammation whereas coping resources such as religiosity and black friends decrease inflammation. These associations were also evident in the present study. Further, our correlational analysis showed that discrimination was negatively related and both religiosity and black friends positively related to TP53 expression. SEM indicated, however, that the effect of these social environmental variables on TP53 was indirect through level of inflammation. These findings suggest that in addition to its established contribution to the onset and progression of cancer through DNA damage and cell proliferation (Korniluk, Koper, Kemona, & Dymicka-Piekarska, 2017; Shalapour & Karin, 2015), inflammation likely increases risk of cancer indirectly by suppressing expression of the TP53 cancer suppressor gene. Although the study sample is still quite young (only 29 years of age), the elevated inflammation and reduced TP53 expression manifested by many of these individuals signals they may be at significant risk for developing cancer later in life.
Importantly, our analyses controlled for a variety of life style factors thought to influence inflammation, and perhaps TP53. Our findings indicated that the social environmental variables included in our study exerted more of an effect on inflammation and TP53 than traditional lifestyle factors such as diet, exercise, and substance use. Only illness and smoking showed associations with the study outcomes. The marginal impact of these lifestyle variables might be attributable, at least in part, to the well-known limitations of using self-reports to measure them. And, it may be that the health consequences of these lifestyle behaviors take a while to build up and will become more evident by the time our respondents enter middle age.
While a strength of the present study was its use of longitudinal data to predict inflammation and TP53, it also contained some important limitations. First, our sample was exclusively African American. In one sense this should be considered a strength given the high rates of disadvantage and poor health, including cancer, suffered by this population (Geronimus et al., 2016; Simons et al., 2016; Williams, 2012). Still, it is essential that our findings regarding the indirect effect of the social environment on TP53 through inflammation be replicated with more ethnically and racially diverse samples. Second, support for our causal arguments would be more compelling if we had had longitudinal assessments of inflammation and TP53 expression. This would have enabled us to examine whether changes in the social environment are associated with alterations in inflammation and TP53. Such data would also facilitate examination of the causal priorities operating in the relationship between inflammation and TP53 expression. Unfortunately, our data set does not contain multiple assessments of these biomarkers. Hopefully, such assessments can be obtained in the coming years.
Past studies point to the importance of reducing inflammation to stave off chronic illness, and findings from the present study provide further evidence that a social environment characterized by low stress and access to coping resources is an avenue for achieving this effect. Past research has shown that meditation, cognitive-behavioral stress management, and gratitude exercises can reduce inflammation in adults, often countering the inflammatory effects of life adversities (Cole, 2014). Given its damaging effects on DNA and promotion of cell proliferation, such reductions in inflammation should decrease a person's chances of developing cancer. Findings from the present study suggest that decreasing inflammation also likely leads to increased expression of the cancer suppressor gene TP53, thereby further decreasing a person's chances of cancer. Future studies need to examine the extent to which reductions in inflammation effected by various stress reduction interventions have the consequence of increasing TP53.
Ethics statement
None of the authors have any conflicts of interests to declare, and none of the findings in this paper have been published elsewhere. The order of authorship reflects the relative level of contribution make by each of the authors. Informed consent was obtained from all of the study respondents, and IRB approval was obtained for all aspects of the project.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute (R01 HL118045), the National Institute of Child Health and Human Development (R01 HD080749), the National Institute on Aging (R01 AG055393), the National Institute on Drug Abuse (R21 DA034457, R01 DA021898) and the National Institute of Mental Health (R01 MH62699, R01 DA018871, R01 MH62666, R01 MH062668). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2019.100389.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aboud F.E., Mendelson M.J., Purdy K.T. Cross-race peer relations and friendship quality. International Journal of Behavioral Development. 2003;27:165–173. [Google Scholar]
- Brighenti E., Calabrese C., Giguori G., Giannone F.A., Trere D., Montanaro L. Interleukin 6 downregulates p53 expression and activity by stimulating ribosome biogensis: A new pathway connecting inflammation to cancer. Oncogene. 2014;33:4396–4406. doi: 10.1038/onc.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G.H., Yu T., Miller G.E., Chen C. Discrimination, racial identity, and cytokine levels among African-American adolescents. Journal of Adolescent Health. 2015;56:496–501. doi: 10.1016/j.jadohealth.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S.W. Human social genomics. PLoS Genetics. 2014;10(8) doi: 10.1371/journal.pgen.1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooks T., Harris C.C., Oren M. Caught in the cross fire: P53 in inflammation. Carcinogenesis. 2014;35:1680–1690. doi: 10.1093/carcin/bgu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S.M., High A.C. Widening the gap: Support in same race versus different race female friendship dyads. Journal of Social and Personal Relationships. 2019;36:187–213. [Google Scholar]
- Fagundes C.P., Way B. Early-life stress and adult inflammation. Current Directions in Psychological Science. 2014;23:277–283. [Google Scholar]
- Feng Z., Liu L., Zhang C., Zheng T., Wang J., Lin M. Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proceedings of the National Academy of Sciences. 2012;109:7013–7018. doi: 10.1073/pnas.1203930109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro K.F., Kim S. Health benefits of religion among black and white older adults? Race, religiosity, and C-reactive protein. Social Science & Medicine. 2014;120:92–99. doi: 10.1016/j.socscimed.2014.08.030. [DOI] [PubMed] [Google Scholar]
- Fredrickson B.L., Grewen K.M., Algoe S.B., Firestine A.M., Arevalo J.M.G., Ma J. Psychological well-being and the human conserved transcriptional response to adversity. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0121839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus A.T., James S.A., Destin M., Graham L.F. Jedi public health: Co-creating an identity-safe culture to promote health equity. Social Science and Health-Population Health. 2016;2:105–116. doi: 10.1016/j.ssmph.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons F.X., Gerrard M., Cleveland M.J., Wills T.A., Brody G. Perceived discrimination and substance use in African American parents and their children: A panel study. Journal of Personality and Social Psychology. 2004;86:517–529. doi: 10.1037/0022-3514.86.4.517. [DOI] [PubMed] [Google Scholar]
- Gudkov A.V., Gurova K.V., Komarova E.A. Inflammation and p53: A tale of two stresses. Genes & Cancer. 2011;2:503–515. doi: 10.1177/1947601911409747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S., Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews: Genetics. 2018;19:371–384. doi: 10.1038/s41576-018-0004-3. [DOI] [PubMed] [Google Scholar]
- Ironson G., Lucette A., Hylton E., Pargament K.I., Krause N. The relationship between religious and psychospiritual measures and an inflammation marker (CRP) in older adults experiencing life event stress. Journal of Religion and Health. 2018 doi: 10.1007/s10943-018-0600-8. [DOI] [PubMed] [Google Scholar]
- Kao G., Joyner K. Do race and ethnicity matter among friends? Activities among interracial, interethnic, and intraethnic adolescent friends. The Sociological Quarterly. 2004;45:557–573. [Google Scholar]
- Kinney A.Y., Bloor L.E., Dudley W.N., Millikan R.C., Marshall E., Martin C. Roles of religious involvement and social support in the risk of colon cancer among Blacks and Whites. American Journal of Epidemiology. 2003 doi: 10.1093/aje/kwg264. [DOI] [PubMed] [Google Scholar]
- Korniluk A., Koper O., Kemona H., Dymicka-Piekarska V. From inflammation to cancer. International Journal of Medical Sciences. 2017;186:57–62. doi: 10.1007/s11845-016-1464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrine H., Klonoff E.A. The schedule of racist events: A measure of racial discrimination and a study of its negative physical and mental health consequences. Journal of Black Psychology. 1996;22:144–168. [Google Scholar]
- Levine A.J. Introduction: The changing directions of p53 research. Genes and Cancer. 2011;2:382–384. doi: 10.1177/1947601911413463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A.J., Oren M. The first 30 years of p53: Growing ever more complex. Nature Reviews Cancer. 2009;9:749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T.T., Aiello A.E., Leurgans S., Kelly J., Barnes L.L. Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain, Behavior, and Immunity. 2010;24:438–443. doi: 10.1016/j.bbi.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey D.S. Why death haunts black lives. Proceedings of the National Academy of Sciences. 2017;114:800–802. doi: 10.1073/pnas.1620083114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill R.K., Way N., Hughes D. Intra- and interracial best friendships during middle school: Links to social and emotional well-being. Journal of Research on Adolescence. 2012;22:722–738. [Google Scholar]
- National Cancer Institute, National Institutes of Health . 2017. Cancer statistics.www.gov/about-cancer/understanding/statistics [Google Scholar]
- Oliver M., Hollstein M., Hainaut P. TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harbor Perspectives on Biology. 2010;2:a001008. doi: 10.1101/cshperspect.a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki T., Nakagawara A. Role of p53 in cell death and human cancers. Cancers. 2011;3:994–1013. doi: 10.3390/cancers3010994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J.A. Can't buy me whiteness: New lessons from the titanic on race, ethnicity, and health. Du Bois Review. 2008;5:27–48. [Google Scholar]
- Pew Research Center . 2009. A religious portrait of African Americans.http://pewforum.Org/2009/1/30/a-religious-portrait-of-african-americans/ [Google Scholar]
- Phelan J.C., Link B.G., Tehranifar P. Social conditions as fundamental causes of health inequalities: Theory, evidence, and policy implications. Journal of Health and Social Behavior. 2010;51:S28–S40. doi: 10.1177/0022146510383498. [DOI] [PubMed] [Google Scholar]
- Powell N.D., Tarr A.,J., Sheridan J.F. Psychosocial stress and inflammation in cancer. Brain, Behavior, and Immunity. 2013;30:541–547. doi: 10.1016/j.bbi.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Rivlin N., Brosh R., Moshe O., Rotter V. Mutations in the p53 tumor suppressor gene: Important milestones at the various steps of tumorigenesis. Genes and Cancer. 2011;2:466–474. doi: 10.1177/1947601911408889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalapour S., Karin M. Immunity, inflammation, and cancer: An eternal fight between good and evil. Journal of Clinical Investigation. 2015;125:3347–3355. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R.L., Lei M.-K., Beach S.R.H., Barr A.B., Simons L.G., Gibbons F.X. Discrimination, segregation, and chronic inflammation: Testing the weathering explanation for the poor health of Black Americans. Developmental Psychology. 2018;54:1993–2006. doi: 10.1037/dev0000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R.L., Lei M.K., Beach S.R.H., Brody G.H., Philibert R.A., Gibbons F.X. Social environment, genes, and aggression: Evidence supporting the differential susceptibility perspective. American Sociological Review. 2011;76:883–912. doi: 10.1177/0003122411427580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R.L., Lei M.-K., Beach S.R., Philibert R., Cutrona C., Gibbons F.X., Barr A. Economic hardship and biological weathering: the epigenetics of aging in a U.S. sample of black women. Social Science and Medicine. 2016;150:191–200. doi: 10.1016/j.socscimed.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G.M., Cole S.W. The emerging field of human social genomics. Clinical Psychological Science. 2013;1:331–348. doi: 10.1177/2167702613478594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R.J., Brown T.N., Hale W.B. Race, socioeconomic position, and physical health: A descriptive analysis. Journal of Health and Social Behavior. 2017;58:23–36. doi: 10.1177/0022146516687008. [DOI] [PubMed] [Google Scholar]
- Walsemann K.M., Goosby B.J., Farr D. Life course SES and cardiovascular risk: Heterogeneity across race/ethnicity and gender. Social Science & Medicine. 2016;152:147–155. doi: 10.1016/j.socscimed.2016.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.R. Miles to go before we sleep: Racial inequities in health. Journal of Health and Social Behavior. 2012;53:279–295. doi: 10.1177/0022146512455804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.