Abstract
The global disease burden of end stage renal disease (ESRD) is growing rapidly, and organ transplantation remains the gold standard for improving both the quality and quantity of life particularly in young adults. It is therefore critical to understand the effect of ESRD and renal transplantation on male fertility. Many men in renal failure exhibit subfertility or infertility due to several factors including hypogonadism, erectile dysfunction (ED), and direct impairment of spermatogenesis with spermatotoxicity and late stage maturational arrest causing oligospermia or azoospermia. Kidney transplantation has been shown to rescue some—but not all—of these defects, with normalization of reproductive hormonal parameters, improvement in semen parameters (sperm count, motility, morphology), and partial restoration of erectile function. This improvement in fertility is sufficient for a subset to father children either naturally or with assisted reproduction. In this review, we summarize the literature regarding fertility in men with chronic kidney disease (CKD) and on dialysis, and we describe the changes associated with renal transplantation.
Keywords: Infertility, chronic kidney disease (CKD), end stage renal failure, transplant, hypogonadism
Introduction
With the rising prevalence of end stage renal disease (ESRD), over 100,000 Americans are now currently listed for kidney transplant. Approximately 13,000 patients received a deceased donor organ in 2016, and half of these recipients had been on dialysis for >5 years (1). Men and women of childbearing age account for nearly 40% of the potential renal transplant recipients (1). It is now well-established that both men and women with chronic kidney disease (CKD) have significant fertility and hormonal deficits associated with uremia, chronic inflammation, and changes in reproductive hormone levels. Taken together, the literature suggests that the paucity of donor organs and the prolonged wait time predisposes young adults with ESRD to a multitude of pathological reproductive changes associated with hemodialysis (HD).
Even without the complications associated with ESRD, approximately 15% of all couples worldwide experience infertility (defined as the inability to conceive after 12 months of timed intercourse). Roughly half of these cases can be attributed to male factors (2). While the causes of male infertility have been well defined from an epidemiology perspective in the general population (3), the ESRD population clearly represents a more challenging cohort. This is primarily due to the fact that male infertility in the dialysis population is multifactorial and often related to underlying comorbidities such as hypertension and diabetes, as well as specific changes associated with CKD and HD. In this brief review, we discuss the association of male infertility with CKD and dialysis. We explore the changes in reproductive hormones, semen quality, erectile dysfunction (ED), and paternity with renal transplantation.
ESRD and reproductive hormones
Hormonal aberrations in ESRD
The hormonal changes associated with ESRD are profound and have significant consequences on a variety of men’s health issues, but the precise physiology underlying changes in the hypothalamic-pituitary-gonadal (HPG) axis remains relatively poorly understood. Testosterone, for example, has been shown to be decreased in ESRD (Figure 1), and the prevalence of hypogonadism in HD-dependent men may surpass 50% (4). The levels of testosterone are inversely related to pro-inflammatory markers such as IL-6, implicating chronic inflammation as a potential contributing factor (5). As expected, the changes in testosterone are accompanied by changes in luteinizing hormone (LH). Mechanistically, there appears to be a disruption of the normal cyclic GnRH release pattern (6). This aberration in turn is thought to lead to decreased testosterone (T) levels via a blunted LH secretory burst, which in turn predisposes CKD men to chronically elevated LH and hypogonadism (hypergonadotropic hypogonadism). This theory is supported by an improvement in all parameters when ESRD patients are administered clomiphene, which improves hypogonadism by acting centrally to stimulate follicle stimulating hormone (FSH) and LH (6). The diminished T production is also likely further exacerbated by Leydig cell dysfunction, resulting in the profound symptomatic hypogonadism seen in many male ESRD patients.
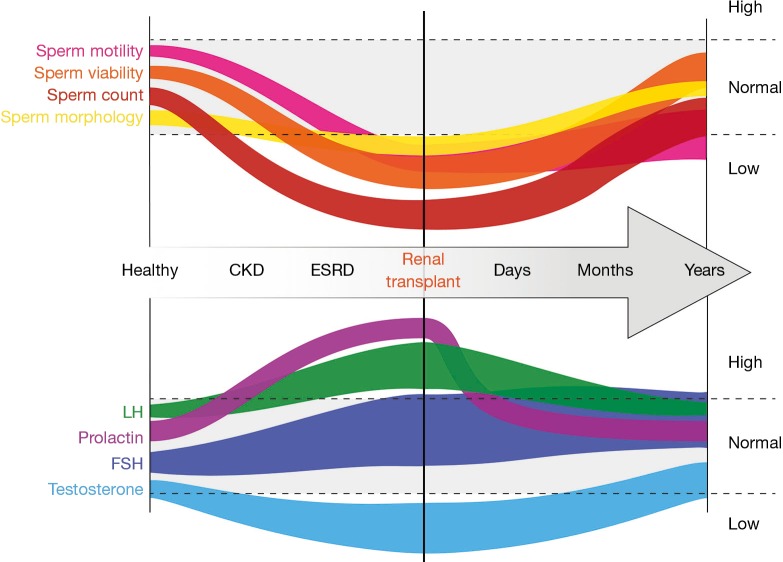
Figure 1.
Graphical representation of hormonal and seminal parameter changes with end stage renal disease and renal transplantation. Note that the width of the graphical elements represents the variability reported in the literature for each respective variable. The gray areas and dotted lines represent the range of values seen in healthy fertile males.
In addition to the above changes, prolactin is also elevated in CKD. This elevation is predominately thought to be due to a loss of the negative feedback mechanism and a modest decrease in renal clearance rate. More recent work has also demonstrated diminished anti-Müllerian hormone (AMH), suggesting a Sertoli cell defect in addition to the Leydig cell suppression (7).
Hormonal changes with transplantation
A growing body of literature has suggested that renal transplantation has a significant effect in rescuing the eugonadal phenotype in at least a subset of recipients. Early work by Lim and colleagues (8) showed that transplantation resulted in a doubling in plasma testosterone levels. More detailed analysis showed an improvement in testosterone accompanied by a decrease in LH, FSH, and prolactin (Figure 1) (9). A large contemporary series by Reinhardt and colleagues published in 2018 showed that of the 40% of ESRD patients with documented hypogonadism, less than half remained so after a year following transplant. The mean testosterone was significantly improved as early as 3 months postoperatively. Interestingly, while FSH and LH remained unchanged, estrogen decreased and prolactin robustly decreased immediately after transplant (4). This is in contrast to Hamdi and colleagues, who noted a significant change in both LH (decreased) and FSH (increased) at 6 months following transplant (10). Finally, Prem et al. showed resolution of hypogonadism and elevated LH in the majority of patients undergoing transplantation, but in this cohort FSH levels remained aberrant (11). It remains to be seen whether these discrepancies represent differences in the underlying etiologies of ESRD, the immunosuppressive regimen employed, or other subtle differences in these populations. It also remains to be proven whether long-term support with a functional renal transplant graft could allow hormone levels to normalize, or whether this population is likely to be hypogonadal long-term.
ESRD and spermatogenesis
Congenital genitourinary anomalies and ESRD/infertility
Many young patients develop CKD at a young age secondary to congenital anomalies which may also predispose to male infertility by distinct mechanisms. For example, autosomal dominant polycystic kidney disease is associated with seminal vesicle cysts, megavesicles, and asthenozoospermia (12). Prune belly syndrome is associated with near-universal refractory male infertility due to undescended testicles and prostatic hypoplasia (13), and paternity is only achieved in approximately 5.0% of patients with exstrophy due to sexual dysfunction and abnormal genitalia (14). Posterior urethral valves may confer increased risk of ED and/or ejaculatory dysfunction (15). Congenital unilateral or bilateral absence of the vas deferens (CUAVD or CBAVD, respectively) is identified in 1% of men undergoing infertility workup (10% of those with azoospermia) and predisposes men to renal insufficiency later in life due to the associated prevalence of solitary kidney (16). Interestingly, while 42% of men with CUAVD are found to have a solitary kidney, this rate falls to only 18% of men with CBAVD (16). Presumably this is due to the fact that if the underlying mechanism bilaterally were to predispose to bilateral renal agenesis, then the developing embryo would have been nonviable. While management of these cases from a CKD perspective can be challenging, management of the infertility component is often equally challenging and requires careful patient-tailored management and in some cases assisted reproduction.
ESRD and impaired spermatogenesis
In addition to the association between a common congenital cause for ESRD and infertility, uremia itself strongly influences a man’s ability to conceive. The mechanisms behind this are multifactorial and include (but are not likely limited to) direct effects on spermatogenesis, ED, and hormonal imbalances. Semen analysis in men with advanced CKD demonstrates decreased volume and oligoasthenozoospermia (17), and testicular pathology can demonstrate Sertoli cell atrophy (6). Xu and colleagues further characterized this phenomenon in patients on HD and noted a roughly 50% decrease in sperm viability, motility, concentration, and normal morphology in ESRD patients compared to controls (18). Men on long-term dialysis have also been shown to have diminished testicular volume that continues to decrease with further time on HD. Biopsy of testicles from these patients has shown increased fibrosis with decreased germ cell proliferation (19). Further mechanistic work demonstrated that sperm motility and normal morphology were the two basic semen parameters most affected by uremia (20). The decrease in motility was directly correlated with the duration of HD. Ultrastructural analysis has shown significant morphological changes including a lack of acrosome with both head and tail abnormalities (21). Biochemical analysis has also shown a downregulation of the cystic fibrosis transmembrane conductance regulator (CFTR) gene below levels found in both healthy and in infertile men (22). Taken together, the data suggests profound changes in spermatogenesis and sperm function in men with CKD.
Structural reproductive consequences of renal transplantation
The potential for reproductive consequences from transplant starts at the time of operation as the retroperitoneal exposure can result in damage to the spermatic cord structures including the vas deferens and testicular blood supply (23). This operation can result in injury to the vas deferens (24) and predispose an already-subfertile man to further decline in fertility. If the man desires future fertility, care must be taken to mobilize the spermatic cord and preserve the testicular bloody supply and vas deferens. It is thus important to address the patient’s fertility concerns preoperatively among the litany of other preoperative variables that are considered prior to renal transplantation.
Following transplantation, two groups (11,25) have characterized the changes in testicular architecture via testicular biopsy. Rodrigues Netto biopsied the testicles of 9 men before and renal transplant. The group quantitatively showed a robust increase in the number of spermatogonia, spermatocytes, spermatids, and spermatozoa, but no change in the number of Sertoli cells was appreciated (25). Prem and colleagues later studied a cohort of 19 young men who underwent living donor renal transplantation; a subset of these men underwent testicular biopsy before and after transplantation with azathioprine-based immunosuppression (11). Pretransplant testicular biopsy revealed late stage maturation arrest and decreased spermatogenesis. While post-transplant testicular biopsy showed improvement in spermatogenesis in a small portion of these patients, the majority continued to demonstrate signs of late stage maturational arrest (11). This data suggests that while some functional recovery may occur with reversal of uremia, the underlying fibrosis and abnormal testicular architecture likely remains and will permanently affect fertility.
Semen quality following renal transplantation
Motivated by early reports of improvement in the hormonal panels of ESRD patients who received a kidney transplant, Lim and colleagues assessed semen parameters before and after transplant (8) in a small cohort of young men. As expected, they saw oligoasthenospermia and azoospermia in the pretransplant population with a gradual improvement in sperm count and motility over the course of ~12 months following transplant (Figure 1). Subsequent studies showed a statistically robust improvement in semen density, but this effect appeared to be limited to a subset of patients with a significant portion of men remaining azoospermic (11). Interestingly, sperm motility in this study diminished significantly following transplantation. Follow-up work by Akbari et al. re-demonstrated the improvement in count, albeit with only modest improvements in motility and normal morphology (9). They found that less than half of dialysis patients had normal semen analysis, whereas this improved to almost 75% after transplant (9). At the 2-year mark, their data suggested that transplant patients demonstrate little-to-no difference in basic semen parameters when compared to healthy age-matched controls.
An elegant study by Eid and colleagues stratified young male renal transplant recipients into fertile and infertile categories and compared these groups to each other and to fertile and infertile patients in the general population (26). They found that the fertile transplant group had sperm concentration and computer-aided sperm analysis (CASA) parameters that compared favorably to the fertile controls, supporting the possibility for complete spermatogenic recovery in some cases. In stark contrast, the infertile transplant cohort exhibited oligospermia and diminished motility that was strikingly similar to the infertile non-transplant control group. Interestingly, the transplant infertile group demonstrated poor flagellar coordination compared to infertile controls, suggesting fundamental mechanistic differences in sperm function. The group postulated that some of the effects may be related to a significant difference in cyclosporine levels, which may be gonadotoxic or spermatotoxic in a dose-dependent fashion (26).
ED in renal failure and transplant
ESRD and ED
While an in-depth discussion on this topic is outside the scope of this review, it is important to acknowledge that the dramatic effect of ESRD on ED is now well documented and related both to the underlying etiology of CKD (e.g., diabetes or hypertension) as well as direct effects of uremia, particularly on the nervous system. Psychogenic ED in the setting of high levels of anxiety and depression, decreased activity, and poor body image associated with patients initiating dialysis is likely also a key driver in this phenomenon (27). Finally, polypharmacy with medications known to impair erections (beta blockers, diuretics, neuromodulators) likely contributes in many cases as well. When combined, these synergistic effects result in a >50% prevalence of ED in the ESRD population (28). This suggests that, even in the absence of changes in hormones and semen quality, a significant proportion of men with ESRD may be unable to conceive due to ED alone.
ED and renal transplant
In parallel with the improvement in hormonal parameters, erectile function also appears to improve with transplantation in a subset of patients (9). The degree of improvement, however, is widely variable in the literature depending on the cohort studied and analysis method, and the degree of reported improvement ranges from no change to improvement in 75% of patients. The most comprehensive study to date (including IIEF, Doppler ultrasound, cavernosometry, and Rigiscan endpoints) supports improvement in the majority (>50%) of patients (29). In stark contrast, a large cohort from Europe was found to have worse—not better—ED via IIEF in young men undergoing transplantation (30). Further work will be needed to better characterize this important aspect of men’s reproductive health as it relates to renal dysfunction.
The effect of immunosuppression on male fertility
In addition to the rapid and profound physiological changes associated with renal transplantation and clearance of uremia itself, the selection of an immunosuppression regimen likely plays a critical role in the recovery of male fertility following renal transplant. Numerous drugs and regimens are now available, and each agent has a side effect profile that must be considered when making this decision.
Calcineurin inhibitors (CNIs)
Representing the backbone of most modern immunosuppressive regimens, CNIs modulate immunity via blocking the nuclear factor of activated T cells (NFAT) dephosphorylation and interrupting IL-2 synthesis. The two main agents in this class in the modern era include cyclosporine (CsA) and tacrolimus. Data from the basic science literature suggests that in a solitary kidney rodent model, tacrolimus exerts minimal detrimental effects on spermatogenesis (31). This is in contrast to regimens containing cyclosporine or sirolimus, both of which caused oligospermia with diminished motility and morphology, decreased testosterone levels, and altered testicular architecture on histological analysis (31). In humans, however, small studies have shown relatively normal semen parameters and successful paternity on male renal transplant recipients on a cyclosporine regimen (18,21,26,32). Another larger contemporary study showed successful paternity in 212 male transplant recipients on a CsA regimen showed relatively normal paternity at most doses. It appears the effects of cyclosporine on sperm parameters occurs in a dose dependent fashion (33). For an excellent more detailed summary of this literature, the reader is directed towards a recent review article by Georgiou and colleagues (34). Despite the widespread use of tacrolimus, there is a paucity of data in the literature on its effects on male reproductive health. Animal studies on relatively high doses of tacrolimus have shown testicular changes including cell death and diminished numbers of Sertoli cells and spermatocytes (35). A small comparative study between cyclosporine and tacrolimus, however, found no differences in hormonal parameters between transplant recipients receiving each regimen (36). While early studies are promising and suggest that paternity is certainly possible on CNIs, further work will be required to fully elucidate whether therapeutic doses of tacrolimus suppress male fertility to a meaningful degree. Interestingly, specific isoforms of calcineurin in sperm have been proposed as a candidate target for a male infertility pill (37), further suggesting this pathway may plan an important role in male infertility.
mTOR inhibitors
Sirolimus, everolimus, and temsirolimus are inhibitors of the mammalian target of rapamycin (mTOR) and PI3K pathways and thus suppresses immunity as well as neoplastic processes. An anti-HPG effect has also been described (38), resulting in modest levels hypogonadism. In theory, both of these effects could have significant deleterious effects on spermatogenesis, and indeed this has been seen in practice. Patients receiving mTORs such as sirolimus appear to have diminished sperm counts, poorer motility, and significantly decreased spontaneous pregnancy rates with unknown additional risk of birth defects (39). Whether these changes are reversible remains controversial (40), but based upon this data, patients should be counseled on the risks of mTOR-based regimens on male fertility.
Antimetabolites
Mycophenolate is a reversible noncompetitive inhibitor of purine synthesis, which exerts its immunosuppressive effect by inhibiting DNA synthesis in lymphocytes. While mycophenolate appears to be teratogenic during pregnancy (41), the effect on spermatogenesis and paternity is less clear. Studies in animal models have demonstrated a decreased sperm count and motility, but this has not been studied in humans. The National Transplantation Pregnancy Registry (NTPR) recently published paternity data on 152 male transplant patients maintained on mycophenolic acid-based medications (42). The rates of spontaneous abortion, prematurity, live births, and structural anomalies were all similar to the general population (42). This finding has been confirmed with another large cohort from Norway, which also found no evidence of adverse events in the setting of mycophenolate (43).
Adolescents and renal transplant
There is clear evidence that renal transplantation is the treatment of choice for young patients with ESRD from the perspectives of cost, quality of life, and mortality. Based upon the above literature, there is now increasing evidence that transplant also confers an improvement in male fertility before and during the window of fatherhood. The development of uremia during the critical window of childhood or puberty, however, appears to have profound and potentially irreversible effects on testicular health and semen quality even decades later. A recent study by Tainio and colleagues examined a small cohort of 24 male patients with ESRD secondary who underwent renal transplantation at an average of 10 years of age and compared them to healthy age-matched controls (44). The group evaluated these young men for the next ~20 years and reported testicular size, endocrine function, and semen analyses. They found a striking 3-fold decrease in average testicular size compared to healthy male controls. This was accompanied by a lower testosterone level (322 vs. 399 pmol/L, respectively), higher LH (7.6 vs. 3.3 IU/L), and equivalent levels of FSH and inhibin B. Semen quality mirrored these changes. The transplant recipients demonstrated a 100-fold decrease in sperm count, with 28% of the patients demonstrating azoospermia. Of note, the majority of their cohort was on a cyclosporine-based regimen with or without mycophenolate. Taken together, this data suggests that either adolescent uremia or long-term immunosuppression (or both) provokes a significant and precipitous decline in male fertility. This group should be counseled accordingly and aggressively managed with urological consultation if paternity is desired.
Paternity following renal transplantation
Spontaneous pregnancy outcomes
Successful paternity during dialysis is challenging, with spontaneous pregnancy rates being decreased by at least 50% and possibly even more (45). Despite the physiologic challenges associated with ESRD and renal transplantation, paternity rates appear to improve significantly following transplant. One large study identified over 200 successful spontaneous pregnancies following renal transplant in the male partner (33). The pregnancies occurred between 1 to 16 years after transplant, and the data suggested that partners who conceive within 2 years of transplant are at risk for modestly decreased birth weights and premature delivery, which occurred in 15% of patients in this cohort (33). This study, however, unfortunately did not provide the incidence of pregnancy or the total number of male transplants during the study period, and thus the actual paternity rate cannot be calculated. Another fascinating population-based retrospective study examined almost 500 children fathered by recipients of solid organ transplants. The majority of men were on a triple immunosuppression regimen including steroids, tacrolimus/cyclosporine, and mycophenolate/azathioprine. Compared to children fathered by this same group prior to transplant, the rates of major malformations and preterm delivery were not significantly different. Surprisingly, however, the odds ratio for developing preeclampsia was 7.4, suggesting a strong linkage between male immunosuppression and this condition (46). While the mechanism underlying this effect and preeclampsia in general is not fully elucidated, the data is tantalizing and suggests that further studies are necessary.
Assisted reproduction
Despite the improvement in semen parameters seen in most patients, some men will persistently demonstrate oligoasthenozoospermia following transplant. If unable to conceive naturally, these men may benefit from additional workup and management including the use of intracytoplasmic sperm injection (ICSI). Two small case series have demonstrated feasibility for this approach. The first showed successful paternity in 2 women whose partners had prior kidney transplants without subsequent recovery in fertility (47); unfortunately the third couple in this report were unable to sustain a pregnancy despite transfer of multiple embryos. The second report described 8 couples who pursued ICSI with resulting successful pregnancies in 4 couples and live births in 3 (48). In addition to these early reports, successful ICSI has been reported in a variety of unique male transplant recipient patient populations including cystinosis (49), hyperoxaluria with cryptorchidism (50), and oligospermic renal transplant male recipient with female liver transplant recipient (51). Taken together, this body of literature suggests that ART is a viable option for couples with male infertility following renal transplant, albeit with mixed success.
Conclusions
It is clear that ESRD exerts profound suppressive effects on male endocrine function, ED, spermatogenesis, resulting in significant reduction in conception rates for men with ESRD. While renal transplantation appears to reverse at least a portion of these changes, subfertility likely represents the rule rather than the exception in this population. The ongoing suppression in spermatogenesis and fertility is likely related to chronic structural changes in the testis, subtle residual perturbations in the HPG axis, and immunosuppression (though the latter still remains poorly studied, particularly with regards to modern regimens based upon tacrolimus). Ultimately, however, many of these men will successfully go on to father healthy offspring either through natural conception or with the assistance of ART. The reproductive outcomes appear to be worse for pediatric and adolescent transplant recipients—the explanation for this is multi-factorial as described above and is related to the underlying disease and its impact on the HPG axis and testis during puberty.
From humble beginnings with little clinical utility, the field of renal transplant has grown immensely in the past half-century and now represents the gold standard for improving the quality and quantity of life in young patients with renal failure. It is now time for the field to further refine this craft to improve secondary outcomes such as male infertility in order to maximize the urological care we as a field can offer our patients.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2016 Annual Data Report: Kidney HHS Public Access. Am J Transplant 2018;18:18-113. 10.1111/ajt.14557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal A, Mulgund A, Hamada A, et al. A unique view on male infertility around the globe. Reprod Biol Endocrinol 2015;13:37. 10.1186/s12958-015-0032-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubin L, Amelar RD. Etiologic factors in 1294 consecutive cases of male infertility. Fertil Steril 1971;22:469-74. 10.1016/S0015-0282(16)38400-X [DOI] [PubMed] [Google Scholar]
- 4.Reinhardt W, Kübber H, Dolff S, et al. Rapid recovery of hypogonadism in male patients with end stage renal disease after renal transplantation. Endocrine 2018;60:159-66. 10.1007/s12020-018-1543-2 [DOI] [PubMed] [Google Scholar]
- 5.Carrero JJ, Qureshi AR, Nakashima A, et al. Prevalence and clinical implications of testosterone deficiency in men with end-stage renal disease. Nephrol Dial Transplant 2011;26:184-90. 10.1093/ndt/gfq397 [DOI] [PubMed] [Google Scholar]
- 6.Holley JL. The hypothalamic-pituitary axis in men and women with chronic kidney disease. Adv Chronic Kidney Dis 2004;11:337-41. 10.1053/j.ackd.2004.07.004 [DOI] [PubMed] [Google Scholar]
- 7.Eckersten D, Giwercman A, Christensson A. Male patients with terminal renal failure exhibit low serum levels of antimüllerian hormone. Asian J Androl 2015;17:149-53. 10.4103/1008-682X.135124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim VS, Fang VS. Gonadal dysfunction in uremic men. A study of the hypothalamo-pituitary-testicular axis before and after renal transplantation. Am J Med 1975;58:655-62. 10.1016/0002-9343(75)90501-X [DOI] [PubMed] [Google Scholar]
- 9.Akbari F, Alavi M, Esteghamati A, et al. Effect of renal transplantation on sperm quality and sex hormone levels. BJU Int 2003;92:281-3. 10.1046/j.1464-410X.2003.04323.x [DOI] [PubMed] [Google Scholar]
- 10.Hamdi SM, Walschaerts M, Bujan L, et al. A prospective study in male recipients of kidney transplantation reveals divergent patterns for inhibin B and testosterone secretions. Basic Clin Androl 2014;24:11. 10.1186/2051-4190-24-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prem AR, Punekar SV, Kalpana M, et al. Male reproductive function in uraemia: efficacy of haemodialysis and renal transplantation. Br J Urol 1996;78:635-8. 10.1046/j.1464-410X.1996.14624.x [DOI] [PubMed] [Google Scholar]
- 12.Luciano RL, Dahl NK. Extra-renal manifestations of autosomal dominant polycystic kidney disease (ADPKD): Considerations for routine screening and management. Nephrol Dial Transplant 2014;29:247-54. 10.1093/ndt/gft437 [DOI] [PubMed] [Google Scholar]
- 13.Kolettis PN, Ross JH, Kay R, et al. Sperm retrieval and intracytoplasmic sperm injection in patients with prune-belly syndrome. Fertil Steril 1999;72:948-9. 10.1016/S0015-0282(99)00388-X [DOI] [PubMed] [Google Scholar]
- 14.Hsieh MH, Hollander A, Lamb DJ, et al. The genetic and phenotypic basis of infertility in men with pediatric urologic disorders. Urology 2010;76:25-31. 10.1016/j.urology.2010.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodhouse CR, Reilly JM, Bahadur G. Sexual function and fertility in patients treated for posterior urethral valves. J Urol 1989;142:586-8; discussion 603-5. 10.1016/S0022-5347(17)38824-9 [DOI] [PubMed] [Google Scholar]
- 16.Mieusset R, Fauquet I, Chauveau D, et al. The spectrum of renal involvement in male patients with infertility related to excretory-system abnormalities: phenotypes, genotypes, and genetic counseling. J Nephrol 2017;30:211-8. 10.1007/s40620-016-0286-5 [DOI] [PubMed] [Google Scholar]
- 17.Lessan-Pezeshki M, Ghazizadeh S. Sexual and reproductive function in end-stage renal disease and effect of kidney transplantation. Asian J Androl 2008;10:441-6. 10.1111/j.1745-7262.2008.00348.x [DOI] [PubMed] [Google Scholar]
- 18.Xu LG, Xu HM, Zhu XF, et al. Examination of the semen quality of patients with uraemia and renal transplant recipients in comparison with a control group. Andrologia 2009;41:235-40. 10.1111/j.1439-0272.2009.00924.x [DOI] [PubMed] [Google Scholar]
- 19.Shiraishi K, Shimabukuro T, Naito K. Effects of Hemodialysis on Testicular Volume and Oxidative Stress in Humans. J Urol 2008;180:644-50. 10.1016/j.juro.2008.04.010 [DOI] [PubMed] [Google Scholar]
- 20.Xu L, Xu H, Zhu X, et al. Effect of Uremia on Semen Quality and Reproductive Function in Humans. Cell Biochem Biophys 2012;62:29-33. 10.1007/s12013-011-9254-9 [DOI] [PubMed] [Google Scholar]
- 21.Xu LG, Shi SF, Qi XP, et al. Morphological characteristics of spermatozoa before and after renal transplantation. Asian J Androl 2005;7:81-5. 10.1111/j.1745-7262.2005.00002.x [DOI] [PubMed] [Google Scholar]
- 22.Xu HM, Li HG, Xu LG, et al. The decline of fertility in male uremic patients is correlated with low expression of the cystic fibrosis transmembrane conductance regulator protein (CFTR) in human sperm. Hum Reprod 2012;27:340-8. 10.1093/humrep/der403 [DOI] [PubMed] [Google Scholar]
- 23.Barry JM. Spermatic cord preservation in kidney transplantation. J Urol 1982;127:1076-7. 10.1016/S0022-5347(17)54233-0 [DOI] [PubMed] [Google Scholar]
- 24.Sheynkin YR, Hendin BN, Schlegel PN, et al. Microsurgical repair of iatrogenic injury to the vas deferens. J Urol 1998;159:139-41. 10.1016/S0022-5347(01)64036-9 [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues Netto NJ, Pecoraro G, Sabbaga E, et al. Spermatogenesis before and after renal transplant. Int J Fertil 1980;25:131-3. [PubMed] [Google Scholar]
- 26.Eid MM, Abdel-Hamid IA, Sobh MA, et al. Assessment of sperm motion characteristics in infertile renal transplant recipients using computerized analysis. Int J Androl 1996;19:338-44. 10.1111/j.1365-2605.1996.tb00524.x [DOI] [PubMed] [Google Scholar]
- 27.Papadopoulou E. Erectile dysfunction in chronic kidney disease: From pathophysiology to management. World J Nephrol 2015;4:379. 10.5527/wjn.v4.i3.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Procci WR, Hoffman KI, Chatterjee SN. Sexual functioning of renal transplant recipients. J Nerv Ment Dis 1978;166:402-7. 10.1097/00005053-197806000-00003 [DOI] [PubMed] [Google Scholar]
- 29.Shamsa A, Motavalli SM, Aghdam B. Erectile function in end-stage renal disease before and after renal transplantation. Transplant Proc 2005;37:3087-9. 10.1016/j.transproceed.2005.08.067 [DOI] [PubMed] [Google Scholar]
- 30.Mirone V, Longo N, Fusco F, et al. Renal Transplantation Does Not Improve Erectile Function in Hemodialysed Patients. Eur Urol 2009;56:1047-53. 10.1016/j.eururo.2008.09.020 [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Zhang Z, Lin Y, et al. Long-term impact of immunosuppressants at therapeutic doses on male reproductive system in unilateral nephrectomized rats: A comparative study. Biomed Res Int 2013;2013:690382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haberman J, Karwa G, Greenstein SM, et al. Male fertility in cyclosporine-treated renal transplant patients. J Urol 1991;145:294-6. 10.1016/S0022-5347(17)38318-0 [DOI] [PubMed] [Google Scholar]
- 33.Xu LG, Yang YR, Wang HW, et al. Characteristics of male fertility after renal transplantation. Andrologia 2011;43:203-7. 10.1111/j.1439-0272.2010.01052.x [DOI] [PubMed] [Google Scholar]
- 34.Georgiou GK, Dounousi E, Harissis HV. Calcineurin inhibitors and male fertility after renal transplantation - a review. Andrologia 2016;48:483-90. 10.1111/and.12477 [DOI] [PubMed] [Google Scholar]
- 35.Caneguim BH, Cerri PS, Spolidório LC, et al. Structural alterations in the seminiferous tubules of rats treated with immunosuppressor tacrolimus. Reprod Biol Endocrinol 2009;7:19. 10.1186/1477-7827-7-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kantarci G, Şahin S, Uras AR, et al. Effects of different calcineurin inhibitors on sex hormone levels in transplanted male patients. Transplant Proc 2004;36:178-9. 10.1016/j.transproceed.2003.11.017 [DOI] [PubMed] [Google Scholar]
- 37.Miyata H, Satouh Y, Mashiko D, et al. Sperm calcineurin inhibition prevents mouse fertility with implications for male contraceptive. Science 2015;350:442-5. 10.1126/science.aad0836 [DOI] [PubMed] [Google Scholar]
- 38.Tondolo V, Citterio F, Panocchia N, et al. Sirolimus impairs improvement of the gonadal function after renal transplantation. Am J Transplant 2005;5:197. 10.1111/j.1600-6143.2005.00604.x [DOI] [PubMed] [Google Scholar]
- 39.Zuber J, Anglicheau D, Elie C, et al. Sirolimus may reduce fertility in male renal transplant recipients. Am J Transplant 2008;8:1471-9. 10.1111/j.1600-6143.2008.02267.x [DOI] [PubMed] [Google Scholar]
- 40.Skrzypek J, Krause W. Azoospermia in a renal transplant recipient during sirolimus (rapamycin) treatment. Andrologia 2007;39:198-9. 10.1111/j.1439-0272.2007.00787.x [DOI] [PubMed] [Google Scholar]
- 41.Perez-Aytes A, Ledo A, Boso V, et al. In utero exposure to mycophenolate mofetil: A characteristic phenotype? Am J Med Genet A 2008;146A:1-7. 10.1002/ajmg.a.32117 [DOI] [PubMed] [Google Scholar]
- 42.Jones A, Clary M, McDermott E, et al. Outcomes of pregnancies fathered by solid-organ transplant recipients exposed to mycophenolic acid products. Prog Transplant 2013;23:153-7. 10.7182/pit2013636 [DOI] [PubMed] [Google Scholar]
- 43.Midtvedt K, Bergan S, Reisæter AV, et al. Exposure to Mycophenolate and Fatherhood. Transplantation 2017;101:e214-7. 10.1097/TP.0000000000001747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tainio J, Jahnukainen K, Nurmio M, et al. Testicular function, semen quality, and fertility in young men after renal transplantation during childhood or adolescence. Transplantation 2014;98:987-93. 10.1097/TP.0000000000000173 [DOI] [PubMed] [Google Scholar]
- 45.Elstein M, Smith EK, Curtis JR. Reproductive potential of patients treated by maintenance haemodialysis. Br Med J 1969;2:734-6. 10.1136/bmj.2.5659.734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morken NH, Diaz-Garcia C, Reisaeter AV, et al. Obstetric and neonatal outcome of pregnancies fathered by males on immunosuppression after solid organ transplantation. Am J Transplant 2015;15:1666-73. 10.1111/ajt.13159 [DOI] [PubMed] [Google Scholar]
- 47.Zeyneloglu HB, Oktem M, Durak T. Male infertility after renal transplantation: Achievement of pregnancy after intracytoplasmic sperm injection. Transplant Proc 2005;37:3081-4. 10.1016/j.transproceed.2005.08.029 [DOI] [PubMed] [Google Scholar]
- 48.Berkkanoglu M, Bulut H, Coetzee K, et al. Intracytoplasmic sperm injection in male renal transplant recipients. Middle East Fertil Soc J 2015;20:127-30. 10.1016/j.mefs.2014.10.002 [DOI] [Google Scholar]
- 49.Veys KR, D’Hauwers KW, van Dongen AJ, et al. First Successful Conception Induced by a Male Cystinosis Patient. JIMD Rep 2018;38:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balmori C, Guillén A, Montans J, et al. Successful ICSI in an azoospermic and kidney transplant man with type 1 primary hyperoxaluria and first histopathological testicular findings described in the literature. Andrologia 2015;47:109-11. 10.1111/and.12230 [DOI] [PubMed] [Google Scholar]
- 51.Case AM, Weissman M, Sermer EM, Greenblatt. Successful twin pregnancy in a dual-transplant couple resulting from in-vitro fertilization and intracytoplasmic sperm injection. Hum Reprod 2000;15:626-8. 10.1093/humrep/15.3.626 [DOI] [PubMed] [Google Scholar]