Abstract
Background
To date, definitions of liver dysfunction (LD) after hepatic resection rely on late postoperative time points. Further, the used parameters are markedly influenced by perioperative management. Thus, we aimed to establish a very early postoperative score to predict postoperative mortality.
Methods
Liver related parameters were evaluated after liver resection in a retrospective evaluation cohort of 228 colorectal cancer patients with liver metastasis (mCRC) and subsequent validation in a prospective set of 482 consecutive patients from 4 independent institutions undergoing hepatic resection was performed.
Results
C-reactive protein (CRP, AUC =0.739, P<0.001) and antithrombinIII-activity (ATIII, AUC =0.844, P<0.001) on the first postoperative day (POD) were found to be elevated in patients with LD. Cut-off values for CRP at 3 mg/dL and for ATIII at 60% significantly identified high-risk patients for postoperative LD and mortality (P<0.001) and thus defined the 3-60 criteria on POD1. The 3-60 criteria showed superior sensitivity and specificity compared to established criteria for LD [3-60 criteria: total positive patients: 26 patients (70% mortality detected), odds ratio (OR): 48.8; International Study Group for Liver Surgery: total positive patients: 43 (70% mortality detected), OR: 23.3; Peak7: total positive patients: 9 (30% mortality detected), OR: 27.8; 50-50: total positive patients: 9 (30% mortality detected), OR: 27.8]. These results could be validated in a multi-center analysis and ultimately the 3-60 criteria remained an independent predictor of postoperative mortality upon multivariable analysis.
Conclusions
The 3-60 criteria on POD1 predict postoperative LD and mortality early after liver resection with a comparable or better accuracy than established criteria, allowing for immediate identification of high-risk patients.
Keywords: Liver surgery, liver dysfunction (LD), mortality, C-reactive protein (CRP), AntithrombinIII-activity (ATIII)
Introduction
Liver resection is frequently used in patients suffering from primary or non-primary liver malignancies (1). Due to the livers unique ability to regenerate and restore its functional capacity, extensive resection removing up to 75–80% of liver volume is possible. However, post-hepatectomy liver dysfunction (LD) is still the main cause for postoperative mortality (2,3). There are a variety of criteria used for assessment of postoperative LD, the three most common being the ones given by the International Study Group of Liver Surgery (ISGLS) (4), the 50-50 criteria (5) and the Mullen or Peak7 criteria (6). In summary, these three definitions of postoperative LD are based on values of serum bilirubin (SB) alone or in combination with prothrombin time (PT) in the postoperative time course. While these parameters have been found to be very useful in clinical routine and as outcome measure after liver resection, they collectively suffer from two major drawbacks. All of them rely on parameters strongly influenced by intraoperative and perioperative management and, most importantly, they are defined by on postoperative day (POD) 5 or even later. As we know from experimental as well as clinical studies, the crucial period of liver regeneration has already taken place on POD5 and the potential for intervention at this late time point seems to be limited (7).
LD after resection is strongly associated with multi organ dysfunction and to this day the standard treatment resembles goal-directed therapy resembling sepsis management. Early initiation of supportive treatment is key to improve outcome of patients suffering from LD. Accordingly, we now aimed to explore the role of markers that are not affected by perioperative management to serve as predictors for liver failure at a very early postoperative stage. Both C-reactive protein (CRP) and AntithrombinIII-activity (ATIII) are primarily produced by the liver (8,9). Further, these factors are easily assessable routine parameters not influenced by transfusion of red blood cells or synthetic coagulation factors, frequently used during the postoperative management of patients undergoing liver resection. We evaluated perioperative CRP and ATIII values in a retrospective cohort of our prospectively maintained institutional database including a homogenous cohort of patients undergoing liver resection for liver metastasized colorectal carcinoma (mCRC). Importantly, we aimed to further validate our results in a multi-institutional validation cohort of consecutively included patients undergoing liver resection, representing a routine clinical setting.
Methods
Study cohorts
A homogenous cohort of patients undergoing liver resection for metastasized colorectal cancer (mCRC) between March 2001 and December 2009 was evaluated using our prospectively maintained institutional database (evaluation cohort). Subsequently, a multi-center validation cohort of patients undergoing liver resection was recruited at the Medical University of Vienna, Rudolfstiftung Hospital Vienna, Medical University of Innsbruck and State Hospital Wiener Neustadt to confirm our findings in a routine clinical setting.
The extent of resection was characterized following the IHPBA Brisbane 2000 nomenclature in minor (<3 liver segments) and major (>3 liver segments) (10). Blood samples were collected routinely prior to surgery (PRE OP) as well as on the first seven PODs (POD1 through POD7) after liver resection. All patients gave written informed consent. The study was approved by the institutional ethics committee (#424/2010; #2032/2013) and registered at a clinical trials registry (ClinicalTrials.gov Identifier: NCT01700231).
Postoperative LD and morbidity
Postoperative LD was assessed according to the three most frequently used classifications—the ISGLS criteria, the 50-50 criteria and the Mullen (Peak7) criteria (4-6). Briefly, for ISGLS the criteria were met if SB concentration and PT did not reach normal levels on POD5, for 50-50 if SB was above 50 mmol/L (3 mg/dL) and PT was lower than 50% on POD5 and for Peak7 if SB was higher than 7 mg/dL within the first 7 days after liver resection. Patients who reached normal SB or PT values before POD5 were considered as “no LD”.
Postoperative mortality was defined as death within 90 days after surgery (11).
Quantification of blood parameters
All perioperative parameters of liver function [SB, PT, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl-transferase (GGT), alkaline phosphatase (AP), and albumin] as well as CRP, fibrinogen and platelet counts were measured in appropriate samples by routine laboratory blood tests. Activity of ATIII was assessed in routine laboratory blood tests and used as a surrogate parameter for absolute levels of ATIII.
Statistical analysis
All analyses are performed using data from subjects with valid marker values only. Statistical analyses were carried out using SPSS software (SPSS, Inc., Chicago, IL, USA, Version 23) and were based on non-parametric tests. A P value below 0.05 was considered statistically significant. Distribution of CRP and ATIII was compared between subjects with and subjects without LD and mortality using Mann-Whitney-U-test. Boxplot illustrations are given without outliers and extreme values to improve resolution of the interquartile ranges. We fit receiver operating characteristic (ROC) curves to assess the predictive potential of both postoperative CRP and ATIII for LD and mortality within 90 days after surgery. A suitable cut-off value for classification of patients with high risk for postoperative LD was assessed using Youden’s J statistics (i.e., the sum of sensitivity and specificity). Moreover, chi-squared tests were performed to evaluate differential incidences within the proposed risk-groups. To assess independency of our proposed cut-offs multivariable analysis model based on logistic regression and stepwise forward selection was fitted. All parameters being significant upon univariate analysis entered multivariable analysis. Of note, given the low incidence of LD in the validation sample, we combined the evaluation and validation cohort for univariate and multivariable analysis to achieve a solid model fit.
Results
Patients and cohorts
A total of 710 patients were included in this study. The evaluation set consisted of a homogenous cohort of 228 mCRC patients, retrospectively analysed from our prospectively maintained institutional database. This homogenous group served as an exploratory cohort to elucidate the role of perioperative CRP in patients undergoing liver resection, as well as to establish the 3-60 criteria for early diagnosis of liver resection, as described in the following. Subsequently, a prospective validation cohort (N=482) was recruited at four different institutions: Medical University of Vienna (N=102), Rudolfstiftung Hospital Vienna (N=74), Medical University of Innsbruck (N=254) and State Hospital Wiener Neustadt (N=52). Of note, the multi-center validation cohort aimed to assess the relevance of our previously described results on CRP and the 3-60 criteria in a routine clinical setting of patients undergoing liver resection. Hence, it consisted of patients treated for mCRC (N=263), hepatocellular carcinoma (HCC, N=76), cholangiocellular carcinoma (CCC, N=100), benign neoplastic entities (N=16) and other liver pathologies requiring resection (N=27). Baseline characteristics of both cohorts are given in Table 1 and comparative analysis was applied. Of note, as the evaluation cohort exclusively contained patients treated for mCRC, there were notable statistical differences in preoperative parameters of liver function and damage. Importantly, the multi-centric validation cohort included consecutive patients undergoing liver resection and thus reflects clinical routine.
Table 1. Patient demographics.
| Parameter | Entire cohort (N=710, %) | Evaluation cohort (N=228, %) | Validation cohort (N=482, %) | P value |
|---|---|---|---|---|
| Gender | 0.585 | |||
| Male | 435 (61.3) | 143 (62.7) | 292 (60.6) | |
| Female | 275 (38.7) | 85 (37.3) | 190 (39.4) | |
| Age, median (range), years | 63 (22.0–89.0) | 62 (28.0–83.0) | 63 (22.0–89.0) | 0.977 |
| Hepatic resection | <0.001 | |||
| Minor (<3 segments) | 362 (51.0) | 151 (66.2) | 211 (43.8) | |
| Major (≥3 segments) | 348 (49.0) | 77 (33.8) | 271 (56.2) | |
| Tumor type | <0.001 | |||
| mCRC | 491 (69.2) | 228 (100.0) | 263 (54.6) | |
| HCC | 76 (10.7) | 0 (0.0) | 76 (15.8) | |
| CCC | 100 (14.1) | 0 (0.0) | 100 (20.7) | |
| Benign | 16 (2.3) | 0 (0.0) | 16 (3.3) | |
| Other | 27 (3.8) | 0 (0.0) | 27 (5.6) | |
| Preoperative parameters, median (range) | ||||
| PDR (%) | 20.0 (3.5–43.0) | 19.0 (3.5–35.8) | 22.0 (8.0–43.0) | <0.001 |
| R15 (%) | 5.0 (0.2–59.2) | 6.0 (0.5–59.2) | 3.7 (0.2–32.0) | <0.001 |
| Platelets (×103/µL) | 198 (43.0–679.0) | 139 (49.0–503.0) | 221 (43.0–679.0) | <0.001 |
| SB (mg/dL) | 0.61 (0.15–18.50) | 0.63 (0.24–2.26) | 0.60 (0.15–18.50) | 0.026 |
| PT (%) | 104 (39.0–150.0) | 107 (45.0–147.0) | 102 (39.0-150.0) | 0.002 |
| AP (U/L) | 99 (14.0–2,005.0) | 108 (42.0–1,111.0) | 95 (14.0-2005.0) | 0.035 |
| GGT (U/L) | 58 (0.0–2,055.0) | 47 (9.0–968.0) | 63 (0.0–2,055.0) | <0.001 |
| AST (U/L) | 29 (5.0–615.0) | 28 (5.0–496.0) | 30 (12.0–615.0) | 0.001 |
| ALT (U/L) | 26 (2.0–497.0) | 22 (2.0–410.0) | 28 (4.0–497.0) | <0.001 |
| Albumin (g/L) | 42.0 (21.0–52.0) | 41.0 (21.0–50.0) | 43.0 (25.5–52.0) | <0.001 |
| ATIII-activity (%) | 105 (51.0–168.0) | 107 (67.0–168.0) | 103 (51.0–136.0) | 0.003 |
| CRP (mg/dL) | 0.40 (0.02–33.58) | 0.5 (0.04–9.67) | 0.32 (0.02–33.58) | 0.004 |
| Postoperative outcome | ||||
| Liver dysfunction (ISGLS) | 67 (9.4) | 26 (11.4) | 41 (8.5) | 0.218 |
| Liver dysfunction (Peak7) | 36 (5.1) | 6 (2.6) | 30 (6.2) | 0.042 |
| Liver dysfunction (50-50) | 18 (2.5) | 6 (2.6) | 12 (2.5) | 0.911 |
| Morbidity (Dindo et al.) | 284 (40.0) | 81 (35.5) | 203 (42.1) | 0.094 |
| ICU stay, median (range), days | 1 (0.0–42.0) | 1 (0.0–42.0) | 1 (0.0–26.0) | <0.001 |
| Total hospitalization, median (range), days | 10 (3.0–117.0) | 9 (4.0–77.0) | 10 (3.0–117.0) | 0.002 |
| Postoperative mortality | 17 (2.4) | 7 (3.1) | 10 (2.1) | 0.418 |
mCRC, metastasized colorectal carcinoma; HCC, hepatocellular carcinoma; CCC, cholangiocellular carcinoma; PDR, plasma disappearance rate; R15, retention rate at 15 minutes; SB, serum bilirubin; PT, prothrombin time; AP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ATIII, antithrombinIII-activity; CRP, C-reactive protein; ISGLS, international study group on liver surgery; ICU, intensive care unit.
CRP increases after liver resection but is affected by the extent of liver resection
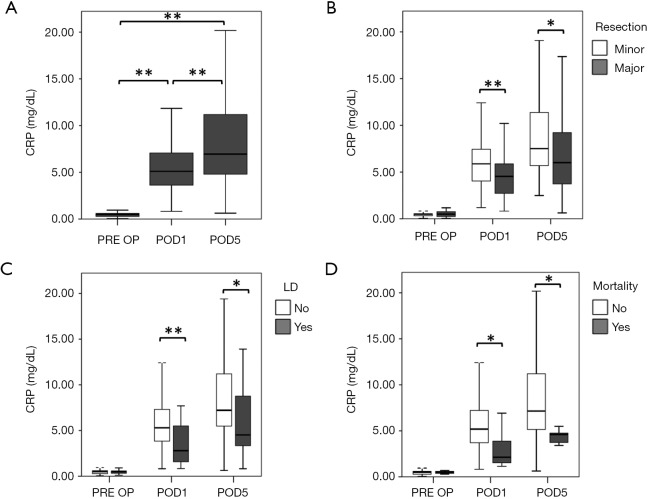
We initially evaluated perioperative dynamics of CRP in our evaluation cohort (Figure 1A). We observed a significant increase in CRP levels on POD1 (median CRP PRE OP =0.50 mg/dL, median CRP POD1 =5.11 mg/dL, P<0.001), followed by an additional increase up to POD5 (median CRP POD5 =6.95 mg/dL, P<0.001). Interestingly, when comparing levels of CRP between patients with minor and major hepatic resection, we found that CRP followed the extent of resection (Figure 1B). While no preoperative difference in CRP was observed (median CRP before minor resection =0.50 mg/dL, median CRP before major resection =0.50 mg/dL, P=0.970; Figure 1B), we found reduced levels of CRP on POD1 and POD5 in patients undergoing major liver resection, as compared to patients undergoing minor resection (POD1: median CRP after minor resection =5.88 mg/dL, median CRP after major resection =4.53 mg/dL, P<0.001; POD5: median CRP after minor resection =7.51 mg/dL, median CRP after major resection =6.01 mg/dL, P=0.036; Figure 1B).
Figure 1.
Perioperative CRP dynamics are associated with clinical outcome after liver resection. CRP was evaluated prior to surgery (PRE OP), as well as on the first (POD1) and fifth (POD5) POD. Perioperative dynamics are shown in (A). Patients were divided in groups according to the extent of their resection (B), postoperative LD (C) and postoperative 90 days mortality (D). *, P<0.05; **, P<0.001. CRP, C-reactive protein; LD, liver dysfunction; POD, postoperative day.
Diminished postoperative levels of CRP are associated with poor clinical outcome after liver resection and remain an independent predictor upon multivariable analysis
We further evaluated whether perioperative CRP dynamics were associated with clinical outcome. Accordingly, we observed that patients suffering from postoperative LD, while starting at comparable CRP levels (median CRP no LD =0.50 mg/dL, median CRP LD =0.50 mg/dL, P=0.914; Figure 1C), had significantly lower CRP on POD1 (median CRP no LD =5.30 mg/dL, median CRP LD =2.80 mg/dL, P<0.001; Figure 1C), and POD5 (median CRP no LD =7.21 mg/dL, median CRP LD =4.52 mg/dL, P=0.037; Figure 1C).
Similarly, postoperative CRP was found to be significantly reduced in patients that died within 90 PODs (POD1: median CRP no mortality =5.19 mg/dL, median CRP mortality =2.13 mg/dL, P=0.012; POD5: median CRP no mortality =7.15 mg/dL, median CRP mortality =4.60 mg/dL, P=0.024; Figure 1D), while there was no difference prior to the operation (median CRP no mortality =0.50 mg/dL, median CRP mortality =0.50 mg/dL, P=0.770; Figure 1D).
To exclude any interfering factors and to investigate whether CRP was able to independently predict postoperative LD and mortality, we performed multivariable analysis. Accordingly, extent of resection, and levels of CRP, SB, PT, AST, ALT, and albumin on POD1 gave P values below 0.05 in the univariate models for LD and were consequently included in the initial multivariable model. After step-wise forward selection extent of resection, CRP, PT and ALT on POD1 remained. The results from the final model fit are shown in Table 2.
Table 2. Multivariable analysis for liver dysfunction.
| Parameter | Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| CRP (mg/dL) on POD1 | 0.648 | 0.519–0.801 | <0.001 | 0.650 | 0.483–0.874 | 0.004 | |
| Age (years) | 1.030 | 0.988–1.075 | 0.167 | – | – | – | |
| Gender | 1.268 | 0.554–2.906 | 0.574 | – | – | – | |
| Extent of hepatic resection | 10.950 | 3.937–30.452 | <0.001 | 5.438 | 1.515–19.519 | 0.009 | |
| Neoadjuvant CTx | NA | NA | 0.999 | – | – | – | |
| Intraoperative RBC | 1.864 | 0.726–4.790 | 0.196 | – | – | – | |
| Steatosis (%) | 0.993 | 0.974–1.013 | 0.512 | – | – | – | |
| Pringle maneuver | 1.187 | 0.327–4.306 | 0.794 | – | – | – | |
| Preoperative parameters | – | – | – | ||||
| PDR (%) | 0.961 | 0.877–1.052 | 0.385 | ||||
| R15 (%) | 0.999 | 0.926–1.078 | 0.985 | ||||
| Platelets (×103/µL) | 0.999 | 0.994–1.005 | 0.858 | ||||
| SB (mg/dL) | 1.368 | 0.480–3.902 | 0.558 | ||||
| PT (%) | 0.992 | 0.972–1.014 | 0.479 | ||||
| AP (U/L) | 1.002 | 0.999–1.005 | 0.161 | ||||
| GGT (U/L) | 1.003 | 0.999–1.006 | 0.109 | ||||
| AST (U/L) | 1.001 | 0.993–1.009 | 0.814 | ||||
| ALT (U/L) | 1.002 | 0.993–1.010 | 0.728 | ||||
| Albumin (g/L) | 0.958 | 0.870-1.056 | 0.389 | ||||
| POD1 parameters | |||||||
| Platelets (×103/µL) | 0.996 | 0.988–1.005 | 0.404 | – | – | – | |
| SB (mg/dL) | 1.824 | 1.376–2.418 | <0.001 | – | – | – | |
| PT (%) | 0.927 | 0.897–0.958 | <0.001 | 0.934 | 0.898–0.917 | 0.001 | |
| AP (U/L) | 1.002 | 0.997–1.008 | 0.437 | – | – | – | |
| GGT (U/L) | 1.006 | 0.999–1.013 | 0.075 | – | – | – | |
| AST (U/L) | 1.001 | 1.000–1.002 | 0.014 | 1.002 | 1.000–1.003 | 0.038 | |
| ALT (U/L) | 1.001 | 1.000–1.003 | 0.046 | – | – | – | |
| Albumin (g/L) | 0.900 | 0.811–0.999 | 0.048 | – | – | – | |
CTx, chemotherapy; PDR, plasma disappearance rate; R15, retention rate at 15 minutes; SB, serum bilirubin; PT, prothrombin time; AP, alcalic phosphatase; GGT, gamma-glutamyltranspeptidase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; POD, postoperative day; CRP, C-reactive protein; OR, odds ratio; CI, confidence interval.
Similarly, for 90 days mortality postoperative CRP, AST and albumin were included in multivariable analysis. Interestingly, after step-wise forward selection exclusively CRP remained significant with an odds ratio (OR) of 0.582 [95% confidence interval (CI): 0.373–0.906, P=0.017; Table 3).
Table 3. Multivariable analysis for mortality.
| Parameter | Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| CRP (mg/dL) on POD1 | 0.582 | 0.373–0.906 | 0.017 | 0.582 | 0.373–0.906 | 0.017 | |
| Age (years) | 1.077 | 0.988–1.175 | 0.092 | – | – | – | |
| Gender | 2.305 | 0.503–10.555 | 0.282 | – | – | – | |
| Extent of hepatic resection | 5.174 | 0.980–27.313 | 0.053 | – | – | – | |
| Neoadjuvant CTx | 0.206 | 0.022–1.948 | 0.168 | – | – | – | |
| Intraoperative RBC | 0.769 | 0.090–6.572 | 0.811 | – | – | – | |
| Steatosis (%) | 1.020 | 0.992–1.049 | 0.166 | – | – | – | |
| Pringle maneuver | 1.508 | 0.173–13.103 | 0.710 | – | – | – | |
| Preoperative parameters | – | – | – | ||||
| PDR (%) | 0.968 | 0.815–1.150 | 0.713 | ||||
| R15 (%) | 0.975 | 0.812–1.172 | 0.789 | ||||
| Platelets (×103/µL) | 1.001 | 0.991–1.011 | 0.815 | ||||
| SB (mg/dL) | 1.096 | 0.152–7.890 | 0.927 | ||||
| PT (%) | 1.017 | 0.978–1.057 | 0.392 | ||||
| AP (U/L) | 0.988 | 0.966–1.011 | 0.295 | ||||
| GGT (U/L) | 1.000 | 0.993–1.008 | 0.988 | ||||
| AST (U/L) | 0.997 | 0.975–1.020 | 0.813 | ||||
| ALT (U/L) | 0.996 | 0.973–1.021 | 0.772 | ||||
| Albumin (g/L) | 0.866 | 0.754–0.995 | 0.042 | ||||
| POD1 parameters | – | – | – | ||||
| Platelets (×103/µL) | 0.992 | 0.976–1.009 | 0.377 | ||||
| SB (mg/dL) | 1.460 | 1.123–1.897 | 0.005 | ||||
| PT (%) | 0.965 | 0.920–1.013 | 0.148 | ||||
| AP (U/L) | 0.996 | 0.975–1.017 | 0.710 | ||||
| GGT (U/L) | 1.007 | 0.998–1.017 | 0.147 | ||||
| AST (U/L) | 1.001 | 0.999–1.003 | 0.368 | ||||
| ALT (U/L) | 1.000 | 0.998–1.003 | 0.753 | ||||
| Albumin (g/L) | 1.051 | 0.859–1.286 | 0.631 | ||||
CTx, chemotherapy; PDR, plasma disappearance rate; R15, retention rate at 15 minutes; SB, serum bilirubin; PT, prothrombin time; AP, alcalic phosphatase; GGT, gamma-glutamyltranspeptidase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; POD, postoperative day; CRP, C-reactive protein; OR, odds ratio; CI, confidence interval.
CRP on POD1 below 3 specifically identifies patients with postoperative LD and mortality
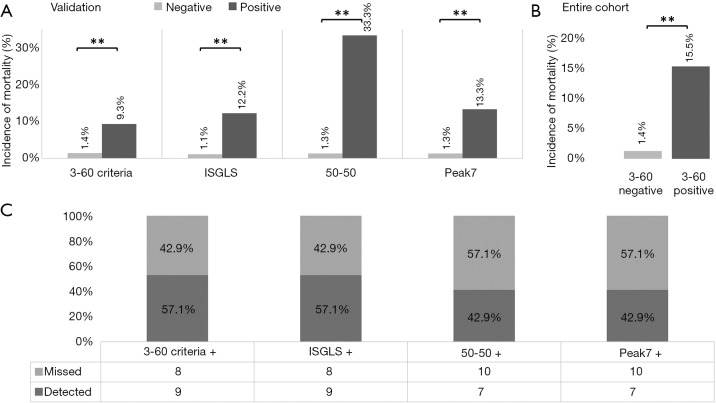
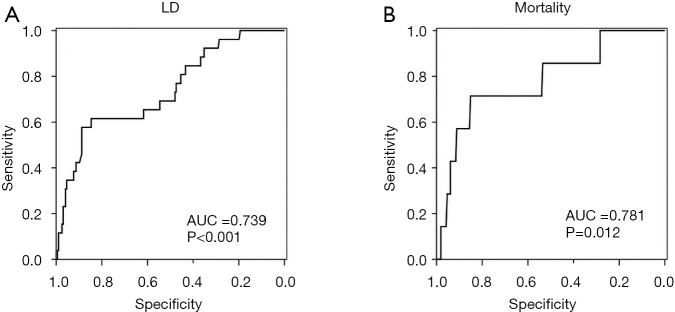
To further assess the ability of CRP to predict LD after liver resection we performed ROC analysis. Accordingly, we found a good discriminatory potential between patients with and without postoperative LD for CRP on POD1 (AUC =0.739, P<0.001, 95% CI: 0.632–0.845, Figure S1A). Moreover, ROC analysis revealed an equally good potential of CRP on POD1 to discriminate between patients that did or did not die within 90 PODs (AUC =0.781, P=0.012, 95% CI: 0.595–0.966, Figure S1B). Based on the ROC analysis for postoperative LD, a cut-off at 2.88 mg/dL was identified using Youden’s J statistic. To render this cut-off more clinically applicable, we rounded it to 3 mg/dL. Indeed, using our cut-off at 3 mg/dL, we were able to identify patients suffering from postoperative LD and 90 days mortality already on POD1 [LD: 11 of 182 (6.0%) in CRPhigh vs. 15 of 40 (37.5%) in CRPlow, P<0.001; mortality: 2 of 182 (1.1%) in CRPhigh vs. 5 of 40 (12.5%) in CRPlow, P<0.001; Figure 2A).
Figure 2.
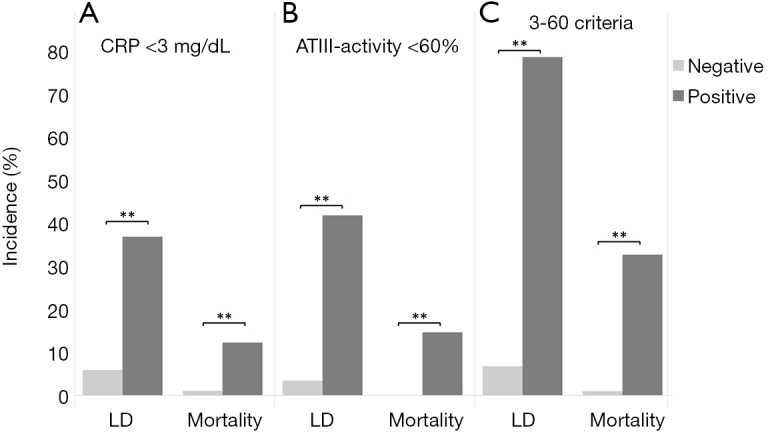
The 3-60 criteria—combination of CRP and ATIII-activity allows specific identification of patients with LD and mortality. Incidences for liver dysfunction (LD) and postoperative mortality are shown according to their postoperative CRP (A), their postoperative ATIII-activity (B), as well as according to the 3-60 criteria (C). **, P<0.001. CRP, C-reactive protein; LD, liver dysfunction; ATIII, AntithrombinIII.
Combination of CRP and ATIII on POD1 increases the predictive value for postoperative LD and mortality synergistically
We previously reported on a close relation of postoperative ATIII and clinical outcome (12). Briefly, a cut-off at 61.5% of ATIII-activity on POD1 was found to identify patients suffering from postoperative LD, mortality and from a reduced overall survival. To render this cut-off more clinically applicable, we rounded it to 60% on POD1. Indeed, also in this cohort, patients below a postoperative value of 60% ATIII, were found to suffer more frequently from postoperative LD [6 of 171 (3.5%) in ATIIIhigh vs. 20 of 47 (42.6%) in ATIIIlow, P<0.001; Figure 2B] and mortality [0 of 171 (0.0%) in ATIIIhigh vs. 7 of 47 (14.9%) in ATIIIlow, P<0.001; Figure 2B]. Accordingly, we defined the 3-60 criteria as patients with both CRP below 3 mg/dL and ATIII-activity below 60% on POD1. Intriguingly, combinations of both markers increased the specificity for detection of both LD (CRP <3 mg/dL =87.2%, ATIII <60%=85.9%, 3-60 criteria =98.4%) and mortality (CRP <3mg/dL =83.7%, ATIII <60%=81.0%, 3-60 criteria =95.2%). Correspondingly, patients that fulfilled the 3-60 criteria displayed significantly increased incidences of postoperative LD [14 of 202 (6.9%) in 3-60 negative vs. 12 of 15 (80.0%) in 3-60 positive, P<0.001; Figure 2C] and postoperative mortality [2 of 202 (1.0%) in 3-60 negative vs. 5 of 15 (33.3%) in 3-60 positive, P<0.001; Figure 2C].
The 3-60 criteria challenge established markers for postoperative mortality after liver resection
To assess the clinical relevance of the 3-60 criteria we compared established predictors for postoperative 90 days mortality after liver resection in our cohort. Accordingly, we observed that all established markers for postoperative LD and the 3-60 criteria were able to predict postoperative mortality as shown in Figure 3A. However, while the 50-50 and the Peak7 criteria were found to be more specific for postoperative mortality than the 3-60 criteria, they missed more than half of patients suffering from postoperative mortality as illustrated in Figure 3B. In particular, the 3-60 criteria were found to have the optimal relation of sensitivity and specificity as compared to all other established predictors of postoperative mortality (Table 4). This was also reflected by the highest AUC of ROC analysis for the 3-60 criteria as compared to other predictors of postoperative mortality (3-60 criteria: AUC =0.833, P=0.003; 50-50: AUC =0.707, P=0.062; Peak7: AUC =0.707, P=0.062; ISGLS: AUC =0.733, P=0.036).
Figure 3.
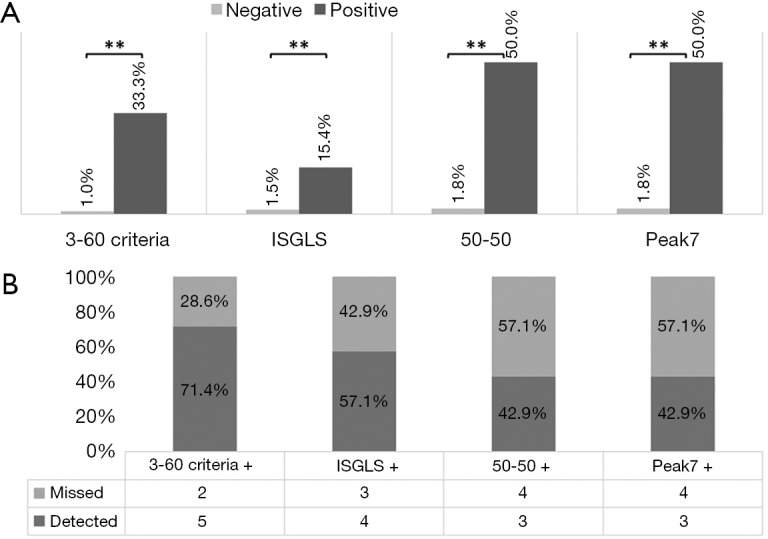
The 3-60 criteria reliably identify patients with postoperative mortality. Incidences of postoperative mortality are shown for test negative and test positive patients, according to the 3-60 criteria, the ISGLS criteria, the 50-50 criteria, and according to Peak7 (A). Further, the percentage of detected patients with postoperative mortality is visualized (B). **, P<0.001. ISGLS, international study group on liver surgery.
Table 4. Evaluation cohort (N=228).
| Parameter | 3-60 criteria | ISGLS | 50-50 | Peak7 |
|---|---|---|---|---|
| Sensitivity | 71.4 | 57.1 | 42.9 | 42.9 |
| Specificity | 95.2 | 90.0 | 98.6 | 98.6 |
| PPV | 33.3 | 15.4 | 50.0 | 50.0 |
| NPV | 99.0 | 98.5 | 98.2 | 98.2 |
ISGLS, international study group on liver surgery; PPV, positive predictive value; NPV, negative predictive value.
Validation of predictive potential of the 3-60 criteria for postoperative 90 days mortality using an independent external validation cohort
Ultimately, we aimed to validate the results of our exploratory cohort in an independent multi-center patient set. Accordingly, we were able to confirm that the 3-60 criteria significantly predicted postoperative 90 days mortality [6 of 431 (1.4%) in 3-60 negative vs. 4 of 43 (9.3%) in 3-60 positive, P<0.001; Figure 4A). Further, also other definitions of LD were able to identify patients that died within 90 PODs [5 of 441 (1.1%) in ISGLS negative vs. 5 of 41 (12.2%) in ISGLS positive, 6 of 470 (1.3%) in 50-50 negative vs. 4 of 12 (33.3%) in 50-50 positive, 6 of 452 (1.3%) in Peak7 negative vs. 4 of 30 (13.3%) in Peak7 positive, P<0.001, respectively; Figure 4A].
Figure 4.
Validation of our results and entire cohort. As in the evaluation cohort, the 3-60 criteria were able to predict postoperative mortality in a multi-center validation set of patients undergoing liver resection representing the clinical routine. Incidences of postoperative mortality are shown for test negative and test positive patients in the entire cohort, according to the 3-60 criteria, the ISGLS criteria, the 50-50 criteria, and according to Peak7 (A). This was also the case after collective analysis of both the evaluation and validation cohort (B). In addition, the percentage of detected patients with postoperative mortality is visualized (C). **, P<0.001. ISGLS, international study group on liver surgery.
To further increase the power of our analysis, we combined our exploration and validation cohort and assessed the predictive potential for detection of patients suffering from postoperative mortality of the 3-60 criteria. Again, the proposed marker was able to identify a significant number of patients that will ultimately die within 90 PODs [8 of 633 (1.3%) in 3-60 negative vs. 9 of 58 (15.5%) in 3-60 positive, P<0.001; Figure 4B]. Interestingly, we observed a higher sensitivity of the 3-60 criteria compared to Peak7 and the 50-50 criteria, that was equal to the sensitivity of the ISGLS criteria (Figure 4C). Importantly, regarding the specificity, positive predictive value (PPV) and negative predictive value (NPV) of the 3-60 criteria on POD 1 performed equally well compared to established markers, as also shown in Table 5.
Table 5. Entire cohort (N=710).
| Parameter | 3-60 criteria | ISGLS | 50-50 | Peak7 |
|---|---|---|---|---|
| Sensitivity | 52.9 | 52.9 | 41.2 | 41.2 |
| Specificity | 92.7 | 91.6 | 98.4 | 95.8 |
| PPV | 15.5 | 13.4 | 38.9 | 19.4 |
| NPV | 98.7 | 98.8 | 98.6 | 98.5 |
ISGLS, international study group on liver surgery; PPV, positive predictive value; NPV, negative predictive value.
When we ultimately performed multivariable analysis in the entire cohort, we observed that next to preoperative ALT and age at time of operation, only the 3-60 criteria and Peak7 remained independent predictors for postoperative 90 days mortality, as illustrated in Table 6. Strikingly, in the ultimate multivariable model for prediction of postoperative mortality, the 3-60 criteria reached the highest OR with a value of 11.073 (95% CI: 3.709–33.657, P<0.001).
Table 6. Multivariable analysis for mortality—entire cohort (N=710).
| Parameter | Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| 3-60 criteria | 14.349 | 5.301–38.841 | <0.001 | 11.073 | 3.709–33.657 | <0.001 | |
| ISGLS | 12.317 | 4.579–33.134 | <0.001 | – | – | – | |
| 50-50 | 43.400 | 13.952–134.999 | <0.001 | – | – | – | |
| Peak7 | 16.028 | 5.694–45.118 | <0.001 | 7.673 | 2.295–25.657 | 0.001 | |
| Age (years) | 1.063 | 1.010–1.119 | 0.019 | 1.066 | 1.004–1.131 | 0.037 | |
| Gender | 1.110 | 0.417–2.952 | 0.834 | – | – | – | |
| Extent of hepatic resection | 2.550 | 0.889–7.315 | 0.082 | – | – | – | |
| Tumor type | 1.330 | 0.921–1.921 | 0.128 | – | – | – | |
| Neoadjuvant CTx | 1.072 | 0.213–5.400 | 0.933 | – | – | – | |
| Intraoperative RBC | 0.820 | 0.100–6.689 | 0.853 | – | – | – | |
| Steatosis (%) | 1.017 | 0.991–1.043 | 0.195 | – | – | – | |
| Pringle maneuver | 1.322 | 0.269–6.502 | 0.731 | – | – | – | |
| Preoperative parameters | |||||||
| PDR (%) | 0.888 | 0.769–1.026 | 0.107 | – | – | – | |
| R15 (%) | 1.041 | 0.959–1.131 | 0.339 | – | – | – | |
| Platelets (×103/µL) | 0.992 | 0.985–0.999 | 0.029 | – | – | – | |
| AP (U/L) | 1.001 | 0.999–1.003 | 0.259 | – | – | – | |
| GGT (U/L) | 1.002 | 1.000–1.003 | 0.007 | – | – | – | |
| AST (U/L) | 1.005 | 1.000–1.009 | 0.033 | – | – | – | |
| ALT (U/L) | 1.007 | 1.002–1.012 | 0.006 | 1.006 | 1.000–1.012 | 0.041 | |
| Albumin (g/L) | 0.854 | 0.762–0.957 | 0.007 | – | – | – | |
| POD1 parameters | – | – | – | ||||
| Platelets (×103/µL) | 0.992 | 0.980–1.005 | 0.247 | ||||
| AP (U/L) | 0.995 | 0.976–1.015 | 0.643 | ||||
| GGT (U/L) | 1.004 | 0.996–1.012 | 0.304 | ||||
| AST (U/L) | 1.000 | 0.999–1.001 | 0.666 | ||||
| ALT (U/L) | 1.000 | 0.998–1.002 | 0.818 | ||||
| Albumin (g/L) | 1.028 | 0.867–1.220 | 0.748 | ||||
CTx, chemotherapy; PDR, plasma disappearance rate; R15, retention rate at 15 minutes; SB, serum bilirubin; AP, alcalic phosphatase; GGT, gamma-glutamyltranspeptidase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; POD, postoperative day; OR, odds ratio; CI, confidence interval.
Discussion
Despite technical refinements in liver surgery over the last decades, post hepatectomy liver failure remains the main cause of morbidity and mortality in patients undergoing liver resection (2,13,14). In this study we were able to show that the 3-60 criteria, a composite score of both reduced CRP and ATIII on POD1, not only independently predicted LD with striking accuracy, but also challenged the three commonly used classifications of LD (ISGLS, 50-50 and Peak7) concerning their ability to predict 90-day mortality (4-6).
In fact, the 3-60 criteria proved to be as accurate in their prediction of postoperative death on POD1 as the ISGLS criteria and significantly more accurate than the 50-50 and Peak7 criteria, with the later three relying on variables assessed not before POD 5 after liver resection.
As reported in previous analysis all established criteria for LD share similar shortcomings (13,15). They exclusively rely on parameters strongly influenced by goal directed perioperative management such as blood and fresh frozen plasma transfusions as well as specific coagulation management. Even more important, they are only available at a time point (at or beyond POD5) where the possibility of therapeutic intervention has often elapsed (16). This study was set up to test parameters routinely available on POD1 that are not influenced by the clinical management of patients after liver resection. Accordingly, we chose CRP and ATIII as candidates for early prediction of adverse outcomes. Of note, while ATIII substitution is sometimes applied in the postoperative management of this patient collective, it is usually initiated as a response to the first postoperative blood withdrawal and would thus not interfere with the evaluation of the 3-60 criteria. Importantly, within this study CRP was found to be a very good predictor of LD and mortality. Based on the good discriminatory potential in the ROC analysis for postoperative LD (AUC =0.739, P<0.001, 95% CI: 0.632–0.845) we were able to identify a clinically applicable cut-off at 3 mg/dL. Similarly, we were able to validate the association of postoperative ATIII with clinical outcome after liver resection, as recently reported by us and further could implement a clinically relevant cut-off at 60 mg/dL (12). While both parameters themselves showed good specificity for LD according to the most commonly used ISGLS criteria (CRP <3 mg/dL =87.2%, ATIII <60%=85.9%) and 90 days mortality (CRP <3 mg/dL =83.7%, ATIII <60%=81.0%), the combination of these two independent markers in the 3-60 criteria substantially increased the specificity for detection of patients that will develop LD (98.4%) and mortality (95.2%), suggesting two independent underlying mechanisms.
Interestingly, even though both CRP and ATIII-activity as markers of liver synthesis show a correlation to the amount of liver tissue resected, the 3-60 criteria on POD 1 showed a predictive potential for postoperative mortality regardless of the extent of liver resection [minor liver resection: 4 of 334 (1.2%) in 3-60 negative vs. 1 of 12 (8.3%) in 3-60 positive, P=0.042; major liver resection: 4 of 299 (1.3%) in 3-60 negative vs. 8 of 46 (17.4%) in 3-60 positive, P<0.001; Figure S2).
Figure S2.
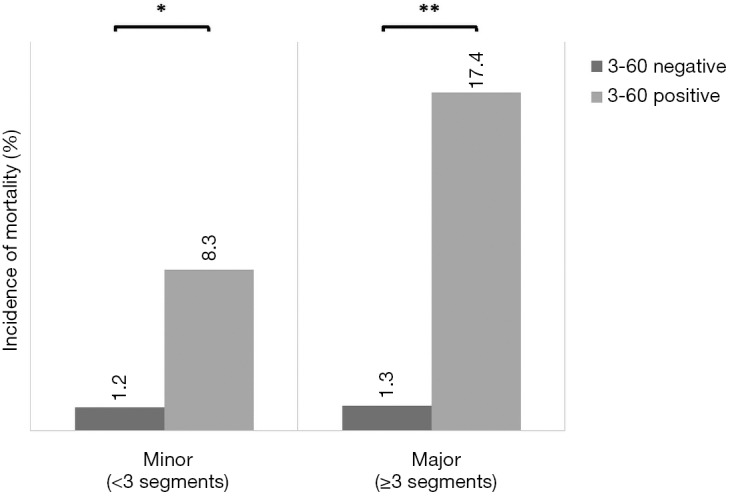
The 3-60 criteria allow prediction of postoperative mortality independent of degree of resection. The incidence of mortality within 90 postoperative days was assessed both in patients undergoing minor or major liver resection in the entire cohort. *, P<0.05; **, P<0.001.
These findings allow for precise and timely identification of patients at risk after liver resection and are of crucial clinical importance. To further assess the linearity of ATIII and CRP with respect to clinical outcome, we chose to assess if an additional cut-off could further increase positive predictive potential for postoperative mortality. Accordingly, the specificity for prediction of postoperative mortality of both CRP and ATIII were set at 90%, leading to a cut-off at 2 mg/dL of CRP and 50% of ATIII. Indeed, patients fulfilling this highly stringent cut-off were found to suffer postoperative mortality in 50% of cases, compared to 2.3% in remaining patients. This documents that the lower ATIII and CRP are on POD1, the worse their clinical outcome can be expected. Therefore, ATIII and CRP values might be directly incorporated into clinical decision making. However, all patients who meet the 3-60 criteria may benefit from extensive microbial sampling and immediate postoperative antibiotic therapy, as the high susceptibility for infectious complications in patients with LD after resection is well known (2,16). Even though there is no level 1 evidence on postoperative antibiotic prophylaxis, some data suggest that antibiotic treatment might improve the outcome of patients suffering from systemic inflammatory response syndrome or liver failure after hepatic resection (17,18). In addition, therapy with the molecular adsorbent recirculating system was shown to attenuate hyperbilirubinemia and hepatic encephalopathy in patients with postoperative LD, even though its impact on survival was limited in more recent analyses (19,20). However, growing evidence suggests that liver support devices are primarily effective, if they are introduced early, which further underlines the importance of a tool for early risk assessment, such as the 3-60 criteria (21,22). Here, the 3-60 criteria might lead to augmented results, as early introduction of the molecular absorbent and recirculation system might be used as a bridging therapy for patients classified as high risk no later than POD1, which in turns could lead to further improvement of patients’ outcome. However, this has to be evaluated in further studies. Nevertheless, early prediction of LD might also path the way for targeted therapeutic interventions supporting liver regeneration.
Importantly, after having identified the 3-60 criteria as a valuable tool for identification of patients with an increased risk for postoperative mortality in a cohort of patients undergoing liver resection for mCRC, we were able to validate our results in an independent cohort of patients from four different institutions. Of note, there were significant differences between the evaluation and the validation cohort, most likely due to distinct differences in preoperative treatment modalities like neoadjuvant chemotherapy and other oncological interventions. Indeed, patients in the evaluation cohort showed reduced liver function when compared to the validation cohort. Nevertheless, the 3-60 criteria proved to be applicable in a mixed set of patients undergoing liver resection, representing the actual clinical situation. This further highlights the robustness of ATIII and CRP as routine clinical markers for LD and undoubtedly renders the results clinically applicable. Of note, the multi-center validation cohort was set up as a consecutive series of patients undergoing liver resection irrespective of indication. Even though this heterogeneity added an additional degree of complexity in terms of prediction of outcome, the 3-60 criteria were able to stratify patients at risk for postoperative mortality.
In addition, the 3-60 criteria remained significant upon multivariable analysis including predictors of LD and confounding factors, whereas the otherwise equally good ISGLS criteria ultimately failed to prove independency. While this data strongly warrants the use of the 3-60 criteria in clinical risk assessment early after liver resection, it also poses one main weakness of this study, as the calculation was performed in the combined set of patients. As the incidence of postoperative mortality in all included institutions was less than 3%, pooling of the data was necessary to allow solid conclusions. However, the 3-60 criteria were found to show a clear association to postoperative mortality in both the evaluation and validation cohort, which renders the results scientifically sound.
In conclusion, the 3-60 criteria on POD1 independently predict postoperative LD and challenge established criteria to predict mortality after liver resection. As the 3-60 criteria are assessed on POD1 they allow adjustment of patients’ management and induction of supportive therapy immediately after liver resection.
Figure S1.
CRP on POD1 allows identification of patients suffering from LD or mortality. ROC curve for CRP on POD1 was performed for detection of patients that developed postoperative LD (A), as well as for patients that died within 90 postoperative days (B). AUC, area under the curve; LD, liver dysfunction; ROC, receiver operating characteristic; CRP, C-reactive protein; POD, postoperative day.
Acknowledgements
None.
Ethical Statement: The study was approved by the institutional ethics committee (#424/2010; #2032/2013) and registered at a clinical trials registry (ClinicalTrials.gov Identifier: NCT01700231).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Starlinger P, Assinger A. Importance of platelet-derived growth factors in liver regeneration. Expert Rev Gastroenterol Hepatol 2016;10:557-9. 10.1586/17474124.2016.1158100 [DOI] [PubMed] [Google Scholar]
- 2.Schindl MJ, Redhead DN, Fearon KC, et al. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut 2005;54:289-96. 10.1136/gut.2004.046524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Broek MA, Olde Damink SW, Dejong CH, et al. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int 2008;28:767-80. 10.1111/j.1478-3231.2008.01777.x [DOI] [PubMed] [Google Scholar]
- 4.Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713-24. 10.1016/j.surg.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 5.Balzan S, Belghiti J, Farges O, et al. The "50-50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg 2005;242:824-8, discussion 828-9. 10.1097/01.sla.0000189131.90876.9e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg 2007;204:854-62; discussion 862-4. 10.1016/j.jamcollsurg.2006.12.032 [DOI] [PubMed] [Google Scholar]
- 7.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. J Hepatol 2012;57:692-4. 10.1016/j.jhep.2012.04.016 [DOI] [PubMed] [Google Scholar]
- 8.Moshage HJ, Roelofs HM, van Pelt JF, et al. The effect of interleukin-1, interleukin-6 and its interrelationship on the synthesis of serum amyloid A and C-reactive protein in primary cultures of adult human hepatocytes. Biochem Biophys Res Commun 1988;155:112-7. 10.1016/S0006-291X(88)81056-8 [DOI] [PubMed] [Google Scholar]
- 9.Heinz S, Braspenning J. Measurement of Blood Coagulation Factor Synthesis in Cultures of Human Hepatocytes. Methods Mol Biol 2015;1250:309-16. 10.1007/978-1-4939-2074-7_23 [DOI] [PubMed] [Google Scholar]
- 10.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 2005;12:351-5. 10.1007/s00534-005-0999-7 [DOI] [PubMed] [Google Scholar]
- 11.Schiergens TS, Dorsch M, Mittermeier L, et al. Thirty-day mortality leads to underestimation of postoperative death after liver resection: A novel method to define the acute postoperative period. Surgery 2015;158:1530-7. 10.1016/j.surg.2015.07.019 [DOI] [PubMed] [Google Scholar]
- 12.Pereyra D, Offensperger F, Klinglmueller F, et al. Early prediction of postoperative liver dysfunction and clinical outcome using antithrombin III-activity. PLoS One 2017;12:e0175359. 10.1371/journal.pone.0175359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Mierlo KM, Schaap FG, Dejong CH, et al. Liver resection for cancer: new developments in prediction, prevention and management of postresectional liver failure. J Hepatol 2016;65:1217-31. 10.1016/j.jhep.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 14.Clavien PA, Petrowsky H, DeOliveira ML, et al. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med 2007;356:1545-59. 10.1056/NEJMra065156 [DOI] [PubMed] [Google Scholar]
- 15.Skrzypczyk C, Truant S, Duhamel A, et al. Relevance of the ISGLS definition of posthepatectomy liver failure in early prediction of poor outcome after liver resection: study on 680 hepatectomies. Ann Surg 2014;260:865-70; discussion 870. 10.1097/SLA.0000000000000944 [DOI] [PubMed] [Google Scholar]
- 16.Hammond JS, Guha IN, Beckingham IJ, et al. Prediction, prevention and management of postresection liver failure. Br J Surg 2011;98:1188-200. 10.1002/bjs.7630 [DOI] [PubMed] [Google Scholar]
- 17.Stravitz RT, Kramer DJ. Management of acute liver failure. Nat Rev Gastroenterol Hepatol 2009;6:542-53. 10.1038/nrgastro.2009.127 [DOI] [PubMed] [Google Scholar]
- 18.Togo S, Tanaka K, Matsuo K, et al. Duration of antimicrobial prophylaxis in patients undergoing hepatectomy: a prospective randomized controlled trial using flomoxef. J Antimicrob Chemother 2007;59:964-70. 10.1093/jac/dkm028 [DOI] [PubMed] [Google Scholar]
- 19.van de Kerkhove MP, de Jong KP, Rijken AM, et al. MARS treatment in posthepatectomy liver failure. Liver Int 2003;23 Suppl 3:44-51. 10.1034/j.1478-3231.23.s.3.2.x [DOI] [PubMed] [Google Scholar]
- 20.Rittler P, Ketscher C, Inthorn D, et al. Use of the molecular adsorbent recycling system in the treatment of postoperative hepatic failure and septic multiple organ dysfunction--preliminary results. Liver Int 2004;24:136-41. 10.1111/j.1478-3231.2004.0898.x [DOI] [PubMed] [Google Scholar]
- 21.Gilg S, Sparrelid E, Saraste L, et al. The molecular adsorbent recirculating system in posthepatectomy liver failure: Results from a prospective phase I study. Hepatol Commun 2018;2:445-54. 10.1002/hep4.1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He GL, Feng L, Duan CY, et al. Meta-analysis of survival with the molecular adsorbent recirculating system for liver failure. Int J Clin Exp Med 2015;8:17046-54. [PMC free article] [PubMed] [Google Scholar]