Abstract
Background
Bile leaks are one of the most common complications after liver resection. The International Study Group of Liver Surgery (ISGLS) established a uniform bile leak definition including a severity grading. However, a risk factor assessment according to ISGLS grading as well as the clinical implications has not been studied sufficiently so far.
Methods
The incidence and grading of bile leaks according to ISGLS were prospectively documented in 501 consecutive liver resections between July 2012 and December 2016. A multivariate regression analysis was performed for risk factor assessment. Association with other surgical complications, 90-day mortality as well as length of hospital stay (LOS) was studied.
Results
The total rate of bile leaks in this cohort was 14.0%: 2.8% grade A, 8.0% grade B, and 3.2% grade C bile leaks were observed. Preoperative chemotherapy or biliary intervention, diagnosis of hilar cholangiocarcinoma, colorectal metastasis, central minor liver resection, major hepatectomy, extended hepatectomy or two-stage hepatectomy, were some of the risk factors leading to bile leaks. The multivariate regression analysis revealed that preoperative chemotherapy, major hepatectomy and biliodigestive reconstruction remained significant independent risk factors for bile leaks. Grade C bile leaks were associated not only with surgical site infection, but also with an increased 90-day mortality and prolonged LOS.
Conclusions
The preoperative treatment as well as the surgical procedure had significant influence on the incidence and the severity of bile leaks. Grade C bile leaks were clinically most relevant, and led to significant increased LOS, rate of infection, and mortality.
Keywords: Bile leakage, post-operative complication, liver resection, International Study Group of Liver Surgery (ISGLS), Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS)
Introduction
Advances in surgical techniques have expanded the boundaries of liver resections towards greater resection dimensions and more complex resection strategies on the one hand (1-3), but also towards reduced invasiveness by laparoscopic approaches or parenchyma-sparing on the other (4-6). Enhanced recovery after surgery protocols (ERAS) promises improved surgical outcome and reduced hospitalization (7,8). Assessment of postoperative complications and identifying risk factors could further optimize the outcome after liver resection.
Bile leaks are one of the most common complications after liver resection with incidence rates between 6.5% and 27.2% (9-14). After resections with biliary reconstruction by biliodigestive anastomoses (BDA), leak rates increase to 36.9% (15), while rates are low when the extrahepatic bile duct preserved (3.6–8.0%) (16-21). Due to discrepancies in international reports and a lack of global standardized classifications, the International Study Group of Liver Surgery (ISGLS) established a uniform bile leak definition including a severity grading in 2010 (22). The ISGLS definition has since been verified as a suitable grading tool and has been associated with postoperative morbidity and mortality (10,12,15).
Since the dimension of bile leakage has proven to be relevant for postoperative outcomes, the goal of our current analysis was to assess risk factors discriminatingly for the three ISGLS grades of bile leaks. At the same time, the aim of this study was to identify patients with a low incidence of bile leaks that would benefit from a precise “no drainage” policy as suggested by ERAS protocol. Our heterogeneous study group comprised straightforward resection, laparoscopic minor resections as well as complex procedures including biliary reconstructions and staged hepatectomy cases [Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS)].
Methods
Study design
Risk factors for bile leaks after liver resections and the clinical patient outcome at our institution were analyzed in a prospective observational cohort study. All perioperative and follow up data of liver resections performed between July 2012 and December 2016 were prospectively entered into a database. Cases involving simultaneous major resections of other organs (e.g., stomach, pancreas, colon) were excluded from the analysis. Postoperative bile leaks were documented and graded according to the definition of ISGLS (22). Risk factors for bile leaks and patient outcome were assessed. The study was approved by the institutional research review committee.
Bile leakage definition
According to the ISGLS definition, total bilirubin levels in the abdominal drain fluid of three times the serum concentration from the third postoperative day (POD) onwards, or the need for interventional or operative treatment of biliary collections, or biliary peritonitis were considered as bile leaks (22). Leaks were categorized in three grades of severity: grade A required no change in patient management and intraoperatively placed drains were left no longer than POD 7; grade B involved therapeutic interventions different from surgery or retaining intraoperatively placed drains for more than 7 days; grade C leaks were surgically treated by reoperation (22).
Surgical procedures
Analyzed procedures comprised both open and laparoscopic liver resections. The extent of the procedures was divided into minor (non-anatomic resection or resections of less than 3 segments) and major resections (resection of three and more liver segments) (23). All laparoscopic procedures were minor resections during the study phase. ALPPS (24) was performed in patients with assumed remnant liver tissue of less than 25% of the total liver volume or less than 0.6% of body weight in normal livers, or less than 40% of total liver volume or 0.8% of body weight in patients with macro-steatosis or fibrosis. Patients with hilar cholangiocarcinoma or liver cirrhosis were not considered for ALPPS procedures.
An ultrasonic cavitation device (CUSA; Valleylab, Boulder, CO, USA) was routinely used for liver parenchymal transection in open procedures. In laparoscopic procedures, energy devices (ultrasonic, bipolar, or combined) were used as standard dissection tool.
A BDA was carried out as mucosa-mucosa hepaticojejunostomy with a retrocolic Roux-en-Y limb when the extrahepatic bile duct was resected. The white-test, an intraoperative bile leak test, was performed routinely in central resections or surgery close to the hilar plate, but not in patients with extrahepatic bile duct resection and BDA due to technical limitations (25). Capillary abdominal drains were routinely used as a fluid collection system.
The management of bile leaks was adapted according to the daily drainage output volume, clinical features as well as the type of biliary tract reconstruction which had been performed. Reoperation was performed in the following situation: (I) bile leak associated peritonitis or sepsis; (II) daily drainage output over 500 mL without decreasing potential despite endoscopic or radiological intervention; (III) uncontrolled bile leak from the incision site.
Data collection
Preoperative data collected in the database included patient age, gender, body-mass index (BMI), diagnosis of the underlying liver disease, medical history including diabetes, previous liver resections, previous endoscopic (ERCP) or interventional radiological (PTCD) treatments, previous chemotherapy (within 4 weeks prior to surgery), and risk of bleeding (defined as anticoagulant or antiplatelet therapy, thrombocytopenia <100 million/mL, and/or increased international normalized ratio, INR >1.5). Additionally, the type of hepatectomy and cases of BDA reconstruction were documented.
Postoperatively histology reports, other postoperative complications including post-hepatectomy liver failure (PHLF), post-hepatectomy hemorrhage (PHH), surgical site infections (SSI), length of hospital stay (LOS), and 90-day mortality were recorded and analyzed. PHLF was defined as an increased INR and concomitant hyperbilirubinemia on or after POD 5 (26). PHH was defined as a drop in hemoglobin level >3 g/dL postoperatively compared to the postoperative baseline level and/or any postoperative transfusion of packed red blood cells for a decreasing hemoglobin and/or the need for radiological intervention and/or re-laparotomy to stop bleeding (27). We used the definition of SSI according to the “Guideline for Prevention of Surgical Site Infection, 1999” by the Centers for Disease Control and Prevention (28,29).
Statistical analysis
Descriptive analyses were performed to compare patient baseline characteristics within groups. Means and standard deviations (SD) were used to describe the distribution of continuous variables and percentages were used for categorical variables. Variables were tested using binary logistic regression. A step-wise forward multivariable logistic regression model was built to predict the probability of bile leaks on the basis of significant co-variables from the univariable analysis. Results are reported as odds ratio (OR) and confidence interval (CI). LOS was analyzed by a log rank test and displayed as Kaplan Meier curve. A P value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS 22 (IBM, Chicago, USA).
Results
Study group characteristics and incidence of bile leaks
The study group included a total of 501 liver resections. The age of patients ranged from 18 to 85 years (61.2±13.7). There were 177 (35.3%) major liver resections and 324 (64.7%) minor liver resections. Sixty-nine patients (13.8%) received a BDA. Fifty-two patients (10.4%) underwent ALPPS procedures. The total rate of bile leaks was 14.0% (2.8% grade A, 8.0% grade B, and 3.2% grade C).
Risk factor analysis for bile leak
Comorbidities and indications for surgery
Patient sex, age, BMI, as well as rates of diabetes mellitus were similar in both patients with and without bile leaks (see Table 1).
Table 1. Preoperative risk factors for bile leaks.
| Preoperative risk factors | Rate of bile leaks | Odds ratio (CI) | P value |
|---|---|---|---|
| Overall | 14.0% | – | – |
| Gender (female) | 11.9% | 1.357 (0.795–2.316) | 0.26 |
| Age | – | 1.015 (0.995–1.035) | 0.14 |
| BMI | – | 0.958 (0.909–1.009) | 0.10 |
| Diabetes mellitus | 11.5% | 0.766 (0.375–1.564) | 0.46 |
| Repeated liver resection | 16.9% | 1.391 (0.817–2.368) | 0.22 |
| Chemotherapy within 4 weeks | 23.0% | 2.298 (1.028–5.137) | 0.04 |
| Biliary intervention (ERC/PTC) | 34.5% | 4.088 (2.182–7.657) | <0.001 |
| Bleeding risk factors | 8.4% | 0.519 (0.229–1.177) | 0.12 |
Chemotherapy before surgery and preoperative biliary interventions increased the bile leak rate significantly. Definition bleeding risk factors: anticoagulant or antiplatelet therapy, thrombocytopenia <100 mio/mL, and/or increased international normalized ratio, INR <1.5. CI, confidence interval; BMI, body-mass index; INR, international normalized ratio.
Bile leaks occurred significantly more often in patients with diseases of the biliary tract (defined as intrahepatic cholangiocarcinoma, peri-hilar cholangiocarcinoma, gallbladder carcinoma, benign biliary tumors, and benign biliary diseases) than in patients with other indications (23.6% vs. 11.3%, P=0.001, Table 2). Grade C bile leaks were most frequent in patients with hilar cholangiocarcinoma (29.4%). Bile leaks were least common in patients with non-biliary benign lesions (4.7%). In case of non-biliary malignant disease, bile leaks were most common in patients with colorectal liver metastases (17.3%, mostly grade A and B bile leaks). Meanwhile, bile leaks were uncommon in patients with hepatocellular carcinoma (7.6%). Moreover, the presence of fibrotic or cirrhotic liver disease was associated with a lower rate of postoperative bile leaks (6.3%, P=0.04).
Table 2. Association of indications for liver resection, underlying liver disease, and rate of bile leaks.
| Diagnosis and liver disease | Rate of bile leaks | Odds ratio (CI) | P value |
|---|---|---|---|
| Biliary | 23.6% | 2.331 (1.422–4.190) | 0.001 |
| Intrahepatic CCA | 18.4% | ||
| Extrahepatic CCA | 29.4% | ||
| Gallbladder carcinoma | 12.5% | ||
| Benign | 31.6% | ||
| Non-biliary | 11.3% | – | – |
| HCC | 7.6% | ||
| CRLM | 17.3% | ||
| Other LM | 11.6% | ||
| Benign | 4.7% | ||
| Fibrosis/cirrhosis | 6.3% | 0.365 (0.142–0.938) | 0.04 |
Biliary diagnoses were associated with an increased rate of bile leaks compared to non-biliary indications. Fibrotic liver deterioration appeared to have a protective effect. CI, confidence interval; CCA, cholangiocarcinoma; CRLM, colorectal liver metastasis; HCC, hepatocellular carcinoma; LM, liver metastasis.
Preoperative treatment (see Table 1)
Previous liver procedures and increased risk for bleeding (coagulation disorder, thrombocytopenia, or anticoagulant treatment) were not found to be risk factors for bile leaks. Patients who had received chemotherapy within four weeks prior to surgery (n=35) had a significantly increased rate of bile leaks (23.0% vs. 13.1%, P<0.05). However, none of these patients developed a grade C bile leak. Of these patients, 31 had colorectal liver metastasis and only two patients had received bile duct reconstruction via BDA. Twenty-four patients had received biological antibody treatment (seven bevacizumab, 17 cetuximab or panitumumab). Biologicals or different chemotherapy regimens were not found to be a risk factor for bile leaks.
Patients who had required non-operative interventional biliary drainage (ERCP/PTCD) before surgery presented a high rate of grade C bile leaks (overall rate of bile leaks 34.5%, grade C leaks 10.9%).
Surgical procedures (see Table 3)
Table 3. Association of operative procedures on the rate of bile leaks.
| Operative procedures | Rate of bile leaks | Odds ratio (CI) | P value |
|---|---|---|---|
| Major liver resection | 23.7% | 3.289 (1.956–5.530) | <0.001 |
| Right hepatectomy | 19.7% | ||
| Left hepatectomy | 15.4% | ||
| Extended right hepatectomy | 30.0% | ||
| Extended left hepatectomy | 24.2% | ||
| Minor liver resection | 8.6% | – | – |
| Left lateral resection | 0.0% | ||
| Peripheral NAR extended | 9.7% | ||
| Peripheral NAR limited | 6.4% | ||
| Central NAR | 15.2% | ||
| ALPPS | 23.0% | 2.022 (1.003–4.079) | 0.049 |
| With BDA | 31.9% | 3.745 (2.079–6.746) | <0.001 |
Major liver resections—especially extended right hepatectomies—were associated with an increased risk for bile leaks. BDA reconstruction had also a higher rate of bile leaks. CI, confidence interval; ALPPS, associating liver partition and portal vein ligation for staged hepatectomy; BDA, biliodigestive anastomosis; NAR, non-anatomic resection.
Major liver resections were associated with significantly higher rates of bile leaks than minor resections (23.7% vs. 8.6%, P<0.01). Within the major resection group, patients with right hemi-hepatectomy had higher rates of grade A and grade B bile leaks (grade A 4.4%, grade B 13.0%) while grade C bile leaks were mostly observed in patients undergoing left or right extended hepatectomy (grade C 11.7%).
Within the minor resection group, central non-anatomic resections stood out with above average bile leak rates (all bile leaks 15.2%, grade A 3.2%, grade B 12.0%, grade C 0%).
ALPPS procedures were associated with significantly increased rates of bile leaks (23.0%, P=0.049).
Furthermore, BDA reconstructions were accompanied by significantly increased bile leak rates in all grades (31.9%, P<0.001), especially grade C bile leaks (11.6%). In comparison, patients without BDA reconstruction had a bile leak rate of 11.1% (grade C 1.9%).
Multivariate analysis (see Table 4)
Table 4. Multivariate analysis of risk factors for bile leaks.
| Risk factors | Odds ratio (CI) | P value |
|---|---|---|
| Chemotherapy within 4 weeks | 2.850 (1.229–6.608) | 0.02 |
| Biliary liver disease | – | 0.64 |
| Fibrosis/cirrhosis | – | 0.07 |
| Major liver resection | 2.550 (1.419–4.584) | 0.002 |
| With BDA | 2.387 (1.230–4.633) | 0.01 |
| ALPPS | – | 0.18 |
Chemotherapy, major liver resections, and BDA reconstructions were independent risk factors in the multivariate analysis. CI, confidence interval; ALPPS, associating liver partition and portal vein ligation for staged hepatectomy; BDA, biliodigestive anastomosis.
In a step-wise forward logistic regression model chemotherapy within 4 weeks before the procedure, major liver resection, and BDA reconstruction remained significant independent risk factors for bile leaks. Of note, the variables BDA reconstruction, disease of the biliary track, and preoperative interventions as ERCP or PTCD had high reciprocal correlation scores and, thus, were interdependent.
Clinical implication of bile leaks (see Table 5)
Table 5. Association of bile leaks and other postoperative complications.
| Complications | Overall (n=501) | No bile leak (n=431) | Bile leak (n=70) | Odds ratio (CI) | P value |
|---|---|---|---|---|---|
| Surgical site infect | 9.4% [47] | 7.7% [33] | 20.0% [14] | 3.015 (1.520–5.981) | 0.002 |
| Superficial | 7.0% [35] | 6.0% [26] | 12.9% [9] | ||
| Deep | 1.6% [8] | 0.9% [4] | 5.7% [4] | ||
| Organ/space | 0.8% [4] | 0.7% [3] | 1.4% [1] | ||
| PHLF | 5.0% [25] | 4.2% [18] | 10.0% [7] | 2.549 (1.024–6.349) | 0.044 |
| Grade A | 0.6% [3] | 0.2% [1] | 2.9% [2] | ||
| Grade B | 3.2% [16] | 2.8% [12] | 5.7% [4] | ||
| Grade C | 1.2% [6] | 1.2% [5] | 1.4% [1] | ||
| PHH | 5.6% [28] | 5.8% [25] | 4.3% [3] | 0.727 (0.214–2.476) | 0.610 |
| Grade A | 2.4% [12] | 2.6% [11] | 1.4% [1] | ||
| Grade B | 0.4% [2] | 0.5% [2] | 0.0% [0] | ||
| Grade C | 2.8% [14] | 2.8% [12] | 2.9% [2] | ||
| 90-day mortality | 2.6% [13] | 1.9% [8] | 7.1% [5] | 4.067 (1.291–12.813) | 0.017 |
The incidence of surgical site infections, PHLF, and 90-day mortality were increased in patients with bile leaks. CI, confidence interval; PHH, post-hepatectomy hemorrhage; PHLF, post-hepatectomy liver failure.
Surgical site infections occurred more often in patients with bile leaks (mainly grade B or C but not grade A). All infection categories were elevated in grade C leaks, while only superficial infections occurred in grade B. Bile leaks were not found to be associated with post-hepatectomy hemorrhage. Grade C bile leaks, mainly after an extended right hepatectomy, were associated with PHLF (OR 2.549, P=0.044).
LOS was increased in patients with bile leaks regardless of the grading (see Figure 1, P<0.05). The overall 90-day mortality in this cohort was 2.6%, with 1.9% in patients without bile leak, nil in grade A, 1/40 (2.5%) in grade B and 4/16 (25%) in grade C bile leak. The patient having grade B bile leak died after cholangio-sepsis after PTC intervention for treatment of the persistent bile leak. One of four patients having grade C bile leak died of fulminant aspiration pneumonia that was not related to the bile leak itself. A relation between the bile leaks and the causes of death in the other three patients cannot be excluded. Two patients died of septic multi organ failure, one of these after several reoperations including a surgical revision of the BDA due to progressive postoperative cholestasis. The last patient died of hemorrhagic multi organ failure after delayed abdominal bleeding. All of these five patients were older than 65 years (three of them were above 75 years) and all had received an extended liver resection (four right and one left extended hepatectomy).
Figure 1.
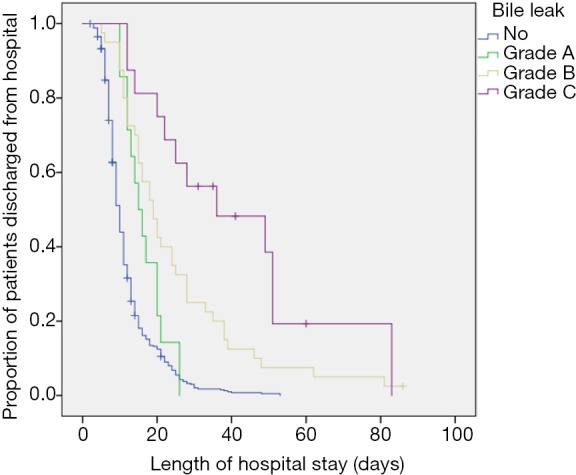
Length of hospital stay. Bile leaks increased the length of hospital stay (P<0.05). The severer the bile leak grading, the longer became the hospital stay. Kaplan-Meier plot.
Discussion
The incidence of bile leaks after liver resection depends greatly on a center’s surgical repertoire, its risk tolerance, and, not least, on its surgical experience. Thus, the reported incidence rates of bile leaks after liver resection diverge significantly between centers (6.5–27.2%) (9-14). Our study confirmed that surgical indications, preoperative treatment as well as the extent of surgical procedures have an influence on the incidence of bile leaks.
The ISGLS severity grading system of bile leaks was developed to allow adequate standardization and comparability for clinical research purposes (22). In our cohort, grade A bile leaks occurred in 2.8%, grade B in 8.0%, and grade C in 3.2% of all cases (literature: grade A 2.2–9.5%, grade B 3.4–27.4%, grade C 0–4.1%) (10-12,15). These heterogeneous results could be primarily attributed to the analyzed patient cohorts and, thus, comparability between centers is usually impractical because of varying patient characteristics. Moreover, treatment strategies especially of severe bile leaks vary significantly between centers and comparability of results remains challenging in spite of the ISGLS grading systems even if a similar patient population could be assumed. According to the ISGLS definition, the incidence of grade C bile leaks is highly influenced by a center’s reoperation policy, especially in patients undergoing BDA. Ferrero et al., e.g., suggested a low indication threshold for revision operations in cases of bile leakage after BDA reconstructions (9). Bile leakage was significantly associated with severe abdominal bleeding as well as an increased mortality rate in their analysis. On the other hand, Taguchi et al. and Hoekstra et al. did not re-operate on any bile leak after BDA reconstruction, thus producing no grade C bile leak at all in this group (15,30). Nevertheless, none of the patients with severe bile leaks associated with BDA reconstruction died in the postoperative course in either of both reports. The grading system is consequently dependent on subjective treatment strategies and has a limited informative value especially distinguishing severity and relevance of grade B and grade C bile leaks. In liver resection without biliary reconstruction on the other hand, there appears to be wider consensus that non-operative interventional treatment of relevant biliary leaks should be the rule and surgical approaches the exception (31-33).
Although comparing incidences and severity of bile leaks between centers appears to be impracticable, a severity grading system is important to identify risk factors and their clinical importance. In our cohort, we were able to identify several risk factors for bile leaks after liver resection.
Patients with a biliary disease, who had required interventions of the biliary track before the operation and who usually received a BDA reconstruction, had a high risk for severe bile leaks. The severity of bile leaks correlates in particular with the site of leakage and the involvement of major bile duct branches (19). Biliary reconstruction with BDA increases the risk of bile leakage substantially. In a large multi-centric cohort, Martin et al. described a bile leak rate of 32% in patients receiving a BDA reconstruction during major liver resections compared to only 10% without biliary reconstruction (14). Nagino et al. reported a comparably low bile leakage rate of 17% after major liver resection with BDA reconstruction. Of note, almost half of these cases were attributed to anastomotic leaks (34). A congested and infected biliary system is certainly impairing the healing process and stability of BDAs and closure sites of bile duct stumps, thus causing severe bile leakage especially after resection of hilar cholangiocarcinoma that had required interventional drainage preoperatively (ERCP or PTCD). Hence, pre- and intraoperative bile cultures and continuous perioperative antibiotic prophylaxis have to be mandatory for patients with high risk of bile leakage. Considering the high incidence of bile leaks after BDA reconstruction, relieving the biliary pressure by a trans-anastomotic drainage or stent might appear feasible. However, the potential benefit of this technique has to outweigh the risk of complications. Furthermore, a clear advantage of trans-anastomotic drains in regards to the incidence of bile leaks remains unclear (35). Hence, trans-anastomotic drains are used very rarely at our clinic.
Patients who had received chemotherapy prior to liver resection suffered more often from grade A and B leaks. In our limited cohort, an addition of biologicals to the medical regimen did not appear to have a major impact within this group. Guillaud et al. suggested in their analysis that healing impairment of minor bile leaks was one possible explanation for an increased bile leak rate after preoperative chemotherapy (16). Analogously to Karoui et al. (36), we have the impression that the predominant cause of bile leaks in soft and fragile post-chemotherapy liver parenchyma is the problematic intraoperative identification and closure of miniature bile ducts. Since neoadjuvant chemotherapy is common in particular for colorectal metastases, this indication group was associated with increased rates of minor bile leaks in our analysis. Although LOS is prolonged for these patients, there is no increase in perioperative mortality. In contrast to soft post-chemotherapy parenchyma, the tougher tissue of fibrotic livers appeared to have a protective effect on the occurrence of bile leaks, although it did not prove to be a significant independent influence factor in the multivariate analysis in this cohort. A similar correlation has been described by Capussotti et al. (17). However, this correlation might be flawed by the generally reduced extent of resection in these pre-damaged livers.
Major liver resections were associated with increased rates of all three grades of bile leaks. In that regards our results resemble the previous literature cited in this paper.
The clinical relevance of bile leaks has rarely been quantified in regards to severity scores. In general, bile leaks have been associated with increased rates of mortality and complications like postoperative bleeding, peritonitis, and organ failure (9,20-22). Furthermore, LOS increased in patients with bile leaks—a plausible effect of the additional treatment period (9,10,17,19-22).
In our analysis, only grade C bile leaks were associated with a higher mortality, while LOS was increased in all grades and gradually rising with severity. In that respect, our results resemble that of Brooke-Smith et al., who found an increased LOS in all bile leaks grades, with grade C bile leaks standing out with almost three times the LOS of grade A and B bile leaks (10). Koch et al., on the other hand, associated an increased LOS with grade B and C bile leaks only, while grade A bile leaks did not face additional treatment time (22).
Additionally, we found surgical site infections to be relevant primarily in grade C bile leaks. Other groups have not investigated this aspect before. Different to less severe bile leaks, grade C leaks are not adequately treated by drains resulting in bile collections that are prone to superinfection. These superinfections entail both superficial and deeper wound infections.
PHLF and mortality were relevantly increased in grad C leaks in our analysis. All of these patients had received extended liver resections and were relatively old; hence, the occurrence of a bile leak itself appears not to be an independent risk factor for poor outcome. Generally, it is well known that perioperative risk increases gradually with age, highlighting the necessity for careful indication of higher risk procedures in this patient group (37-41).
Techniques for intraoperative detection of bile leaks, e.g., the white-test (25), have the potential to reduce bile leak rates significantly; however the clinical effect remains controversial. Yamashita et al. found an advantage in using an intraoperative bile leakage test and suggested it especially for high-risk surgical procedures (20). Ijichi et al., on the other hand, did not find intraoperative bile leak tests to have a benefit in their cohort of liver resections (42). However, neither of those study groups used such a test in cases including biliary reconstruction. Especially these cases proved to have the highest risk for bile leaks in our analysis. The staining fluid of a detection test is usually introduced to the biliary tract via a small branch like the cystic duct. Finding an appropriate access can be very challenging, especially in extended liver resections. Nevertheless, we suggest a higher dedication in carrying out such a test especially in difficult cases and recommend its use whenever possible.
In contrast to high risk procedures mentioned above, we have identified low risk constellations (e.g., left lateral resections and resection of benign non biliary lesions) with minimal bile leak rates. In times of ERAS, this group of patients would definitely benefit from a “no drainage” policy after liver resection.
An important limitation of our study is the relatively small number of laparoscopic liver resections, which are increasingly performed. Even major laparoscopic hepatectomies have become safe and feasible (43). Still, BDA reconstructions and staged hepatectomies appear to be technical demanding for laparoscopic procedures. Hence, the promising results of laparoscopic liver resection with lower bile leak rate have to be perceived with great caution
In conclusion, surgical indications, preoperative treatment as well as surgical procedure have been found to not only have an influence on the incidence of bile leaks but also the severity of bile leaks. Grade C bile leaks are clinically most relevant and are associated with malignant and benign biliary diseases that require preoperative biliary interventions as well as BDA reconstruction. These patients in particular require increased awareness peri-operatively, e.g., by intraoperative bile leakage tests.
Acknowledgements
None.
Ethical Statement: The study was approved by the institutional research review committee.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Giglio MC, Giakoustidis A, Draz A, et al. Oncological Outcomes of Major Liver Resection Following Portal Vein Embolization: A Systematic Review and Meta-analysis. Ann Surg Oncol 2016;23:3709-17. 10.1245/s10434-016-5264-6 [DOI] [PubMed] [Google Scholar]
- 2.Schadde E, Schnitzbauer AA, Tschuor C, et al. Systematic review and meta-analysis of feasibility, safety, and efficacy of a novel procedure: associating liver partition and portal vein ligation for staged hepatectomy. Ann Surg Oncol 2015;22:3109-20. 10.1245/s10434-014-4213-5 [DOI] [PubMed] [Google Scholar]
- 3.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002;236:397-406; discussion 406-7. 10.1097/00000658-200210000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciria R, Cherqui D, Geller DA, et al. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg 2016;263:761-77. 10.1097/SLA.0000000000001413 [DOI] [PubMed] [Google Scholar]
- 5.Jin B, Chen MT, Fei YT, et al. Safety and efficacy for laparoscopic versus open hepatectomy: A meta-analysis. Surg Oncol 2018;27:A26-34. 10.1016/j.suronc.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 6.He J, Amini N, Spolverato G, et al. National trends with a laparoscopic liver resection: results from a population-based analysis. HPB (Oxford) 2015;17:919-26. 10.1111/hpb.12469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouxel P, Beloeil H. Enhanced recovery after hepatectomy: A systematic review. Anaesth Crit Care Pain Med 2019;38:29-34. 10.1016/j.accpm.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 8.Ratti F, Cipriani F, Reineke R, et al. Impact of ERAS approach and minimally-invasive techniques on outcome of patients undergoing liver surgery for hepatocellular carcinoma. Dig Liver Dis 2016;48:1243-8. 10.1016/j.dld.2016.06.032 [DOI] [PubMed] [Google Scholar]
- 9.Ferrero A, Russolillo N, Vigano L, et al. Safety of conservative management of bile leakage after hepatectomy with biliary reconstruction. J Gastrointest Surg 2008;12:2204-11. 10.1007/s11605-008-0586-8 [DOI] [PubMed] [Google Scholar]
- 10.Brooke-Smith M, Figueras J, Ullah S, et al. Prospective evaluation of the International Study Group for Liver Surgery definition of bile leak after a liver resection and the role of routine operative drainage: an international multicentre study. HPB (Oxford) 2015;17:46-51. 10.1111/hpb.12322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panaro F, Hacina L, Bouyabrine H, et al. Risk factors for postoperative bile leakage: a retrospective single-center analysis of 411 hepatectomies. Hepatobiliary Pancreat Dis Int 2016;15:81-6. 10.1016/S1499-3872(15)60424-6 [DOI] [PubMed] [Google Scholar]
- 12.Rahbari NN, Elbers H, Koch M, et al. Bilirubin level in the drainage fluid is an early and independent predictor of clinically relevant bile leakage after hepatic resection. Surgery 2012;152:821-31. 10.1016/j.surg.2012.03.012 [DOI] [PubMed] [Google Scholar]
- 13.Yokoo H, Miyata H, Konno H, et al. Models predicting the risks of six life-threatening morbidities and bile leakage in 14,970 hepatectomy patients registered in the National Clinical Database of Japan. Medicine (Baltimore) 2016;95:e5466. 10.1097/MD.0000000000005466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin AN, Narayanan S, Turrentine FE, et al. Clinical Factors and Postoperative Impact of Bile Leak After Liver Resection. J Gastrointest Surg 2018;22:661-7. 10.1007/s11605-017-3650-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taguchi Y, Ebata T, Yokoyama Y, et al. The determination of bile leakage in complex hepatectomy based on the guidelines of the International Study Group of Liver Surgery. World J Surg 2014;38:168-76. 10.1007/s00268-013-2252-x [DOI] [PubMed] [Google Scholar]
- 16.Guillaud A, Pery C, Campillo B, et al. Incidence and predictive factors of clinically relevant bile leakage in the modern era of liver resections. HPB (Oxford) 2013;15:224-9. 10.1111/j.1477-2574.2012.00580.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capussotti L, Ferrero A, Vigano L, et al. Bile leakage and liver resection: Where is the risk? Arch Surg 2006;141:690-4; discussion 695. 10.1001/archsurg.141.7.690 [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa K, Tanaka K, Nojiri K, et al. Predictive factors for bile leakage after hepatectomy for hepatic tumors: a retrospective multicenter study with 631 cases at Yokohama Clinical Oncology Group (YCOG). J Hepatobiliary Pancreat Sci 2017;24:33-41. 10.1002/jhbp.411 [DOI] [PubMed] [Google Scholar]
- 19.Nagano Y, Togo S, Tanaka K, et al. Risk factors and management of bile leakage after hepatic resection. World J Surg 2003;27:695-8. 10.1007/s00268-003-6907-x [DOI] [PubMed] [Google Scholar]
- 20.Yamashita Y, Hamatsu T, Rikimaru T, et al. Bile leakage after hepatic resection. Ann Surg 2001;233:45-50. 10.1097/00000658-200101000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erdogan D, Busch OR, van Delden OM, et al. Incidence and management of bile leakage after partial liver resection. Dig Surg 2008;25:60-6. 10.1159/000118024 [DOI] [PubMed] [Google Scholar]
- 22.Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680-8. 10.1016/j.surg.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 23.Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333-39. HPB (Oxford) 2002;4:99; author reply 99-100. 10.1080/136518202760378489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Ewald F, Gulati A, et al. Associating liver partition and portal vein ligation for staged hepatectomy: From technical evolution to oncological benefit. World J Gastrointest Surg 2016;8:124-33. 10.4240/wjgs.v8.i2.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Malago M, Sotiropoulos GC, et al. Intraoperative application of "white test" to reduce postoperative bile leak after major liver resection: results of a prospective cohort study in 137 patients. Langenbecks Arch Surg 2009;394:1019-24. 10.1007/s00423-008-0455-7 [DOI] [PubMed] [Google Scholar]
- 26.Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713-24. 10.1016/j.surg.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 27.Rahbari NN, Garden OJ, Padbury R, et al. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB (Oxford) 2011;13:528-35. 10.1111/j.1477-2574.2011.00319.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 1999;20:250-78; quiz 279-80. 10.1086/501620 [DOI] [PubMed] [Google Scholar]
- 29.Togo S, Matsuo K, Tanaka K, et al. Perioperative infection control and its effectiveness in hepatectomy patients. J Gastroenterol Hepatol 2007;22:1942-8. 10.1111/j.1440-1746.2006.04761.x [DOI] [PubMed] [Google Scholar]
- 30.Hoekstra LT, van Gulik TM, Gouma DJ, et al. Posthepatectomy bile leakage: how to manage. Dig Surg 2012;29:48-53. 10.1159/000335734 [DOI] [PubMed] [Google Scholar]
- 31.Dechêne A, Jochum C, Fingas C, et al. Endoscopic management is the treatment of choice for bile leaks after liver resection. Gastrointest Endosc 2014;80:626-33.e1. 10.1016/j.gie.2014.02.1028 [DOI] [PubMed] [Google Scholar]
- 32.Schaible A, Schemmer P, Hackert T, et al. Location of a biliary leak after liver resection determines success of endoscopic treatment. Surg Endosc 2017;31:1814-20. 10.1007/s00464-016-5178-1 [DOI] [PubMed] [Google Scholar]
- 33.Viganò L, Ferrero A, Sgotto E, et al. Bile leak after hepatectomy: predictive factors of spontaneous healing. Am J Surg 2008;196:195-200. 10.1016/j.amjsurg.2007.08.062 [DOI] [PubMed] [Google Scholar]
- 34.Nagino M, Nishio H, Ebata T, et al. Intrahepatic cholangiojejunostomy following hepatobiliary resection. Br J Surg 2007;94:70-7. 10.1002/bjs.5531 [DOI] [PubMed] [Google Scholar]
- 35.Suzuki H, Shimura T, Mochhida Y, et al. To Stent or Not To Stent Hepaticojejunostomy--Analysis of Risk Factors for Postoperative Bile Leaks and Surgical Complication. Hepatogastroenterology 2014;61:920-6. [PubMed] [Google Scholar]
- 36.Karoui M, Penna C, Amin-Hashem M, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg 2006;243:1-7. 10.1097/01.sla.0000193603.26265.c3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phan K, An VV, Ha H, et al. Hepatic resection for malignant liver tumours in the elderly: a systematic review and meta-analysis. ANZ J Surg 2015;85:815-22. 10.1111/ans.13211 [DOI] [PubMed] [Google Scholar]
- 38.van Tuil T, Dhaif AA, Te Riele WW, et al. Systematic Review and Meta-Analysis of Liver Resection for Colorectal Metastases in Elderly Patients. Dig Surg 2019;36:111-23. 10.1159/000487274 [DOI] [PubMed] [Google Scholar]
- 39.Okinaga H, Yasunaga H, Hasegawa K, et al. Short-Term Outcomes following Hepatectomy in Elderly Patients with Hepatocellular Carcinoma: An Analysis of 10,805 Septuagenarians and 2,381 Octo- and Nonagenarians in Japan. Liver Cancer 2018;7:55-64. 10.1159/000484178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sultana A, Brooke-Smith M, Ullah S, et al. Prospective evaluation of the International Study Group for Liver Surgery definition of post hepatectomy liver failure after liver resection: an international multicentre study. HPB (Oxford) 2018;20:462-9. 10.1016/j.hpb.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 41.Gwiasda J, Schrem H, Kaltenborn A, et al. Introduction of the resection severity index as independent risk factor limiting survival after resection of colorectal liver metastases. Surg Oncol 2017;26:382-8. 10.1016/j.suronc.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 42.Ijichi M, Takayama T, Toyoda H, et al. Randomized trial of the usefulness of a bile leakage test during hepatic resection. Arch Surg 2000;135:1395-400. 10.1001/archsurg.135.12.1395 [DOI] [PubMed] [Google Scholar]
- 43.Kasai M, Cipriani F, Gayet B, et al. Laparoscopic versus open major hepatectomy: a systematic review and meta-analysis of individual patient data. Surgery 2018;163:985-95. 10.1016/j.surg.2018.01.020 [DOI] [PubMed] [Google Scholar]


