Abstract
Based in the anatomical concept of the mesoesophagus, that at subcarinal level all the vessels come through a by-layer connective tissue plane from the aorta to the esophagus whereas supracarinally these structures will come from both sides, with vagal and recurrent laryngeal nerves, a minimally invasive mesoesophageal (MIME) resection model may be described. Based on this surgical plane concept, dissection of esophagus and mediastinal lymphadenectomy can be performed along these structures establishing clear anatomical modules for an adequate oncological resection.
Keywords: Minimally invasive esophagectomy (MIE), mesoesophagus, mediastinal lymphadenectomy
Introduction
In the search for the ideal approach for minimally invasive esophagectomy (MIE) there are two important developments. These are the systematic use of the transthoracic approach for an adequate oncological resection (1,2) and the description of the surgical anatomy of the esophagus based on findings gathered during MIE (3-5).
For squamous cell cancer (SCC) the transthoracic approach has always been the chosen approach, but for intrathoracic located adenocarcinomas (Adcs) the systematic use of the transthoracic approach in the West is based on the findings of the HIVEX trial that have shown that the transthoracic approach has a better long-term survival than the transhiatal approach (6,7). Consequently, adopting the thoracoscopy as minimally invasive procedure, question arise which of the two approaches lateral decubitus or prone position will be chosen. Moreover, it seems that the prone position may offer advantages over the lateral approach (8,9), remaining as technical questions how extensive will be the supracarinal lymphadenectomy in the different types of esophageal cancer (EC) and the role of the semiprone position to accomplish this (10).
Anatomical concepts
The anatomical definition of the mesoesophagus or peri-esophageal connective layers—clinically and by magnetic resonance imaging (MRI)—has helped to standardize in many aspects the proper anatomy of the esophagus and the surgical planes for a standardized esophageal resection for cancer. The mesoesophagus has been described by using the findings gathered during MIE and confirmed by cadaver studies and MRI. It is a bi-layered connective tissue plane coursing from the aorta to the left lateral aspect of the esophagus. It divides the posterior mediastinum into two distinct compartments. The first is an anterior compartment containing the esophagus, infracarinal lymph nodes (LN) and vagus nerves; the second is a posterior compartment containing the azygos vein and thoracic duct. This “distal” mesoesophagus concept is especially useful for surgery of the esophagus up to the level of the carina (Figure 1) (3,4). Concerning the supracarinal anatomy, this is different than the infracarinal because of upper vascularization and locations of the vagal and recurrent laryngeal nerves are bilateral (5,11). This difference between the supracarinal and infracarinal areas probably has its origin in the embryological development of the esophagus. The esophagus develops from the foregut. This foregut develops the respiratory diverticulum that forms the two lung buds. In this way the foregut is the origin of the trachea and the lungs. After this, the trachea is separated from the pharynx and the esophagus. The place where the esophagus forms the two lung buds probably marks the difference between the infracarinal and supracarinal esophagus (12). Moreover, it seems that the “proximal” mesoesophagus can also be traced at the supracarinal level on the left side of the esophagus. Japanese surgeons performing the lymphadenectomy of the left laryngeal recurrent nerve, by retraction of the divided proximal esophagus, have described an esophageal “mesenteriolum” on this side containing the left recurrent nerve and LN (13-16).
Figure 1.
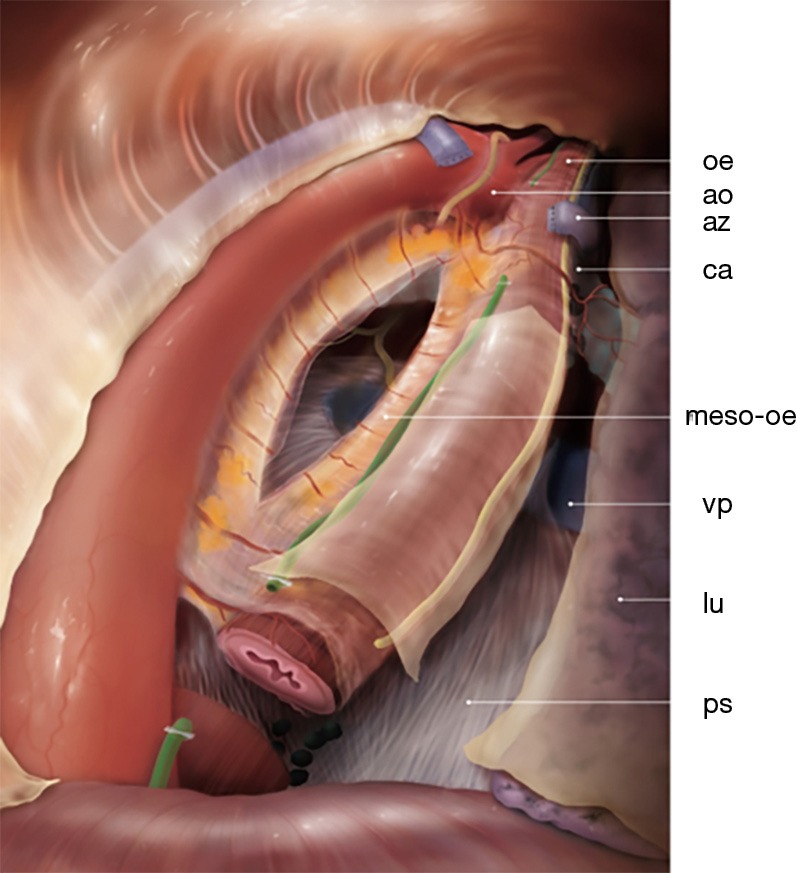
General view of esophagus in prone position with the mesoesophagus. oe, esophagus; ao, aorta; az, azygos vein; ca, carina; meso-oe, mesoesophagus; vp, right pulmonal vein; lu, right lung; ps, pericard sac.
An adequate knowledge of the surgical anatomy of the thoracic esophagus is paramount in order to determine the adequate planes of the resection in order to achieve an adequate and sufficient oncological resection. In all oncological abdominal resections, optimal dissection is performed along adequate planes that help to define and fix the borders of the oncological resection.
Different types of EC, extension of mediastinal lymphadenectomy and increased use of MIE
Different types of EC can be distinguished, two by histopathological diagnosis: the esophageal SCC, and the Adc. Concerning its location in the esophagus, four locations can be differentiated: upper, middle, lower thoracic and gastroesophageal junction (EGJ) cancer (17,18). In the EGJ cancers only the types 1 and 2 of Siewert can be considered as ECs. As example of the regional differences, differences between two countries can be marked: Japan in Asia and the Netherlands in North Europe and these are relevant to know. The incidence of EC is higher in Asia (at least more than twofold) than in Europe. In Europe the incidence rate of esophageal Adc is steadily increasing to more than 80% of all EC, whereas in Asia the rate of SCC is more than 90%. Moreover, in Europe the distally located EC and EGJ tumors account for 86.5%, whereas in Japan tumors located in the upper and middle esophagus account for more than 65% (19).
Extension of the mediastinal lymphadenectomy. In 1994 and following an update in 2003, the International Society for Diseases of the Esophagus (ISDE) defined different types of mediastinal lymphadenectomy currently still in use: (i) standard: lymphadenectomy up to the subcarinal LN; (ii) extended type: also includes paratracheal lymphadenectomy on the right side; (iii) total mediastinal: including both paratracheal and both recurrent nerves LN; and (iv) the three-field lymphadenectomy (10). In the Netherlands, the majority of esophageal surgeons carry out the so-called two-field lymphadenectomies in different extensions; they never—as in Japan—carry out the total mediastinal lymphadenectomy. Currently, in Japan, the three-field lymphadenectomy is used for upper and middle SCC (20).
Based on the potential advantages of MIE, there is an increasing use of MIE all over the world. All forms of esophageal resections have been performed by MIE. The most used are the transthoracic 2 stage Ivor Lewis, the 3 stage McKeown and the transhiatal resection. Moreover, most surgeons use the total MIE by thoracoscopy and laparoscopy, but also the hybrid forms of MIE using laparoscopy in combination with thoracoscopy, or the thoracoscopy in combination with laparotomy are frequently performed and currently the robot assisted MIE (RAMIE) is increasingly introduced (2,21).
Based on these considerations and the knowledge acquired by using this concept we will try to standardize this MIE mesoesophageal resection.
Different types of minimally invasive mesoesophageal (MIME) resection based on the localization of the tumor
There are two types of MIME resection: the “total” mesoesophageal resection of the thoracic esophagus (the McKeown procedure) followed by cervical anastomosis and the “partial” mesoesophageal resection followed by intrathoracic anastomosis, the so-called Ivor Lewis procedure.
By thoracoscopy in prone position, three or four trocars can be used. In our practice, all the trocars are introduced between de scapula and the spine. A 30-grade thoracoscope is introduced at the point of the scapula. Insufflation of 7 mmHg is used (2).
Total mesoesophageal resection
Step by step:
open the pleura along the right lung, the right bronchus, azygos vein and along the right vagal nerve (Figure 2);
dissect the subcarinal LN (remains attached to the esophagus);
division of the right vagal nerve distal of the level of the right bronchus;
division of the azygos vein by means of stapler and the right bronchial artery with a sealing device (Figure 3);
the right recurrent laryngeal nerve (RRLN) is visualized over de right subclavian artery (gentle pulling of the stump of the right vagal nerve may help). Dissection of the RRLN LN (send them separately to pathology) (Figure 4);
open the pleura at the lateral edge of the upper mediastinum along the azygos vein up to the hiatus (Figure 5);
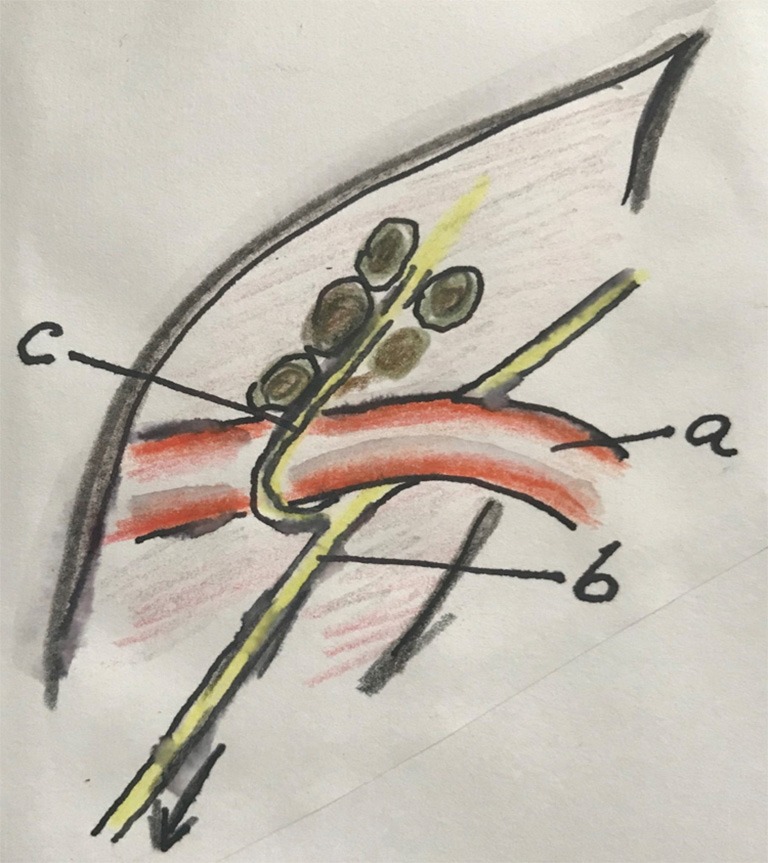
put an external loop around the proximal esophagus and suspend it by traction. In this way, the proximal mesoesophagus will be tented and dissection of the left recurrent laryngeal nerve (LRLN) can adequately be performed (the LN may remain attached to the esophagus or send separately to pathology) (Figure 6). (Other possibility is to dissection between the esophagus and trachea to create an ample window, localize the LRLN and perform a lymphadenectomy);
complete dissection of the esophagus at the upper mediastinum by traction of the esophagus to right. Visualize the LRLN up to the aorta arch. Lymphadenectomy of the aorta arch is performed;
dissection of the space between the thoracic aorta and the azygos vein to localize and resect the thoracic duct;
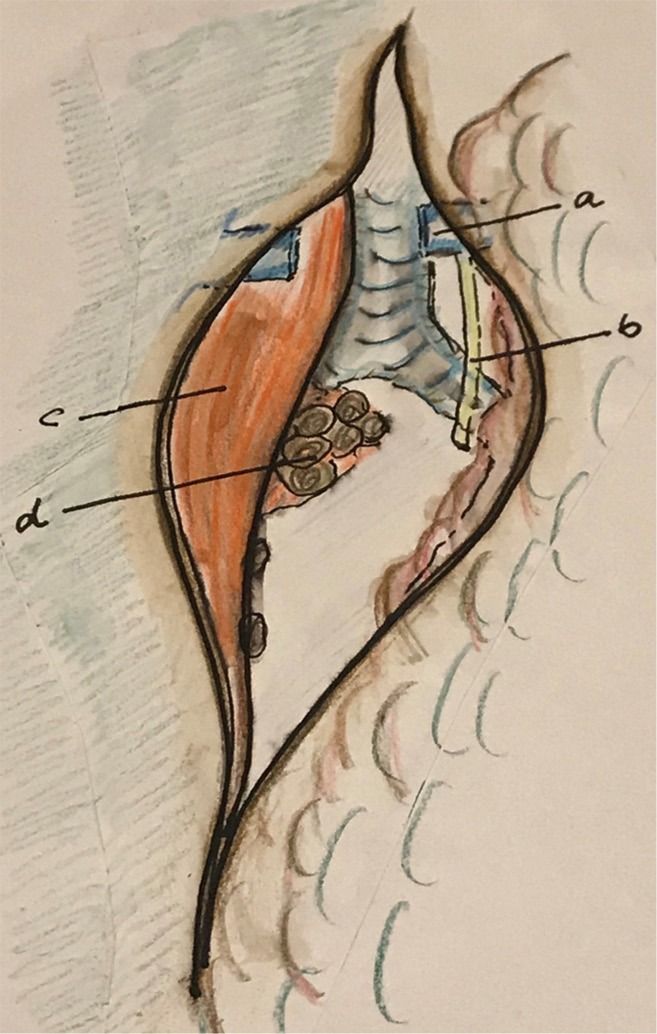
by traction of the esophagus to the right, dissection between the descending aorta and the esophagus will show the “distal” mesoesophagus. Division of the distal mesoesophagus from proximal to distal (Figure 7);
complete de dissection of the esophagus from upper thorax to hiatus. Periesophageal tissues, the mesoesophagus and the infracarinal LN are attached to the esophageal specimen.
Figure 2.
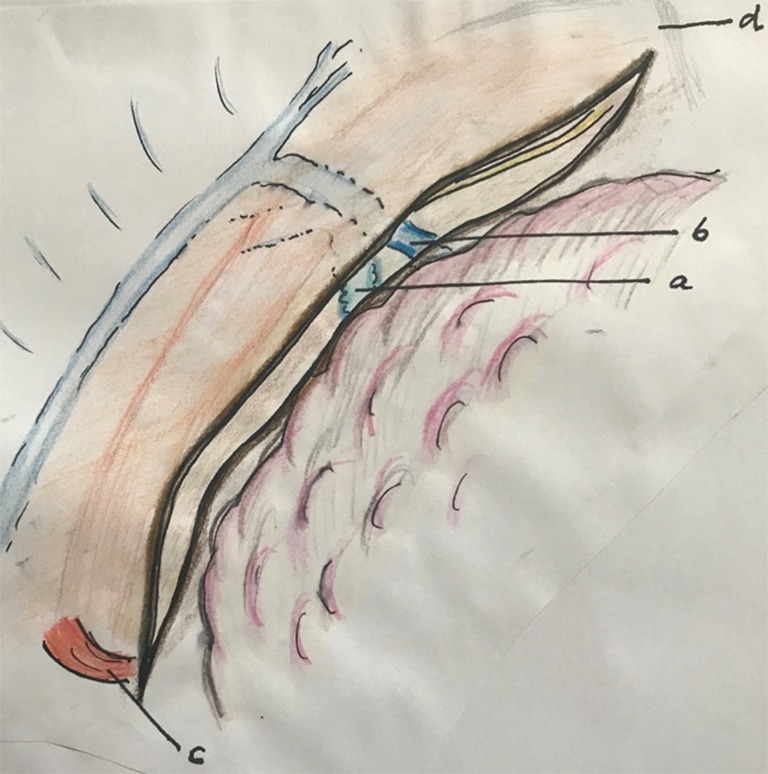
Opening the pleura along the right lung, right bronchus, azygos vein and along the right vagal nerve. a, right bronchus; b, azygos vein; c, hiatus; d, apex thorax.
Figure 3.
With retraction of the esophagus to the left, the azygos vein and right vagal nerve are divided and infracarinal lymphadenectomy is performed. a, divided azygos vein; b, divided right vagal nerve; c, esophagus; d: infracarinal LN. LN, lymph nodes.
Figure 4.
Approach of the right recurrent laryngeal nerve and lymphadenectomy. a, right subclavian artery; b, right vagal nerve; c, right laryngeal recurrent nerve and LN. LN, lymph nodes.
Figure 5.
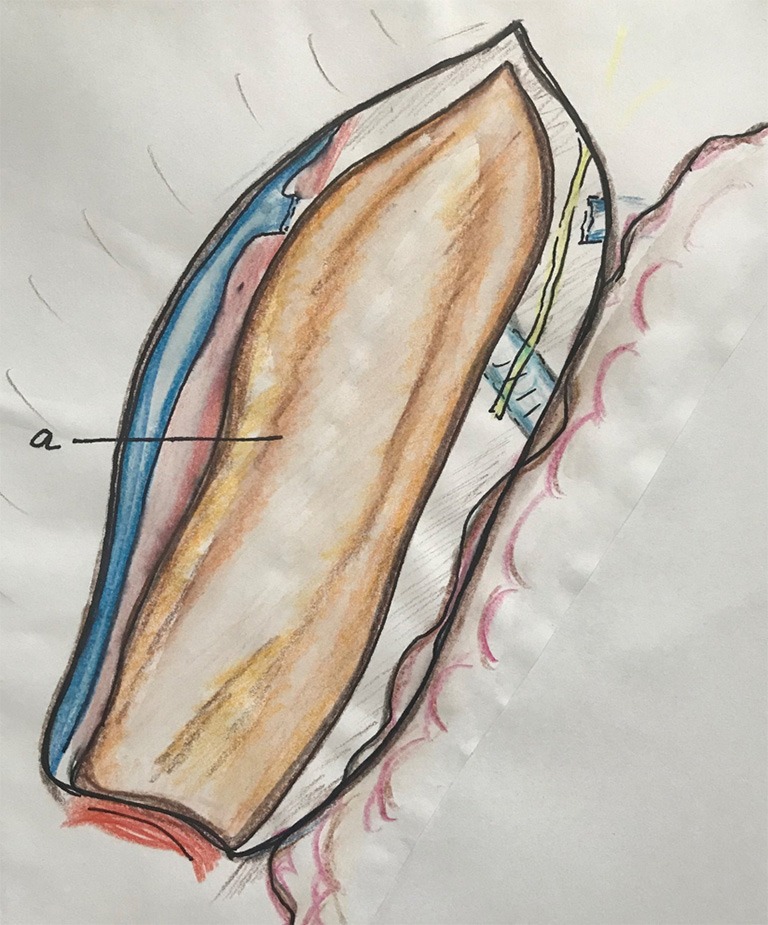
The pleura along the azygos vein is opened to distal. a, the whole incision in the mediastinal pleura is done around the esophagus covered by mediastinal pleura.
Figure 6.
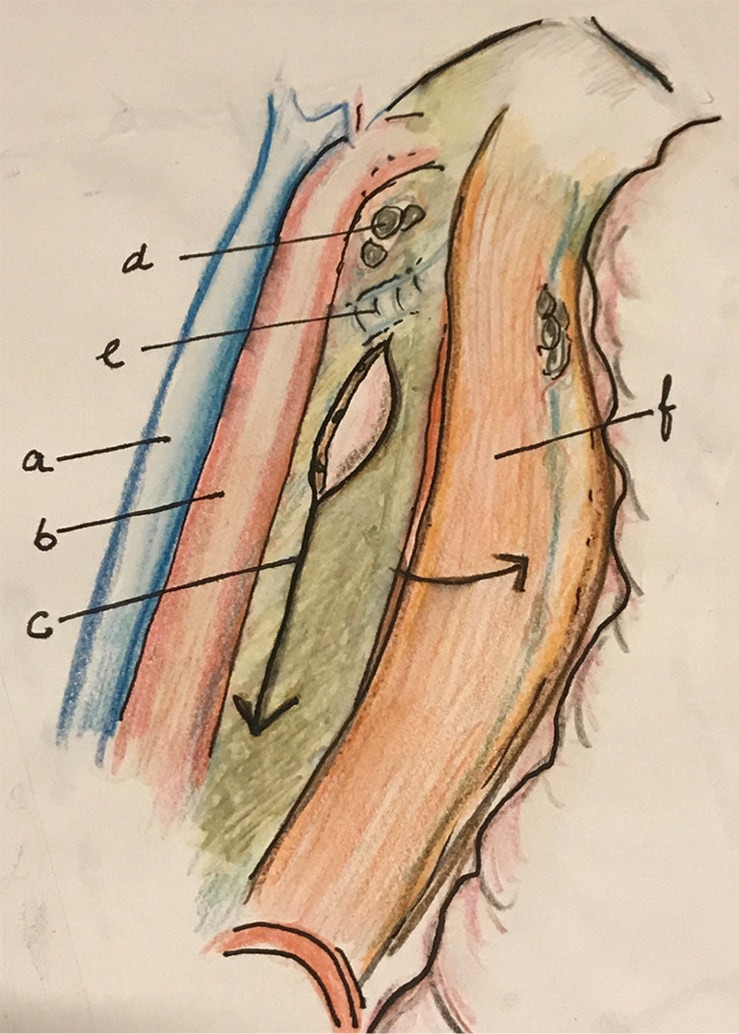
After dissection along the descending aorta and dissection and resection of the thoracic duct, the esophagus is retracted to right to expose and divide the lower mesoesophagus. a, azygos vein; b, aorta; c, mesoesophagus (distal); d, subaortic LN; e, left bronchus; f, esophagus. LN, lymph nodes.
Figure 7.
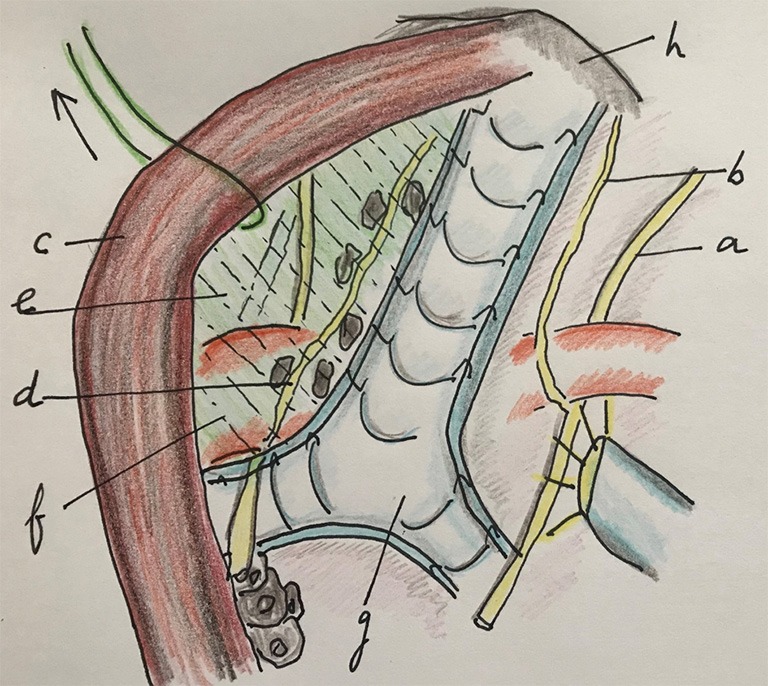
The area of the left recurrent laryngeal nerve is exposed by suspending the proximal esophagus from the trachea. In this way the “upper mesoesophagus” is exposed, dissected and lymphadenectomy is performed. a, right vagal nerve; b, RRLN; c, esophagus; d, left recurrent laryngeal nerve; e, mesoesophagus (upper) under traction of the proximal esophagus; f, aorta arch; g, trachea; h, apex thorax. RRLN, right recurrent laryngeal nerve.
Partial mesoesophageal resection
Step by step:
open the pleura along the right lung following the right bronchus, azygos vein and along the right vagal nerve (Figure 2);
dissect the infracarinal LN (remains attached to the esophagus);
division of the right vagal nerve distal of the level of the right bronchus;
section of the azygos vein by means of stapler and the right bronchial artery (Figure 3);
dissection of the paratracheal LN between the vagal nerve and the trachea (send them separately to pathology);
open the pleura at the lateral edge of the mediastinum along the azygos vein up to the hiatus (Figure 5);
dissection of the space between the descending thoracic aorta and the azygos vein to localize and resect the thoracic duct;
dissection and division of the “distal” mesoesophagus (Figure 7);
complete de dissection of the esophagus and divide it at the level of the azygos vein.
If the surgeon wants to sample or perform a lymphadenectomy of the RRLN and the LRLN this can be performed in the same way as in the total mesoesophageal resection, first the right side and then the left side. The only difference is that the dissection of the LRLN should be performed along the paratracheal groove without proximal mobilization of the esophagus without disturbing its vascularization.
Discussion
The whole abdominal oncology follows the general principle of dissecting the affected organ along the planes as defined by embryology, in order to determine the adequate borders of the resection. A model serving for this has been the mesorectal excision for rectal cancer (22).
Definition of the concept of mesoesophagus by the findings gathered during thoracoscopic esophageal resection and confirmed by MRI has helped to establish standardization of the esophageal resection. The mesoesophagus is a clear bi-fascial structure located between the ventral side of the descending aorta and the esophagus. In this bi-fascial structures vessels and lymph vessels can be found going to and from the esophagus, such as the esophageal arteries and veins (3-5).
In this review paper, we denote this mesoesophagus as the “distal” mesoesophagus. The “proximal” can be defined by traction of the proximal esophagus to posterior before the LRLN lymphadenectomy (14-16).
The esophageal resection can be performed either by total (McKeown) or by the limited (Ivor Lewis) MIME. The choice is basically based on the type and location of the EC. There are important differences in EC, Adc or SCC as found in the Western and the Eastern world (19). The distal and EGJ Adcs account for more than 80% of all cancers in the West and usually are resected after neoadjuvant therapy by a MIE Ivor Lewis with intrathoracic anastomosis. During this intervention, some surgeons perform a limited form of mediastinal lymphadenectomy along the right paratracheal area. This involves the issue whether by extending the upper mediastinal lymphadenectomy they could damage the vascularization of the proximal esophagus that is necessary for an optimal anastomosis. Although this practice is not frequent, during the Ivor Lewis procedure they may upon indicate to perform a total mediastinal lymphadenectomy. It must be noted that regarding mediastinal lymphadenectomy consensus has not been established about the extent, and that the surgeons know that if the supracarinal LN are positive for cancer, after neoadjuvant therapy, the prognosis will be dismal (23). These factors probably form the main reasons for using this conservative approach towards these distal and EGJ Adcs. For the more proximal and middle located SCC—as happens in Asia—it is clear that a total mesoesophageal resection with total mediastinal lymphadenectomy is the standard approach.
In order to perform a standard MIE, the surgeon has to know what constitutes the standard oncological resection and its borders. Doing this standard resection in all situations she/he knows what to do and that doing it, she/he knows is acting properly for every patient. Consensus about the extension of lymphadenectomy in all cases of EC has to be reached.
Furthermore, the use of neoadjuvant therapy is currently extended in stages 2 and 3 EC and there is evidence that its use does not increase the number of postoperative complications (24,25).
Conclusions
According with the knowledge of the surgical anatomy of the esophagus, the concept of the mesoesophagus can help to define the plane of dissection during esophageal resection. Two types of mesoesophageal resection, the total and the partial will be defined. Consensus has to be reached concerning the extent of mediastinal lymphadenectomy in esophageal Adcs.
Acknowledgements
None.
Footnotes
Conflict of Interest: The author has no conflicts of interest to declare.
References
- 1.Cuschieri A. Thoracoscopic subtotal esophagectomy. Endosc Surg Allied Technol 1994;2:21-5 [PubMed] [Google Scholar]
- 2.Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. 10.1016/S0140-6736(12)60516-9 [DOI] [PubMed] [Google Scholar]
- 3.Cuesta MA, Weijs TJ, Bleys RL, et al. A new concept of the anatomy of the thoracic esophagus: the meso-oesophagus. Observational study during thoracoscopic oesophagectomy. Surg Endosc 2015;29:2576-82. 10.1007/s00464-014-3972-1 [DOI] [PubMed] [Google Scholar]
- 4.Weijs TJ, Goense L, van Rossum PS, et al. The periesophageal connective tissue layers and related compartments: visualization by histology and magnetic resonance imaging. J Anat 2017;230:262-71. 10.1111/joa.12552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuesta MA, van der Wielen N, Weijs TJ, et al. Surgical anatomy of the supracarinal esophagus based on a minimally invasive approach: vascular and nervous anatomy and technical steps to resection and lymphadenectomy. Surg Endosc 2017;31:1863-70. 10.1007/s00464-016-5186-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. 10.1056/NEJMoa022343 [DOI] [PubMed] [Google Scholar]
- 7.Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007;246:992-1000. 10.1097/SLA.0b013e31815c4037 [DOI] [PubMed] [Google Scholar]
- 8.Markar SR, Wiggins T, Antonowicz S, et al. Minimally invasive esophagectomy: Lateral decubitus vs. prone positioning; systematic review and pooled analysis. Surg Oncol 2015;24:212-9. 10.1016/j.suronc.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 9.Kawakubo H, Takeuchi H, Kitagawa Y. Current status and future perspectives on minimally esophagectomy. Korean J Thorac cardiovasc Surg 2013;46:241-8. 10.5090/kjtcs.2013.46.4.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bumm R, Wong J. More or less surgery for esophageal cancer: extent of lymphadenectomy for squamous cell carcinoma. How much is necessary. Dis Esophagus 1995;8:78. [Google Scholar]
- 11.Osugi H, Takemura M, Higashino M, et al. A comparison of video-assisted thoracoscopic oesophagectomy and radical lymph node dissection for squamous cell cancer of the oesophagus with open operation. Br J Surg 2003;90:108-13. 10.1002/bjs.4022 [DOI] [PubMed] [Google Scholar]
- 12.Carlson BM. Human Embriology and Development Biology. Maryland Heights: Mosby; 2004. [Google Scholar]
- 13.Noshiro H, Iwasaki H, Kobayashi K, et al. Lymphadenectomy along the left recurrent laryngeal nerve by a minimally invasive esophagectomy in the prone position for thoracic esophageal cancer. Surg Endosc 2010;24:2965-73. 10.1007/s00464-010-1072-4 [DOI] [PubMed] [Google Scholar]
- 14.Makino H, Yoshida H, Maruyama H, et al. An original technique for lymph node dissection along the left recurrent laryngeal nerve after stripping the residual esophagus during video-assisted thoracoscopic surgey of esophagus. J Visual Surg 2016;2:1-6. 10.21037/jovs.2016.11.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oshikiri T, Yasuda T, Harada H, et al. A new method (the “bascule method”) for lymphadenectomy along the left recurrent laryngeal nerve during prone esophagectomy for esophageal cancer. Surg Endosc 2015;29:2442-50. 10.1007/s00464-014-3919-6 [DOI] [PubMed] [Google Scholar]
- 16.Lin M, Shen Y, Feng M, et al. Minimally invasive esophagectomy: Chinese experiences. J Vis Surg 2016;2:134. 10.21037/jovs.2016.07.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Japan Esophageal Society. Japanese Classification of Esophageal Cancer, tenth edition: part I. Esophagus 2009;6:1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siewert JR. Adenocarcinoma of the esophago-gastric junction. Gastric Cancer 1999;2:87-8. 10.1007/s101200050028 [DOI] [PubMed] [Google Scholar]
- 19.Cuesta MA, van der Peet DL, Gisbertz SS, et al. Mediastinal lymphadenectomy for esophageal cancer: Differences between two countries, Japan and the Netherlands. Ann Gastroenterol Surg 2018;2:176-81. 10.1002/ags3.12172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus 2017;14:1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuesta MA, van der Wielen N, Straatman J, van der Peet DL. Video-assisted thoracoscopic esophagectomy: Keynote lecture. Gen Thorac Cardiovasc Surg 2016;64:380-5. 10.1007/s11748-016-0650-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1:1479-82. 10.1016/S0140-6736(86)91510-2 [DOI] [PubMed] [Google Scholar]
- 23.Parry K, Haverkamp L, Bruijnen RC, et al. Surgical treatment of adenocarcinomas of the gastro-esophageal junction. Ann Surg Oncol 2015;22:597-603. 10.1245/s10434-014-4047-1 [DOI] [PubMed] [Google Scholar]
- 24.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 25.Nederlof N, Slaman AE, van Hagen P, et al. using the Comprehensive Complications Index to assess the impact of neoadjuvant chemoradiotherapy on complication severity after esophagectomy for cancer. Ann Surg Oncol 2016;23:3964-71. 10.1245/s10434-016-5291-3 [DOI] [PMC free article] [PubMed] [Google Scholar]


