Abstract
Esophagectomy with gastric tube reconstruction is a highly complex surgical procedure. With regard to mobilization of the stomach and optimal gastric tube preparation and anastomosis, there are several important intraoperative steps that can influence the outcome of the operation. This study aims to describe the optimal mobilization of the stomach for gastric tube reconstruction and explore the best place in the gastric tube for intrathoracic anastomosis after esophagectomy. A search of the literature was performed and results are described in a descriptive review. Based on literature and our own experience we describe important operating steps for laparoscopic stomach mobilisation for gastric tube reconstruction. Steps to create additional length include preserving the left gastroepiploic artery, transecting the right gastric artery, extended duodenal mobilization, and duodenal diversion with roux-Y reconstruction. Several techniques for intrathoracic anastomosis are described in literature. Several imaging techniques, of which fluorescence imaging is the most commonly used, are available to assess the vascularization of the gastric tube and to assist in determining the best place in the gastric tube for intra thoracic anastomosis. Although there is little evidence of exact technique on stomach mobilization and location for an intrathoracic anastomosis, many techniques are used by different authors with varying results.
Keywords: Esophagectomy, intrathoracic anastomosis, gastric tube
Introduction
Esophageal cancer is currently the 9th most prevalent cancer worldwide, and the 6th most frequent cause of cancer-related death (1). Despite the advent of (neo-)adjuvant therapies, surgery with the aim of a radical resection remains the mainstay of treatment (2). Radical resection usually involves open or minimally invasive esophagectomy with lymphadenectomy, and reconstruction is mostly performed by creating a gastric conduit with a cervical or intrathoracic esophago-gastrostomy (3). In recent decades, minimally invasive surgery has become increasingly popular. It has been shown to reduce pulmonary complications and length of hospital stay (4). In the Netherlands, the majority of patients are now operated minimally invasively, with an intrathoracic anastomosis (Ivor Lewis procedure) (5).
Esophagectomy with gastric tube reconstruction and intrathoracic anastomosis is a highly complex surgical procedure associated with considerable morbidity. Optimal surgical technique is important to reduce short term complications such as anastomotic leakage, but also to facilitate good long-term functional outcome. This descriptive review aims to describe the process of stomach mobilization and formation of the intrathoracic anastomosis. With regards to mobilization of the stomach, the goal is to create a well vascularized and functional gastric tube with maximal length for reconstruction. Key steps in the operation are described, as well as several options to gain additional length for a safe and well vascularized anastomosis. For the creation of the intrathoracic anastomosis, several methods are described and evidence for the optimal place in the gastric tube for the anastomosis is reviewed.
Optimal mobilization of the stomach
Key steps in mobilization of the stomach
In laparoscopic mobilization of the stomach, usually an optical trocart is placed in the midline above the umbilicus, with three working trocarts subcostally (two on the left side and one on the right) and a Nathanson liver retractor via a separate subxyphoidal incision. Firstly, the hepatogastric ligament is opened close to the liver; all lymph nodes from the ligament will be included in the resection specimen. The right gastric artery is preserved along the smaller curvature of the stomach to the point where the gastric angle is prepared for tube formation by stapling. Any adhesions now visible in the omental bursa are released. This will facilitate adequate exposure of the celiac trunk. Subsequently a lymphadenectomy is performed in the hepatoduodenal ligament, along the common hepatic artery, along the splenic artery towards the splenic hilum, and around the celiac trunk. The left gastric artery is transected. Arterial branches coming from the splenic artery to the posterior stomach will be transected. The smaller curvature is now completely mobilized.
The next step is to transect the gastrocolic ligament to open the greater sac without damaging the right gastroepiploic artery. The gastrocolic ligament is completely transected to the right side until the proximal duodenum is reached. Subhepatic adhesions of the proximal duodenum occur frequent and should be freed. The proximal duodenum is mobilized on the dorsal side until the gastroduodenal artery is visualized and the base of the right gastroepiploic artery is separated from the mesentery of the transverse colon. To the left side, the gastrocolic ligament is transected completely and all short gastric vessels are transected. Along the greater curvature a part of the greater omentum is included in the resection to serve as an omental flap to cover the anastomosis during reconstruction. Hereby also the left gastroepiploic artery will remain intact. An anatomical study by Buunen et al. showed an arterial anastomosis between the right and left gastroepiploic artery in 70% of patients (6). Leaving the left gastroepiploic artery in situ and transecting it at the splenic hilum gave a 5 cm increase in feeding arterial arcade-length of the gastric tube. Anatomical structures of the omental bursa are depicted in Figure 1.
Figure 1.
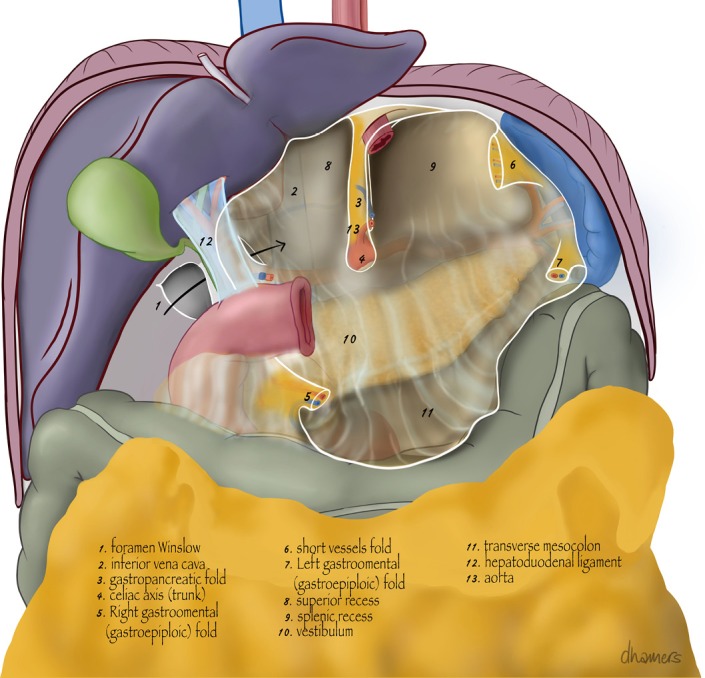
Anatomical structures of the omental bursa.
After the distal esophagus is dissected free from the diaphragm the stomach is completely mobilized, and ready for tube reconstruction. A review by Akkerman et al. previously reported lower rates of delayed gastric emptying of gastric tube reconstruction compared to gastric pull up reconstruction (7). In the included studies of the meta-analysis gastric tubes with a width of 3–4 cm were created. Gastric tube reconstruction is also associated with less reflux esophagitis compared to whole gastric reconstruction (8). From the gastric angle on the smaller curvature, approximately 5 cm proximal to the pyloric ring, a stapling device is used to create the gastric tube. Powered tri-stapling devices are very suitable for tube formation and create a reliable staple line. The potentially weakest point of the staple line is the crossing point of the subsequent staple lines; these are reinforced with additional stitches. We then create a gastric tube of approximately 4–5 centimeters wide. No consensus exists on the optimal width of the gastric tube; some authors suggest that the width is not of great importance; however, in literature the width usually varies between 2 and 6 centimeters (7,9). After creating the gastric tube it is useful to test the mobility of the pyloric ring. Preferably the pyloric ring can be brought towards, but not above, the level of the esophageal hiatus. A complete intrathoracic gastric tube with the pylorus situated above the diaphragm may result in redundant gastric tube length, resulting in delayed emptying, which may even need surgical correction (10). In our experience, the abovementioned steps allow for sufficient cephalad retraction of the stomach.
Another important aspect of gastric mobilization is to avoid damage to the stomach as much as possible; the so called no touch technique. Especially the fundus of the stomach is fragile and prone to tears if grabbed during the laparoscopic part of the procedure. Therefore it is important to touch this part of the stomach as little as possible.
Additional mobilizing steps
In case of insufficient length of the gastric tube or the inability to stretch the pyloric ring towards the diaphragm, additional mobilization steps can be performed.
The tension on the right gastric artery originating from the proper hepatic artery is one of the things that could be explored. A vascular anatomy study showed that the arterial contribution of the right gastric artery of the gastric tube is negligible (11). This seems especially true for narrow gastric tubes (12). If necessary, transection of the right gastric artery at its origin can provide additional length of the gastric tube. No literature is available on the specific amount of gained length or consequences to the vascularization or postoperative complication rate.
Another option to gain length is an extended duodenal mobilization (Kocher maneuver). Hereby all lateral peritoneal attachments of the duodenum are transected. The retroperitoneal adhesions are released and posteriorly the inferior caval vein is exposed. The duodenum and the head of the pancreas can then be retracted to the patients left facilitating additional upward movement of the gastric tube. Some authors routinely perform the Kocher maneuver in the process of stomach mobilization to attain additional length of the gastric conduit (13-15). However, the more extended mobilization of the duodenum may increase the risk of delayed gastric emptying; in our clinical practice we therefore do not routinely perform the Kocher maneuver but reserve it for selected cases.
In case of a short gastric tube, for example in case of patients with an irradiated gastric fundus, a more radical alternative for extended duodenal mobilization is to perform duodenal transection. In this procedure described by Kosumi et al. the duodenum is transected distally from the pyloric ring (16). The right gastroepiploic artery remains intact and serves as the feeding artery of the gastric tube. After performing the esophago-gastric anastomosis, the distal end of the gastric tube is anastomosed to the upper jejunum using a Roux-Y reconstruction. In this manner a short gastric tube could be mobilized to the level of the chin in their series. Another small series from Japan suggested better functional outcomes after duodenal diversion with Roux-Y reconstruction than after conventional gastric tube reconstruction, i.e., less gastroduodenal content reflux and delayed gastric emptying (17).
The best place in the gastric tube for intrathoracic anastomosis
Key steps in the creation of intrathoracic anastomosis
After completion of the abdominal phase, including the construction of a feeding jejunostomy, the patient is positioned in prone position. A four port right sided thoracoscopy is performed. The esophagus is dissected and mobilized, including the thoracic duct. Para-esophageal, lower and middle mediastinal, subcarinal and paratracheal lymphadenectomy is performed. The gastric tube is brought into the thorax and the esophagus is transected just superior to the carina, or—on indication—more proximally, but never more distally, in order to be able to bury the anastomosis behind the pleural flap after completion. It is also important to take the radiation field into account when transecting the esophagus: irradiated tissue should not be used for the anastomosis. During transection, the nasogastric tube can be retracted under vision and be placed in the proximal esophagus for proper reinsertion in the gastric tube after completion of the anastomosis. After transection the diameter of the esophagus is assessed to determine the correct stapler size.
The specimen is brought outside the patient via a mini-thoracotomy at the most caudal trocart point and separated from the stomach with the powered stapling device, after which the gastric tube is completed (Figure 2). The distal part of the stapling line is reinforced with interrupted sutures, which serve as an aid in thoracoscopic identification of the optimal place for the anastomosis. Thoracoscopy is resumed and the anvil of a circular stapler is inserted into the esophagus and a purse string suture is made with an endoscopic suturing device.
Figure 2.
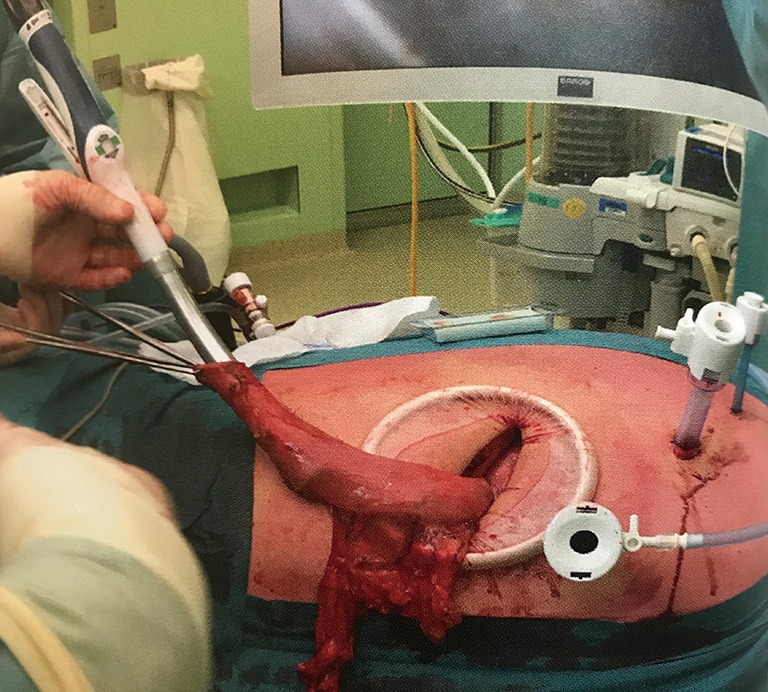
The specimen is brought extracorporeal through a mini-thoracotomy, after which the gastric tube is completed and a circular stapler can be introduced.
Then the place of the anastomosis in the gastric tube is determined. It is important to carefully position the gastric tube in a straight, tensionless manner, without rotation.
After this step, the vascularization or perfusion of the gastric tube is tested by fluorescent imaging using indocyanine green (ICG). During this procedure we note the time from ICG injection to lighting up (‘time to enhancement’) of the lung (as reference), proximal gastric tube, intended anastomotic site, and tip of the gastric tube. In case of doubt about the quality of perfusion at the intended anastomotic site, we will explore the possibility of a more proximal, better part of the gastric tube for site of anastomosis. Then the anastomosis is created in an end-to-side fashion with a conventional circular stapler after a purse string suture on the esophageal stump, at the mesenteric side of the gastric tube (Figure 3). The remnant gastric tube is stapled off with the linear tri-stapler, after which the anastomosis is reinforced with two standing sutures and then buried behind the pleural flap with an additional suture (Figure 4). As final step, the omental flap is wrapped around the anastomosis (‘flap and wrap’-technique) (Figure 5).
Figure 3.
End-to-side technique for intrathoracic anastomosis in Ivor Lewis esophagectomy.
Figure 4.
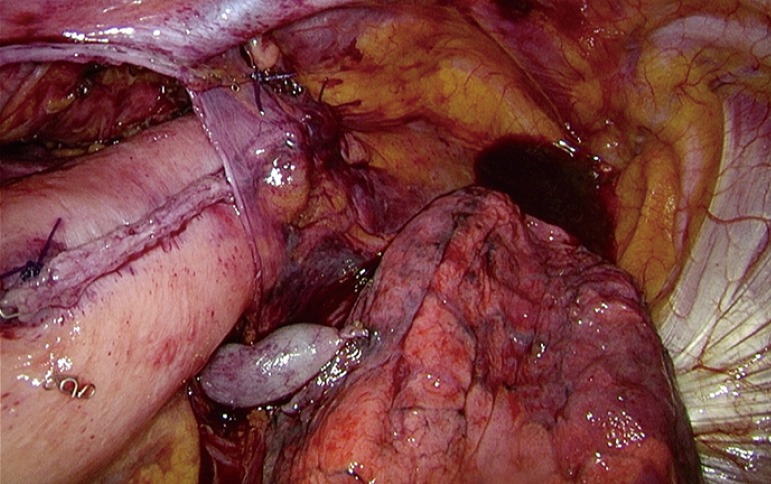
Anastomosis is buried under a pleural flap.
Figure 5.
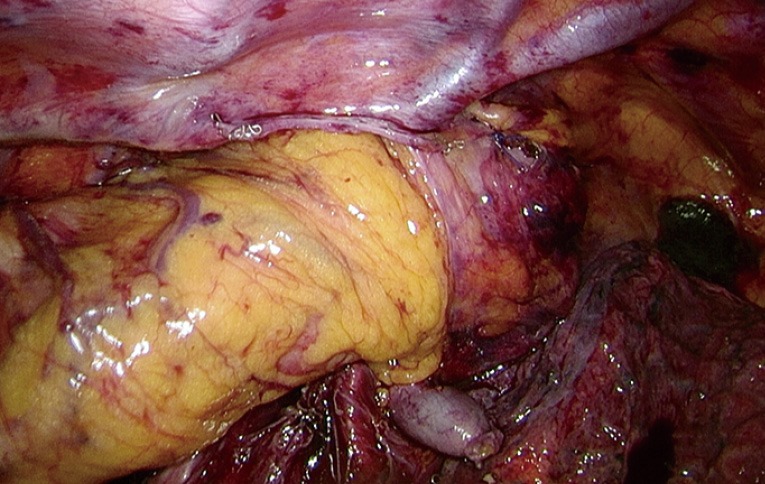
Anastomosis is covered by omental wrap.
Alternative techniques for the creation of intrathoracic anastomosis
Several alternative techniques are available for the creation of the intrathoracic anastomosis. With regard to the circular stapler, one can choose for transthoracic or transoral application of the anvil (Orvil device®). A review comparing these two most frequently used stapling methods found no differences in outcomes such as anastomotic leakage or stenosis (18). In our experience the mini-thoracotomy, which is necessary to retrieve the specimen, provides for an excellent route for introduction of both anvil and stapler. Furthermore, the application of the anvil with a suturing device prevents a double stapling line in the anastomosis.
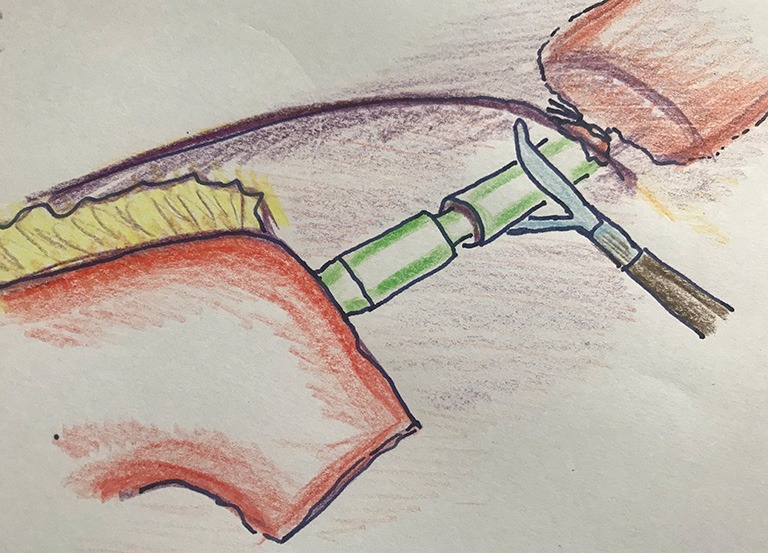
An alternative method for the intrathoracic anastomosis is a side-to-side anastomosis with a linear stapler (Figure 6). The remaining defect can be closed with a suture (e.g., V-locR suture), or by stapling. This method creates a larger luminal diameter, and is functionally comparable to an end-to-end anastomosis (19).
Figure 6.
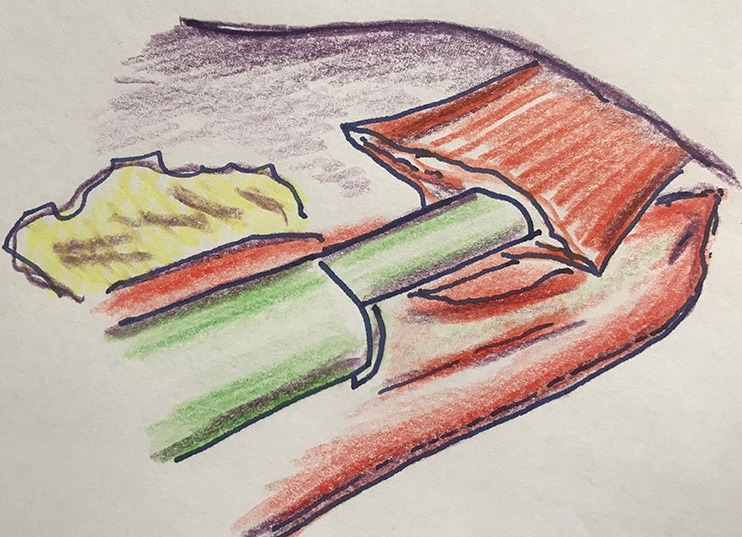
Side-to-side technique for intrathoracic anastomosis in Ivor Lewis esophagectomy.
A third method, next to circular and linear stapling techniques, is the handsewn end-to-side anastomosis. Single or double layer suturing can be used. Especially in robotic-assisted minimally invasive esophagectomy, the (robotic assisted) handsewn technique is commonly used (20).
Comparisons between the different methods usually show no significant differences in leak rates, but some authors suggest that anastomotic stenosis occurs more frequently after handsewn anastomosis (19). In our opinion the major advantage of the circular stapling technique is that is a very reproducible technique. Furthermore, different diameters can be applied (mostly 25 or 29 mm) and it produces a clear proximal resection margin, namely the proximal (esophageal) stapling ‘donut’.
The best place in the gastric tube for intrathoracic anastomosis
Regarding the best place in the gastric tube for the anastomosis there are several choices to be made. Firstly one has to choose between the mesenteric and antimesenteric side of the gastric tube (Figure 7). In our opinion, the advantage of the mesenteric side is that it is probably the better perfused side, and mesenteric stapling will also facilitate easier transthoracic introduction of the stapler. Another advantage of mesenteric placement of the stapler is that the anastomosis will not contain a cross staple line. Kesler and colleagues recently described a novel intrathoracic anastomotic technique on the antimesenteric side, where a linear stapler is fired cutting through and restapling the lesser curvature staple line (21). In their series of 278 open esophagectomies this technique led to excellent results, with a leak rate of only 2.9%. However, the reproducibility in minimally invasive esophagectomy is yet to be demonstrated.
Figure 7.
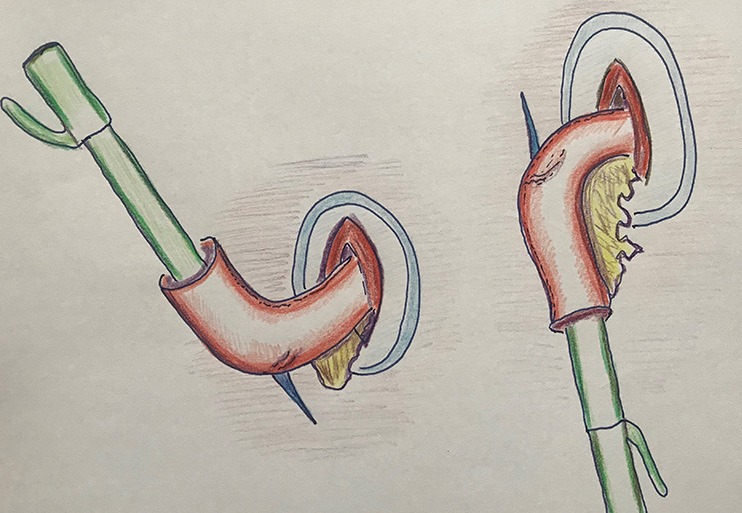
Stapling on the mesenterial side of the gastric tube (left) and on the antimesenterial side (right).
Regardless of the type of intrathoracic anastomosis, the following principles should always be respected: adequate vascularized, watertight and tension free. Important is to determine the level in the gastric tube for the anastomosis. Previously the quality and vascularization of the gastric tube was primarily judged subjectively based on palpation and visual inspection. Nowadays several imaging techniques are available to assist in determining the quality of the intended anastomotic site in the gastric tube. Laser speckle contrast imaging, optical coherence imaging, sidestream dark field microscopy, fluorescence imaging and near infrared spectroscopy are all optical techniques currently under investigation for perfusion analysis of gastric tubes after esophagectomy, as shown in a review from our group (22). Fluorescence imaging is the most commonly used technique in current practice. The influx of ICG is used as a surrogate for arterial perfusion. Quantification remains an issue in fluorescence imaging and needs further clinical investigation. However, even without quantification, the visual assessment of arterial perfusion of the gastric tube has been shown to correlate with the likelihood of an anastomotic leak (23). Future studies are needed to confirm these results and to investigate whether fluorescence imaging can contribute to lower incidence of anastomotic leakage. With regard to quantification of perfusion, ‘time to enhancement’ is the most easy and reproducible parameter and can be applied without additional software (24). Time-fluorescence intensity curves analysis and ICG flow speed are alternative quantification parameters that may be of use in the future. With our current experience, we feel that the combination of intraoperative visualization and the ‘time to enhancement’ parameter provides sufficient information regarding perfusion at the intended anastomotic site and is useful in the decision making process.
Conclusions
In summary, this descriptive review described the optimal mobilization of the stomach for gastric tube reconstruction. Several mobilization steps can result in additional length of the conduit, including preserving the left gastroepiploic artery, transecting the right gastric artery and extended duodenal mobilization. Several techniques for a minimally invasive intrathoracic anastomosis are known and whether the preferred anastomotic site in the gastric tube should be mesenteric or antimesenteric remains unclear based on current literature. Several imaging techniques are nowadays available to assess vascularization of the gastric tube. Fluorescence imaging has been shown to affect decision making while performing the esophagogastric anastomosis and may be helpful in determining the best place for the anastomosis in the gastric tube.
Acknowledgements
None.
Disclosure: The drawings (Figures 1,3,6,7) are made by MA Cuesta and the perioperative photos (Figures 2,4,5) are made by SS Gisbertz.
Footnotes
Conflicts of Interest: MI van Berge Henegouwen—Educational grant Stryker, Research grant Olympus, Consultant for Medtronic; SS Gisbertz—Research grant Olympus, Consultant for Medtronic. The other authors have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Lagergren J, Smyth E, Cunningham D, et al. Oesophageal cancer. Lancet 2017;390:2383-96. 10.1016/S0140-6736(17)31462-9 [DOI] [PubMed] [Google Scholar]
- 3.Gisbertz SS, Hagens ERC, Ruurda JP, et al. The evolution of surgical approach for esophageal cancer. Ann N Y Acad Sci 2018;1434:149-55. 10.1111/nyas.13957 [DOI] [PubMed] [Google Scholar]
- 4.Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. 10.1016/S0140-6736(12)60516-9 [DOI] [PubMed] [Google Scholar]
- 5.Seesing MFJ, Gisbertz SS, Goense L, et al. A Propensity Score Matched Analysis of Open Versus Minimally Invasive Transthoracic Esophagectomy in the Netherlands. Ann Surg 2017;266:839-46. 10.1097/SLA.0000000000002393 [DOI] [PubMed] [Google Scholar]
- 6.Buunen M, Rooijens PP, Smaal HJ, et al. Vascular anatomy of the stomach related to gastric tube construction. Dis Esophagus 2008;21:272-4. 10.1111/j.1442-2050.2007.00771.x [DOI] [PubMed] [Google Scholar]
- 7.Akkerman RD, Haverkamp L, van Hillegersberg R, et al. Surgical techniques to prevent delayed gastric emptying after esophagectomy with gastric interposition: a systematic review. Ann Thorac Surg 2014;98:1512-9. 10.1016/j.athoracsur.2014.06.057 [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Yu D, Peng J, et al. Gastric-tube versus whole-stomach esophagectomy for esophageal cancer: A systematic review and meta-analysis. PLoS One 2017;12:e0173416. 10.1371/journal.pone.0173416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabira Y, Sakaguchi T, Kuhara H, et al. The width of a gastric tube has no impact on outcome after esophagectomy. Am J Surg 2004;187:417-21. 10.1016/j.amjsurg.2003.12.008 [DOI] [PubMed] [Google Scholar]
- 10.Farnes I, Johnson E, Johannessen HO. Management of gastric conduit retention following hybrid and minimally invasive esophagectomy for esophageal cancer: Two retrospective case series. Int J Surg Case Rep 2017;41:505-10. 10.1016/j.ijscr.2017.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liebermann-Meffert DM, Meier R, Siewert JR. Vascular anatomy of the gastric tube used for esophageal reconstruction. Ann Thorac Surg 1992;54:1110-5. 10.1016/0003-4975(92)90077-H [DOI] [PubMed] [Google Scholar]
- 12.Ndoye JM, Dia A, Ndiaye A, et al. Arteriography of three models of gastric oesophagoplasty: the whole stomach, a wide gastric tube and a narrow gastric tube. Surg Radiol Anat 2006;28:429-37. 10.1007/s00276-006-0129-5 [DOI] [PubMed] [Google Scholar]
- 13.Orringer MB. Transhiatal Esophagectomy: How I Teach It. Ann Thorac Surg 2016;102:1432-7. 10.1016/j.athoracsur.2016.09.044 [DOI] [PubMed] [Google Scholar]
- 14.Yoshida T, Inoue H, Iwai T. Hand-assisted laparoscopic surgery for the abdominal phase in endoscopic esophagectomy for esophageal cancer: an alteration on the site of minilaparotomy. Surg Laparosc Endosc Percutan Tech 2000;10:396-400. 10.1097/00129689-200012000-00012 [DOI] [PubMed] [Google Scholar]
- 15.Barreto JC, Posner MC. Transhiatal versus transthoracic esophagectomy for esophageal cancer. World J Gastroenterol 2010;16:3804-10. 10.3748/wjg.v16.i30.3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosumi K, Baba Y, Watanabe M, et al. Pedunculated gastric conduit interposition with duodenal transection after salvage esophagectomy: an option for increasing the flexibility of the gastric conduit. J Am Coll Surg 2012;214:e31-3. 10.1016/j.jamcollsurg.2012.01.048 [DOI] [PubMed] [Google Scholar]
- 17.Yano M, Motoori M, Tanaka K, et al. Prevention of gastroduodenal content reflux and delayed gastric emptying after esophagectomy: gastric tube reconstruction with duodenal diversion plus Roux-en-Y anastomosis. Dis Esophagus 2012;25:181-7. 10.1111/j.1442-2050.2011.01229.x [DOI] [PubMed] [Google Scholar]
- 18.Maas KW, Biere SS, Scheepers JJ, et al. Minimally invasive intrathoracic anastomosis after Ivor Lewis esophagectomy for cancer: a review of transoral or transthoracic use of staplers. Surg Endosc 2012;26:1795-802. 10.1007/s00464-012-2149-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackmon SH, Correa AM, Wynn B, et al. Propensity-matched analysis of three techniques for intrathoracic esophagogastric anastomosis. Ann Thorac Surg 2007;83:1805-13; discussion 1813. [DOI] [PubMed]
- 20.Grimminger PP, van der Horst S, Ruurda JP, et al. Surgical robotics for esophageal cancer. Ann N Y Acad Sci 2018;1434:21-6. 10.1111/nyas.13676 [DOI] [PubMed] [Google Scholar]
- 21.Kesler KA, Ramchandani NK, Jalal SI, et al. Outcomes of a novel intrathoracic esophagogastric anastomotic technique. J Thorac Cardiovasc Surg 2018;156:1739-45 e1. [DOI] [PubMed]
- 22.Jansen SM, de Bruin DM, van Berge Henegouwen MI, et al. Optical techniques for perfusion monitoring of the gastric tube after esophagectomy: a review of technologies and thresholds. Dis Esophagus 2018;31. [DOI] [PubMed] [Google Scholar]
- 23.Zehetner J, DeMeester SR, Alicuben ET, et al. Intraoperative Assessment of Perfusion of the Gastric Graft and Correlation With Anastomotic Leaks After Esophagectomy. Ann Surg 2015;262:74-8. 10.1097/SLA.0000000000000811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalton BGA, Ali AA, Crandall M, et al. Near infrared perfusion assessment of gastric conduit during minimally invasive Ivor Lewis esophagectomy. Am J Surg 2018;216:524-7. 10.1016/j.amjsurg.2017.11.026 [DOI] [PubMed] [Google Scholar]


