Abstract
Esophagectomy with en-bloc lymphadenectomy after neoadjuvant chemo(radio)therapy is the standard of care for resectable locally advanced esophageal cancer. Postoperative complications may have a significant impact on the duration of hospital stay and quality of life. Early recognition and treatment of complications may reduce failure to rescue rates and improve postoperative outcomes. New-onset atrial fibrillation (AF) after esophagectomy for cancer is frequently observed, and may be related to other postoperative complications. AF could function as an early warning sign for other complications in the postoperative course after esophagectomy and may thus be of clinical value. This review discusses the pathophysiology and possible risk factors of AF, the association between AF and other postoperative complications, and the influence of AF on postoperative outcomes after esophagectomy for cancer. Furthermore, clinical recommendations for the management of new-onset AF after esophagectomy for cancer are provided.
Keywords: Esophageal, cancer, esophagectomy, atrial fibrillation (AF)
Introduction
Esophageal cancer is an aggressive disease and is currently the sixth leading cause of cancer-related mortality worldwide (1,2). Curative therapy for locally advanced esophageal cancer comprises of neoadjuvant chemo(radio)therapy followed by esophagectomy with adequate lymphadenectomy (3,4). Despite significant improvements in surgical techniques and patient selection over the years, the risk of postoperative complications remains substantial (40–60%), with pulmonary complications, anastomotic leakage and atrial fibrillation (AF) being the most common (5-7).
New-onset AF after esophagectomy occurs in 12–37% of patients (7-17). AF is characterized by rapid and non-functional contractions of the atria and is a clinical concern as it may directly increase the risk of thromboembolic events, as well as indirectly increase the onset of other complications through hemodynamic instability and organ hypoperfusion (8-11). Furthermore, AF may be related to other postoperative (infectious) complications and has been significantly associated with prolonged hospital stay, increased reoperation rates and increased risk of postoperative mortality (8-11,17).
This review discusses the pathophysiology and possible risk factors of AF, the association between AF and other postoperative complications, and the influence of AF on postoperative outcomes after esophagectomy for cancer. Additionally, clinical recommendations for the management of new-onset postoperative AF after esophagectomy will be provided.
Pathophysiology
The pathophysiology of AF is a complicated, multifactorial process, and may occur in diverse clinical settings (17). New-onset AF after esophagectomy may be caused by perioperative factors, however, the exact pathophysiology remains unclear. Intra-operatively, the pleural surfaces and pericardium are exposed (especially during transthoracic esophagectomy), which might cause direct trauma to the atrium or autonomic nerve fibers and thus may result in AF (18). Also, the heart has a high rate of adenosine triphosphate (ATP) consumption, being seriously dependent on mitochondrial oxidative phosphorylation (19). Intra-operative (single lung) ventilation causes oxidative stress, which subsequently leads to a mismatch between atrial ATP production and use. This may cause mitochondrial dysfunction, potentially leading to AF (19). Furthermore, infections may create an oxidative stress reaction, potentially leading to AF.
Postoperative factors, such as hypo- and hypervolemia, may also play a role in the pathophysiology of new-onset AF. In a state of hypovolemia, which may be caused by fluid loss, anesthesia-induced cardiovascular depression or sepsis, a decreased venous return to the right atrium reduces the cardiac output. Release of endogenous catecholamines is stimulated by decreased tissue oxygen delivery (20). This sympathetic hyperactivity has a proarrhythmic effect on the heart and may cause AF. In contrast, hypervolemia may cause AF through an increase in atrium diastolic volume and reduced compliance, which leads to an altering of electrical properties of atrial cells (20).
Lastly, the presence of preoperatively undetected heart diseases potentially contributes to the development of post-operative AF.
Risk factors
A multitude of risk factors for AF have been described in the literature, varying from patient-specific risk factors such as male gender, comorbidities such as congestive heart failure, peripheral vascular disease and diabetes mellitus, as well as surgery related factors such as an open surgical approach, the need for an intraoperative blood transfusion and an extended surgical field in case of a large tumor (21-23). Some studies also demonstrate the administration of neo-adjuvant chemo(radio)therapy to be significantly associated with new-onset AF (12). In a study by Vaporciyan et al. (12) the risk of AF was 34% in elderly patients receiving chemo(radio)therapy followed by surgery versus 18% in the group that underwent esophagectomy alone (P<0.01). This might be attributed to the cardiotoxic effects of both the radio- and chemotherapy, which are frequently administered during neoadjuvant treatment for locally advanced resectable esophageal cancer.
It is important to realize the clinical relevance of the identification of these risk factors. First, some studies suggest that it may guide the prophylactic use of anti-arrhythmic agents in high-risk patients (23-25). The effect of prophylactic treatment with either rhythm- or rate control of new-onset AF after esophagectomy has been studied in several randomized controlled clinical trials, with significant favorable results for the patients in the intervention group compared to the placebo group in terms of AF incidence rates after esophagectomy (23-25). In order to be able to study whether lower incidence rates of AF result in better overall outcomes, data regarding other postoperative outcomes is warranted. Of the mentioned randomized controlled clinical trials, only one study reported data regarding other postoperative complications. In that particular study, 100 patients were randomized between arrhythmic prophylaxis with landiolol or a placebo (24). New-onset AF occurred in 10% of the patients receiving landiolol versus 30% of the patients receiving the placebo (P<0.05). Postoperative pneumonia (6% in the patients receiving landiolol versus 14% in the placebo group, P=0.318) and anastomotic leakage rates (4% in the patients receiving landiolol versus 14% in the placebo group, P=0.160) did not differ between the two groups. Even though this could be due to a lack of power in this relatively small study, AF to date may rather be considered an early warning sign for other (infectious) complications than the cause of these complications. Therefore, prophylactic treatment for AF might only mask an early clinical sign of other postoperative complications and possibly delays treatment. Second, it remains questionable whether AF needs to be prevented, since it is relatively easy to treat and generally resolves fast after treatment initiation. Nevertheless, the identification of risk factors may have an impact on patient selection, since it offers the opportunity to (medically) manage these risk factors and optimally prepare the patient for surgery.
Association with other postoperative complications
Recently, the role of pathophysiologic pathways that involve inflammatory and oxidative processes are under investigation. As inflammation might lead to AF, and AF might promote inflammation as well, a vicious cycle might be created.
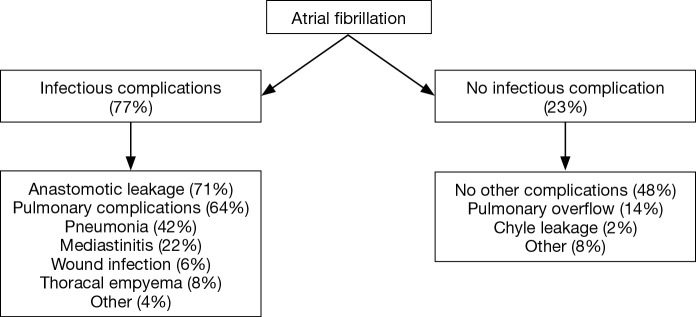
Multiple studies report significant associations between new-onset AF and other postoperative complications (8-13,17). A recently published prospective cohort study reported a significant association between new-onset AF and other postoperative infectious complications in general [odds ratio (OR): 3.00; 95% confidence interval (CI): 1.73–5.21], as well as pulmonary complications, pneumonia and anastomotic leakage specifically [OR (95% CI): 2.06 (1.29–3.30), 2.41 (1.48–3.91) and 3.00 (1.80–4.99), respectively] (17). As such, AF might function as an early warning sign for the occurrence of other postoperative complications but this only applies when AF actually precedes these postoperative complications, which was demonstrated in the same study. New-onset AF was diagnosed at a median of 4 days before the diagnosis of anastomotic leakage, whereas pneumonia and AF were mostly diagnosed on the same day. Likewise, Stawicki et al. (11) demonstrated that new-onset AF was significantly associated with anastomotic leakage and also reported a time interval of 4 days between the diagnosis of new-onset AF and the occurrence of anastomotic leakage. In the largest cohort study published to date it was found that in less than 10% of the patients, new-onset AF after esophagectomy was not accompanied by other complications (17). This suggests that AF is a surrogate marker for other postoperative complications and could therefore be of clinical importance (Figure 1). As such, clinicians should carefully evaluate patients who are diagnosed with new-onset AF shortly after esophagectomy to diagnose or exclude other postoperative complications. This evaluation should include at least a thorough physical examination including assessment of vital functions and body temperature, blood tests to screen for signs of infection (such as increased leukocyte counts or C-reactive protein levels) and a subsequent CT scan of the thorax and abdomen when appropriate.
Figure 1.
Flowchart: new-onset atrial fibrillation after esophagectomy [based on Seesing et al. (17)].
Conclusions and clinical consequences
New-onset AF after esophagectomy for cancer is a common postoperative complication and may be associated with other, severe, postoperative complications such as pneumonia, respiratory failure and anastomotic leakage. Since AF seldomly occurs without other postoperative complications, physicians have to be aware that other complications may occur. Furthermore, the prophylactic use of use of anti-arrhythmic agents is not indicated since it may mask an early warning signal for other complications.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol 2013;19:5598-606. 10.3748/wjg.v19.i34.5598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N Engl J Med 2012;366:2074-84. 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 4.Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. 10.1016/S1470-2045(15)00040-6 [DOI] [PubMed] [Google Scholar]
- 5.Low DE. Evolution in Surgical Management of Esophageal Cancer. Dig Dis 2013;31:21-9. 10.1159/000343650 [DOI] [PubMed] [Google Scholar]
- 6.Reynolds JV, Donohoe CL, McGillycuddy E, et al. Evolving progress in oncologic and operative outcomes for esophageal and junctional cancer: Lessons from the experience of a high-volume center. J Thorac Cardiovasc Surg 2012;143:1130-7.e1. 10.1016/j.jtcvs.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 7.Blencowe NS, Strong S, McNair AG, et al. Reporting of Short-Term Clinical Outcomes After Esophagectomy. Ann Surg 2012;255:658-66. 10.1097/SLA.0b013e3182480a6a [DOI] [PubMed] [Google Scholar]
- 8.Stippel DL, Taylan C, Schröder W, et al. Supraventricular tachyarrhythmia as early indicator of a complicated course after esophagectomy. Dis Esophagus 2005;18:267-73. 10.1111/j.1442-2050.2005.00487.x [DOI] [PubMed] [Google Scholar]
- 9.Rao VP, Addae-Boateng E, Barua A, et al. Age and neo-adjuvant chemotherapy increase the risk of atrial fibrillation following oesophagectomy. Eur J Cardiothorac Surg 2012;42:438-43. 10.1093/ejcts/ezs085 [DOI] [PubMed] [Google Scholar]
- 10.Murthy SC, Law S, Whooley BP, et al. Atrial fibrillation after esophagectomy is a marker for postoperative morbidity and mortality. J Thorac Cardiovasc Surg 2003;126:1162-7. 10.1016/S0022-5223(03)00974-7 [DOI] [PubMed] [Google Scholar]
- 11.Stawicki SP, Prosciak MP, Gerlach AT, et al. Atrial fibrillation after esophagectomy: An indicator of postoperative morbidity. Gen Thorac Cardiovasc Surg 2011;59:399-405. 10.1007/s11748-010-0713-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaporciyan AA, Correa AM, Rice DC, et al. Risk factors associated with atrial fibrillation after noncardiac thoracic surgery: analysis of 2588 patients. J Thorac Cardiovasc Surg 2004;127:779-86. 10.1016/j.jtcvs.2003.07.011 [DOI] [PubMed] [Google Scholar]
- 13.Mc Cormack O, Zaborowski A, King S, et al. New-onset atrial fibrillation post-surgery for esophageal and junctional cancer: incidence, management, and impact on short- and long-term outcomes. Ann Surg 2014;260:772-8; discussion 778. 10.1097/SLA.0000000000000960 [DOI] [PubMed] [Google Scholar]
- 14.Van Der Sluis PC, Verhage RJ, Van Der Horst S, et al. A new clinical scoring system to define pneumonia following esophagectomy for cancer. Dig Surg 2014;31:108-16. 10.1159/000357350 [DOI] [PubMed] [Google Scholar]
- 15.Weijs TJ, Seesing MF, van Rossum PS, et al. Internal and External Validation of a multivariable Model to Define Hospital-Acquired Pneumonia After Esophagectomy. J Gastrointest Surg 2016;20:680-7. 10.1007/s11605-016-3083-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy. Ann Surg 2015;262:286-94. 10.1097/SLA.0000000000001098 [DOI] [PubMed] [Google Scholar]
- 17.Seesing MF, Scheijmans JC, Borggreve AS, et al. The predictive value of new-onset atrial fibrillation on postoperative morbidity after esophagectomy. Dis Esophagus 2018;31. [DOI] [PubMed] [Google Scholar]
- 18.Mathisen DJ, Grillo HC, Wilkins EW, Jr, et al. Transthoracic esophagectomy: a safe approach to carcinoma of the esophagus. Ann Thorac Surg 1988;45:137-43. 10.1016/S0003-4975(10)62424-1 [DOI] [PubMed] [Google Scholar]
- 19.Montaigne D, Marechal X, Lefebvre P, et al. Mitochondrial dysfunction as an arrhythmogenic substrate: a translational proof-of-concept study in patients with metabolic syndrome in whom post-operative atrial fibrillation develops. J Am Coll Cardiol 2013;62:1466-73. 10.1016/j.jacc.2013.03.061 [DOI] [PubMed] [Google Scholar]
- 20.Chelazzi C, Villa G, De Gaudio A. Postoperative Atrial Fibrillation. ISRN Cardiol 2011;2011:203179. 10.5402/2011/203179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chin JH, Moon YJ, Jo JY, et al. Association between Postoperatively Developed Atrial Fibrillation and Long-Term Mortality after Esophagectomy in Esophageal Cancer Patients: An Observational Study. PLoS One 2016;11:e0154931. 10.1371/journal.pone.0154931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tisdale JE, Wroblewski HA, Wall DS, et al. A randomized, controlled study of amiodarone for prevention of atrial fibrillation after transthoracic esophagectomy. J Thorac Cardiovasc Surg 2010;140:45-51. 10.1016/j.jtcvs.2010.01.026 [DOI] [PubMed] [Google Scholar]
- 23.Ojima T, Nakamori M, Nakamura M, et al. Randomized clinical trial of landiolol hydrochloride for the prevention of atrial fibrillation and postoperative complications after oesophagectomy for cancer. Br J Surg 2017;104:1003-9. 10.1002/bjs.10548 [DOI] [PubMed] [Google Scholar]
- 24.Horikoshi Y, Goyagi T, Kudo R, et al. The suppressive effects of landiolol administration on the occurrence of postoperative atrial fibrillation and tachycardia, and plasma IL-6 elevation in patients undergoing esophageal surgery: a randomized controlled clinical trial. J Clin Anesth 2017;38:111-6. 10.1016/j.jclinane.2017.01.036 [DOI] [PubMed] [Google Scholar]
- 25.Bobbio A, Caporale D, Internullo E, et al. Postoperative outcome of patients undergoing lung resection presenting with new-onset atrial fibrillation managed by amiodarone or diltiazem. Eur J Cardiothorac Surg 2007;31:70-4. 10.1016/j.ejcts.2006.10.020 [DOI] [PubMed] [Google Scholar]