Abstract
Purpose: Seborrheic dermatitis (SD) is a chronic inflammatory skin disorder that mainly affects areas rich in sebaceous glands, such as the scalp. Although the exact cause of SD is not clearly understood, it seems that skin colonization with Malassezia fungus and the inflammatory responses of the immune system to this fungus play an important role in the pathology of SD. Recently a growing body of evidence has shown anti-inflammatory and anti-fungal effects of statins. Thus, this study aimed to evaluate the efficacy of topical atorvastatin in the treatment of scalp SD.
Patients and methods: In this double-blind, clinical trial, 86 patients with mild-to-moderate scalp SD were divided into either atorvastatin (n=45) or betamethasone groups (n=41) by block randomization method. In addition to the ketoconazole 2% shampoo (3 times per week), the atorvastatin group received atorvastatin 5% lotion and the betamethasone group received betamethasone 0.1% lotion daily for 4 weeks. The SD severity of each patient was determined by Symptom Scale of Seborrheic Dermatitis (SSSD) at baseline and 4 weeks after treatment. Also, the patient’s satisfaction of the treatment and adverse effects were investigated through individual reporting.
Results: After 4 weeks of treatment, the score of SD severity decreased significantly in both groups, while changes of SSSD score from baseline to the fourth week of treatment were comparable in the two groups (P-value=0.476). Regarding patient’s satisfaction of the treatment, results demonstrated the non-inferiority of atorvastatin as compared to betamethasone. Topical atorvastatin was also well-tolerated in almost all patients.
Conclusion: Although preliminary, the results of the present study showed that topical atorvastatin has a comparable effect to topical betamethasone and can be considered as an alternative therapeutic modality in the treatment of scalp SD. However, these results need to be confirmed in future studies while taking into consideration the improvement of topical statin formulations.
Keywords: seborrheic dermatitis, anti-inflammatory effects, topical statin, skin disorders
Introduction
Seborrheic dermatitis (SD) is a common inflammatory skin condition that can affect body sites with increased numbers of sebaceous glands such as the scalp, face, chest, upper trunk, external ear, axillae, and inguinal folds. The prevalence of SD is about 3%, and young men are affected more frequently than women. In addition to physical discomfort, SD has a negative impact on the psycho-social function of affected patients.1–3 The etiology of SD is not completely known, but it seems that skin colonization with harmless yeast called Malassezia is implicated in the etiology of SD. M. restricta and M. globosa appear to be the most commonly isolated species of Malassezia in SD patients.4 However, the degree of colonization with this fungus in individuals with SD is not different from the normal population.5,6 Several lines of evidence demonstrate that, besides the pathogenic role for Mallassezia in SD, the host’s immune responses to Malassezia or its byproducts appear to have a causal link to the development and maintenance of SD. Malassezia by its lipase activity can hydrolyze human sebum triglycerides and release some metabolites that can disrupt epidermal barrier function and activate inflammatory responses.7,8 Further findings in favor of the role of inflammation in the pathogenesis of SD are the elevated levels of some inflammatory cytokines such as interleukins (in particular IL-1b, IL-6, IL-8), and tumor necrosis factor alpha (TNF-α) in the skin of patients suffering from SD.9 Furthermore, most of the effective therapeutic medications commonly used for SD, including azole antifungal agents, topical preparations of lithium, and topical corticosteroids have anti-inflammatory effects.10,11
SD is usually characterized by well-delimited plaques with scaling, itching, and erythematous looking, with severity of disease varying from mild to very severe.12 Dandruff is the mildest and most common form of SD that is restricted to the scalp with fine white or greasy scales without significant erythema or irritation.13 Depending on the severity of the disease, topical agents are commonly used for mild-to-moderate cases, while systemic antifungal agents may be a therapeutic option for severe cases.14,15
Statins are competitive inhibitors of 3-hydroxy-3-methyl glutaryl-coenzyme A reductase (HMG-CoA reductase), which are commonly used for the prevention and treatment of atherosclerosis and cardiovascular diseases.16 Recently, accumulating evidence has demonstrated anti-inflammatory and immonomudolatory effects of statins, and preliminary studies have showed statins may be effective in the treatment of inflammatory skin diseases, such as acne, vitiligo, psoriasis, and dermatitis.17,18 Additionally, since many fungal species depend on a functional HMGCoA reductase for cell wall synthesis, growing in vitro evidence has also demonstrated that statins can have antifungal activity. By inhibiting HMG-CoA reductase Class I and inhibition of cholesterol synthesis, statins can cause a reduction in the production of ergosterol that appears to have a critical role in the survival of the fungi.19
Atorvastatin is one of the most effective agents among currently available statins in cholesterol reduction, which has exhibited potent anti-inflammatory effects in clinical studies.20 On the other hand, atorvastatin is administered as the active hydroxy form. So, it does not need activation by intracellular esterases.18 Thus, considering the role of Malassezia fungus and inflammation in the pathogenesis of SD and at the same time the anti-inflammatory and anti-fungal effects of statins, this study was conducted to investigate the effectiveness of topical Atorvastatin 5% vs topical betamethasone 0.1% in the treatment of scalp SD.
Materials and methods
This study was conducted as a randomized double-blind, 4-week control trial from May 2018 to August 2018 in an outpatient dermatology clinic affiliated to Hamadan University of Medical Sciences, Hamadan, Iran. This study was approved by the Ethics committee of Hamadan University of Medical Sciences (Hamadan, Iran), and was performed in accordance with the rules laid down in the Declaration of Helsinki and its later amendments. It was registered in Iranian Registry of Clinical Trials (IRCT2017070922965N11. www.irct.ir). All participants were informed about the study’s aims, and informed consent forms were signed by all the participating patients.
One hundred and two patients with mild-to-moderate scalp SD were randomly assigned to atorvastatin or betamethasone groups using the block randomization method. Neither patients nor physician and data collector were aware of group assignment. In addition to Ketoconazole 2% shampoo (3 times a week), patients were asked to apply 10 ml (or one half of a capful) of the topical medication (atorvastatin 5% (W/V) lotion or betamethasone 0.1% lotion according to the patients’ assigned groups) on his/her dry scalp once daily for a period of 4 weeks. Patients were instructed to spread it thoroughly onto their scalp and, after 24 hours, the hair and scalp needed to be washed completely.
Betamethasone lotion was manufactured by Najo Pharmaceutical Company (Tehran, Iran), and atorvastatin 5% topical lotion (W/V) was prepared by solubilizing atorvastatin powder in ethanol and glycerol, and then homogenized with about 50,000 rounds. Selection of the 5% concentration of atorvastatin was based on a previous study that topical atorvastatin 5% was used in the treatment of diabetic wounds.21
To evaluate the stability of topical lotion, it was kept in a germination with 60% of humidity and a temperature of 60°C for 6 months. Results showed a stability of 96.4% at the end of the first month, and 91.8% at the end of the second month.
Sampling
Patients were included in this study if the following inclusion criteria were met at the baseline of the study: (a) aged between 18–65 years; (b) clinical diagnosis of SD of the scalp; (c) mild-to-moderate SD based on Symptom Scale of Seborrheic Dermatitis (SSSD); (d) not receiving any topical or systemic treatment for SD during 1 month before enrollment; and (e) absence of any disorder that was associated or exacerbated SD, such as HIV and Parkinson’s disease. Exclusion criteria at baseline and during the study were as follows: (a) patients with severe SD (SSSD score greater than 10); (b) pregnancy or lactation or expecting to get pregnant during the treatment; (c) poor adherence to the treatment; (d) presence of any adverse effects resulting in patients’ intolerance or complications; and (e) unwilling or unable to follow the study protocol.
The severity of SD in each patient was determined based on SSSD criteria at baseline (visit 1) and 4 weeks (visit 2) after treatment. SSSD score was determined according to severity of three cardinal symptoms of SD including erythema, scaling, and itching. The symptoms of erythema and scaling are scored on a scale varying from 0–5 (0=no signs, 1=first sign, 2=mild, 3=moderate, 4=severe, and 5=very severe), and the severity of itching was evaluated by the visual analog scale (VAS) that was scored from 0 (denoting no itch) to 100 (denoting worst possible imaginable itch), and which was also categorized in six steps as follows: ≤10 mm=0, 11– 20 mm=1, 21–40 mm=2, 41–60 mm=3, 61–80 mm=4, and 81–100 mm=5. The clinical severity score of SD was determined by summing up the scores and was classified as follows: Mild SI (0–6), Moderate SI (7–9), and Severe SI (10–15). In addition, after 4 weeks of treatment, the patient’s satisfaction with the treatment was investigated according to a 4-point scale ranging from 0–3 as follows: 0=non, 1=mild, 2=moderate, and 3=good.23
Participants data, including demographic data and SSSD scores, are available on request from the corresponding author for up to 2 years after publication.
Assessment of adverse effects
To evaluate the adverse effects of medications, all patients were asked at each visit if they had experienced one or more of the possible adverse effects, such as itching, burning, and erythema, and exacerbation of them if such symptoms were present prior to the treatment. The type and severity (mild, moderate, or severe) of the adverse effects were recorded.
Data analysis
Per protocol analysis was exploited to analyze data of all individuals who completed the study. Data were analyzed by SPSS software (version 16.0, SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov test was used to assess the normal distribution of continuous variables. Normally- and non-normally distributed continuous data were expressed as mean (standard deviation [Std. Dev.]) and median (interquartile range: IQ), respectively. Categorical variables were reported as percentages. Mean (Std. Dev.) and median (IQ) of continues variables were compared between two groups using independent t-test and Mann-Whitney U-test, respectively. The distribution of categorical variables between two groups was compared using Chi-square or Fisher exact test (if more than 20% of the categories were expected to have frequencies less than 5). Also, P-value<0.05 was considered as the significant level.
Results
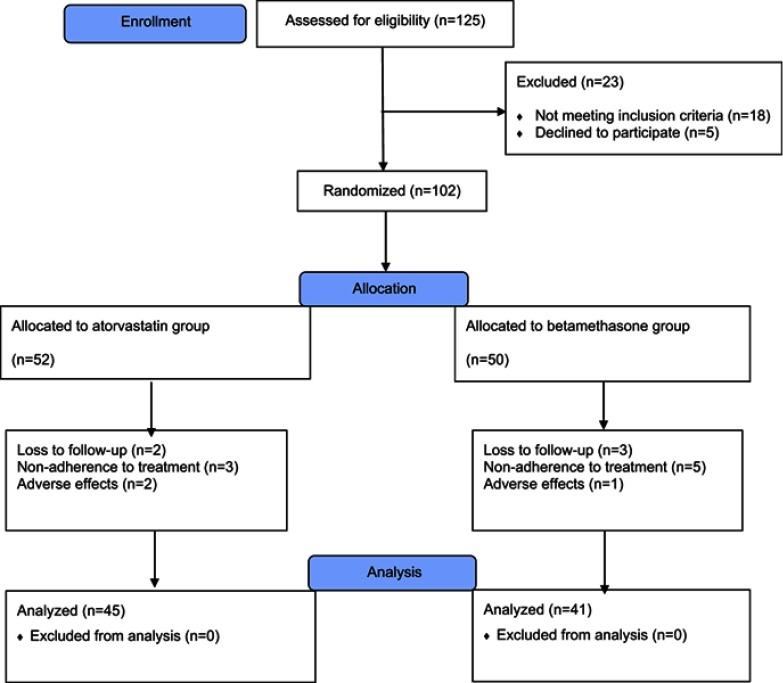
As shown in the patients’ flow diagram (Figure 1), 125 patients were screened for recruitment. Among them, 18 patients did not meet the inclusion criteria, and five patients did not agree to participate in the study. The remaining 102 patients were randomly allocated into two groups to receive either topical atorvastatin 5% lotion or betamethasone 0.1% lotion (52 and 50 patients in each group, respectively).
Figure 1.
Clinical trial flowchart of the study.
In the atorvastatin group, seven patients were excluded during the study period due to the patients’ unwillingness to continue the new medication (three patients), experiencing intolerable adverse effects (two patients), and loss to follow-up (two patients). In the betamethasone group, nine patients were excluded during the study period, due to patients’ unwillingness to continue the new medication (five patients), experiencing intolerable adverse effects (one patient), and loss to follow-up (three patients). Finally, 86 subjects, including 45 and 41 patients in the atorvastatin and betamethasone groups, respectively, completed the 4 weeks of the study. Causes of patient loss during the study period were comparable between the two groups (P=0.39). All of the following results were related to 86 patients who completed the 4 weeks of the study.
Baseline demographic and background characteristics
The patients’ demographic characteristics, such as age, sex, body mass index (BMI), and duration of SD are shown in Table 1. There were no significant differences between the atorvastatin and betamethasone groups regarding the demographic characteristics at the baseline. Sixty-four percent of the study patients were males, and the mean age of the patients in the atorvastatin and betamethasone groups was 30.37±7.24 and 28.09±7.22 years, respectively. Also, participants in both groups were comparable considering the duration of disease. Regarding the SD severity at baseline, disease severity was comparable in both treatment groups, as measured by the SSSD score (6.66±0.93 points in the atorvastatin group vs 7.12±1.26 points in the betamethasone group, with the P-value=0.064).
Table 1.
Comparison of baseline background and demographic variables between two groups
| Variable | Atorvastatin group (n=45) |
Betamethasone group (n=41) |
|---|---|---|
| Sex | ||
|
Male, n (%) Female, n (%) |
29 (64.5%) 16 (35.5%) |
26 (63.4%) 15 (36.6%) |
| Age, years (mean ± Std. Dev.) | 30.37±7.24 | 28.09±7.22 |
| BMI, kg/m2 (mean ± Std. Dev.) | 23.28±3.2 | 24.39±3.3 |
| Duration of disease, months (mean ± Std. Dev.) | 18±8.2 | 21.6±4.5 |
| SSSD score (mean ± Std. Dev.) | 6.66±0.93 | 7.12±1.26 |
Abbreviations: BMI, Body Mass Index; SSSD, Symptom Scale of Seborrheic Dermatitis; Std.Dev., Standard Deviation.
Efficacy results
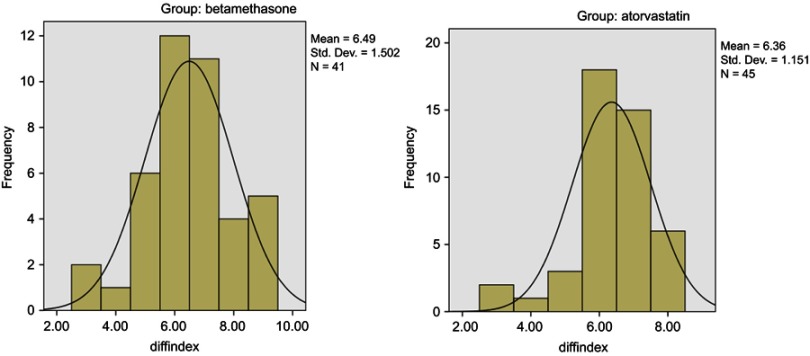
The comparing results of improvement in clinical assessment score intra- and inter-groups after 4 weeks of treatment are shown in Tables 2 and 3. The results showed that the score of SSSD severity as compared with baseline values significantly decreased in the two groups after 4 weeks of treatment, while the changes in SSSD from baseline to week 4 demonstrated no significant difference between the two groups (P-value=0.65). The adjusted difference in treatment effect between the atorvastatin and betamethasone groups was 0.13 SSSD score points (95% confidence interval=-0.48–0.70 SSSD-points). Therefore, according to the obtained results, the topical atorvastatin was as effective as topical betamethasone in the treatment of scalp SD. Furthermore, the histogram of the two groups represents the normal distribution of the patients in both groups (Figure 2).
Table 2.
Mean changes in SSSD score from baseline (visit 1) to week 4 (visit 2) by treatment group
| Atorvastatin group (n=45) |
Betamethasone group (n=45) |
Difference, atorvastatin–betamethasone |
P-value | |
|---|---|---|---|---|
| Mean [two-sided 95% CI] |
−6.35 [−6.04,−6.95] |
−6.48 [−6.02,−6.69] |
0.13 [−0.48,0.70] |
0.65 |
Abbreviation: SSSD, Symptom Scale of Seborrheic Dermatitis.
Table 3.
Summary of the effects of interventions in the severity of seborrheic dermatitis (mean ± Std. Dev.)
| Variables | Groups | P-value | |
|---|---|---|---|
| Atorvastatin | Betamethasone | ||
| Baseline severity | 6.66±0.92 | 7.12±1.26 | 0.06 |
| Endpoint severity | 0.31±0.56 | 0.63±1.04 | 0.08 |
| P-value | <0.001 | <0.001 | |
| Difference between endpoint and baseline severity | 6.35±1.15 | 6.48±1.50 | 0.65 |
Figure 2.
Changes in SSSD score from baseline to week 4 by treatment group (histogram). The frequency in figure 2 is number of patients and the diffindex is changes in SSSD score from baseline to week 4.
Abbreviations: SSSD, Symptom Scale of Seborrheic Dermatitis.; Std. Dev., standard deviation.
Assessment of satisfaction and adverse events
Comparison of patients’ frequency distribution considering satisfaction after the treatment in the atorvastatin and betamethasone groups is showed in Table 4. As shown, the groups did not differ significantly in the degree of satisfaction with treatment (P=0.499), and results demonstrated the non-inferiority of atorvastatin as compared to betamethasone.
Table 4.
Patients’ frequency distribution while considering after-treatment satisfaction on the 28th day of treatment
| Group | Satisfaction frequency of treatment | Total | P-value | |||
|---|---|---|---|---|---|---|
| Non | Mild | Moderate | Good | |||
| Betamethasone (n) | 1 (2.4%) | 9 (22.0%) | 10 (24.4%) | 21 (51.2%) | 41 | 0.49 |
| Atorvastatin (n) | 1 (2.2%) | 6 (13.3%) | 8 (17.8%) | 30 (66.7%) | 45 | |
| Total (n) | 2 (2.3%) | 15 (17.4%) | 18 (20.9%) | 51 (59.3%) | 86 (100%) | |
Regarding the occurrence of adverse effects, seven (13.47%) and six (12%) patients in the atorvastatin and betamethasone groups experienced adverse events, such as itching or irritation, while none of the patients experienced severe and systemic adverse effects. Two patients in the atorvastatin group and one patient in the betamethasone group left the study due to the intolerable adverse effects, none of which was serious or caused any complication for the patients. Therefore, there were no significant differences regarding the occurrence of adverse effects between the two groups, and the topical atorvastatin was well-tolerated in almost all patients.
Discussion
Based on our best knowledge, this is the first randomized, double-blind, control trial evaluating the efficacy of topical statins in the treatment of SD. The results of the current study showed that atorvastatin lotion as an adjuvant therapy had efficacy comparable to betamethasone lotion in the treatment of scalp SD and was well-tolerated.
Since SD is a common disease and requires chronic treatment, it is necessary to use an effective treatment with acceptable safety and tolerability.1,2 The etiology and pathogenesis of SD are not completely understood, and there is much controversy in this regard. Although there are strong casual association between skin colonization with Malassezia fungus and SD, recent studies provide compelling evidence that inflammation plays an important role in the pathogenesis of SD.19,24 Actually, in some patients SD may result from the host’s immune responses to Malassezia or to its byproducts. Malassezia was shown to react with triglycerides released from the sebaceous glands and produced metabolites that can alter the epidermal permeability barrier function leading to cycles of immune stimulation.25,26 Also, fungus lipid-layer causes keratinocytes to produce pro-inflammatory mediators that can elicit skin inflammation via recruitment of inflammatory cells and vasodilation.8 In skin biopsy of patients with SD, increased local production of some inflammatory cytokines such as IL-1a, IL-10, NK11, CD161, as well as in the regulatory interleukins for both T helper 1 and T helper 2 cells were observed in SD lesions.7 Additionally, the serum level of total IgA and IgG antibodies has increased in some SD patients.27 Accordingly, due to mentioned pathological pathways, agents with antifungal and anti-inflammatory properties can be effective in the treatment of SD.11 Though topical corticosteroids and antifungal agents are widely used for the treatment of SD, due to the concern regarding the adverse effects such as skin atrophy and telangiectasia in long-term use of topical corticosteroids and ineffectiveness of antifungal drugs in some cases, the identification of safe and effective alternative treatments can be valuable in these patients.24
In clinical practice, statins are routinely used in the treatment of cardiovascular disease. Currently ample evidence from both experimental and clinical studies demonstrated anti-inflammatory properties of statins and showed that these agents are useful in treating some inflammatory and autoimmune diseases. Anti-inflammatory effects of statins are independent of lowering serum level cholesterol. It was shown that statins by diverse effects on innate and adaptive immune system can reduce inflammatory responses.28 A main mechanism of anti-inflammatory of statins is inhibition of isoprenoid synthesis through interfering with cholesterol biosynthesis. Available evidence has demonstrated that statins through antioxidant effects on blood vessels and inhibitory effects on function of some inflammatory factors such as IL-6 and CRP can regulate innate immune response. Also, through inhibitory effects on the expression of adhesion molecules as well as blocking function of lymphocyte function-associated antigen (LFA)-1, which is crucial for lymphocyte recirculation, antigen-specific T-cell activation, and transendothelial migration of immunocytes, statins suppress activation and function of lymphocytes.28 Due to their anti-inflammatory effects, recently topical and systemic statins are employed in the treatment of a variety of skin disorders, while relevant studies reported conflicting results in this regard. While results of some studies demonstrated that treatment with oral statins in psoriasis was associated with significant improvement of Psoriasis Area and Severity Index (PASI),29 the results of other studies showed that not only any significant clinical improvement was seen in psoriasis patients,30 but also the exacerbation of psoriasis skin symptoms have been reported in some cases treated by statins for hypercholesterolemia.31 Conflicting effects of statins in treatment of psoriasis in the mentioned studies can be attributed to the diversity of the patient population, which had relatively different baseline PASI scores. It seems that clinical benefits of statins would be likely to occur in patients with more severe psoriasis. Some evidence also showed that oral statins via their immunomodulatory and antioxidant activities can be effective in treating other autoimmune skin disorders such as vitiligo and alopecia areata.18 The utility of topical or systemic statins in the treatment acne vulgaris has also been investigated in some studies. Results of one study showed that oral simvastatin, in combination with oral contraceptive pills in patients’ with poly-cystic ovary syndrome, was able to reduce serum level of testosterone and improved acne severity more effectively as compared to contraceptive pills alone.32 Also, in agreement with the beneficial effects of statins in the treatment of acne vulgaris, the results obtained from our recent clinical trial study showed that topical and systemic simvastatin have profound alleviating effects on the acne lesions.33 In contrast to these findings, the results of the study by Mikhael et al, probably due to small sample size and short duration of their study, showed that topical atorvastatin had similar effects to topical placebo in the treatment of papulopustular acne lesions.34 There are some other relevant studies in which the effectiveness of topical application of statins have been investigated in some skin condition. In this regard, although topical lovastatin was well-tolerated, application of it before chemotherapy was only modestly effective in the prevention of chemotherapy-induced alopecia.35 The potential therapeutic value of statins in controlling inflammatory symptoms of contact dermatitis also investigated in some animal studies and, according to these studies, the topical statins have inhibitory effects comparable to topical corticosteroids on the acute model of irritant contact dermatitis.36 Conflicting results regarding effectiveness of statins in treatment of inflammatory dermatological diseases in conducted studies, in addition to differences in sample size and duration of studies, may contribute to several other factors including lipophilicity of the study agents and their concentration, particularly in topical application, as well as severity and chronicity of study skin diseases. Furthermore, it seems that combination therapy of statins may be a more effective therapeutic modality than treatment as a single agent in the treatment of skin disorders. Results of our current study also showed that topical atorvastatin as adjunct therapy was effective in improving symptoms of scalp SD.
Despite the beneficial effects of statins in the treatment of skin disorders, there are some concerns implying that statins can induce or exacerbate some skin autoimmune diseases. Although long-term exposure to statins may associate to some skin adverse effects, especially in patients with pre-existing skin barrier dysfunction and genetic predispositions, the risk of occurrence of cutaneous adverse reactions to statins seems to be relatively low.37
In addition to their inflammatory effects, recently there is preliminary in vitro evidence demonstrating statins in combination with commonly antifungal agents can exhibit potent synergistic effects against many clinically relevant yeast and mold species, including dermatophytes that are common fungal species in skin infections.19,38,39 Actually, statins by inhibiting HMG-CoA Reductase and reduction in synthesis of important terpenoid derivatives, which is involved in the synthesis of ergosterol in fungi, exhibit their own fungicidal or fungistatic effects in a dose dependent-manner.19 However, it seems that anti-inflammatory effects of statins on immune host cells also have a crucial role in their effectiveness in fungal infections. Therefore, in our study, in addition to its anti-inflammatory effects, beneficial effects of atorvastatin on symptoms of scalp SD at least in part can be linked to its antifungal properties. Nevertheless, given that statins exhibit antifungal activity at supraphysiological concentrations and there is little clinical evidence in this regard, high-quality future clinical studies are needed to confirm the antifungal effects of these agents.
The limitations of our study include the small sample size and the mild-to-moderate severity of SD in the study population that can affect generalization of our results. We used atorvastatin as adjutant therapy in treatment of scalp SD, so the effects of other topical statins as well as their therapeutic effects as mono-therapy on clinical outcomes need to be addressed in future studies. Also, a formulation of topical atorvastatin that has been used in our study was a basic and raw formulation that needs to be improved in future studies. Since in this study instruction by the researcher on how to take topical atorvastatin had a positive impact on compliance to the treatment, the compliance of our study population might have over-estimated the overall compliance of patients.
Conclusion
Although preliminary, according to the results of the present study, topical application of statins, drugs with anti-inflammatory and antifungal properties, can have a valuable place in the treatment of inflammatory skin diseases, such as SD of the scalp, while concern about the uncommon but serious systemic adverse effects, such as myopathy and hepatotoxicity, do not matter in the chronic application of topical statins.
Acknowledgments
This study was supported by the Vice-Chancellor of Research and Technology of Hamadan University of Medical Sciences, Hamadan, Iran (number 9607184522). The authors thank all patients for helping and participating in the study.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol. 2013;6(2):44. [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta A, Bluhm R. Seborrheic dermatitis. J Eur Acad Dermatol Venereol. 2004;18(1):13–26. doi: 10.1111/jdv.2004.18.issue-1 [DOI] [PubMed] [Google Scholar]
- 3.Soeprono FF, Schinella RA, Cockerell CJ, Comite SL. Seborrheic-like dermatitis of acquired immunodeficiency syndrome: a clinicopathologic study. J Am Acad Dermatol. 1986;14(2):242–248. [DOI] [PubMed] [Google Scholar]
- 4.Burton J, Pye R. Seborrhoea is not a feature of seborrhoeic dermatitis. Br Med J (Clin Res Ed). 1983;286(6372):1169–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pechère M, Krischer J, Remondat C, Bertrand C, Trellu L, Saurat JH. Malassezia spp carriage in patients with seborrheic dermatitis. J Dermatol. 1999;26(9):558–561. [DOI] [PubMed] [Google Scholar]
- 6.Falk MHS, Linder MT, Johansson C, et al. The prevalence of Malassezia yeasts in patients with atopic dermatitis, seborrhoeic dermatitis and healthy controls. Acta Derm Venereol. 2005;85(1):17–23. doi: 10.1080/00015550410022276 [DOI] [PubMed] [Google Scholar]
- 7.Faergemann J, Bergbrant IM, Dohse M, Scott A, Westgate G. Seborrhoeic dermatitis and pityrosporum (Malassezia) folliculitis: characterization of inflammatory cells and mediators in the skin by immunohistochemistry. Br J Dermatol. 2001;144(3):549–556. [DOI] [PubMed] [Google Scholar]
- 8.Riciputo R, Oliveri S, Micali G, Sapuppo A. Phospholipase activity in Malassezia furfur pathogenic strains: phospholipase‐Aktivität an patientenisolaten von Malassezia furfur. Mycoses. 1996;39(5‐6):233–235. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe S, Kano R, Sato H, Nakamura Y, Hasegawa A. The effects of Malassezia yeasts on cytokine production by human keratinocytes. J Invest dermatol. 2001;116(5):769–773. doi: 10.1046/j.1523-1747.2001.01321.x [DOI] [PubMed] [Google Scholar]
- 10.Ballanger F, Tenaud I, Volteau C, Khammari A, Dréno B. Anti-inflammatory effects of lithium gluconate on keratinocytes: a possible explanation for efficiency in seborrhoeic dermatitis. Arch Dermatol Res. 2008;300(5):215. doi: 10.1007/s00403-007-0806-1 [DOI] [PubMed] [Google Scholar]
- 11.Kastarinen H, Oksanen T, Okokon EO, et al. Topical anti-inflammatory agents for seborrhoeic dermatitis of the face or scalp. Cochrane Database Syst Rev. 2014;(5):CD009446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dessinioti C, Katsambas A. Seborrheic dermatitis: etiology, risk factors, and treatments:: facts and controversies. Clin Dermatol. 2013;31(4):343–351. doi: 10.1016/j.clindermatol.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 13.Soares RC, Zani MB, Arruda ACBB, de Arruda LHF, Paulino LC. Malassezia intra-specific diversity and potentially new species in the skin microbiota from Brazilian healthy subjects and seborrheic dermatitis patients. PLoS One. 2015;10(2):e0117921. doi: 10.1371/journal.pone.0117921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kastarinen H, Okokon EO, Verbeek JH. Topical anti-inflammatory agents for seborrheic dermatitis of the face or scalp: summary of a cochrane review. JAMA dermatol. 2015;151(2):221–222. doi: 10.1001/jamadermatol.2014.3186 [DOI] [PubMed] [Google Scholar]
- 15.Shuster S, Meynadier J, Kerl H, Nolting S. Treatment and prophylaxis of seborrheic dermatitis of the scalp with antipityrosporal 1% ciclopirox shampoo. Arch Dermatol. 2005;141(1):47–52. doi: 10.1001/archderm.141.1.47 [DOI] [PubMed] [Google Scholar]
- 16.Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352(1):29–38. doi: 10.1056/NEJMoa042000 [DOI] [PubMed] [Google Scholar]
- 17.Wierzbicki AS, Poston R, Ferro A. The lipid and non-lipid effects of statins. Pharmacol Ther. 2003;99(1):95–112. doi: 10.1016/S0163-7258(03)00055-X [DOI] [PubMed] [Google Scholar]
- 18.Jowkar F, Namazi MR. Statins in dermatology. Int J Dermatol. 2010;49(11):1235–1243. doi: 10.1111/j.1365-4632.2010.04579.x [DOI] [PubMed] [Google Scholar]
- 19.Bergman PW, Björkhem-Bergman L. Is there a role for statins in fungal infections? Expert Rev Anti Infect Ther. 2013;11(12):1391–1400. doi: 10.1586/14787210.2014.856755 [DOI] [PubMed] [Google Scholar]
- 20.Khurana S, Gupta S, Bhalla H, Nandwani S, Gupta V. Comparison of anti-inflammatory effect of atorvastatin with rosuvastatin in patients of acute coronary syndrome. J Pharmacol Pharmacother. 2015;6(3):130. doi: 10.4103/0976-500X.162011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toker S, Gulcan E, Caycı MK, Olgun EG, Erbilen E, Özay Y. Topical atorvastatin in the treatment of diabetic wounds. Am J Med Sci. 2009;338(3):201–204. doi: 10.1097/MAJ.0b013e3181aaf209 [DOI] [PubMed] [Google Scholar]
- 22.Buechner SA. Multicenter, double-blind, parallel group study investigating the non-inferiority of efficacy and safety of a 2% miconazole nitrate shampoo in comparison with a 2% ketoconazole shampoo in the treatment of seborrhoeic dermatitis of the scalp. J Dermatolog Treat. 2014;25(3):226–231. doi: 10.3109/09546634.2013.782092 [DOI] [PubMed] [Google Scholar]
- 23.Goldust M, Rezaee E, Raghifar R. Treatment of seborrheic dermatitis: comparison of sertaconazole 2% cream versus pimecrolimus 1% cream. Ir J Med Sci. 2013;182(4):703–706. doi: 10.1007/s11845-013-0960-8 [DOI] [PubMed] [Google Scholar]
- 24.Hay R. Malassezia, dandruff and seborrhoeic dermatitis: an overview. Br J Dermatol. 2011;165:2–8. doi: 10.1111/j.1365-2133.2011.10570.x [DOI] [PubMed] [Google Scholar]
- 25.McGinley KJ, Leyden JJ, Marples RR, Path M, Kligman AM. Quantitative microbiology of the scalp in non-dandruff, dandruff, and seborrheic dermatitis. J Invest Dermatol. 1975;64(6):401–405. [DOI] [PubMed] [Google Scholar]
- 26.Heng MC, Henderson CL, Barker DC, Haberfelde G. Correlation of pityosporum ovale density with clinical severity of seborrheic dermatitis as assessed by a simplified technique. J Am Acad Dermatol. 1990;23(1):82–86. [DOI] [PubMed] [Google Scholar]
- 27.Bergbrant IM, Johansson S, Robbins D, Scheynius A, Faergemann J, Söderström T. An immunological study in patients with seborrhoeic dermatitis. Clin Exp Dermatol. 1991;16(5):331–338. [DOI] [PubMed] [Google Scholar]
- 28.Antonopoulos AS, Margaritis M, Lee R, Channon K, Antoniades C. Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr Pharm Des. 2012;18(11):1519–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naseri M, Hadipour A, Sepaskhah M, Namazi M. The remarkable beneficial effect of adding oral simvastatin to topical betamethasone for treatment of psoriasis: a double-blind, randomized, placebo-controlled study. Niger J Med. 2010;19(1):58–61. [DOI] [PubMed] [Google Scholar]
- 30.Faghihi T, Radfar M, Mehrabian Z, Ehsani AH, Hemami MR. Atorvastatin for the treatment of plaque‐type psoriasis. Pharmacotherapy. 2011;31(11):1045–1050. doi: 10.1592/phco.31.11.1045 [DOI] [PubMed] [Google Scholar]
- 31.Jacobi T, Highet A. A clinical dilemma while treating hypercholesterolaemia in psoriasis. Br J Dermatol. 2003;149(6):1305–1306. [DOI] [PubMed] [Google Scholar]
- 32.Lanham M, Lebovic D, Domino S. Contemporary medical therapy for polycystic ovary syndrome. Int J Gynecol Obstet. 2006;95(3):236–241. doi: 10.1016/j.ijgo.2006.08.004 [DOI] [PubMed] [Google Scholar]
- 33.Ahmadvand A, Yazdanfar A, Yasrebifar F, Mohammadi Y, Mahjub R, Mehrpooya M. Evaluating the effects of oral and topical simvastatin in the treatment of acne vulgaris: a double-blind, randomized, placebo-controlled clinical trial. Curr Clin Pharmacol. 2018;13(4):279–283. doi: 10.2174/1574884713666180821143545 [DOI] [PubMed] [Google Scholar]
- 34.Bracht L, Caparroz‐Assef SM, Magon T, Ritter AMV, Cuman RKN, Bersani‐Amado CA. Topical anti‐inflammatory effect of hypocholesterolaemic drugs. J Pharm Pharmacol. 2011;63(7):971–975. doi: 10.1111/j.2042-7158.2011.01302.x [DOI] [PubMed] [Google Scholar]
- 35.Joshi R, Olver I, Keefe D, Marafioti T, Smith K. A phase I study to assess the safety and activity of topical lovastatin (FP252S) for the prevention of chemotherapy-induced alopecia. Support Care Cancer. 2007;15(9):1109–1112. doi: 10.1007/s00520-007-0267-2 [DOI] [PubMed] [Google Scholar]
- 36.Otuki MF, Pietrovski EF, Cabrini DA. Topical simvastatin: preclinical evidence for a treatment of skin inflammatory conditions. J Dermatol Sci. 2006;44(1):45–47. doi: 10.1016/j.jdermsci.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 37.Noel B. Lupus erythematosus and other autoimmune diseases related to statin therapy: a systematic review. J Eur Acad Dermatol Venereol. 2007;21(1):17–24. doi: 10.1111/j.1468-3083.2006.01838.x [DOI] [PubMed] [Google Scholar]
- 38.Cabral ME, Figueroa LI, Fariña JI. Synergistic antifungal activity of statin–azole associations as witnessed by Saccharomyces cerevisiae-and Candida utilis-bioassays and ergosterol quantification. Rev Iberoam Micol. 2013;30(1):31–38. doi: 10.1016/j.riam.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 39.Galgóczy L, Lukács G, Nyilasi I, Papp T, Vágvölgyi C. Antifungal activity of statins and their interaction with amphotericin B against clinically important Zygomycetes. Acta Biol Hung. 2010;61(3):356–365. doi: 10.1556/ABiol.61.2010.3.11 [DOI] [PubMed] [Google Scholar]