Abstract
Objectives: To update the epidemiology and susceptibility of hospital-acquired (HA) and community-acquired (CA), as well as intensive care unit (ICU) vs non-ICU-derived intra-abdominal infection (IAI) and urinary tract infection (UTI) pathogens in Chinese hospitals.
Methods: A total of 2,546 Gram-negative isolates from IAIs and 1,947 isolates from UTIs collected in 16 hospitals and 7 regions of China from 2016 to 2017 were analyzed.
Results: E. coli and K. pneumoniae were the most common pathogens identified in HA (40.7%, 21.9%) and CA (49.2%, 21.3%) IAIs and in HA (59.0%, 17.3%) and CA (64.3%, 12.7%) UTIs, respectively. The overall rates of extended-spectrum β-lactamase (ESBL)-positive strains were 48.2% for E. coli and 26.4% for K. pneumoniae. The rates of ESBL-positive E. coli and K. pneumoniae strains were significantly higher in HA than in CA IAIs (51.7% vs 42.4%, P=0.016 and 22.0% vs 20.6%, P<0.001). IAI E. coli ESBL-producing isolates were most susceptible to IPM (97.2%) and AMK (93.9%), and UTI-associated E. coli ESBL-producers were 94.74% susceptible to amikacin (AMK), 97.02% to imipenem (IPM), and 91.4% to ertapenem (ETP). IAI K. pneumoniae ESBL-producing isolates were most susceptible to AMK (84.43%) and IPM (82.79%), and UTI-associated K. pneumoniae ESBL-producers were 88.39% susceptible to AMK, 87.5% to IPM, and 82.14% to ETP. Overall, percentages of susceptible strains to ETP, IPM, AMK, and Piperacillin-Tazobactam (TZP) were in the range of 82.0% to 96.4%, to 5 cephalosporins in the range of 31.4%-69.6% and to 2 fluoroquinolones in the range of 37.8%-45.5% for E. coli and 65.5%-90.7%, 37.7%-75.3%, and 43.9%-73.2% for K. pneumoniae, respectively.
Conclusion: E. coli and K. pneumoniae continued to be the main pathogens in Chinese UTIs and IAIs with high ESBL-positive rates between 2016 and 2017. Carbapenem- or amikacin-based therapies were the most effective to combat IAI and UTI pathogens.
Keywords: IAI; UTI; ESBL, E. coli, K. pneumoniae
Introduction
The availability of current resistance data, generated through ongoing antimicrobial susceptibility surveillance, is important for the medical community, antibiotic developers, and government policymakers, among other interested agencies. The aim of the present study was to satisfy this need by investigating and reporting on the susceptibility patterns of recent Gram-negative pathogens isolated from hospitalized patients with intra-abdominal infections (IAIs) and urinary tract infections (UTIs) in 7 regions from 16 Chinese hospitals between 2016 and 2017. Isolates were collected for the Study for Monitoring Antimicrobial Resistance Trends (SMART) global surveillance program, which was established in China in 2002 for IAIs and in 2012 for UTIs 2008 to monitor in vitro antimicrobial susceptibility profiles of clinical isolates collected from Chinese patients with IAIs and UTIs.1 In addition, surveillance data describing susceptibility patterns may provide guidance for accurate empirical antimicrobial therapy for selected infections and patient types (eg, ICU- and non-ICU-), and may stimulate antimicrobial stewardship efforts.2,3 James et al reported that, during 2013–2015, Gram-negative ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) isolated from patients in Asia–Pacific countries demonstrated reduced in vitro susceptibilities to parenterally administered advanced-generation cephalosporins (cefepime, ceftazidime, and ceftriaxone), piperacillin–tazobactam and fluoroquinolones (levofloxacin), with isolates from UTIs even less susceptible than isolates from IAIs. These findings demonstrated higher rates of antimicrobial resistance than those observed in North American and European studies, and identified the majority of pathogens isolated from ICUs in a number of Asia–Pacific countries.3,4 Previously reported SMART epidemiological survey results revealed that IPM and ETP were effective in vitro against Enterobacteriaceae isolated from IAIs and UTIs between 2014 and 2015, but susceptibility to carbapenems for UTIs markedly decreased in 2015. Thus, global antimicrobial resistance, particularly for Gram-negative bacteria in China, as well as the variability in antibiotic susceptibility and the prevalence of extended-spectrum β-lactamase (ESBL)-producers in different hospital departments (both ICU and non-ICU) have been considered essential for guiding effective empirical therapy for clinical practice in recent years.
This report describes the epidemiology and susceptibility of pathogens (including ESBL-producers) from HA vs CA UTIs and IAIs sampled in ICUs and non-ICUs in China from 2016 to 2017 as an update of the SMART surveillance program.
Materials and methods
Isolates from IAI and UTI patients
From 2016 to 2017, the SMART surveillance survey collected 4,493 isolates of Gram-negative bacilli from patients in 16 hospitals with IAI (n=2,546) and UTI (n=1,947), including 2,254 E. coli and 866 K. pneumoniae strains from 7 regions in China (northeast, north, east, central, south, and the southwest). The majority of the intra-abdominal specimens were obtained during surgery, with some paracentesis specimens, and were derived from abscesses, appendix, colon, gall bladder, liver, pancreas, peritoneal fluid, rectum, small intestine, and stomach. The urinary tract infection isolates were obtained from clean-catch midstream urine, the urinary bladder, ureter, kidney, urethra, and the prostate gland. Isolates were identified using local site procedures and then shipped to the central clinical microbiology laboratory of the Peking Union Medical College Hospital for analyses and re-identification using MALDI-TOF MS (Vitek MS, BioMérieux, France). All duplicate isolates (the same genus and species from the same patient) were excluded from the study. Isolates were considered to be community-associated (CA) if they were recovered from a specimen taken <48 hrs after the patient was admitted to a hospital or HA if the specimen was taken ≥48 hrs after hospital admission, as previously described (Hawser et al, 2009b).
Antimicrobial susceptibility test method
Antimicrobial susceptibility testing was performed at the Peking Union Medical College Hospital center laboratory using panels purchased from ThermoFisher Scientific (Cleveland, OH, USA). Minimum inhibitory concentrations (MIC)90/MIC50 were determined using susceptibility interpretations based on CLSI clinical breakpoints.5
Twelve antimicrobial agents commonly used to treat IAIs and UTI were tested (Ampicillin-Sulbactam (SAM), Piperacillin-Tazobactam (TZP), Ceftriaxone (CRO), Cefotaxime (CTX), Ceftazidime (CAZ), Cefoxitin (FOX), Cefepime (FEP), Ciprofloxacin (CIP), Levofloxacin (LVX), Amikacin (AMK), Imipenem (IPM), and Ertapenem (ETP)).
The above antimicrobial agents tested by the SMART global surveillance program are those recommended by the Surgical Infection Society and the Infectious Diseases Society of America in their guidelines for the diagnosis and management of complicated IAIs and UTIs.6 Reference strains, E. coli ATCC (American Type Culture Collection) 25,922, Pseudomonas aeruginosa ATCC 27,853, and K. pneumoniae ATCC 700,603 (positive ESBL control), were used as quality control (QC) strains for each batch of MIC tests. The results were only included in the analysis when the corresponding quality control isolate test results were in accordance with CLSI guidelines and therefore within an acceptable range.
Detection of ESBLs
Phenotypic identification of ESBL production among E. coli, K. pneumoniae, P. mirabilis, and K. oxytoca was detected by disc diffusion. If the cefotaxime zone was ≤27 mm or the ceftazidime zone ≤22 mm, ESBL production was defined as a ≥5 mm increase in a zone diameter for 30 µg cefotaxime or ceftazidime tested in combination with 10 µg clavulanic acid per disc compared to the zone diameter of the agent when tested alone.
Statistical analysis
The susceptibility of all Gram-negative isolates combined was calculated using breakpoints appropriate for each species and assuming 0% susceptibility for species with no breakpoints for any given antibiotic. The 95% confidence intervals were calculated using the adjusted Wald method; comparison of ESBL rates was assessed using a chi-squared test. P-values <0.05 were considered statistically significant.
Results
The distribution of major isolates in IAIs and UTIs in 2016–2017
In 2016–2017, the major IAI and UTI pathogens were E. coli and K. pneumoniae, from which most E. coli were collected from UTIs (60.5%) and most K. pneumoniae appeared in IAIs (21.8%). Although the fermenting bacteria Pseudomonas aeruginosa and Acinetobacter baumannii were the third and fourth ranking isolates in IAIs and UTIs during 2016–2017, their total proportion (n=629) was only 14.0% of all isolates (n=4,493), and the majority of them were collected from HA infections (Table 1). In addition, from all IAI and UTI isolates, 4 ESBL-positive species, including E. coli, K. pneumoniae, P. mirabilis, and K. oxytoca, were analyzed and among them, IAI E. coli ESBL-positive (n=532) and K. pneumoniae ESBL-positive (n=120) isolates accounted for 96.9% of all ESBL-positive IAI isolates (n=673), whereas the ESBL-positive strains of E. coli (n=554) and K. pneumoniae (n=109) from UTIs accounted for 97.2% of all UTI ESBL-positive strains (n=682). In contrast to UTIs, in IAIs the percentage of ESBL-positive E. coli and K. pneumoniae strains were significantly higher (P=0.016; P<0.001) in HA than in CA infections (Table 2).
Table 1.
Distribution of the IAI and UTI pathogens in China during 2016–2017
| Name of pathogen (n, %) | IAI(/All) | HA | CA | UTI(/All) | HA | CA |
|---|---|---|---|---|---|---|
| Escherichia coli (n=2,254, 50.2) | 1,076(42.3) | 845(40.7) | 224(49.2) | 1,178(60.5) | 815(59.0) | 361(64.3) |
| Klebsiella pneumoniae (n=866, 19.3) | 555(21.8) | 454(21.9) | 97(21.3) | 311(16.0) | 239(17.3) | 71(12.7) |
| Pseudomonas aeruginosa (n=347, 7.7) | 203(8.0) | 178(8.6) | 25(5.5) | 144(7.4) | 111(8.0) | 33(5.9) |
| Acinetobacter baumannii (n=282, 6.3) | 211(8.3) | 182(8.8) | 28(6.2) | 71(3.6) | 60(4.3) | 11(2.0) |
| Enterobacter cloacae (n=196, 4.4) | 143(5.6) | 128(6.2) | 15(3.3) | 53(2.7) | 37(2.7) | 16(2.9) |
| Proteus mirabilis (n=100, 2.2) | 44(1.7) | 36(1.7) | 8(1.8) | 56(2.9) | 33(2.4) | 22(3.9) |
| Klebsiella aerogenes (n=74, 1.6) | 53(2.1) | 43(2.1) | 9(2.0) | 21(1.1) | 13(0.9) | 8(1.4) |
| Citrobacter freundii (n=49, 1.1) | 32(1.3) | 22(1.1) | 9(2.0) | 17(0.9) | 10(0.7) | 7(1.2) |
| Klebsiella oxytoca (n=49, 1.1) | 37(1.5) | 30(1.4) | 7(1.5) | 12(0.6) | 8(0.6) | 4(0.7) |
| Stenotrophomonas maltophilia (n=50, 1.1) | 41(1.6) | 36(1.7) | 5(1.1) | 9(0.5) | 6(0.4) | 3(0.5) |
| Serratia marcescens (n=44, 1.0) | 25(1.0) | 19(0.9) | 6(1.3) | 19(1.0) | 14(1.0) | 5(0.9) |
| Others (n=182, 4.1) | 126(4.9) | 103(5.0) | 22(4.8) | 56(2.9) | 35(2.5) | 20(3.6) |
| All (n=4493,100) | 2,546(56.7) | 2,076(81.5) | 455(17.9) | 1,947(43.3) | 1,381(70.9) | 561(28.8) |
Abbreviations: HA, hospital-acquired; CA, community-acquired; IAI, intraabdominalinfection; UTI, urinary tract infection.
Table 2.
Distribution of ESBL-producing strains in China during 2016–2017
| IAI (n=2,546) | UTI (n=1,947) | Total (n=4,493) | ||||
|---|---|---|---|---|---|---|
| HA (n=2,076) | CA (n=455)* | HA (n=1,381) | CA (n=561)* | HA (n=3,457) | CA (n=1,016)* | |
| Total ESBL + (% of HA or CA) | 557 (26.8) | 116 (25.5) | 478 (34.6) | 204 (36.4) | 1035 (29.9) | 320 (31.5) |
| E. coli | 845 (40.7) | 224 (49.2) | 815 (59.0) | 361 (64.3) | 1660 (48.0) | 585 (57.6) |
| ESBL + (% of E. coli HA or CA) | 437(51.7)# | 95 (42.4) | 378 (46.4) | 176 (48.8) | 815 (49.1) | 271 (46.3) |
| K. pneumoniae | 454 (21.9) | 97 (21.3) | 239 (17.3) | 71 (12.7) | 693 (20.9) | 168 (16.5) |
| ESBL + (% of K. pneumoniae HA or CA) | 100 (22.0)& | 20 (20.6) | 86 (36.0) | 23 (32.4) | 186 (26.8) | 43 (25.6) |
| P. mirabilis | 36 (1.7) | 8 (1.8) | 33 (2.4) | 22 (3.9) | 69 (2.0) | 30 (3.0) |
| ESBL + (% of P. mirabilis HA or CA) | 13 (36.1) | 0 (0) | 10 (30.3) | 5 (22.7) | 23 (33.3) | 5 (16.7) |
| K. oxytoca | 30 (1.4) | 7 (1.5) | 8 (0.6) | 4 (0.7) | 38 (1.1) | 11 (1.1) |
| ESBL + (% of K. oxytoca HA or CA) | 7 (23.3) | 1 (14.3) | 4 (50) | 0 (0) | 11 (28.9) | 1 (9.1) |
Notes: *Missing isolates are those whose hospitalization was not specified. #P=0.016 (IAI HA vs CA); &P<0.001 (IAI HA vs UTI HA).
Abbreviations: HA, hospital-acquired; CA, community-acquired; IAI, intraabdominalinfection; UTI, urinary tract infection; IPM, Carbapenems: Imipenem; ETP, Ertapenem; AMK, Aminoglycoside: Amikacin; TZP, Piperacillin-Tazobactam; FOX, Cephalosporins: Cefoxitin; CAZ, Ceftazidime; CRO, Ceftriaxone; CTX, Cefotaxime; FEP, Cefepime; LVX, Fluoroquinolones: Levofloxacin; CIP, Ciprofloxacin.
The susceptibility of ESBL-positive isolates of E. coli and K. pneumoniae in IAIs and UTIs to the 12 most commonly used antibiotics in 2016–2017
The susceptibility of E. coli ESBL-positive isolates from IAIs, including HA and CA, was >90% only for AMK and IPM, while the susceptibility of E. coli ESBL-positive isolates from UTI, including HA and CA, were >90% for AMK and IPM, but also for ETP.
For K. pneumoniae ESBL-positive isolates in CA and HA IAIs and UTIs, no antibiotics showed >90% susceptibility, but >80% susceptibilities were found for AMK and IPM (Table 3). Except for E. coli and K. pneumoniae, the other 2 ESBL-positive strains, P. mirabilis and K. oxytoca, accounted for only for a small portion in both IAIs and UTIs. P. mirabilis was only isolated from HA IAI infections and showed high susceptibilities (>90%) to AMK, FOX, CAZ, and ETP, but interestingly, only 1 of 13 strains was still susceptible to IPM. In UTIs, only AMK was effective in >90% of the isolates.
Table 3.
ESBL positivity and antibiotic susceptibility rates (%) for E. coli and K. pneumoniae isolates from HA vs CA IAIs & UTIs
| E. coli ESBL +(n=1,094) | K. pneumoniae ESBL +(n=231) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IAI | UTI | IAI | UTI | |||||||||
| All (n=538) | HA (n=437) | CA (n=95) | All (n=556) | HA (n=378) | CA (n=176) | All (n=122) | HA (n=100) | CA (n=20) | All (n=109) | HA (n=86) | CA (n=23) | |
| IPM | 97.21 | 97.02 | 97.89 *** | 97.02 | 96.95 | 97.16 | 82.79 | 82 | 90 | 87.5 | 87.64 | 86.96 |
| ETP | 90.71 | 91.08 | 88.42 | 91.4 | 90.08 | 94.29 *** | 77.05 | 76 | 85 | 82.14 | 85.39 | 69.57 *** |
| AMK | 93.87 | 94.05 | 92.63 *** | 94.74 | 94.4 | 95.43 ** | 84.43 | 85 | 85 | 88.39 | 88.76 | 86.96 *** |
| TZP | 82.34 | 81.92 | 86.32 | 88.97 | 89.06 | 88.64 | 57.38 | 55 | 75 | 61.61 | 62.92 | 56.52 |
| FOX | 57.81 | 57.67 | 58.95 | 62.35 | 62.34 | 61.93 | 51.64 | 49 | 65 | 60.71 | 64.05 | 47.83 |
| CAZ | 31.97 | 31.58 | 34.74 | 37.19 | 37.91 | 35.43 | 23.77 | 23 | 30 | 24.11 | 28.09 | 8.7 |
| CRO | 0 | 0 | 0 | 1.58 | 1.28 | 2.27 | 0 | 0 | 0 | 1.79 | 2.25 | 0 |
| CTX | 0 | 0 | 0 | 1.05 | 0.76 | 1.71 | 0 | 0 | 0 | 2.68 | 3.37 | 0 |
| FEP | 3.35 | 4.12 | 0 | 3.86 | 3.32 | 5.11 | 3.28 | 3 | 5 | 2.68 | 3.37 | 0 |
| LVX | 28.44 | 29.29 | 25.26 | 25.09 | 24.94 | 24.57 | 37.7 | 37 | 45 | 33.93 | 37.08 | 21.74 |
| CIP | 26.95 | 27.92 | 23.16 | 22.94 | 22.9 | 22.16 | 34.43 | 34 | 40 | 30.36 | 32.58 | 21.74 |
Notes: **P<0.01; ***P<0.001, HA vs CA.
Abbreviations: HA, hospital-acquired; CA, community-acquired; IAI, intraabdominalinfection; UTI, urinary tract infection; IPM, Carbapenems: Imipenem; ETP, Ertapenem; AMK, Aminoglycoside: Amikacin; TZP, Piperacillin-Tazobactam; FOX, Cephalosporins: Cefoxitin; CAZ, Ceftazidime; CRO, Ceftriaxone; CTX, Cefotaxime; FEP, Cefepime; LVX, Fluoroquinolones: Levofloxacin; CIP, Ciprofloxacin.
From K. oxytoca ESBL positive HA IAI isolates <50% were still susceptible to all cephalosporins and only 71.43% and 57.14% to IPM and ETP, with the highest percentage susceptible to AMK (85.7%). (Table S1).
Table S1.
ESBL positivity and antibiotic susceptibility rates (%) for P. mirabilis and K. oxytoca isolates combined in HA vs CA IAIs and UTIs
| P. mirabilis ESBL +(n=28) | K. oxytoca ESBL +(n=23) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IAI | UTI | IAI | UTI | |||||||||
| All (n=13) | HA (n=13) | CA (n=0) | All (n=15) | HA (n=10) | CA (n=5) | ALL (n=8) | HA (n=7) | CA (n=1) | All (n=4) | HA (n=4) | CA (n=0) | |
| IPM | 7.69 | 7.69 | NA | 33.33 | 50 | 0 | 75 | 71.43 | 100 | 100 | 100 | NA |
| ETP | 92.31 | 92.31 | NA | 80 | 80 | 80 | 62.5 | 57.14 | 100 | 100 | 100 | NA |
| AMK | 92.31 | 92.31 | NA | 100 | 100 | 100 | 87.5 | 85.71 | 100 | 100 | 100 | NA |
| TZP | 84.62 | 84.62 | NA | 86.67 | 90 | 80 | 62.5 | 57.14 | 100 | 50 | 50 | NA |
| FOX | 92.31 | 92.31 | NA | 66.67 | 70 | 60 | 37.5 | 42.86 | 0 | 100 | 100 | NA |
| CAZ | 92.31 | 92.31 | NA | 80 | 80 | 80 | 12.5 | 14.29 | 0 | 25 | 25 | NA |
| CRO | 0 | 0 | NA | 6.67 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| CTX | 0 | 0 | NA | 6.67 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| FEP | 15.38 | 15.38 | NA | 6.67 | 10 | 0 | 12.5 | 0 | 100 | 0 | 0 | NA |
| LVX | 23.08 | 23.08 | NA | 33.33 | 50 | 0 | 50 | 57.14 | 0 | 25 | 25 | NA |
| CIP | 15.38 | 15.38 | NA | 13.33 | 20 | 0 | 50 | 57.14 | 0 | 25 | 25 | NA |
Abbreviations: HA, hospital-acquired; CA, community-acquired; IAI, intraabdominalinfection; UTI, urinary tract infection; IPM, Carbapenems: Imipenem; ETP, Ertapenem; AMK, Aminoglycoside: Amikacin; TZP, Piperacillin-Tazobactam; FOX, Cephalosporins: Cefoxitin; CAZ, Ceftazidime; CRO, Ceftriaxone; CTX, Cefotaxime; FEP, Cefepime; LVX, Fluoroquinolones: Levofloxacin; CIP, Ciprofloxacin; NA, not available.
Susceptibilities of E. coli and K. pneumoniae ESBL-producing isolates from ICU- and non-ICU-derived strains to 12 common antibiotics
Most susceptibilities of IAI-derived E. coli ESBL-positive isolates in ICUs were slightly lower than that of E. coli ESBL-positive isolates from non-ICUs, but without significance. Also for other isolates, there was no significant difference in susceptibilities between ICU and non-ICU samples (all P>0.05). E. coli ESBL-producing strains acquired from IAIs and UTIs were 85.6% to 97.5% susceptible to IPM, ETP, and AMK, whereas 70% to 90.0% of K. pneumoniae ESBL-producing strains from IAIs and UTIs were susceptible to IPM, ETP, and AMK. Susceptibilities to TZP and FOX were intermediate, and all other tested antibiotics were only effective for 0% - 38.46% of the isolates (Table 4).
Table 4.
ESBL producing E. coli and K. pneumoniae susceptibility rates from Gram-negative IAI & UTI isolates obtained from ICU and non-ICU units
| IPM | ETP | AMK | TZP | FOX | CAZ | CRO | CTX | FEP | LVX | CIP | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IAI-E. coli ESBL +(n=538) | ||||||||||||
| ICU (n=97) | 95.88 | 85.57 | 91.75 | 77.32 | 56.7 | 34.02 | 0 | 0 | 4.12 | 24.74 | 25.77 | |
| Non-ICU (n=441) | 97.50 | 91.84 | 94.33 | 83.45 | 58.05 | 31.52 | 0 | 0 | 3.17 | 29.25 | 27.21 | |
| P-value | 0.326 | 0.079 | 0.350 | 0.185 | 0.821 | 0.632 | 0.547 | 0.456 | 0.900 | |||
| IAI-K. pneumoniae ESBL +(n=122) | ||||||||||||
| ICU (n=20) | 80.00 | 70.00 | 90.00 | 55.00 | 65.00 | 15.00 | 0.00 | 0.00 | 5.00 | 35.00 | 30.00 | |
| Non-ICU (n=102) | 83.33 | 78.43 | 83.33 | 57.84 | 49.02 | 25.49 | 0.00 | 0.00 | 2.94 | 38.24 | 35.29 | |
| P-value | 0.748 | 0.398 | 0.736 | 0.810 | 0.227 | 0.399 | 0.516 | 1.000 | 0.799 | |||
| UTI-E. coli ESBL +(n=556) | ||||||||||||
| ICU (n=39) | 94.87 | 92.31 | 92.31 | 87.18 | 64.1 | 38.46 | 0.00 | 0.00 | 0.00 | 28.21 | 25.64 | |
| Non-ICU (n=517) | 97.18 | 91.34 | 94.92 | 89.10 | 62.22 | 37.10 | 1.69 | 1.13 | 4.14 | 24.86 | 22.74 | |
| P-value | 0.339 | 1.000 | 0.447 | 0.603 | 0.733 | 0.865 | 1.000 | 1.000 | 1.000 | 0.702 | 0.694 | |
| UTI-K. pneumoniae ESBL +(n=109) | ||||||||||||
| ICU (n=13) | 85.71 | 85.71 | 78.57 | 57.14 | 50.00 | 7.14 | 0.00 | 0.00 | 0.00 | 28.57 | 28.57 | |
| Non-ICU (n=96) | 87.76 | 81.63 | 89.8 | 62.24 | 62.24 | 26.53 | 2.04 | 3.06 | 3.06 | 34.69 | 30.61 | |
| P-value | 0.673 | 1.000 | 0.186 | 0.558 | 0.365 | 0.184 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
Abbreviations: ICU, intensive care unit; IAI, intraabdominalinfection; UTI, urinary tract infection; IPM, Carbapenems: Imipenem; ETP, Ertapenem; AMK, Aminoglycoside: Amikacin; TZP, Piperacillin-Tazobactam; FOX, Cephalosporins: Cefoxitin, CAZ, Ceftazidime, CRO, Ceftriaxone, CTX, Cefotaxime, FEP, Cefepime; LVX, Fluoroquinolones: Levofloxacin; CIP, Ciprofloxacin.
Overall susceptibilities of the major CA and HA IAI and UTI isolates E. coli and K. pneumoniae to 12 common antibiotics from 2016 to 2017
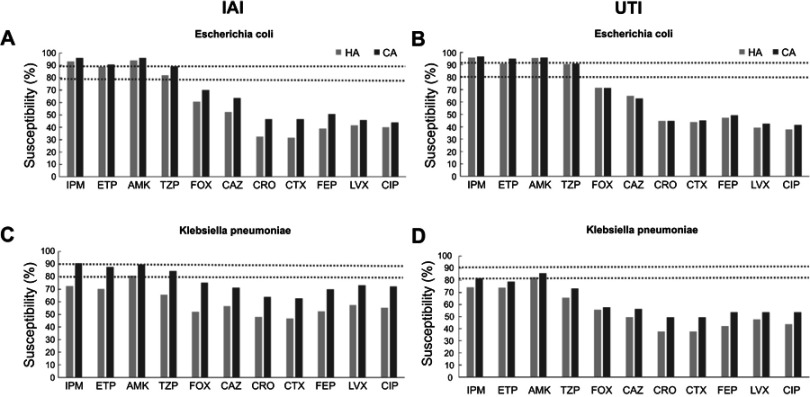
IAI and UTI E. coli and K. pneumoniae isolates from HA infections were generally less susceptible to 12 common antibiotics, compared to CA infections. The overall percentages of susceptible E. coli strains to ETP, IPM, AMK, and Piperacillin-Tazobactam (TZP) were in the range of 82.0% to 96.4%, to 5 cephalosporins in the range of 31.4%-69.6% and to 2 fluoroquinolones in the range of 37.8%–45.5% and 65.5%–90.7%, 37.7%–75.3%, and 43.9%–73.2% for K. pneumoniae, respectively (Figure 1).
Figure 1.
Susceptibilities of E. coli and K. pneumonia isolates from HA and CA IAIs and UTIs. (A) E. coli isolates from IAIs, (B) E. coli isolates from UTIs, (C) K. pneumonia isolates from IAIs, and (D) K. pneumonia isolates from UTIs.
Abbreviations: HA, hospital-acquired; CA, community-acquired; IAI, intraabdominalinfection; UTI, urinary tract infection; IPM: Carbapenems: Imipenem; ETP, Ertapenem; AMK, Aminoglycoside: Amikacin; TZP, Piperacillin-Tazobactam; FOX, Cephalosporins: Cefoxitin, CAZ, Ceftazidime, CRO, Ceftriaxone, CTX, Cefotaxime, FEP, Cefepime; LVX, Fluoroquinolones: Levofloxacin; CIP, Ciprofloxacin.
Discussion
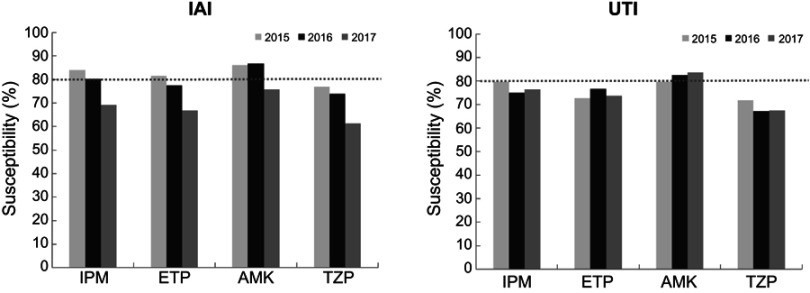
In 2016 and 2017, the major pathogens of Chinese IAIs and UTIs were E. coli and K. pneumoniae, which is in accordance with previous years and studies abroad.7 The rate of ESBL-producing E. coli and K. pneumoniae strains was higher in HA IAIs than in CA IAIs, which is in accordance with a previous Chinese study, but the former percentages in 2010–2011 were essentially higher than in 2016–2017.8 However, though ESBL rates have dropped in 2016–2017, compared to 2010–2011, the overall susceptibilities of IAI and UTI-derived E. coli and K. pneumoniae strains remained low to cephalosporins, especially in HA infections (Figure 1). Since cefoxitin (FOX) is also considered to be effective in ESBL-producing Enterobacteriaceae,9 the relatively low susceptibilities of E. coli and K. pneumoniae to FOX also indicate other resistance mechanisms than ESBL production.10 Also the low susceptibility rate of ESBL-positive P. mirabilis strains from HA IAI infections to IPM might be explained by alterations in penicillin-binding proteins, which has been also described for Acinetobacter baumannii and Pseudomonas aeruginosa.11,12 In 2017 there was an obvious trend of lowered susceptibility to carbapenems in K. pneumoniae IAI isolates, which is in line with other studies from China.13,14 However, there was no significant difference between susceptibilities to carbapenems in ICU and non-ICU departments, which is in contrast to a multicenter study about carbapenem-nonsusceptible GNB ICU infections in the US in 2017; however, the authors indicated that most ICU-related infections were from respiratory tracts and skin wounds, and the highest carbapenem resistance rates were found in Acinetobacter spp. and P. aeruginosa.15 A trend toward lower susceptibilities in ICU-derived E. coli and K. pneumoniae strains was also visible in the present study, which might be explained by the fact that more frequent previous carbapenem administrations have been applied for ICU patients.16 In addition, some susceptibilities of ESBL-positive E. coli strains from IAI and UTI patients to carbapenems were significantly lower in HA compared to CA infections (Table 3), which is in line with the literature in which infection rates with carbapenem and multidrug-resistant Enterobacteriaceae were highest in long-term acute-care hospitals.17–19 The overall susceptibilities were generally higher in CA than in HA IAIs and UTIs (Figure 1), which is in accordance with data from 2015.20 On the other hand, susceptibilities of ESBL-producing K. pneumoniae strains from CA UTIs were significantly lower than HA UTIs, particularly to ETP, which needs further investigation. Comparing the overall susceptibilities of HA and CA IAI and UTI-derived E. coli and K. pneumoniae strains from 2015 with 2016/2017, susceptibilities to all cephalosporins and fluoroquinolones were far less than 80% for both bacterial species in both time periods. For E. coli isolates from IAIs and UTIs, susceptibilities to IPM, ETP, AMK, and TZP were in the range of 80%–94% in both time periods, without essential changes. However, overall susceptibilities to IPM, ETP, AMK, and TZP gradually dropped for K. pneumoniae isolates, especially from IAIs during 2015–2017 (Figure 2).
Figure 2.
Percentages of susceptible K. pneumoniae IAI and UTI isolates to IMP, ETP, AMK, and TZP from 2015 to 2017.
Abbreviations: IAI, intraabdominalinfection; UTI, urinary tract infection; IPM: Carbapenems: Imipenem; ETP, Ertapenem; AMK, Aminoglycoside: Amikacin; TZP, Piperacillin-Tazobactam.
Limitations of the present study include the fact that molecular mechanisms of resistance have not been identified.
Conclusion
E. coli and K. pneumoniae were the main pathogens in Chinese UTIs and IAIs, with high ESBL-positive rates and low susceptibilities to cephalosporins and fluoroquinolones between 2016 and 2017, and K. pneumoniae showed a trend toward lower susceptibility in HA, compared to CA IAI and UTI infections. Imipenem, ertapenem, and amikacin were the most effective agents against UTIs and IAIs, but the susceptibilities of IAI-derived K. pneumoniae strains to these antibiotics became lower in 2017, compared to 2015 and 2016.
Acknowledgments
Medical writing and editorial assistance were provided by Shanghai BIOMED Science Technology (Shanghai, China) through funding provided by MSD China. The authors were solely responsible for the conception and implementation of this study and for writing the manuscript. This study was supported by a grant from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Further support was granted from the National Key Research and Development Program of China (2018YFC1200100, 2018YFC1200105), the Chinese Academy of Medical Sciences (CAMS) Initiative for Innovative Medicine (Grant No. 2016-I2M-3-014), the CAMS Innovation Fund for Medical Sciences (CIFMS) (Grant No. 2016-I2M-1-014) and the Outstanding Talents Training Funding Project of Dongcheng District, Beijing (2017).
Ethics approval and consent to participate
The protocol has been reviewed by the human research ethics committee of the Institutional Review Board (IRB) of the Peking Union Medical College Hospital and since the project falls under the category observational study and all bacterial strains were from residual samples used in clinical diagnosis or were strains from their subcultures, it covers patient data confidentiality and compliance with the declaration of Helsinki. Since the data do not include any patient’s information and demographic data it has been determined to meet the criteria for exemption. After consultation with the IRB of the Peking Union Medical College Hospital, formal ethical approval was reviewed and written informed consents from patients was not required (Ethics Approval Number: S-K238).
Availability of data and materials
The SMART database is not public and only accessible for SMART investigators, but the data that support the findings of this study are directly available from MSD China or from the authors upon reasonable request and with permission of Merck Sharp & Dohme (China) Co., Ltd.
Author contributions
Conceptualization: HZ, AJ, QWY, YCX; data collection: HZ, AJ, GZ, YY, JJZ, DXL, SMD, QWY, YCX; data analysis: HZ, QWY, YCX; writing original draft: HZ; writing review and editing: HZ, AJ, YY, JJZ, DXL, SMD, GZ, QWY, YCX. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
AJ receives financial support in the form of salaries from International Health Management Associates, which receives funding from MSD to administer the SMART program and for SMART-related travel and consultation expenses. The authors report no other conflicts of interest in this work.
Supplementary material
References
- 1.Biedenbach D, Bouchillon S, Hackel M, et al. Dissemination of NDM metallo-β-lactamase genes among clinical isolates of Enterobacteriaceae collected during the SMART global surveillance study from 2008 to 2012. Antimicrob Agents Chemother. 2015;59:826–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paterson DL, Rossi F, Baquero F, et al. In vitro susceptibilities of aerobic and facultative Gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: the 2003 Study for Monitoring Antimicrobial Resistance Trends (SMART). J Antimicrob Chemother. 2005;55:965–973. doi: 10.1093/jac/dki117 [DOI] [PubMed] [Google Scholar]
- 3.Lu PL, Liu YC, Toh HS, et al. Epidemiology and antimicrobial susceptibility profiles of Gram-negative bacteria causing urinary tract infections in the Asia-Pacific region: 2009–2010 results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). Int J Antimicrob Agents. 2012;40(Suppl):S37–43. doi: 10.1016/S0924-8579(12)70008-0 [DOI] [PubMed] [Google Scholar]
- 4.Jean SS, Coombs G, Ling T, et al. Epidemiology and antimicrobial susceptibility profiles of pathogens causing urinary tract infections in the Asia-Pacific region: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART), 2010–2013. Int J Antimicrob Agents. 2016;47:328–334. doi: 10.1016/j.ijantimicag.2016.01.008 [DOI] [PubMed] [Google Scholar]
- 5.Institute CaLS. Performance Standards for Antimicrobial Susceptibility Testing. 26thed. Wayne, PA: Clinical and Laboratory Standards Institute; 2016. CLSI supplement M100S. [Google Scholar]
- 6.Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the surgical infection society and the infectious diseases society of America. Clin Infect Dis. 2010;50:133–164. doi: 10.1086/649554 [DOI] [PubMed] [Google Scholar]
- 7.Morrissey I, Hackel M, Badal R, et al. A review of ten years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011. Pharmaceuticals (Basel). 2013;6:1335–1346. doi: 10.3390/ph6111335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Q, Zhang H, Wang Y, et al. A 10 year surveillance for antimicrobial susceptibility of Escherichia coli and Klebsiella pneumoniae in community- and hospital-associated intra-abdominal infections in China. J Med Microbiol. 2013;62:1343–1349. doi: 10.1099/jmm.0.059816-0 [DOI] [PubMed] [Google Scholar]
- 9.Demonchy E, Courjon J, Ughetto E, et al. Cefoxitin-based antibiotic therapy for extended-spectrum beta-lactamase-producing Enterobacteriaceae prostatitis: a prospective pilot study. Int J Antimicrob Agents. 2018;51:836–841. doi: 10.1016/j.ijantimicag.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 10.Cardarella S, Ogino A, Nishino M, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res. 2013;19:4532–4540. doi: 10.1158/1078-0432.CCR-13-0657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villar HE, Danel F, Livermore DM. Permeability to carbapenems of Proteus mirabilis mutants selected for resistance to imipenem or other beta-lactams. J Antimicrob Chemother. 1997;40:365–370. [DOI] [PubMed] [Google Scholar]
- 12.Neuwirth C, Siebor E, Duez JM, et al. Imipenem resistance in clinical isolates of Proteus mirabilis associated with alterations in penicillin-binding proteins. J Antimicrob Chemother. 1995;36:335–342. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Zou MX, Wang HC, et al. An outbreak of infections caused by a Klebsiella pneumoniae ST11 clone coproducing Klebsiella pneumoniae carbapenemase-2 and RmtB in a Chinese teaching hospital. Chin Med J (Engl). 2016;129:2033–2039. doi: 10.4103/0366-6999.189049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng B, Dai Y, Liu Y, et al. Molecular epidemiology and risk factors of carbapenem-resistant Klebsiella pneumoniae infections in Eastern China. Front Microbiol. 2017;8:1061. doi: 10.3389/fmicb.2017.01061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCann E, Srinivasan A, DeRyke CA, et al. Carbapenem-nonsusceptible Gram-negative pathogens in ICU and Non-ICU settings in US hospitals in 2017: a multicenter study. Open Forum Infect Dis. 2018;5:ofy241. doi: 10.1093/ofid/ofy241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Routsi C, Pratikaki M, Platsouka E, et al. Risk factors for carbapenem-resistant Gram-negative bacteremia in intensive care unit patients. Intensive Care Med. 2013;39:1253–1261. doi: 10.1007/s00134-013-2914-z [DOI] [PubMed] [Google Scholar]
- 17.Livorsi DJ, Chorazy ML, Schweizer ML, et al. A systematic review of the epidemiology of carbapenem-resistant Enterobacteriaceae in the United States. Antimicrob Resist Infect Control. 2018;7:55. doi: 10.1186/s13756-018-0346-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez F, Van Duin D. Carbapenem-resistant Enterobacteriaceae: A menace to our most vulnerable patients. Cleve Clin J Med. 2013;80:225–233. doi: 10.3949/ccjm.80a.12182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Fallon E, Kandel R, Schreiber R, et al. Acquisition of multidrug-resistant gram-negative bacteria: incidence and risk factors within a long-term care population. Infect Control Hosp Epidemiol. 2010;31:1148–1153. doi: 10.1086/656590 [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Kong H, Yu Y, et al. Carbapenem susceptibilities of Gram-negative pathogens in intra-abdominal and urinary tract infections: updated report of SMART 2015 in China. BMC Infect Dis. 2018;18:493. doi: 10.1186/s12879-018-3109-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The SMART database is not public and only accessible for SMART investigators, but the data that support the findings of this study are directly available from MSD China or from the authors upon reasonable request and with permission of Merck Sharp & Dohme (China) Co., Ltd.