Abstract
Background: Colorectal cancer (CRC) is a common form of cancer associated with a high mortality rate and poor prognosis. Given the limited efficacy of current therapies for CRC, interest in novel therapeutic agents isolated from natural sources has increased. We studied the anticancer properties of isobavachalcone (IBC), a flavonoid isolated from the herb Psoralea corylifolia, which is used in traditional Chinese medicine, in an in vitro model of CRC.
Materials and methods: Cell viability and growth of CRC cells were determined by Cell Counting Kit-8 and colony formation assays following treatment with varying concentrations of IBC, respectively. Apoptosis was examined by 4′,6-diamidino-2-phenylindole staining and flow cytometry with Annexin V/propidium iodide double staining. Western blot analysis was used to analyze expression of apoptosis-associated protein pathway and the AKT/GSK-3β/β-catenin signaling pathway.
Results: Initial experiments showed that IBC inhibited proliferation and colony formation of human CRC cell lines in dose- and time-dependent manners. The antiproliferative effect of IBC resulted from induction of apoptosis, as evidenced by morphological changes in the nucleus, flow cytometry analysis, upregulation of cleaved caspase-3 and cleaved PARP, changes in the ratio of the anti-apoptotic protein Bcl-2 and the pro-apoptotic protein Bax, translocation of Bax from the cytosol to the mitochondria, and decreased expression of two inhibitors of apoptosis family proteins, XIAP, and survivin. Western blot analysis of signaling pathway proteins demonstrated that IBC downregulated Wnt/β-catenin signaling, which has previously been associated with CRC, by inhibiting the AKT/GSK-3β signaling pathway.
Conclusion: This study demonstrated that IBC inhibited cell proliferation and induced apoptosis through inhibition of the AKT/GSK-3β/β-catenin pathway in CRC. These results suggest the potential of IBC as a novel therapeutic agent for the treatment of CRC.
Keywords: colorectal cancer, isobavachalcone, apoptosis, AKT, GSK-3β, β-catenin signaling
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors of the gastrointestinal tract and has a high incidence and a high mortality rate.1 In recent years, the incidence of CRC has maintained a rapid upward trend in China because of changes in dietary structure and lifestyle.2 Approximately 25% of the patients with CRC present with metastases at initial diagnosis and nearly 50% will develop metastases.3 First-line chemotherapy to treat metastatic CRC is consists of 5-fluorouracil alone or in combination with cytotoxic agents such as irinotecan and oxaliplatin.4 However, despite recent advances in therapeutic approaches, the efficacy of current treatments is compromised by a high recurrence rate, and prognosis remains poor, as evidenced by a 5-year survival rate of less than 13% for patients with metastatic CRC.5 Therefore, novel anticancer drugs with a safe profile are urgently needed to improve treatment outcomes for CRC patients.
Plant-derived natural products or their isolated components have been considered suitable candidates to extend the range of therapeutic options for cancer treatment.6 Isobavachalcone (IBC) is a prenylated chalcone of the flavonoid subclass isolated from Psoralea corylifolia, an annual herb used in traditional Chinese medicine. IBC exerts a wide spectrum of various pharmacological and physiological activities, including antifungal, antibacterial and antimycobacterial, anticancer, antireverse transcriptase, and antiparasitic activity.7 Studies have indicated that IBC induces apoptosis and inhibits metastasis in cells derived from a variety of cancers. For example, IBC inhibited proliferation and induced apoptosis via abrogation of AKT signaling in cells derived from ovarian cancer, prostate cancer, lung carcinoma, breast carcinoma, gastric cancer, and tongue squamous cell carcinoma.8–10 IBC has also been shown to induce apoptosis via the mitochondrial pathway and PKCδ activation in myeloma cells.11 However, IBC has little effect on normal human cells such as hepatocytes, umbilical vascular endothelial cells, and cerebellar granule cell.8,12 An in vivo study confirmed the anticancer activity of orally administered IBC without obvious toxicity.13 These studies indicated that IBC is a promising anticancer agent. However, the anticancer properties and underlying molecular mechanisms of IBC in CRC remain unclear.
Aberrant activation of the Wnt/β-catenin signaling contributes to initiating events during carcinogenesis of CRC.14 In the cytoplasm, β-catenin combines with a destruction complex consisting of axis inhibition protein (AXIN), adenomatous polyposis coil (APC), and glycogen synthase kinase 3β (GSK3β), and becomes phosphorylated, resulting in targeting for ubiquitination-dependent proteolysis. When Wnt signaling stimulation is present, GSK3β is phosphorylated, resulting in the removal of the suppressive function of the destruction complex, leading to accumulation of non-phosphorylated active β-catenin in the cytoplasm.15 Approximately 80% of all CRCs contain active β-catenin.16 Activated β-catenin translocates from the cytosol into the nucleus, where it forms a ternary complex with transcription factors TCF/LEF to activate Wnt/β-catenin-responsive genes, which regulate cancer cell proliferation and apoptosis.15 Several studies have shown that phosphorylation Ser9 of GSK-3β by phosphorylated AKT inhibits the kinase activity of GSK-3β, resulting in degradation of β-catenin during carcinogenesis.17 Based on these findings, AKT/GSK-3β/β-catenin signaling has emerged as a promising target for the treatment of CRC.
The purpose of this study was to investigate the underlying mechanisms of the functional effects of IBC in a model of CRC. We demonstrated that IBC could induce apoptosis of CRC cells in vitro. We also revealed that this apoptotic effect was mainly dependent on inactivation of the AKT/GSK-3β/β-catenin signaling pathway.
Materials and methods
Reagents and antibodies
IBC was purchased from Selleck (Houston, TX, USA). The structure of IBC is shown in Figure 1A. A stock solution of IBC (100 mM) was dissolved in dimethyl sulfoxide (DMSO) and diluted to the appropriate concentration with a maximum final DMSO concentration less than 0.5%. Z-DEVD-FMK, necrosulfonamide (NSA), and ferrostatin-1 (Fer-1) were obtained from Selleck (Houston, TX, USA). Antibodies against β-catenin, phospho-β-catenin, GSK-3β, phospho-GSK-3β, AKT, and phospho-AKT were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies against cleaved caspase-3, cleaved PARP, Bcl-2, Bax, XIAP, survivin, COX-IV, and β-actin were purchased from Abcam (Cambridge, UK).
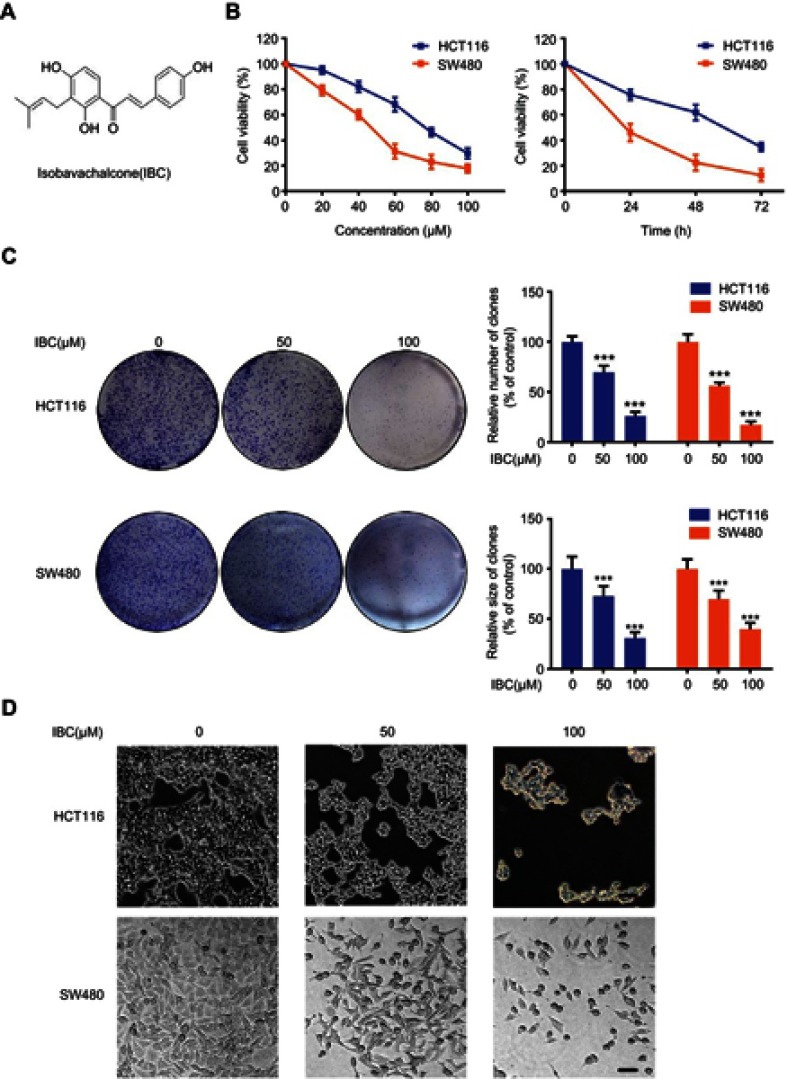
Figure 1.
IBC inhibited proliferation and colony formation of human CRC cells. (A) Chemical structure of IBC. (B) HCT116 and SW480 cells were treated with different concentrations of IBC (0–100 µmol/L) for 24, 48, and 72 hrs, and cell viability was measured using the CCK-8 assay. (C) HCT116 and SW480 cells were incubated with different concentrations of IBC (0, 50, and 100 µM) for 24 hrs and assayed for colony formation. The upper panel shows representative images of colony formation after treatment with IBC for 24 hrs and results of quantitative analyses of colony number. The lower panel shows the results of quantitative analyses of colony size. (D) Morphological changes of HCT116 and SW480 cells were observed using contrast microscopy after treatment with the indicated concentration of IBC for 24 hrs. Scale bars =20 µm. The results shown in panels B and D are represented as mean ± SD of three independent experiments. ***p<0.001 compared with control group.
Cell lines and culture
The human CRC cell lines HCT116 and SW480 were purchased from the Shanghai Institute for Biological Sciences (Shanghai, China). As recommended, HCT116 and SW480 cells were cultured in the McCoy’s 5A and L15 (Invitrogen, Carlsbad, CA, USA) respectively, containing 10% FBS (Invitrogen), 100 U/mL penicillin, and 100 µg/mL streptomycin in a humidified atmosphere of 95% air and 5% CO2 at 37°C.
Cell viability assay
Cell viability was evaluated using the CCK-8 assay kit (Dojindo Laboratories Tokyo, Japan). Briefly, cells (1×104 cells/well) were seeded into 96-well tissue culture plates. Once adhered, cells were treated with the indicated concentrations of IBC. At 24, 48, and 72 hrs after treatment, 10 µL of CCK-8 solution was added to the 100 µL of culture medium to measure cell viability. After incubating for 1 hr, the absorbance of each well was measured at 450 nm using a microplate reader (Bio-Rad Laboratories, Richmond, CA, USA).
Colony formation assay
For colony formation assays, cells (1×103 cells/well) were plated in six-well cell culture plates and treated with indicated concentration of IBC for 48 hrs. After culturing for an additional 14 days, cells were fixed by ethyl alcohol for 15 mins and then stained with 0.1% crystal violet. After incubation for 10 mins, the cells were washed three times with cold PBS and photographed. The number and size of colonies were detected at 40⨰ magnification using a Nikon inverted microscope (Tokyo, Japan).
4′,6-diamidino-2-phenylindole (DAPI) staining
Cells were seeded in six-well tissue culture plates with a glass slide in each well and treated with the indicated concentrations of IBC for 24 hrs. After treatment, the slides were rinsed with PBS and fixed in 3.7% paraformaldehyde for 30 mins at room temperature. Then, the slides were stained with DAPI (Sigma–Aldrich St Louis, MO, USA) to evaluate morphology changes of the nuclei. The stained nuclei were visualized using a fluorescence microscope (Olympus, Tokyo, Japan) with the appropriate filter.
Annexin V/propidium iodide (PI) double staining assay
Apoptotic cell death was detected using the FITC Annexin V Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA). Briefly, cells (1×105 cells/well) were seeded in six-well tissue culture plates. After exposure to IBC for 24 hrs, attached and floating cells were collected. Cells were washed with PBS and diluted in 100 µL of binding buffer. Then, samples were incubated with Annexin V-FITC and PI at room temperature for 15 mins in the dark. After addition of 400 µL of binding buffer to each sample, the stained samples were analyzed using a flow cytometer (FACScan, Becton Dickinson, Franklin Lakes, NJ, USA). Data were analyzed using FlowJo software.
Mitochondria/cytosol fractionation
Mitochondrial fraction was isolated from the cytosolic fraction of CRC cells using a mitochondria/cytosol fractionation kit (Biovision, Mountain View, CA) according to the manufacturer’s protocol. After treatment with IBC, cells (5×107) were collected and washed twice with ice-cold PBS. The cells were resuspended in 500 μL of cytosol extraction buffer, then homogenized using a Dounce tissue grinder on ice for 30–50 passes. Unbroken cells, debris, and nuclei were separated by centrifugation at 700×g for 10 mins at 4°C and discarded. The supernatants were collected and centrifuged at 10,000×g for 30 mins at 4°C. The collected supernatants contained the cytosolic fractions. The collected pellet contained the mitochondrial fractions and was resuspended in 100 μL of mitochondrial extraction buffer.
Plasmids transfection
For β-catenin overexpression, cells were transfected with 4 µg of pCMV-Flag, or pCMV-Flag-β-catenin (human β-catenin pcDNA3 plasmid, from Sino Biological Inc., Beijing, China) using Lipofectamine® 3000 Transfection Reagent (Thermo Scientific, USA) according to the manufacturer’s instructions. Western blot and rescue experiments were performed at 48 hrs post-transfection.
Western blot analysis
After treatment with the indicated concentrations of IBC, total protein was extracted in ice-cold RIPA buffer (Beyotime, Nanjing, China) containing protease inhibitors and analyzed by western blot. Briefly, equal amounts of protein of each group were separated by SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore., Atlanta, USA). The PVDF membranes were blocked with 5% non-fat dry milk for 1 hr at room temperature, and probed with a 1:1,000 dilution of primary antibodies overnight at 4°C. Subsequently, incubation with HRP-conjugated secondary antibodies (1:5,000; Abcam), and developed using ECL Western Blotting Substrate Solution (Bio-Rad Laboratories, Hercules, CA, USA) and images were obtained using an instrument of ECL chemiluminescence instrument (Tanon Science and Technology Co., Ltd., Shanghai, China). Quantification of band density was performed by Image J software.
Statistical analysis
Statistical evaluation was performed using the two-tailed Student’s t-test and one-way ANOVA to evaluate differences between the groups. Values were expressed as the mean ± standard deviation (SD) of at least three independent experiments. P-values of <0.05 was considered to indicate a statistically significant difference.
Results
IBC inhibited proliferation and colony formation of CRC cells
In this study, we first evaluated the cytotoxic effect of IBC against two CRC cell lines (HCT116 and SW480). Cells were incubated with a range of IBC concentrations (20–100 µM) for 24, 48, and 72 hrs, and cytotoxicity of IBC was analyzed by CCK-8 assay. IBC significantly decreased CRC cell viability in a dose- and time-dependent manner (Figure 1B). The IC50 values were 75.48 µM at 24 hrs in HCT116 cells, and 44.07 µM at 24 hrs in SW480 cells. Using effective IBC concentrations based on these data (0, 50, 100 µM), the antiproliferative activity of IBC was further evaluated using a colony formation assay. IBC treatment reduced colony number and colony size in CRC cells in a dose-dependent manner (Figure 1C). IBC-induced changes in morphology of CRC cells were visualized using a microscope. After treatment with IBC, cells number was low and cells exhibited a decreased rate of cellular attachment. Single cells exposed to IBC exhibited cell shrinkage and condensed cytoplasm (Figure 1D). These results indicated that IBC inhibited proliferation of CRC cells in a dose-dependent manner.
IBC induced apoptosis in CRC cells
We next determined whether the antiproliferative activity of IBC resulted from induction of apoptosis. CRC cells were treated with increasing concentrations of IBC for 24 hrs and nuclei were stained with DAPI and visualized using a microscope. As shown in Figure 2A, the number of apoptotic cells, as evidenced by condensed and fragmented nuclei, increased significantly in an IBC dose-dependent manner. To further investigate whether the decrease in proliferation and viability were associated with increased apoptosis, we examined the effect of IBC on the induction of apoptosis in CRC cells by Annexin V/PI double staining and flow cytometric analysis. The results showed that IBC-treated cells demonstrated a dramatic dose-dependent increase in Annexin V-positive cells (Figure 2B and C). These results demonstrate that the apoptosis played a pivotal role in the antiproliferative effect of IBC on CRC cells.
Figure 2.
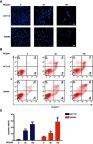
IBC induced apoptosis in CRC cells. CRC cells were treated with selected concentrations of IBC for 24 hrs. (A) Nuclear morphological changes characteristic of apoptosis were observed using DAPI staining. Scale bars =20 µm. (B) Cells were stained with Annexin V and PI and flow cytometry analysis was performed. (C) Graphical representation of the percentages of Annexin V positive cells. The percentages of cells in the Q2 (Annexin V+/PI–) together with Q3 (Annexin V+/PI+) quarters were calculated as Annexin V positive cells for statistical analysis. Data are presented as mean ± SD of three independent experiments. *p<0.05, **p<0.01, ***p<0.001 vs control group.
IBC induced apoptosis via the regulation of apoptosis-associated proteins in CRC cells
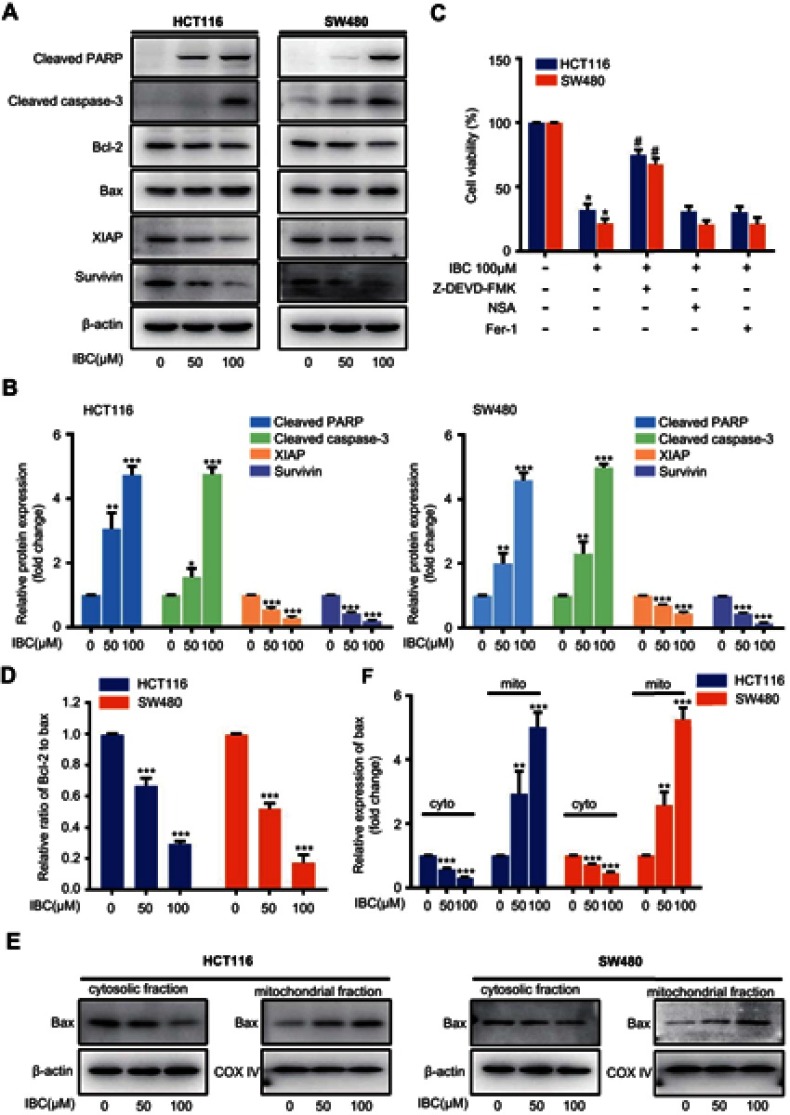
To investigate the potential molecular mechanisms responsible for IBC-induced apoptosis in CRC cells, we measured the expression of apoptosis-related proteins. As cleavage of caspase-3 and PARP is considered a hallmark of apoptosis. As such, we measured expression levels of these proteins in CRC cells by western blot. IBC treatment resulted in upregulation of cleaved caspase-3 and cleaved PARP in a dose-dependent manner (Figure 3A and B). To further evaluate the significance of caspase-3 activation, the caspase-3 inhibitor Z-DEVD-FMK was used. As shown in Figure 3C, IBC-induced cytotoxicity was significantly suppressed by pre-treatment of CRC cells with Z-DEVD-FMK. Furthermore, addition of NSA, an inhibitor of necroptosis, and Fer-1, an inhibitor of ferroptosis, failed to inhibit IBC-induced cytotoxicity against CRC cells (Figure 3C). Apoptosis is controlled by multiple proapoptotic and antiapoptotic proteins, such as Bax and Bcl-2, respectively, which are the major regulators of the caspase cascade. After exposure to increasing concentrations of IBC for 24 hrs, protein expression levels of Bax were significantly increased, whereas levels of Bcl-2 were significantly decreased (Figure 3A), resulting in a significantly decreased Bcl-2 to Bax ratio compared with the control group (Figure 3D). Furthermore, Bax translocated from cytosol to mitochondria in CRC cells following treatment with IBC (Figure 3E and F). In addition to the Bcl-2 family, the inhibitors of apoptosis (IAP) family of proteins also regulates caspase activity, thus affecting apoptosis. Therefore, we measured the expression of two IAP family proteins, XIAP, and survivin. As demonstrated in Figure 3A and B, levels of XIAP and survivin protein were markedly reduced in CRC cells after IBC treatment. In summary, IBC promoted apoptosis through regulation of apoptosis-associated protein expression in CRC cells.
Figure 3.
IBC induced apoptosis through regulation of apoptosis-associated proteins in CRC cells. After treatment with the indicated concentration of IBC for 24 hrs, cell lysates of CRC cells were prepared for western blotting. (A) Expression of cleaved caspase-3, cleaved PARP, Bax, Bcl-2, XIAP, and survivin. β-actin was used as a loading control. (B) Histogram showing the relative protein expression levels. The bands corresponding to each protein were quantified and normalized relative to band intensities for the control group. (C) CRC cells were pre-treated for 1 hr with Z-DEVD-FMK (25 μM), NSA (1 μM) or Fer-1 (2 μM), followed by IBC (100 μM) treatment for 24 hrs, cell viability was analyzed using the CCK-8 assay. (D) The ratio of Bcl-2 to Bax was calculated based on band density. (E) Translocation of Bax form cytosol to mitochondria was further assessed by subcellular (cytoplasmic and mitochondrial) fractionation and western immunoblot after treatment with IBC in CRC cells. β-actin was used as a cytoplasmic control. COX IV was used as a mitochondrial control. (F) Histogram showing the relative protein expression levels. The bands corresponding to each protein were quantified and normalized relative to band intensities for the control group. The results are shown as mean ± SD compared with the control. **P<0.01 and ***p<0.001 vs control group. #p<0.05 compared with IBC treated group.
IBC downregulated Wnt/β-catenin signaling
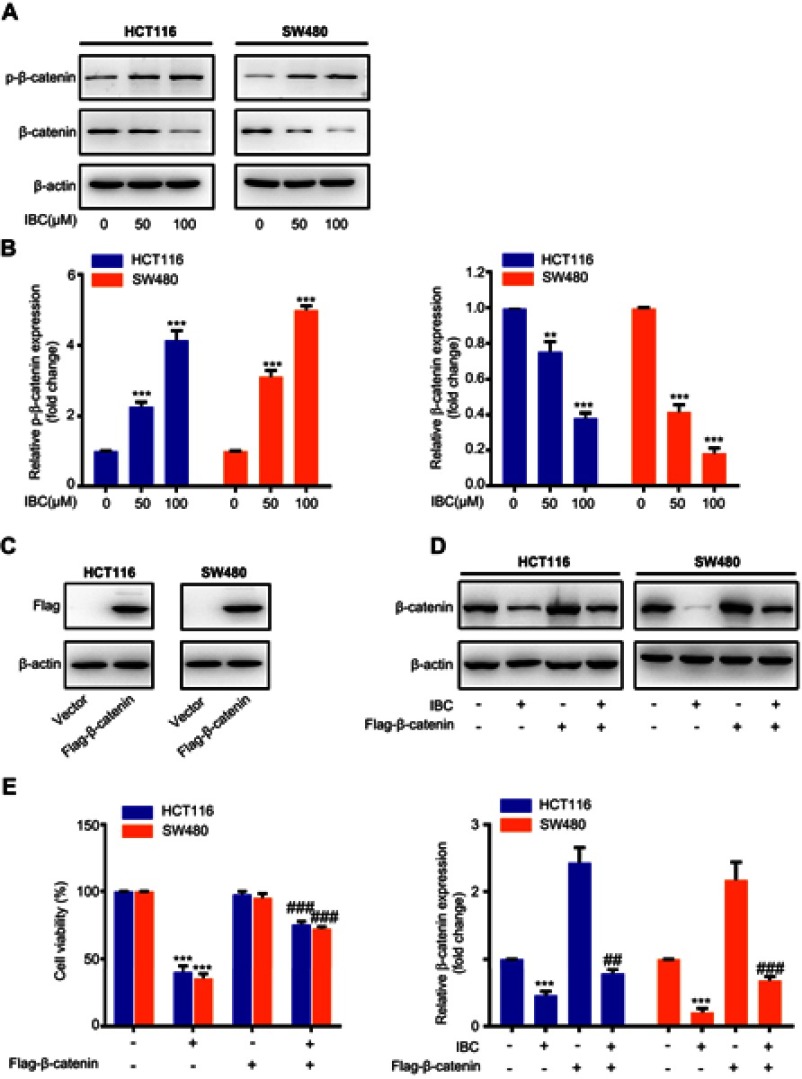
β-catenin plays a key role in CRC carcinogenesis. To explore Wnt/β-catenin inhibition in CRC cells after treatment with IBC, we measured β-catenin expression by western blot. Expression of total β-catenin was inhibited by IBC treatment, but its phosphorylation level was increased, in a dose-dependent manner (Figure 4A and B). To verify whether inhibiting β-catenin pathway was responsible for the antitumor activity of IBC, overexpression of β-catenin by transiently transfecting was used (Figure 4C). In response to IBC, there was a slight change on β-catenin levels in the overexpressed cells (Figure 4D). Furthermore, we found that overexpression of β-catenin significantly abolished the inhibitory effect of IBC on cell viability in CRC cells (Figure 4E). Collectively, our results demonstrate that inhibition of the β-catenin signaling pathway is closely involved in IBC-induced anticancer effect on CRC cells.
Figure 4.
Expression and activation of Wnt/β-catenin signaling in IBC-treated cells. (A) Cells were treated with increasing concentrations of IBC for 24 hrs. Cell lysates were prepared, and the expression of total and phosphorylated β-catenin was analyzed by western blotting. β-actin was used as a loading control. (B) Histogram showing the relative phosphorylated and total β-catenin expression levels. The bands corresponding to each protein were quantified and normalized relative to band intensities for the control group. (C) CRC cells were transfected with pCMV-Flag or pCMV-Flag-β-catenin. Forty-eight hours after transfection, cells were treated with 100 μM IBC for 24 hrs. Total protein was isolated and analyzed by western blot to determine Flag expression. (D) After transfection for 48 hrs, total protein was obtained for western blot to determine β-catenin expression with IBC treatment. β-actin was used as a loading control. Histogram showing the relative protein expression levels (lower panel). (E) After overexpression of β-catenin, cell viability was measured by CCK-8 assay with IBC treatment. Data are represented as means ± SD. **p<0.01, and ***p<0.001 vs control group. ##p<0.01, and ###p<0.001 compared with IBC treated group.
Inhibition of AKT/GSK-3β signaling was involved in the degradation of β-catenin induced by IBC
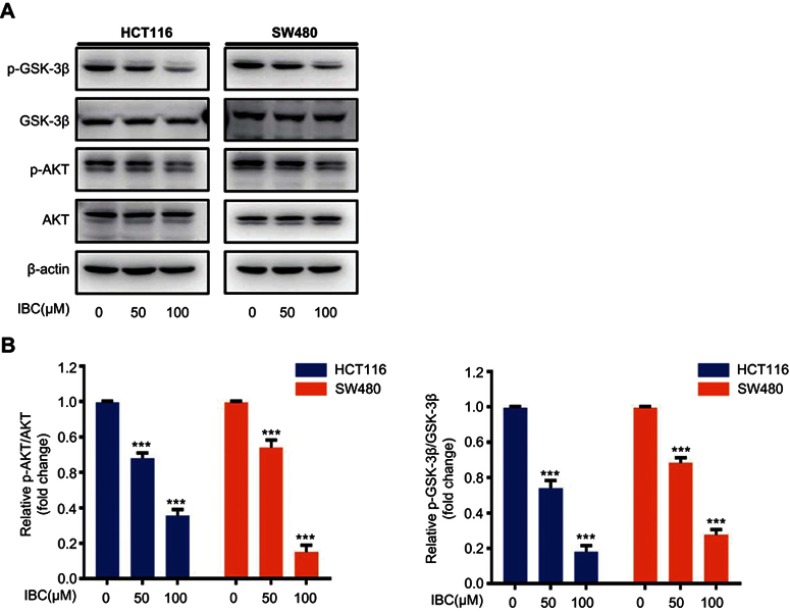
To investigate how IBC affects the expression of β-catenin we examined the role of upstream proteins in the β-catenin pathway. GSK-3β plays a critical role in Wnt/β-catenin signaling, which is abnormally inactivated during CRC development. As shown in Figure 5A and B, IBC inhibited the phosphorylation of GSK-3β with no change in total GSK-3β. The phosphorylation status of AKT, an upstream kinase directly responsible for phosphorylation and subsequent inhibition of GSK-3β, was also decreased by IBC in a dose-dependent manner (Figure 5A and B). These findings indicated that IBC downregulated Wnt/β-catenin signaling by inhibiting the AKT/GSK-3β signaling pathway.
Figure 5.
IBC inhibited activation of AKT/GSK-3β signaling. (A) Cells were treated with increasing concentrations of IBC for 24 hrs. Cell lysates were prepared, and the expression of total and phosphorylated forms of GSK-3β and AKT was analyzed by western blotting. β-actin was used as a loading control. (B) Histogram showing the relative protein expression levels. The bands corresponding to each protein were quantified and normalized relative to band intensities for the control group. Data are represented as means ± SD. ***p<0.001 vs control group.
Discussion
A gap in addressing the killing of all cancer cells through chemotherapy is highlighted in the number of fatalities seen with CRC.18 When evaluating the chemotherapeutic agents used to treat CRC, a number of studies have evaluated natural products, which tend to present fewer adverse effects.19–21 One such example is an active molecule called IBC, which belongs to the group of flavonoids that is present in the well-known traditional Chinese medicinal herb Psoralea corylifolia. Previous studies demonstrated that IBC can induce cell death in tumor cells of several lineages, and reduces the proliferation, migration, and invasion of cancer cells. The current study focuses on the antitumor effects of IBC on human CRC cell to open up potential avenues for therapeutic use of this compound.
We performed cell viability and clonogenic assays to confirm the cytotoxicity of IBC against CRC cell lines. The growth of SW480 and HCT116 cells was slowed by IBC in duration- and dose- dependent manner. Cells exposed to IBC also displayed changes in morphology compared with controls. Consistent with previous reports showing that IBC inhibited tumor cell growth, our findings demonstrated that IBC inhibited the growth of CRC cells. Induction of apoptosis by external signals leads to a decrease in cell viability and increased cell atrophy.22 We, therefore, hypothesized that the cell death and growth inhibition of CRC cell lines caused by IBC could be linked to apoptosis. Apoptosis is associated with characteristic features such as condensation of chromatin and changes in nuclear morphology.23 Such changes were observed in cells exposed to IBC treatment, while the controls exhibited normal morphology. Annexin V-FITC/PI double staining provided further evidence for apoptosis of IBC-treated CRC cell lines. Thus, IBC impaired growth and reduced cell viability through induction of apoptosis.
Cancer cells avoid apoptosis and gain an advantage in survival and proliferation by promoting antiapoptotic mechanisms and downregulating proapoptotic programs.24 One such proapoptotic signaling pathway involves cysteine proteases called caspases that target several substrate molecules. Caspase-3 is an important signaling molecule in this pathway. Activation of caspase-3 is necessary for efficient apoptosis.25 Our study revealed that caspase-3 was activated in tumor cell lines following exposure to IBC, followed by cleavage of PARP, an important target molecule. The mechanisms of apoptosis depending on the balance between proteins that favor and inhibit apoptosis.26 Several natural products have been reported to alter expression of the Bcl-2 family of proteins in cancer cells, resulting in apoptosis.27 Proteins in the Bcl-2 family prevent efflux of cytochrome c from the outer mitochondrial membrane and support cell survival of cells whereas Bax protein, mainly localized in the cytoplasm, translocates to the mitochondrial membrane and bind to the same site to release proteins that lead to apoptosis.28 This led us to evaluate at expression levels of Bcl2 and Bax in response to IBC treatment. Furthermore, we evaluated the distribution of Bax. We found that IBC induced a change in Bcl2 and Bax levels, and resulted in translocation of Bax from the cytosol to mitochondria. Moreover, IAPs inhibited apoptosis by directly binding to activate effector caspases, such as caspase-3. Our results showed that IBC could downregulate the expression of two IAP proteins XIAP and survivin in CRC cells. These results indicated that IBC-induced cell death through the mitochondrial apoptosis pathway in CRC cells.
Carcinogenesis of CRC involves a number of signal pathways, among which the Wnt/β-catenin pathway is strongly implicated.29 β-catenin has been linked to several aspects of tumorigenesis such as proliferation of cells, cell migration, and adhesion between cells.30 A reduction in the growth of CRC has been observed with the use of natural products that adversely influence the β-catenin pathway. Regulation of this pathway was observed after resveratrol treatment that inhibited the growth of human CRC cells.31 Another natural molecule, quercetin, caused a decrease in β-catenin in CRC cells.32 Curcumin suppresses the growth of CRC cells through miR-130a-mediated inhibition of Wnt/β-catenin.33 In the current study, exposure to IBC caused a decrease in the amount of total β-catenin and thus inhibited the Wnt/β-catenin pathway, in line with previous reports. Phosphorylation of Ser33/37/Thr41 by a complex comprised of APC, Axin, and GSK-3β resulted in β-catenin degradation.34 Our study showed that exposure of CRC cells to IBC led to phosphorylation of β-catenin at the same sites. Furthermore, overexpression of β-catenin, blocked the inhibitory effect of IBC on CRC cell viability, indicating that β-catenin signaling participated in the anticancer effect of IBC. Our results demonstrate that IBC inhibited CRC cell proliferation by inhibiting β-catenin signaling.
Phosphorylation of β-catenin by GSK-3β leads to proteasomes-mediated degradation of β-catenin in the cytosol. GSK-3β can also be phosphorylated, resulting in inactivation. One of the best-characterized regulatory molecules of GSK-3β is AKT. Targeting the AKT/GSK-3β pathway might suppress the tumor progression.35–37 Previous research showed a partial role of defective AKT/GSK-3β signaling in IBC-mediated induction of apoptosis in prostate and ovary cancer cells.8 Based on this finding, we explored the role of AKT/GSK-3β signaling in IBC-induced downregulated Wnt/β-catenin signaling. Consistent with previous studies, phosphorylation of AKT and GSK-3β was reduced by IBC in a concentration-dependent manner. These results showed that IBC suppressed of AKT, and consequently activated of GSK-3β, resulting in β-catenin cleavage and subsequent inhibition.
Conclusion
In this study, we showed that IBC inhibited growth and induced apoptosis in CRC cells through activation of caspase-3 and PARP via upregulation of the ratio of Bax/Bcl-2, alterations in Bax distribution and downregulation of XIAP and survivin, resulting from attenuation of AKT/GSK-3β/β-catenin signaling. Our findings demonstrated the therapeutic potential of IBC for the treatment of CRC.
Acknowledgments
This work was funded by the Clinical Capability Construction Project for Liaoning Provincial Hospitals (LNCCC-D44-2015).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii1–9. doi: 10.1093/annonc/mdu260 [DOI] [PubMed] [Google Scholar]
- 4.Carlomagno C, De Stefano A, Rosanova M, et al. Multiple treatment lines and prognosis in metastatic colorectal cancer patients. Cancer Metastasis Rev. 2018. doi: 10.1007/s10555-018-9748-7 [DOI] [PubMed] [Google Scholar]
- 5.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117. doi: 10.3322/caac.21220 [DOI] [PubMed] [Google Scholar]
- 6.HemaIswarya S, Doble M. Potential synergism of natural products in the treatment of cancer. Phytother Res. 2006;20(4):239–249. doi: 10.1002/ptr.1841 [DOI] [PubMed] [Google Scholar]
- 7.Kuete V, Sandjo LP. Isobavachalcone: an overview. Chin J Integr Med. 2012;18(7):543–547. doi: 10.1007/s11655-012-1142-7 [DOI] [PubMed] [Google Scholar]
- 8.Jing H, Zhou X, Dong X, et al. Abrogation of Akt signaling by Isobavachalcone contributes to its anti-proliferative effects towards human cancer cells. Cancer Lett. 2010;294(2):167–177. doi: 10.1016/j.canlet.2010.01.035 [DOI] [PubMed] [Google Scholar]
- 9.Jin X, Shi YI. Isobavachalcone induces the apoptosis of gastric cancer cells via inhibition of the Akt and Erk pathways. Exp Ther Med. 2016;11(2):403–408. doi: 10.3892/etm.2015.2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Y. Wu WZ1, Huo A, et al. Isobavachalcone inhibits the proliferation and invasion of tongue squamous cell carcinoma cells. Oncol Lett. 2017;14(3):2852–2858. doi: 10.3892/ol.2017.6517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao S, Ma CM, Liu CX, et al. Autophagy inhibition enhances isobavachalcone-induced cell death in multiple myeloma cells. Int J Mol Med. 2012;30(4):939–944. doi: 10.3892/ijmm.2012.1066 [DOI] [PubMed] [Google Scholar]
- 12.Nishimura R, Tabata K, Arakawa M, et al. Isobavachalcone, a chalcone constituent of angelica keiskei, induces apoptosis in neuroblastoma. Biol Pharm Bull. 2007;30(10):1878–1883. [DOI] [PubMed] [Google Scholar]
- 13.Wu D, Wang W, Chen W, et al. Pharmacological inhibition of dihydroorotate dehydrogenase induces apoptosis and differentiation in acute myeloid leukemia cells. Haematologica. 2018;103(9):1472–1483. doi: 10.3324/haematol.2018.188185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han YF, Cao GW. Linking Colorectal cancer to Wnt signaling. World J Gastroenterol. 2012;18(47):6865–6873. doi: 10.3748/wjg.v18.i47.6865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta A, Verma A, Mishra AK, Wadhwa G, Sharma SK, Jain CK. The Wnt pathway: emerging anticancer strategies. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(2):138–147. [DOI] [PubMed] [Google Scholar]
- 16.Rowan AJ, Lamlum H, Ilyas M, et al. APC mutations in sporadic colorectal tumors: a mutational “hotspot” and interdependence of the “two hits”. Proc Natl Acad Sci U S A. 2000;97(7):3352–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagini S, Sophia J, Mishra R. Glycogen synthase kinases: moonlighting proteins with theranostic potential in cancer. Semin Cancer Biol. 2018;pii: S1044–579X(17)30179–7. [DOI] [PubMed] [Google Scholar]
- 18.Van der Jeught K, Xu HC, Li YJ, et al. Drug resistance and new therapies in colorectal cancer. World J Gastroenterol. 2018;24:3834–3848. doi: 10.3748/wjg.v24.i34.3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith E, Palethorpe HM, Tomita Y, et al. The purified extract from the medicinal plant bacopa monnieri, bacopaside II, inhibits growth of colon cancer cells in vitro by inducing cell cycle arrest and apoptosis. Cells. 2018;7(7):pii:E81. doi: 10.3390/cells7070081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad Hidayat AF, Chan CK, Mohamad J, Abdul Kadir H. Dioscorea bulbifera induced apoptosis through inhibition of ERK 1/2 and activation of JNK signaling pathways in HCT116 human colorectal carcinoma cells. Biomed Pharmacother. 2018;104:806–816. doi: 10.1016/j.biopha.2018.05.073 [DOI] [PubMed] [Google Scholar]
- 21.Buhrmann C, Yazdi M, Popper B, et al. Resveratrol chemosensitizes TNF-beta-induced survival of 5-FU-treated colorectal cancer cells. Nutrients. 2018;10(7):pii:E888. doi: 10.3390/nu10070888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prokhorova EA, Zamaraev AV, Kopeina GS, et al. Role of the nucleus in apoptosis: signaling and execution. Cell Mol Life Sci. 2015;72(23):4593–4612. doi: 10.1007/s00018-015-2031-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2(4):277–288. doi: 10.1038/nrc776 [DOI] [PubMed] [Google Scholar]
- 25.Jänicke RU, Sprengart ML, Wati MR, et al. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273(16):9357–9360. [DOI] [PubMed] [Google Scholar]
- 26.Schultz DR, Harrington WJ Jr. Apoptosis: programmed cell death at a molecular level. Semin Arthritis Rheum. 2003;32(6):345–369. doi: 10.1053/sarh.2003.50005 [DOI] [PubMed] [Google Scholar]
- 27.Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov. 2008;7(12):989–1000. doi: 10.1038/nrd2658 [DOI] [PubMed] [Google Scholar]
- 28.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281(5381):1322–1326. [DOI] [PubMed] [Google Scholar]
- 29.Pandurangan AK, Divya T, Kumar K, et al. Colorectal carcinogenesis: insights into the cell death and signal transduction pathways: a review. World J Gastrointest Oncol. 2018;10(9):244–259. doi: 10.4251/wjgo.v10.i9.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doerks T, Copley RR, Schultz J, Ponting CP, Bork P. Systematic identification of novel protein domain families associated with nuclear functions. Genome Res. 2002;12(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu YZ, Wu K, Huang J, et al. The PTEN/PI3K/Akt and Wnt/beta-catenin signaling pathways are involved in the inhibitory effect of resveratrol on human colon cancer cell proliferation. Int J Oncol. 2014;45(1):104–112. doi: 10.3892/ijo.2014.2392 [DOI] [PubMed] [Google Scholar]
- 32.Park CH, Chang JY, Hahm ER, et al. Quercetin, a potent inhibitor against beta-catenin/Tcf signaling in SW480 colon cancer cells. Biochem Biophys Res Commun. 2005;328(1):227–234. doi: 10.1016/j.bbrc.2004.12.151 [DOI] [PubMed] [Google Scholar]
- 33.Dou H, Shen R, Tao J, et al. Curcumin suppresses the colon cancer proliferation by inhibiting Wnt/beta-catenin pathways via miR-130a. Front Pharmacol. 2017;8:877. doi: 10.3389/fphar.2017.00877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhuang YW, Wu CE, Zhou JY, et al. Solasodine inhibits human colorectal cancer cells through suppression of the AKT/glycogen synthase kinase-3beta/beta-catenin pathway. Cancer Sci. 2017;108(11):2248–2264. doi: 10.1111/cas.13354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo H, Luo H, Yuan H, et al. Litchi seed extracts diminish prostate cancer progression via induction of apoptosis and attenuation of EMT through Akt/GSK-3beta signaling. Sci Rep. 2017;7:41656. doi: 10.1038/srep41656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saiprasad G, Chitra P, Manikandan R, et al. Hesperidin induces apoptosis and triggers autophagic markers through inhibition of Aurora-A mediated phosphoinositide-3-kinase/Akt/mammalian target of rapamycin and glycogen synthase kinase-3 beta signalling cascades in experimental colon carcinogenesis. Eur J Cancer. 2014;50(14):2489–2507. doi: 10.1016/j.ejca.2014.06.013 [DOI] [PubMed] [Google Scholar]