Abstract
Besides seminal functions in angiogenesis and blood pressure regulation, microvascular pericytes possess a latent tissue regenerative potential that can be revealed in culture following transition into mesenchymal stem cells. Endowed with robust osteogenic potential, pericytes and other related perivascular cells extracted from adipose tissue represent a potent and abundant cell source for refined bone tissue engineering and improved cell therapies of fractures and other bone defects. The use of diverse bone formation assays in vivo, which include mouse muscle pocket osteogenesis and calvaria replenishment, rat and dog spine fusion, and rat non-union fracture healing, has confirmed the superiority of purified perivascular cells for skeletal (re)generation. As a surprising observation though, despite strong endogenous bone-forming potential, perivascular cells drive bone regeneration essentially indirectly, via recruitment by secreted factors of local osteo-progenitors.
Keywords: Pericyte, Blood vessel, Osteogenesis, Mesenchymal stem cell, Bone, Spinal fusion, Non-union, Tunica adventitia, Perivascular cell, Stem cell
Introduction
Pericytes are stellate cells in close contact with endothelial cells and embedded within a basal lamina, which form a discontinuous layer in capillaries (<10 μm diameter), and continuous one around microvessels (diameter 10–100 μm) [1]. First described in 1873 by C. Rouget as, visionarily, contractile cells regulating blood flow, it is Zimmermann who coined the term pericyte in 1923 to describe cells, also known as mural cells, structurally supporting the vasculature [2]. Promotion of angiogenesis, blood vessel diameter regulation [3], and maintenance of vascular integrity and permeability [4] are the main functions attributed to pericytes, through direct cell contact and communication.
As early as the 1970s were pericytes suggested to be also involved in tissue regeneration [5]. It was, however, not before the first decade of this century that definitive experimental evidence was gained that pericytes are native ancestors of mesenchymal stem cells (MSC), the existence of which had been previously documented exclusively in long-term cultures of vascularized organs [6]. Pericytes can differentiate into chondrocytes, adipocytes and osteocytes, regardless of their tissue of origin [6–8], as well as skeletal and cardiac muscle [6, 9], and myofibroblasts at the origin of pathologic fibrosis [10, 11]. Pericytes also support hematopoiesis [12–16] and can modulate immune-inflammatory reactions [17].
Among the potential uses of pericytes/progenitor cells for tissue engineering, the application to bone tissue is most commonly studied [18]. The bone is a richly vascularized organ, and the reaction to bone injury includes the processes of osteogenesis and vasculogenesis that go hand in hand. The theory that mural cells participate in endogenous bone tissue repair has long been posited. Before the advent of cell lineage tracing, the use of intravascular dyes that label mural cells suggested that pericytes participate in osteochondral repair [1, 19]. Later studies using smooth muscle actin (SMA) reporter animals also suggested that endogenous mural cells give rise to bone cells after fracture [20]. SMA is a non-specific marker which labels some pericytes, smooth muscle, and fibroblasts/myofibroblasts. Therefore, to our knowledge, the direct participation of bone-associated pericytes in repair has never been definitely shown. Nevertheless, these observations of the reparative potential of endogenous SMA+ cells, combined with the known mesenchymal progenitor cell properties of human pericytes [6], gave impetus for the use of exogenous pericytes for bone tissue repair. The osteogenic potential of human pericytes and other perivascular cells has been examined in both ectopic and orthotopic models. These findings are briefly reviewed below.
Identification and Purification of Perivascular Cells for Bone Repair
The possibility of using human pericytes/perivascular progenitor cells to speed bone repair was made possible by prior studies that used the cell surface marker CD146, also known as Mel-CAM (melanoma cell adhesion molecule), for the identification and purification of pericytes ([21, 22]; see also [23] for a review). Of note, CD146 expression is by no means specific to pericytes, and as a heterophilic cell-cell adhesion molecule, it is often upregulated when diverse cell types adopt a location on the outside aspect of the endothelial cell [24]. CD146 is also expressed by endothelial cells [25], vascular smooth muscle cells (VSMC) [26], fractions of lymphocytes [27], and tumor cells [28]. Therefore fluorescence-activated cell sorting for a combination of cell surface markers (CD146+CD34-CD31-CD45-) is most commonly used by our group to identify a pericyte among uncultured stromal populations [6, 29, 30]. The potential non-specific identity of CD146+ progenitors has led some investigators to favor the term tissue-specific progenitor cells over pericytes. A presumably analogous CD146+ progenitor cell can also be identified among culture-derived cell populations [22]. CD146+ pericytes/progenitors have been examined for their bone-forming potential alone [31] or in combination with other perivascular mesenchymal progenitor cells derived from the tunica adventitia and typified by CD34 expression and absence of other endothelial cell and pericyte markers (termed adventitial cells or adventitial progenitor cells) [29, 32]. When CD146+ pericytes and CD34+ adventitial progenitor cells are used in combination, they are most commonly referred to as perivascular stem cells or perivascular stromal cells (PSCs) [29], referring to their shared perivascular location. At least in the context of bone tissue engineering, PSCs are most commonly derived from subcutaneous adipose tissue [33]. The rationale for adipose derivation is based principally on the easy access and dispensability of this tissue depot. Once in culture, PSCs are able to undergo differentiation toward multiple mesenchymal lineages under appropriate culture conditions (Fig. 3.1), including osteoblastogenic (Fig. 3.1a–c) and adipo-cytic cell fates (Fig. 3.1d). Importantly the umbrella term of PSC is used, despite the clear understanding that these perivascular progenitor cells differ in their location and cellular morphology within the vascular wall, markers for in situ detection, frequency within different tissues, and gene network profiles [34]. The functional relevance of cellular differences between CD146+ pericytes and CD34+ adventitial progenitor cells for bone repair outcomes is as yet not known.
Fig. 3.1.
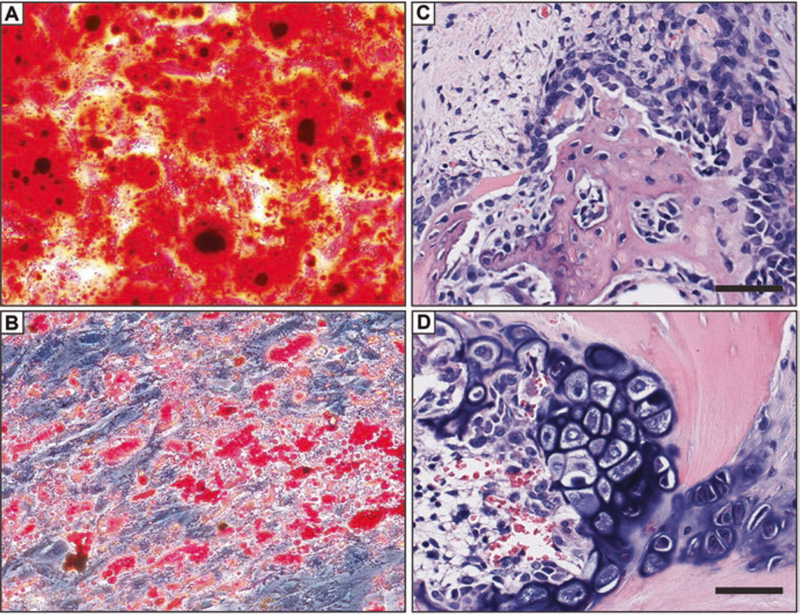
Differentiation of human perivascular stem cells in vitro and stimulation of an osteochondrogenic program in vivo. (a, b) PSCs are a multipotent progenitor cell type in vitro. (a) Human PSCs were cultured in the presence of osteogenic differentiation medium. Frank confluent mineralization was observed among PSC under inductive culture conditions (Alizarin red staining shown). (b) Conversely, intracellular lipid accumulation can be visualized within PSC under appropriate adipogenic conditions (Oil red O staining shown). (c, d) PSC implantation in a rat spinal fusion model induces a combination of intramembranous and endochondral bone formation. (c) Woven bone formation, and prominent bone lining osteoblasts in areas of intramembranous bone formation by PSC. (d) Chondrocyte hypertrophy and mineralization in areas of endochondral bone formation induced by PSC. Scale bar: 25 um
We have described methods general to all human organs: fresh tissues undergo mechanical and enzymatic digestion prior to cell isolation and immunolabeling for FACS, with at least one pericyte, endothelial, and hematopoietic cell marker. Sorted populations are seeded in endothelial growth medium 2 (EGM-2) in gelatin-coated plates and passaged using 20% FCS-supplemented Dulbecco’s modified Eagle’s medium [6]. The immunophenotype CD146+CD34-CD31-CD45-CD56- successfully isolates pericytes in multiple tissues including the skeletal muscle, bone marrow, white adipose tissue, placenta, pancreas, umbilical cord, heart, kidneys, infrapatellar fat pad, and liver [6, 9, 14, 18, 29, 30, 35–38, 39–41]. Of note, the same immunophenotype has been used to isolate pericytes from other mammalian species, including dog [42], sheep [43], and horse [44, 45], offering large animal models of perivascular cell-mediated tissue regeneration.
Pericytes and Ectopic Bone Formation
Animal models of ectopic bone formation have been used to confirm the capacity for in vivo osteogenic differentiation of implanted human pericytes. Human adipose tissue (AT)-derived CD146+ pericytes have been observed to directly ossify when implanted in a SCID (severe combined immunodeficiency) mouse muscle pouch [23]. Inconspicuous bone is produced when AT pericytes are implanted on a collagen sponge carrier, which represents a relatively inert substance with little osteoinductive properties [29]. In contrast, when AT pericytes are implanted intramuscularly using an osteoinductive demineralized bone matrix (DBM) carrier, robust bone formation is observed [29]. In somewhat similar observations, other groups have shown that CD146+ AT-derived progenitor cells do not form significant bone when implanted in a subcutaneous ossicle model [22]. In contrast, bone-associated (bone marrow or periosteum) CD146+ progenitor cells drive robust bone formation in the same subcutaneous ossicle model [22]. Head-to-head comparisons of AT-derived pericytes and adventitial cells from the same patient sample have been performed [23]. Both perivascular cell types induce vascularized ectopic bone and without substantive differences in the degree of bone formation [23]. This pilot study demonstrated that pericytes and adventitial cells have a similar bone-forming potential and laid the framework for later studies in which these two cell populations were combined. Next, experiments have been performed in which uncultured AT-derived PSCs (combined pericytes + adventitial cells) were implanted intramuscularly and compared with an unsorted/uncultured stromal population from the same patient’s adipose sample (termed stromal vascular fraction, SVF) [29]. Here, a DBM scaffold was again used. Results showed that independent of cell number, AT-derived PSC led to more robust intramuscular ossification in comparison to SVF from the same patient sample using quantitative metrics of bone formation by micro-computed tomography, histomorphometry, and select immunohistochemical markers of the bone [29]. Increased bone formation among AT-PSC implants was accompanied by a significant increase in vascularity of the implant site, accompanied by increased elaboration of VEGF (vascular endothelial growth factor) [38]. Ectopic bone formation induced by AT-PSC was also associated with an altered inflammatory milieu within the early wound environment [17]. Overall, these studies showed that AT-derived pericytes or AT-derived adventitial cells either alone or combined result in significant ectopic bone formation. Moreover, and for the first time, it was observed that these FACS-purified cell populations outcompete unpurified stromal cell populations from the same patient sample in terms of bone-forming efficacy.
Pericytes in Calvarial Defect Regeneration
The extent to which AT-derived PSC can induce bone repair was first examined in a mouse calvarial defect model [33]. Here, equal numbers of unpurified SVF or PSC from the same patient’s adipose tissue were implanted in a non-healing, circular, full-thickness calvarial defect of the parietal bone. Cells were implanted on a hydroxyapatite-coated polymeric scaffold for an additional osteoinductive effect. Similar to intramuscular implants, radiographic and histologic analysis showed AT-derived PSC led to a significant increase in bone regenerate at the defect site over an 8-week time course. In comparison, unpurified SVF from the same patient had no statistically appreciable benefit in comparison to a scaffold without cells. In this xenograft model, sparse but present human-specific antigens were detectable within the healing bone defect. Again, and in similarity to intramuscular studies, bone defect vascularity was significantly increased with PSC treatment. Thus, across both ectopic and bone repair models, AT-derived human PSCs have conserved features upon transplantation, including pro-osteogenic/pro-vasculogenic effects of a greater magnitude than unpurified stromal cell fractions. Whether these findings correlated with the enrichment of osteoinductive PSC, or conversely the elimination of an inhibitory cell type within the heterogeneous stroma of SVF, is still a matter of conjecture.
Pericytes in Spinal Fusion
Spinal fusion represents a more functionally demanding environment for a bone graft substitute and represents an assay for the production of contiguous and biome-chanically sound bone tissue. The use of AT-derived human PSC as a cellular therapy for bone grafting has been validated in a rat posterolateral lumbar spinal fusion model. In these studies, human AT-PSC implantation was performed across three cell densities in rats, using a DBM scaffold as a moldable carrier. PSC demonstrated a dose-dependent increase in ossification, increase in bone deposition, increase in measurements of bone strength, and complete fusion between lumbar bone segments in all rats [46]. In this model, both intramembranous (Fig. 3.1c) and endochondral bone formation (Fig. 3.1d) was spurred on by PSC implantation. Like in other studies, new bone regenerate was observed to be a product of both direct osteodifferentiation and host osteoblastogenesis. Like the calvarial defect model, paracrine-mediated bone formation of rat origin predominated [46]. In follow-up studies, Lee et al. extended these observations to rats rendered osteoporotic by ovariectomy. Here, increased numbers of implanted human AT-PSC were required to surmount the hormonal changes of estrogen withdrawal [47].
Pericytes for Non-union Fracture Healing
Atrophic non-union is associated with biological failure of fracture healing. Animal studies have shown the vascular ingrowth within atrophic non-union is much reduced at early timepoints [48]. In combination with the observation, the mesenchymal progenitor cell content within fibrous non-unions is reduced, and the proliferative and osteogenic capabilities of these non-union derived cells are likewise reduced [49]. CD146+ AT pericytes were examined in a well-established model of rat tibial atrophic non-union [48, 50]. Human AT pericytes were percutaneously injected 3 weeks after the establishment of fibrous non-union. Results showed that pericyte injection increased fracture callus size and increased mineralization, eventually resulting in increased bone union [50]. Like in other models, the efficacy of pericyte treatment was primarily a paracrine phenomenon, and in fact species-specific immunohistochemistry failed to later identity residual human cells. These data suggest that at least in the inhospitable microenvironment of atrophic nonunion, the benefit of pericytes primarily resides in their trophic abilities.
Discussion
Pericytes have crossed the limits of vascular biology and entered the field of regenerative medicine via their mesenchymal stem cell-cultured progeny. Advantages of using conventional MSCs include the simplicity of the derivation method and possibility to obtain large numbers of cells. On the negative side, MSCs are the cultured product of a heterogeneous mixture of unseparated cells, and in vitro growth involves cell exposure to animal proteins, hence chances of xenogeneic immunization, and entails risks of bacterial contamination and genetic instability. There have been occasional reports of MSC malignant transformation [51]; principally, it is increasingly accepted that MSC recruitment to the tumor stroma can favor cancer development [52]. For all these reasons, it might be beneficial to use purified, non-cultured perivascular cells in place of culture-derived MSCs for cell therapies. Bone repair has been the first envisioned therapeutic use of pericytes and adventitial perivascular cells. Bone structure is relatively simple, and targeted interventions, such as non-union fracture reduction or spine fusion, are usually not life-threatening, providing convenient models in which to gain a proof-of-concept demonstration of the therapeutic usability of perivascular presumptive MSCs. Importantly, PSCs also appear to represent a reliable source of autologous therapeutic cells, regardless of age, gender, and body mass index [30]. Experimentally, as described in this article, pericytes and adventitial cells purified from human or canine adipose tissue exhibited dramatic bone-forming potential in all autologous and xenogeneic in vivo assays performed, including calvarial regeneration and muscle pouch osteogenesis in mice, spine fusion in rats and dogs, and non-union fracture repair in rats. In these tests, PSCs performed at least as well as conventional MSCs are significantly better than the plain stromal vascular fraction. The bone produced following PSC transplantation was histologically normal and mechanically competent. These data illustrate the propensity of perivascular cells to differentiate along the bone cell lineage: culturing human adipose tissue-derived pericytes on a hard hydrogel substrate was sufficient to induce osteogenesis [53], and transcriptome analysis in single adventitial cells revealed expression of genes associated with osteogenic commitment and differentiation [34], which may have an important significance in cardiovascular pathology since adventitial progenitor cells have been shown in the mouse to drive blood vessel calcification, also known as arteriosclerosis [54]. However, even though PSCs are clearly endowed with strong osteogenic potential, a paradoxical yet recurrent observation is that over time little chimerism can be detected in newly developed bone following xenogeneic PSC transplantation, suggesting these perivascular progenitors merely mediate bone formation by recruiting local osteogenic cells and reinforcing the growing belief that MSCs and related tissue regenerative cells function largely via trophic/chemotactic factor secretion [55]. Do pericytes and adventitial cells, which all contribute to MSC cultures and are arranged along blood vessels as a hierarchy of regenerative cells [34], play distinct roles as either osteoblastic progenitors or trophic secretory cells during osteogenesis? This important question is currently under investigation in experiments where either perivascular cell subset or the combination of the two is administered in the same injury setting.
Although recognized in all tissues with canonical markers and characteristic perivascular distribution, pericytes and adventitial cells represent heterogeneous cell populations which also exhibit organ-restricted anatomic, phenotypic, and functional specializations, the complexity of which is being gradually uncovered [11]. Regarding bone formation, we have recently identified novel surface markers which typify PSC subsets endowed with higher osteogenic potential (Ding, Meyers et al., unpublished results), as was already recently done for pro-fibrotic ability [10] and chondrogenic capacity [56]. Ongoing studies will converge to explain the bone healing effect of pericytes and other regenerative perivascular cells, both natively in situ and following purification and transplantation, and contribute to the development of a refined therapeutic product (Table 3.1).
Table 3.1. Summary of in vivo orthopedic models of PSC application.
| Model | Species/strain | Human cell used |
|---|---|---|
| Intramuscular implant | SCID mouse | AT pericyte, AT-adventitial cell, AT-PSC |
| Calvarial bone defect | SCID mouse | AT-PSC |
| Spinal fusion | Athymic rat | AT-PSC |
| Non-union | SD rat | AT pericyte |
Acknowledgments
The present work was supported by the NIH/NIAMS (R01 AR070773, K08 AR068316), NIH/NIDCR (R21 DE027922), USAMRAA through the Peer Reviewed Medical Research Program (W81XWH-180109121, PR170115), and Department of Defense through the Broad Agency Announcement (BA160256), American Cancer Society (Research Scholar Grant, RSG-18–027-01-CSM), the Maryland Stem Cell Research Foundation, the Musculoskeletal Transplant Foundation, the California Institute for Regenerative Medicine, the British Heart Foundation, and Medical Research Council.
Contributor Information
Carolyn A. Meyers, Department of Pathology, Johns Hopkins University, Baltimore, MD, USA
Joan Casamitjana, MRC Center for Regenerative Medicine, University of Edinburgh, Edinburgh, UK.
Leslie Chang, Department of Pathology, Johns Hopkins University, Baltimore, MD, USA.
Lei Zhang, Department of Pathology, Johns Hopkins University, Baltimore, MD, USA.
Aaron W. James, Department of Pathology, Johns Hopkins University, Baltimore, MD, USA Orthopaedic Hospital Research Center, University of California, Los Angeles, CA, USA awjames@jhmi.edu.
Bruno Péault, Orthopaedic Hospital Research Center, University of California, Los Angeles, CA, USA; MRC Center for Regenerative Medicine, University of Edinburgh, Edinburgh, UK bpeault@mednet.ucla.edu.
References
- 1.Diaz-Flores L, Gutierrez R, Gonzalez P, Varela H (1991) Inducible perivascular cells contribute to the neochondrogenesis in grafted perichondrium. Anat Rec 229(1):1–8. 10.1002/ar.1092290102 [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann KW (1923) Der feinere Bau der Blutkapillaren. Z Anat 68:29–109 [Google Scholar]
- 3.Gerhardt H, Betsholtz C (2003) Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res 314(1):15–23 Epub 2003 Jul 22 [DOI] [PubMed] [Google Scholar]
- 4.Stallcup WB, You WK, Kucharova K, Cejudo-Martin P, Yotsumoto F (2016) NG2 proteoglycan-dependent contributions of pericytes and macrophages to brain tumor vascularization and progression. Microcirculation 23(2):122–133. 10.1111/micc.12251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross R, Everett NB, Tyler R (1970) Wound healing and collagen formation. VI. The origin of the wound fibroblast studied in parabiosis. J Cell Biol 44(3):645–654 PMID: PMCID: PMC2107958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3(3):301–313 [DOI] [PubMed] [Google Scholar]
- 7.Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE (2004) Chondrogenic and adipogenic potential of microvascular pericytes. Circulation 110(15):2226–2232 Epub 2004 Oct 4 [DOI] [PubMed] [Google Scholar]
- 8.Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, Lin CS (2008) Defining stem and progenitor cells within adipose tissue. Stem Cells Dev 17(6):1053–1063. 10.1089/scd.2008.0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen WC, Baily JE, Corselli M, Díaz ME, Sun B, Xiang G, Gray GA, Huard J, Péault B (2015) Human myocardial pericytes: multipotent mesodermal precursors exhibiting cardiac specificity. Stem Cells 33(2):557–573. 10.1002/stem.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray IR, Gonzalez ZN, Baily J, Dobie R, Wallace RJ, Mackinnon AC, Smith JR, Greenhalgh SN, Thompson AI, Conroy KP, Griggs DW, Ruminski PG, Gray GA, Singh M, Campbell MA, Kendall TJ, Dai J, Li Y, Iredale JP, Simpson H, Huard J, Péault B, Henderson NC (2017) αv integrins on mesenchymal cells regulate skeletal and cardiac muscle fibrosis. Nat Commun 8(1):1118 10.1038/s41467-017-01097-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw I, Rider S, Mullins J, Hughes J, Péault B (2018) Pericytes in the renal vasculature: roles in health and disease. Nat Rev Nephrol 14(8):521–534. 10.1038/s41581-018-0032-4 [DOI] [PubMed] [Google Scholar]
- 12.Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS (2013) Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502(7473):637–643. 10.1038/nature12612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding L, Saunders TL, Enikolopov G, Morrison SJ (2012) Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481(7382):457–462. 10.1038/nature10783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corselli M, Chin CJ, Parekh C, Sahaghian A, Wang W, Ge S, Evseenko D, Wang X, Montelatici E, Lazzari L, Crooks GM, Péault B (2013) Perivascular support of human hematopoietic stem/progenitor cells. Blood 121(15):2891–2901. 10.1182/blood-2012-08-451864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sá da Bandeira D, Casamitjana J, Crisan M (2017) Pericytes, integral components of adult hematopoietic stem cell niches. Pharmacol Ther 171:104–113. https://doi.org/10.1016Zj.phar-mthera.2016.11.006 Epub 2016 Nov 28 [DOI] [PubMed] [Google Scholar]
- 16.Chin CJ, Li S, Corselli M, Casero D, Zhu Y, He CB, Hardy R, Péault B, Crooks GM (2018) Transcriptionally and functionally distinct mesenchymal subpopulations are generated from human pluripotent stem cells. Stem Cell Reports 10(2):436–446. 10.1016/j.stemcr.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyers CA, Xu J, Zhang L, Asatrian G, Ding C, Yan N, Broderick K, Sacks J, Goyal R, Zhang X, Ting K, Peault B, Soo C, James AW (2018) Early immunomodulatory effects of implanted human perivascular stromal cells during bone formation. Tissue Eng Part A 24(5–6):448–457. 10.1089/ten.TEA.2017.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James AW, Zara JN, Corselli M, Chiang M, Yuan W, Nguyen V, Askarinam A, Goyal R, Siu RK, Scott V, Lee M, Ting K, Péault B, Soo C (2012) Use of human perivascular stem cells for bone regeneration. J Vis Exp (63):e2952 10.3791/2952 PubMed PMID: ; PMCID: PMC3466949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz-Flores L, Gutierrez R, Lopez-Alonso A, Gonzalez R, Varela H (1992) Pericytes as a supplementary source of osteoblasts in periosteal osteogenesis. Clin Orthop Relat Res 275:280–286 PubMed PMID: [PubMed] [Google Scholar]
- 20.Matthews BG, Grcevic D, Wang L, Hagiwara Y, Roguljic H, Joshi P, Shin DG, Adams DJ, Kalajzic I (2014) Analysis of alphaSMA-labeled progenitor cell commitment identifies notch signaling as an important pathway in fracture healing. J Bone Miner Res 29(5):1283–1294. 10.1002/jbmr.2140 PubMed PMID: ; PMCID: PMC4864015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P (2007) Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131(2):324–336. PMID: 10.1016/j.cell.2007.08.025 [DOI] [PubMed] [Google Scholar]
- 22.Sacchetti B, Funari A, Remoli C, Giannicola G, Kogler G, Liedtke S, Cossu G, Serafini M, Sampaolesi M, Tagliafico E, Tenedini E, Saggio I, Robey PG, Riminucci M, Bianco P (2016) No identical “mesenchymal stem cells” at different times and sites: human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Reports 6(6):897–913. 10.1016/j.stemcr.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James AW, Hindle P, Murray IR, West CC, Tawonsawatruk T, Shen J, Asatrian G, Zhang X, Nguyen V, Simpson AH, Ting K, Soo C (2017) Pericytes for the treatment of orthopedic conditions. Pharmacol Ther 171:93–103. 10.1016/j.pharmthera.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mravic M, Asatrian G, Soo C, Lugassy C, Barnhill RL, Dry SM, Peault B, James AW (2014) From pericytes to perivascular tumours: correlation between pathology, stem cell biology, and tissue engineering. Int Orthop 38(9):1819–1824. 10.1007/s00264-014-2295-0 [DOI] [PubMed] [Google Scholar]
- 25.Sers C, Riethmüller G, Johnson JP (1994) MUC18, a melanoma-progression associated molecule, and its potential role in tumor vascularization and hematogenous spread. Cancer Res 54(21):5689–5694 PMID: [PubMed] [Google Scholar]
- 26.Tormin A, Li O, Brune JC, Walsh S, Schütz B, Ehinger M, Ditzel N, Kassem M, Scheding S (2011) CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood 117(19):5067–5077. 10.1182/blood-2010-08-304287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Covas DT, Panepucci RA, Fontes AM, Silva WA Jr, Orellana MD, Freitas MC, Neder L, Santos AR, Peres LC, Jamur MC, Zago MA (2008) Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol 36(5):642–654. 10.1016/j.exphem.2007.12.015 [DOI] [PubMed] [Google Scholar]
- 28.Shih IM (1999) The role of CD146 (Mel-CAM) in biology and pathology. J Pathol 189(1):4–11. PMID: [DOI] [PubMed] [Google Scholar]
- 29.James AW, Zara JN, Zhang X, Askarinam A, Goyal R, Chiang M, Yuan W, Chang L, Corselli M, Shen J, Pang S, Stoker D, Wu B, Ting K, Peault B, Soo C (2012) Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl Med 1(6):510–519. 10.5966/sctm.2012-0002 PubMed PMID: ; PMCID: PMC3659717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.West CC, Hardy WR, Murray IR, James AW, Corselli M, Pang S, Black C, Lobo SE, Sukhija K, Liang P, Lagishetty V, Hay DC, March KL, Ting K, Soo C, Péault B (2016) Prospective purification of perivascular presumptive mesenchymal stem cells from human adipose tissue: process optimization and cell population metrics across a large cohort of diverse demographics. Stem Cell Res Ther 7:47 10.1186/s13287-016-0302-7 PubMed PMID: ; PMCID: PMC4815276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Péault B, Chen W, Li W, Corselli M, James AW, Lee M, Siu RK, Shen P, Zheng Z, Shen J, Kwak J, Zara JN, Chen F, Zhang H, Yin Z, Wu B, Ting K, Soo C (2011) The Nell-1 growth factor stimulates bone formation by purified human perivascular cells. Tissue Eng Part A 17(19–20):2497–2509. 10.1089/ten.TEA.2010.0705 PubMed PMID: ; PMCID: PMC3179623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Peault B (2012) The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev 21(8):1299–1308. 10.1089/scd.2011.0200.Epub2011/08/25. PubMed PMID: ; PMCID: 3353742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James AW, Zara JN, Corselli M, Askarinam A, Zhou AM, Hourfar A, Nguyen A, Megerdichian S, Asatrian G, Pang S, Stoker D, Zhang X, Wu B, Ting K, Peault B, Soo C (2012) An abundant perivascular source of stem cells for bone tissue engineering. Stem Cells Transl Med 1(9):673–684. Epub 2012/12/01. 10.5966/sctm.2012-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardy WR, Moldovan NI, Moldovan L, Livak KJ, Datta K, Goswami C, Corselli M, Traktuev DO, Murray IR, Peault B, March K (2017) Transcriptional networks in single perivascular cells sorted from human adipose tissue reveal a hierarchy of mesenchymal stem cells. Stem Cells 35(5):1273–1289. 10.1002/stem.2599 [DOI] [PubMed] [Google Scholar]
- 35.Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Péault B, Rubin JP, Donnenberg AD (2010) Stromal vascular progenitors in adult human adipose tissue. Cytometry A 77(1):22–30. 10.1002/cyto.a.20813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park TS, Gavina M, Chen CW, Sun B, Teng PN, Huard J, Deasy BM, Zimmerlin L, Péault B (2011) Placental perivascular cells for human muscle regeneration. Stem Cells Dev 20(3):451–463. 10.1089/scd.2010.0354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerlach JC, Over P, Turner ME, Thompson RL, Foka HG, Chen WC, Péault B, Gridelli B, Schmelzer E (2012) Perivascular mesenchymal progenitors in human fetal and adult liver. Stem Cells Dev 21(18):3258–3269. 10.1089/scd.2012.0296 [DOI] [PubMed] [Google Scholar]
- 38.Askarinam A, James AW, Zara JN, Goyal R, Corselli M, Pan A, Liang P, Chang L, Rackohn T, Stoker D, Zhang X, Ting K, Peault B, Soo C (2013) Human perivascular stem cells show enhanced osteogenesis and vasculogenesis with Nel-like molecule I protein. Tissue Eng Part A 19(11–12): 1386–1397. 10.1089/ten.TEA.2012.0367 Epub 2013/02/15. PubMed PMID: ; PMCID: 3638559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hindle P, Khan N, Biant L, Peault B (2017) The infrapatellar fat pad as a source of perivascular stem cells with increased chondrogenic potential for regenerative medicine. Stem Cells Transl Med 6(1):77–87. 10.5966/sctm.2016-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stefanska A, Kenyon C, Christian HC, Buckley C, Shaw I, Mullins JJ, Péault B (2016) Human kidney pericytes produce renin. Kidney Int 90(6):1251–1261. 10.1016/j.kint.2016.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eliasberg CD, Dar A, Jensen AR, Murray IR, Hardy WR, Kowalski TJ, Garagozlo CA, Natsuhara KM, Khan AZ, McBride OJ, Cha PI, Kelley BV, Evseenko D, Feeley BT, McAllister DR, Péault B, Petrigliano FA (2017) Perivascular stem cells diminish muscle atrophy following massive rotator cuff tears in a small animal model. J Bone Joint Surg Am 99(4):331–341. 10.2106/JBJS.16.00645 [DOI] [PubMed] [Google Scholar]
- 42.James AW, Zhang X, Crisan M, Hardy WR, Liang P, Meyers CA, Lobo S, Lagishetty V, Childers MK, Asatrian G, Ding C, Yen YH, Zou E, Ting K, Peault B, Soo C (2017) Isolation and characterization of canine perivascular stem/stromal cells for bone tissue engineering. PLoS One 12(5):e0177308. 10.1371/journal.pone.0177308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hindle P, Baily J, Khan N, Biant LC, Simpson AH, Péault B (2016) Perivascular mesenchymal stem cells in sheep: characterization and autologous transplantation in a model of articular cartilage repair. Stem Cells Dev 25(21):1659–1669. 10.1089/scd.2016.0165 Epub 2016 Aug 23 [DOI] [PubMed] [Google Scholar]
- 44.Esteves CL, Sheldrake TA, Mesquita SP, Pesántez JJ, Menghini T, Dawson L, Péault B, Donadeu FX (2017) Isolation and characterization of equine native MSC populations. Stem Cell Res Ther 8(1):80 10.1186/s13287-017-0525-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esteves CL, Sheldrake TA, Dawson L, Menghini T, Rink BE, Amilon K, Khan N, Péault B, Donadeu FX (2017) Equine mesenchymal stromal cells retain a pericyte-like phenotype. Stem Cells Dev 26(13):964–972. 10.1089/scd.2017.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung CG, James AW, Asatrian G, Chang L, Nguyen A, Le K, Bayani G, Lee R, Stoker D, Pang S, Zhang X, Ting K, Peault B, Soo C (2015) Human perivascular stem cell-based bone graft substitute induces rat spinal fusion. Stem Cells Transl Med 4(5):538 10.5966/sctm.2014-0027erratum PubMed PMID: ; PMCID: PMC4414212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee S, Zhang X, Shen J, James AW, Chung CG, Hardy R, Li C, Girgius C, Zhang Y, Stoker D, Wang H, Wu BM, Peault B, Ting K, Soo C (2015) Brief report: human perivascular stem cells and Nel-like Protein-1 synergistically enhance spinal fusion in osteoporotic rats. Stem Cells 33(10):3158–3163. 10.1002/stem.2103 PubMed PMID: ; PMCID: PMC4831713s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reed AA, Joyner CJ, Isefuku S, Brownlow HC, Simpson AH (2003) Vascularity in a new model of atrophic nonunion. J Bone Joint Surg (Br) 85(4):604–610 [DOI] [PubMed] [Google Scholar]
- 49.Bajada S, Marshall MJ, Wright KT, Richardson JB, Johnson WE (2009) Decreased osteogenesis, increased cell senescence and elevated Dickkopf-1 secretion in human fracture non union stromal cells. Bone 45(4):726–735. https://doi.org/10.1016Zj.bone.2009.06.015 [DOI] [PubMed] [Google Scholar]
- 50.Tawonsawatruk T, West CC, Murray IR, Soo C, Péault B, Simpson H (2016) Adipose derived pericytes rescue fractures from a failure of healing – non-union. Sci Rep 6:22779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chapelin F, Khurana A, Moneeb M, Gray Hazard FK, Chan CFR, Nejadnik H, Gratzinger D, Messing S, Erdmann J, Gaur A, Daldrup-Link HE (2018) Tumor formation of adult stem cell transplants in rodent arthritic joints. Mol Imaging Biol. 10.1007/s11307-018-1218-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee HY, Hong IS (2017) Double-edged sword of mesenchymal stem cells: cancer-promoting versus therapeutic potential. Cancer Sci 108(10):1939–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alakpa E, Jayawarna V, Burgess K, West CC, Bakker S, Roy S, Javid N, Fleming S, Lamprou D, Yang J, Miller A, Urquhart A, Frederix P, Hunt N, Peault B, Ulijn RV, Dalby M (2016) Tuneable supramolecular hydrogels for selection of lineage guiding metabolites in stem cell cultures. Chem (Cell) 1:1–22 [Google Scholar]
- 54.Kramann R, Goettsch C, Wongboonsin J, Iwata H, Schneider RK, Kuppe C, Kaesler N, Chang-Panesso M, Machado FG, Gratwohl S, Madhurima K, Hutcheson JD, Jain S, Aikawa E, Humphreys BD (2016) Adventitial MSC-like cells are progenitors of vascular smooth muscle cells and drive vascular calcification in chronic kidney disease. Cell Stem Cell 19(5):628–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caplan AI (2017) Mesenchymal stem cells: time to change the name! Stem Cells Transl Med 6(6):1445–1451. 10.1002/sctm.17-0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dickinson SC, Sutton CA, Williams RL, West CC, Evseenko D, Wu L, Brady K, Pang S, Ferro de Godoy R, Goodship AE, Peault B, Blom AW, Kafienah W, Hollander AP (2017) The Wnt5a receptor ROR2 is a predictive cell surface marker of human mesenchymal stem cells with an enhanced capacity for chondrogenic differentiation. Stem Cells 35(11):2280–2291. 10.1002/stem.2691 [DOI] [PMC free article] [PubMed] [Google Scholar]


