Abstract
Objectives: A significant proportion of non-small cell lung cancer (NSCLC) patients are never-smokers. However, the clinical impact of spirometrically diagnosed chronic obstructive pulmonary disease (COPD) on the prognosis of never-smoking NSCLC has not been evaluated in the context of treatment modalities and other cancer-related factors. In the present study, we evaluated the clinical impact of COPD in non-smoking NSCLC patients, and correlations between COPD and other previously unevaluated clinical variables.
Materials and methods: Lung cancer patients (stages I to IV) diagnosed with NSCLC between January 2008 and December 2015 at six university hospitals were enrolled in the study cohort and retrospectively evaluated. Clinical parameters were compared between spirometrically diagnosed COPD and non-COPD groups. Correlations between COPD status and other variables were evaluated. In order to reduce the effect of potential confounders and selection bias, we performed adjustment for differences in baseline parameters by using propensity score matching (PSM). After PSM, clinical variables were evaluated for their effects on overall survival (OS).
Results: Of the 345 patients enrolled in the study, 277 were categorized as non-COPD and 68 as COPD. Old age, male gender, and wild-type EGFR were significantly correlated with COPD. By univariate analysis of 218 patients in a propensity score matched cohort, not receiving active anticancer treatment, advanced stage, and COPD were significantly associated with shorter OS. Multivariate analysis showed that not receiving active anticancer treatment, advanced cancer stage, and COPD (P=0.044, HR: 1.526, 95% CI: 1.012–2.300) were significant predictors of shorter OS.
Conclusion: In the present study, never-smoker NSCLC patients with COPD had shorter OS times, compared to non-COPD never-smoker NSCLC patients.
Keywords: never smoker, overall survival, risk factor
Introduction
Lung cancer is a major cause of cancer-related deaths worldwide.1 While tobacco smoking is known to be the main cause of lung cancer,2 a significant proportion of patients are never-smokers. In the United States, 10–15% of lung cancer patients are never-smokers,3 and the proportion is much higher in East Asian populations. In Korea, Singapore, and Japan, 32–34% of NSCLC patients have never smoked.4,5,6 Among lung cancer subtypes, the proportion of never-smokers is high in non-small cell lung cancer (NSCLC) patients, particularly in those with adenocarcinomas.7
Compared to NSCLC patients with significant smoking histories, non-smoker NSCLC patients are younger and more likely to be female.8,9 Furthermore, previous publications have reported that survival times are longer for non-smoker NSCLC patients than for smokers with NSCLC,4 and that epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) translocations are more frequent in non-smokers with NSCLC.10
In most cases, tobacco smoking is the major contributing factor in the occurrence of both COPD and lung cancer. However several factors other than cigarette smoking, such as biomass smoke exposure, undiagnosed asthma, radon exposure, second-hand smoke, and occupational history are also reported to be risk factors for COPD, especially among never-smokers.11,12,13 Moreover, family history of lung cancer was reported to be an important predictor of lung cancer in the non-smoking population.14 Nevertheless, few studies have evaluated correlations between COPD and cancer-related factors in non-smoking lung cancer patients.
Several studies have evaluated the predictive value of COPD in lung cancer. However, results differed among study populations. A meta-analysis by Gao et al showed that COPD and emphysema are predictors of poor survival in patients with lung cancer, with shorter overall survival (OS) in lung cancer patients with COPD.15 In EGFR mutation-negative NSCLC, COPD was associated with shorter OS among smokers.16 Other studies, however, found that COPD was not a significant risk factor for OS in lung cancer.2,17,18 Lung cancer-related mortality has been evaluated in a never smoker general population,12 and in a never-smoker cohort of patients with obstructive lung disease.19 The study by Turner et al showed that lung cancer mortality was significantly associated with emphysema in lifelong never smokers.20 Nevertheless, in these previous studies, the clinical impact of COPD on the prognosis for never-smoker NSCLC patients was not adjusted to reflect the issues of whether patients received active cancer treatment, what modalities of treatment were used, the presence of driver mutations, or other cancer-related factors.
We assumed that cancer-related factors would also be associated with spirometrically diagnosed COPD in never-smoker NSCLC patients. Furthermore, we hypothesized that airflow limitation would have a significant clinical impact on the survival of those patients.
In the present study, we evaluated the clinical impact of COPD in non-smoker NSCLC patients, as well as correlations among other clinical variables.
Materials and methods
Patient selection
Lung cancer patients (stages I to IV), diagnosed with NSCLC between January 2008 and December 2015 at seven university hospitals (Seoul St. Mary’s Hospital, Yeouido St. Mary’s Hospital, Incheon St. Mary’s Hospital, Bucheon St. Mary’s Hospital, St. Paul’s Hospital, St. Vincent’s Hospital, and Uijeongbu St. Mary’s Hospital) were consecutively enrolled in the study cohort. All patients included had undergone a baseline pulmonary function test.
Definition of smoker and never-smoker
Both ex-smokers and current smokers with at least 100 lifetime cigarettes were defined as smokers. Patients who smoked fewer than 100 lifetime cigarettes were classified in the never-smoker group.2
Clinicopathological data
Patients’ data including age, sex, histological feature, smoking status, cancer stage, Eastern Cooperative Oncology Group Performance Status Scale (ECOG PS), EGFR mutation status, body mass index (BMI), spirometric measurements, survival time, and modalities of first line treatment were acquired from the electronic medical records. The variables were evaluated for correlations with COPD.
Chemotherapy
First-line chemotherapy included cisplatin or carboplatin in combination with one of the following agents: pemetrexed (for nonsquamous histology), vinorelbine, gemcitabine, or paclitaxel. Patients who received pemetrexed maintenance therapy after induction by the same agent were included in the study. Patient age, performance status, and underlying disease were also taken into consideration with respect to selection of the chemotherapy regimen. Toxicity of chemotherapy was carefully followed, and the regimens were changed or stopped if toxicity occurred. Adverse events (AEs) were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events, NCICTC 3.0. In the event of a Grade 3 AE, the dose was reduced until it had dropped to Grade 0 or 1, and treatment was restarted at one dose level lower. The dose was adjusted if any of the following AEs occurred: (1) absolute neutrophil count nadir <500/mm3 for 4 days or more; (2) neutropenia with fever; (3) thrombocytopenia with a bleeding event or requirement for platelet transfusion; (4) platelet nadir <50,000/mm3 for 4 days or more, or (5) grade 3 or higher non-hematological toxicity other than nausea, vomiting, or alopecia.
Spirometry and definition of COPD
Acquired spirometry values are postbronchodilator values. Spirometry tests were performed by qualified technicians, following the criteria for the standardization of pulmonary function tests recommended by the American Thoracic Society/European Respiratory Society (ATS/ERS) Task Force.21 The Morris reference equation, which is widely used in Asian populations, was applied to calculate normal predictive values for spirometric data.22 According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, the post-bronchodilator fixed criteria [forced expiratory volume in 1 s divided by forced vital capacity (FEV1/FVC)<0.7] was applied to define COPD.
Statistical analysis
Comparisons of continuous variables between COPD and non-COPD groups were performed using unpaired t tests. Pearson’s χ2 test was used to compare categorical variables between the two groups. Logistic regression analysis was performed to evaluate correlations between COPD and other categorical variables. The variables that were statistically significant in univariate analysis were entered into the multiple logistic regression analysis. Odds ratios (ORs) and 95% CIs were estimated.
In order to reduce the effect of potential confounders and selection bias, we performed adjustment for differences in baseline parameters by using propensity score matching (PSM).23 To calculate the propensity score for non-COPD and COPD groups, a logistic regression model was used. And age, sex, cancer stage, BMI, driver mutation, modality of 1st line treatment, ECOG score, and pathology were included in the model. Since the sample size of non-COPD group is more than four times that of COPD group, we tried to make as many match as possible to reduce the loss of information. As a result, 155 patients of non-COPD group were matched against 63 patients of COPD group. The optimal matching algorithm with a caliper size of within 0.25 times the pooled estimate of the common standard deviation of the logits of the propensity score was used to construct a matched-paired sample.
After PSM, Kaplan–Meier survival curves were used to evaluate the OS of patients, and differences were assessed using log-rank tests. The follow-up time for survival analysis was defined as the time (in months) from diagnosis of lung cancer to the date of death or to the date of the last outpatient visit, and OS was defined as the time duration from the date of diagnosis to the date of death.
Independent risk factors for mortality were analyzed using a Cox proportional hazards regression model, after adjustment for confounding variables. Variables that were statistically significant in univariate analysis were entered into the multivariate analysis. Continuous variables including BMI and age were changed to categorical variables for survival analysis: cutoffs for BMI and age were 23 and 65 years, respectively.24,25 Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were estimated. For all tests, P <0.05 was considered to indicate statistical significance. Statistical analyses were performed using SPSS for Windows software (ver. 18.0; SPSS Inc., Chicago, IL, USA). Propensity score matching was performed using the PSMATCH Procedure of Statistical Analysis System (SAS) statistical software package (ver. 9.4, SAS Institute Inc., Cary, NC, USA).
Ethics statement
The study was approved by the ethics committee at each center (XC14OIMI0070). The enrolled hospitals were as follows: Seoul St. Mary’s Hospital, Yeouido St. Mary’s Hospital, Incheon St. Mary’s Hospital, Bucheon St. Mary’s Hospital, St. Paul’s Hospital, St. Vincent’s Hospital, and Uijeongbu St. Mary’s Hospital. The requirement for informed consent was waived due to the retrospective nature of this study. Patient information was anonymized and de-identified before analysis. The conduct of this study was in accordance with the ethical principles of the Declaration of Helsinki.
Results
Patients’ characteristics
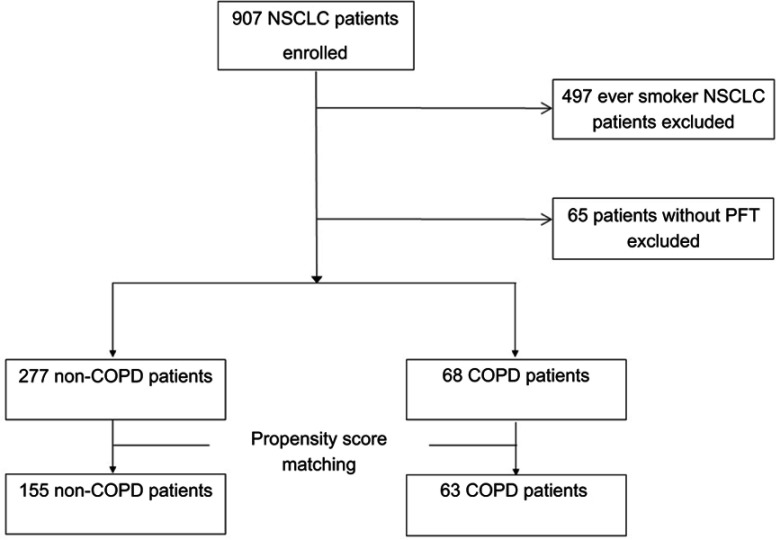
Of the 345 patients enrolled in the study (Figure 1), 277 patients were categorized as non-COPD and 68 patients were categorized as COPD, according to spirometric results from initial pulmonary function tests (PFT). Table 1 shows a comparison of clinical characteristics between the non-COPD and COPD groups. The proportion of male patients was higher in the COPD group (44.1% vs 19.5%), with statistical significance. Median age was higher in the COPD group. Overall cancer stage, T, N, and M stages showed no significant differences between the two groups. Median OS was 29.8 months and 16.7 months for the non-COPD group and the COPD group, respectively (P=0.002). The fraction of patients positive for EGFR mutations was significantly higher in the non-COPD group (P=0.006). More patients received surgery and targeted therapy in the non-COPD group than in the COPD group. No statistically significant difference existed in the proportion of patients who received 2nd line treatment, but more were present in the non-COPD group (48.4% vs 35.3%). More patients with ECOG scores ≥2 were found in the COPD group (P=0.007). Values for FEV1% predicted, FEV1/FVC, and the diffusing capacity of the lung for carbon monoxide (DLCO) were all significantly lower in the COPD group.
Figure 1.
Flowchart of the patients enrolled in the study.
Abbreviations: COPD, chronic obstructive pulmonary disease; NSCLC, non-small cell lung cancer; PFT, pulmonary function test.
Table 1.
Clinical characteristics of subgroups by non-COPD and COPD among never smoker NSCLC (n=345)
| Non-COPD (n=277) (n,%) | COPD (n=68) (n,%) | p-value | |
|---|---|---|---|
| Sex | <0.001 | ||
| Male | 54 (19.5) | 30 (44.1) | |
| Female | 223 (80.5) | 38 (55.9) | |
| Median age (year) | 64.2 (30–92) | 75.2 (48–89) | |
| Stage | 0.783 | ||
| I | 72 (26.1) | 15 (22.1) | |
| II | 23 (8.3) | 5 (7.4) | |
| III | 42 (15.2) | 9 (13.2) | |
| IV | 139 (50.4) | 39 (57.4) | |
| T stage T1/T2/T3/T4 | 68(31.2)/74(33.9)/25(11.5)/51(23.4) | 18(36.7)/13(26.5)/4(8.2)/14(28.6) | 0.594 |
| N stage N0/N1/N2/N3 | 91(41.6)/26(11.9)/44(20.1)/58(26.5) | 19(38.8)/3(6.1)/11(22.4)/16(32.7) | 0.581 |
| M stage M0/M1 | 135(50.4)/133(49.6) | 29(46.0)/34(54.0) | 0.535 |
| Median overall survival (months) | 29.8 (22.2–37.3) | 16.7 (11.2–22.2) | 0.002 |
| BMI (kg/m2) | 23.5±3.5 | 22.9±3.5 | 0.233 |
| EGFR mutation* | 129 (49.0) | 20 (30.3) | 0.006 |
| Number tested (%) | 263 (94.9) | 66 (97.1) | |
| ALK mutation* | 15 (6.2) | 3 (4.7) | 0.638 |
| Number tested (%) | 240 (86.6) | 64 (94.1) | |
| 1st line treatment | 0.038 | ||
| Surgery | 104 (37.7) | 16 (23.9) | |
| Chemotherapy | 94 (34.1) | 27 (40.3) | |
| Targeted therapy | 53 (19.2) | 10 (14.9) | |
| Radiotherapy alone | 3 (1.1) | 2 (3.0) | |
| Supportive care | 22 (8.0) | 12 (17.9) | |
| Undergone 2nd line treatment | 134 (48.4) | 24 (35.3) | 0.052 |
| ECOG | 0.007 | ||
| 0–1 | 244 (88.7) | 51 (76.1) | |
| ≥2 | 31 (11.3) | 16 (23.9) | |
| Pathology | 0.302 | ||
| Squamous | 12 (4.3) | 5 (7.4) | |
| Non-squamous | 265 (95.7) | 63 (92.6) | |
| FEV1 (liter) | 2.01±0.69 | 1.59±0.56 | <0.001 |
| FEV1 (% predicted) | 94.2±23.2 | 78.4±20.2 | <0.001 |
| FVC (liter) | 2.53±0.76 | 2.56±0.85 | 0.786 |
| FVC (% predicted) | 88.8±21.3 | 90.1±21.5 | 0.667 |
| FEV1/FVC (% predicted) |
78.7±6.9 | 62.5±8.0 | <0.001 |
| DLCO (abs) | 14.2±7.1 | 12.2±5.3 | 0.032 |
| DLCO (%) | 80.0±21.0 | 74.6±26.6 | 0.085 |
Notes: *The number in the brackets indicates a proportion of patients with positive mutation among the tested patients.
Abbreviations: ALK: anaplastic lymphoma kinase; BMI: body mass index; COPD: chronic obstructive pulmonary disease; DLCO: diffusing capacity of the lung for carbon monoxide; ECOG: Eastern Cooperative Oncology Group; EGFR: epidermal growth factor receptor; FEV1; forced expiratory volume in 1 s; FVC: forced vital capacity; OS: overall survival.
Risk factors for COPD in non-smokers with NSCLC
Age, gender, histology, EGFR mutation status, ECOG status, cancer stage, BMI, and whether patients received active first line treatment were evaluated for correlations with COPD. Old age, male gender, wild-type EGFR, and poor ECOG showed significant correlations from univariate logistic regression. These significant variables were entered into multiple logistic regression. Old age, male gender, and wild-type EGFR showed significant correlations with COPD (OR: 2.255, 95% CI: 1.206–4.217, OR: 3.202, 95% CI: 1.756–5.839, and OR: 1.939, 95% CI: 1.047–3.591, respectively) (Table 2).
Table 2.
Risk factors for nonsmoker COPD among NSCLC patients (univariate and multivariate analysis)
| Univariate | Multiple | |||||
|---|---|---|---|---|---|---|
| Characteristics | OR | 95% CI | P | OR | 95% CI | P |
| Male/Female | 3.791 | 1.856–5.727 | <0.001 | 3.202 | 1.756–5.839 | <0.001 |
| Age (≤65/>65) | 2.752 | 1.541–4.914 | 0.001 | 2.255 | 1.206–4.217 | 0.011 |
| ECOG (0–1/≥2) | 2.469 | 1.258–4.848 | 0.009 | 1.767 | 0.850–3.671 | 0.127 |
| EGFR mutation (negative/positive) | 0.452 | 0.253–0.805 | 0.007 | 0.516 | 0.278–0.955 | 0.035 |
| Histology (nonsquamous/squamous) | 1.753 | 0.596–5.155 | 0.308 | - | - | - |
| BMI (≥23/<23) | 1.332 | 0.780–2.274 | 0.294 | - | - | - |
| Stage (I-IIIA/IIIB-IV) | 1.573 | 0.902–2.742 | 0.110 | - | - | - |
Abbreviations: BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; OR, odds ratio.
Baseline characteristics of the propensity score matched cohort
In the propensity score matched cohort, there were no significant differences between the groups regarding age, cancer stage, BMI, EGFR and ALK mutations, 1st line treatment, ECOG, and pathology. The proportion of male patients was significantly higher in the COPD group than in the non-COPD group (P=0.015). Mean values of FEV1, FEV1/FVC and DLCO (%) were significantly decreased in the COPD group than in the non-COPD group (Table 3).
Table 3.
Comparison of clinical characteristics between the non-COPD and COPD groups in never smoker NSCLC after propensity score matching
| Non-COPD (n=155) (n,%) | COPD (n=63) (n,%) | p-value | |
|---|---|---|---|
| Sex | 0.015 | ||
| Male | 47 (30.3) | 30 (47.6) | |
| Female | 108 (69.68) | 33 (52.4) | |
| Median age (year) | 69 (30–92) | 75 (48–89) | |
| Stage | 0.905 | ||
| I | 33 (21.3) | 14 (22.2) | |
| II | 15 (9.7) | 5 (7.9) | |
| III | 27 (17.4) | 9 (14.3) | |
| IV | 80 (51.6) | 35 (55.6) | |
| T stage T1/T2/T3/T4 | 30(24.6)/42(34.4)/18(14.8)/32(26.2) | 15(34.1)/12(27.3)/4(9.1)/13(29.6) | 0.475 |
| N stage N0/N1/N2/N3 | 45(36.9)/18(14.8)/23(18.9)/36(29.5) | 17(38.6)/3(6.8)/11(25.0)/13(29.6) | 0.526 |
| M stage M0/M1 | 74(49.3)/76(50.7) | 28(48.3)/30(51.7) | 0.891 |
| Median overall survival (months) | 14.7 (0.4–70.4) | 13.7 (0.1–63.9) | 0.287 |
| BMI (kg/m2) | 23.4±3.6 | 22.7±3.2 | 0.197 |
| EGFR mutation | 60 (38.7) | 19 (30.2) | 0.234 |
| ALK mutation | 10 (7.3) | 3 (4.8) | 0.523 |
| 1st line treatment | 0.357 | ||
| Surgery | 53 (34.2) | 16 (25.4) | |
| Chemotherapy | 52 (33.6) | 25 (39.7) | |
| Targeted therapy | 28 (18.1) | 9 (14.3) | |
| Radiotherapy alone | 2 (1.3) | 2 (3.2) | |
| Supportive care | 17 (11.0) | 11 (17.5) | |
| Undergone 2nd line treatment | 61 (39.4) | 24 (38.1) | 0.863 |
| ECOG | 0.269 | ||
| 0–1 | 126 (81.3) | 47 (74.6) | |
| ≥2 | 29 (18.7) | 16 (25.4) | |
| Pathology | 0.561 | ||
| Squamous | 9 (5.8) | 5 (7.9) | |
| Non-squamous | 146 (94.2) | 58 (92.1) | |
| FEV1 (liter) | 1.9±0.8 | 1.6±0.6 | 0.001 |
| FEV1 (% predicted) | 95.8±23.2 | 77.6±19.6 | <0.001 |
| FVC (liter) | 2.5±0.9 | 2.6±0.9 | 0.405 |
| FVC (% predicted) | 88.3±21.5 | 90.3±22.0 | 0.534 |
| FEV1/FVC (% predicted) |
78.4±5.5 | 62.3±8.1 | <0.001 |
| DLCO (abs) | 14.1±9.0 | 12.3±5.5 | 0.159 |
| DLCO (%) | 80.7±21.7 | 73.4±26.2 | 0.042 |
Abbreviations: ALK: anaplastic lymphoma kinase; BMI: body mass index; COPD: chronic obstructive pulmonary disease; DLCO: diffusing capacity of the lung for carbon monoxide; ECOG: Eastern Cooperative Oncology Group; EGFR: epidermal growth factor receptor; FEV1; forced expiratory volume in 1 s; FVC: forced vital capacity; OS: overall survival.
Association of OS and COPD in the propensity score matched never-smoker NSCLC
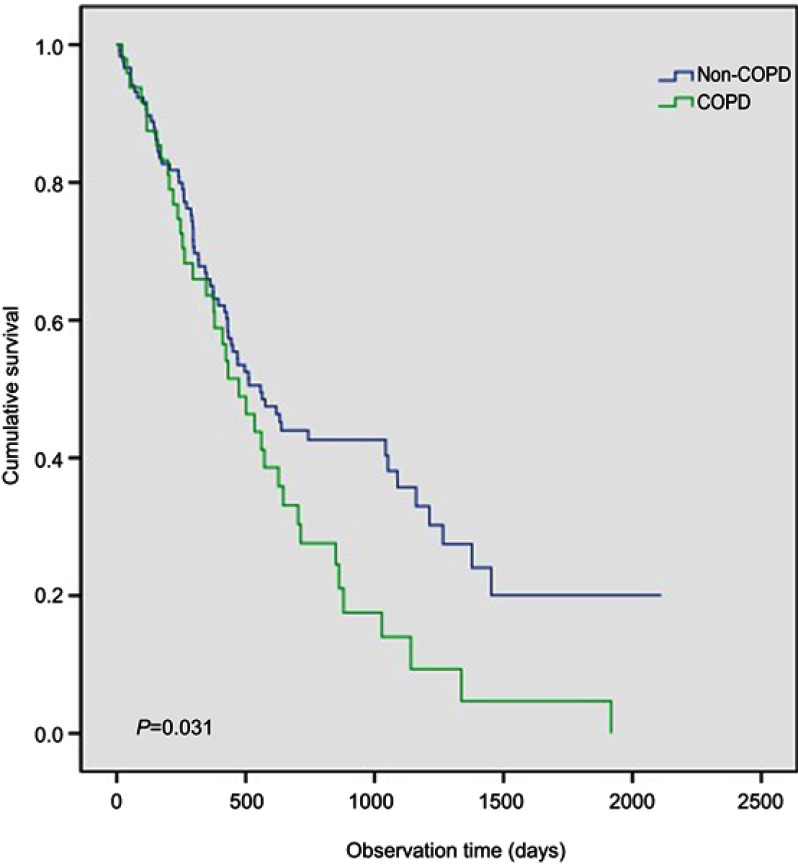
Age, gender, histology, EGFR mutation status, ECOG status, cancer stage, BMI, whether patients received active first-line treatment, and COPD were evaluated for their predictive value for OS in the propensity score matched cohort. From the univariate analysis, not receiving active anticancer treatment, advanced stage, and COPD (Figure 2) were significant factors associated with shorter OS (P=0.031).
Figure 2.
Comparison of overall survival between the COPD and non-COPD group in the never-smoker non-small cell lung cancer patients after propensity score matching.
The variables significant from the univariate analyses, age, and sex were included in a multivariate analysis. Not receiving active anticancer treatment, advanced stage, and COPD were significant factors for shorter OS (P=0.014, HR:2.271, 95% CI: 1.177–4.380; P=0.047, HR: 1.585, 95% CI: 1.006–2.496; P=0.044, HR:1.526, 95% CI: 1.012–2.300, respectively) (Table 4).
Table 4.
Variables analyses for mortality in the 218 patients with NSCLC after propensity score matching
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Characteristics | P | HR | 95% CI | P | HR | 95% CI |
| Age (≤65/>65) | 0.218 | 1.297 | 0.858–1.961 | 0.139 | 1.374 | 0.902–2.091 |
| Male/Female | 0.924 | 1.020 | 0.682–1.524 | 0.857 | 1.039 | 0.685–1.575 |
| Histology (nonsquamous/squamous) | 0.789 | 0.910 | 0.458–1.811 | |||
| EGFR mutation (positive/negative) | 0.114 | 1.393 | 0.923–2.102 | |||
| ECOG (0–1/≥2) | 0.220 | 1.332 | 0.843–2.104 | |||
| First line treatment (active/supportive) | 0.019 | 2.183 | 1.135–4.200 | 0.014 | 2.271 | 1.177–4.380 |
| Stage (I-IIIA/IIIB-IV) | 0.033 | 1.625 | 1.040–2.540 | 0.047 | 1.585 | 1.006–2.496 |
| COPD (non-COPD/COPD) | 0.032 | 1.545 | 1.037–2.302 | 0.044 | 1.526 | 1.012–2.300 |
| BMI (≥23/<23) | 0.587 | 1.113 | 0.756–1.638 | - | - | - |
Abbreviations: BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; HR, hazard ratio; OS, overall survival.
Discussion
The present study has evaluated the clinical impact of COPD in never-smoker NSCLC patients. COPD was a significant predictor of shorter OS in all-stage NSCLC. We performed propensity score matching to minimize effects of confounding factors before survival analysis.
The impact of COPD on OS in our study should be discussed from various perspectives, considering that all the study patients were never-smokers. In the COPD group, decreased DLCO suggests that more emphysematous lungs may be present. In a previous study of never-smokers, lung cancer mortality was significantly associated with both emphysema and chronic bronchitis.20 Moreover, a study by de Torres et al showed that DLCO <60% was an independent risk factor for lung cancer-related mortality among patients with COPD,26 while FEV1 was a significant prognostic factor in advanced NSCLC.27 We assume that decreased lung function and emphysema contributed to the shorter survival time in the COPD group.
Another explanation for shorter survival with COPD is a decreased effect of anticancer treatment. Increased DNA damage results from chronic inflammation in COPD, which in turn, leads to a greater probability of lung carcinogenesis,28,29 and the pro-inflammatory microenvironment of COPD reduces treatment effects in lung cancer.30 These features in the molecular background contribute to a unique and more aggressive phenotype of lung cancer in NSCLC patients with COPD. Our study results show that this negative impact of COPD on prognosis in NSCLC is also present in the never-smoker population.15,16
Our initial hypothesis was that both environmental factors and cancer-related factors contributed to diagnosis of COPD among never-smoker NSCLC patients. Lung parenchymal distortion or extrinsic compression of the bronchial tree from a lung mass could be manifested as spirometrically diagnosed COPD. However, this scenario was not supported by our findings. Overall cancer staging had no significant effect on the diagnosis of COPD in our analyses, nor was T, N, or M stage significantly associated with COPD. Thus, we assume that initial patient factors, such as airflow limitation from non-tobacco causes or natural aging, have more significant associations with the diagnosis of COPD in never-smoker NSCLC patients.
Besides tobacco smoking, biomass fuel burning, air pollution, or undiagnosed asthma could have contributed to COPD among never-smoker NSCLC patients.11,31,32 However, data on exposure history, to pollution or to indoor cooking, were not acquired from this study’s patients, and could not be analyzed.
From the risk-factor association analysis in our study patients, old age, male gender, and wild-type EGFR status were significant factors related to COPD. Natural aging could have contributed to the occurrence and progression of COPD. Previous studies have shown that, with increasing age, the ratio of FEV1/FVC tends to decline, and that decreases in chest wall compliance and in the ability to eliminate carbon dioxide follow these decreases in lung function.33,34 Whether the application of a fixed ratio in the diagnosis of COPD is appropriate is discussed below.
Male gender was a risk factor for COPD in never-smoker NSCLC patients. This is consistent with previous studies, which found that male gender is an associated risk factor for non-smoker COPD among non-cancer patients.35,36,37 This gender difference could be due to differences in lifestyle, or to occupation-related factors,35,37 but it remains unclear why male gender was a significant factor related to the occurrence of COPD in our study.
One could argue that diagnosis of COPD according to GOLD criteria (FEV1/FVC <0.7) might not have been appropriate for this study. Some clinicians have asserted that applying the FEV1/FVC <0.7 criterion in screening for COPD could result in overdiagnosis of airflow limitation among elderly patients.38 Several studies have shown a progressive reduction in FEV1, and a reduction in FEV1/FVC ratio, that were associated with aging.39,40 Thus, it is possible that some of our COPD cases diagnosed from spirometry reflected natural aging.
Another point to consider is that the prevalence of EGFR mutation was lower in our COPD group than in the non-COPD group. EGFR mutation frequency is higher in never-smokers than in patients with significant smoking history.10 Our results are consistent with those of a previous study, which also showed that EGFR mutation was significantly less common in a group of COPD patients comprising never-smokers.41 Thus, even in non-smoking patients, the prevalence of EGFR mutations can differ according to COPD status.
Several limitations exist in our study. First, patients without spirometric data were excluded, and selection bias could have occurred. However, the number of patients excluded came to less than 10%. Second, no occupational history or air pollutant exposure history had been acquired for data analysis, making it difficult to confirm how many patients had been exposed to non-tobacco factors. Finally, selection bias may be present due to the retrospective nature of the study, although we enrolled patients from multiple treatment centers, in consecutive order.
Conclusions
In the present study, never-smoker NSCLC patients with COPD had shorter OS than did non-COPD NSCLC patients. Future studies of larger populations, collecting detailed occupational histories, will be necessary to evaluate full risk factors associated with spirometrically diagnosed COPD among never-smoker patients with NSCLC.
Acknowledgments
We want to acknowledge HyunKyung Park (CC&I) for assistance with statistical analysis.
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see:
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Daniels MG, Bowman RV, Yang IA, Govindan R, Fong KM. An emerging place for lung cancer genomics in 2013. J Thorac Dis. 2013;5(Suppl 5):S491–497. doi: 10.3978/j.issn.2072-1439.2013.10.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SJ, Lee J, Park YS, et al. Impact of chronic obstructive pulmonary disease on the mortality of patients with non-small-cell lung cancer. J Thorac Oncol. 2014;9(6):812–817. doi: 10.1097/JTO.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 3.The 2004 United States Surgeon General’s report: the health consequences of smoking. N S W Public Health Bull. 2004;15(5–6):107. [PubMed] [Google Scholar]
- 4.Cho J, Choi SM, Lee J, et al. Proportion and clinical features of never-smokers with non-small cell lung cancer. Chin J Cancer. 2017;36(1):20. doi: 10.1186/s40880-017-0187-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yano T, Miura N, Takenaka T, et al. Never-smoking nonsmall cell lung cancer as a separate entity: clinicopathologic features and survival. Cancer. 2008;113(5):1012–1018. doi: 10.1002/cncr.23679 [DOI] [PubMed] [Google Scholar]
- 6.Toh CK, Gao F, Lim WT, et al. Never-smokers with lung cancer: epidemiologic evidence of a distinct disease entity. J Clin Oncol. 2006;24(15):2245–2251. doi: 10.1200/JCO.2005.04.8033 [DOI] [PubMed] [Google Scholar]
- 7.Thu KL, Vucic EA, Chari R, et al. Lung adenocarcinoma of never smokers and smokers harbor differential regions of genetic alteration and exhibit different levels of genomic instability. PLoS One. 2012;7(3):e33003. doi: 10.1371/journal.pone.0033003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skarin AT, Herbst RS, Leong TL, Bailey A, Sugarbaker D. Lung cancer in patients under age 40. Lung Cancer. 2001;32(3):255–264. [DOI] [PubMed] [Google Scholar]
- 9.Kuo CW, Chen YM, Chao JY, Tsai CM, Perng RP. Non-small cell lung cancer in very young and very old patients. Chest. 2000;117(2):354–357. [DOI] [PubMed] [Google Scholar]
- 10.Saito S, Espinoza-Mercado F, Liu H, Sata N, Cui X, Soukiasian HJ. Current status of research and treatment for non-small cell lung cancer in never-smoking females. Cancer Biol Ther. 2017;18(6):359–368. doi: 10.1080/15384047.2017.1323580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva GE, Sherrill DL, Guerra S, Barbee RA. Asthma as a risk factor for COPD in a longitudinal study. Chest. 2004;126(1):59–65. doi: 10.1378/chest.126.1.59 [DOI] [PubMed] [Google Scholar]
- 12.Thun MJ, Henley SJ, Burns D, Jemal A, Shanks TG, Calle EE. Lung cancer death rates in lifelong nonsmokers. J Natl Cancer Inst. 2006;98(10):691–699. doi: 10.1093/jnci/djj187 [DOI] [PubMed] [Google Scholar]
- 13.Hagstad S, Bjerg A, Ekerljung L, et al. Passive smoking exposure is associated with increased risk of COPD in never smokers. Chest. 2014;145(6):1298–1304. doi: 10.1378/chest.13-1349 [DOI] [PubMed] [Google Scholar]
- 14.Lin KF, Wu HF, Huang WC, Tang PL, Wu MT, Wu FZ. Propensity score analysis of lung cancer risk in a population with high prevalence of non-smoking related lung cancer. BMC Pulm Med. 2017;17(1):120. doi: 10.1186/s12890-017-0500-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao YH, Guan WJ, Liu Q, et al. Impact of COPD and emphysema on survival of patients with lung cancer: a meta-analysis of observational studies. Respirology. 2016;21(2):269–279. doi: 10.1111/resp.12661 [DOI] [PubMed] [Google Scholar]
- 16.Lim JU, Yeo CD, Rhee CK, et al. Overall survival of driver mutation-negative non-small cell lung cancer patients with COPD under chemotherapy compared to non-COPD non-small cell lung cancer patients. Int J Chron Obstruct Pulmon Dis. 2018;13:2139–2146. doi: 10.2147/COPD.S167372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izquierdo JL, Resano P, El Hachem A, Graziani D, Almonacid C, Sanchez IM. Impact of COPD in patients with lung cancer and advanced disease treated with chemotherapy and/or tyrosine kinase inhibitors. Int J Chron Obstruct Pulmon Dis. 2014;9:1053–1058. doi: 10.2147/COPD.S68766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omote N, Hashimoto N, Morise M, et al. Impact of mild to moderate COPD on feasibility and prognosis in non-small cell lung cancer patients who received chemotherapy. Int J Chron Obstruct Pulmon Dis. 2017;12:3541–3547. doi: 10.2147/COPD.S149456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung CC, Lam TH, Yew WW, et al. Obstructive lung disease does not increase lung cancer mortality among female never-smokers in Hong Kong. Int J Tuberc Lung Dis. 2012;16(4):546–552. doi: 10.5588/ijtld.11.0573 [DOI] [PubMed] [Google Scholar]
- 20.Turner MC, Chen Y, Krewski D, Calle EE, Thun MJ. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med. 2007;176(3):285–290. doi: 10.1164/rccm.200612-1792OC [DOI] [PubMed] [Google Scholar]
- 21.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 22.Morris JF, Koski A, Johnson LC. Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis. 1971;103(1):57–67. doi: 10.1164/arrd.1971.103.1.57 [DOI] [PubMed] [Google Scholar]
- 23.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281. [DOI] [PubMed] [Google Scholar]
- 24.Lim JU, Lee JH, Kim JS, et al. Comparison of World Health Organization and Asia-Pacific body mass index classifications in COPD patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2465–2475. doi: 10.2147/COPD.S141295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajan SS, Cai Y, Yi M, Tsai CL, Du XL. Use of hematopoietic growth factors in elderly lung cancer patients receiving chemotherapy: a SEER-medicare-based study. American Journal of Clinical Oncology. 2017;40(1):66–74. doi: 10.1097/COC.0000000000000104 [DOI] [PubMed] [Google Scholar]
- 26.de-Torres JP, Marin JM, Casanova C, et al. Identification of COPD patients at high risk for lung cancer mortality using the COPD-LUCSS-DLCO. Chest. 2016;149(4):936–942. doi: 10.1378/chest.15-1868 [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Song EM, Sim YS, Ryu YJ, Chang JH. Forced expiratory volume in one second as a prognostic factor in advanced non-small cell lung cancer. J Thorac Oncol. 2011;6(2):305–309. doi: 10.1097/JTO.0b013e318201884b [DOI] [PubMed] [Google Scholar]
- 28.Caramori G, Adcock IM, Casolari P, et al. Unbalanced oxidant-induced DNA damage and repair in COPD: a link towards lung cancer. Thorax. 2011;66(6):521–527. doi: 10.1136/thx.2010.156448 [DOI] [PubMed] [Google Scholar]
- 29.Sohal SS, Reid D, Soltani A, et al. Evaluation of epithelial mesenchymal transition in patients with chronic obstructive pulmonary disease. Respir Res. 2011;12:130. doi: 10.1186/1465-9921-12-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekine Y, Hata A, Koh E, Hiroshima K. Lung carcinogenesis from chronic obstructive pulmonary disease: characteristics of lung cancer from COPD and contribution of signal transducers and lung stem cells in the inflammatory microenvironment. Gen Thorac Cardiovasc Surg. 2014;62(7):415–421. doi: 10.1007/s11748-014-0386-x [DOI] [PubMed] [Google Scholar]
- 31.Camp PG, Ramirez-Venegas A, Sansores RH, et al. COPD phenotypes in biomass smoke- versus tobacco smoke-exposed Mexican women. Eur Respir J. 2014;43(3):725–734. doi: 10.1183/09031936.00206112 [DOI] [PubMed] [Google Scholar]
- 32.Po JY, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax. 2011;66(3):232–239. doi: 10.1136/thx.2010.147884 [DOI] [PubMed] [Google Scholar]
- 33.Polkey MI, Harris ML, Hughes PD, et al. The contractile properties of the elderly human diaphragm. Am J Respir Crit Care Med. 1997;155(5):1560–1564. doi: 10.1164/ajrccm.155.5.9154857 [DOI] [PubMed] [Google Scholar]
- 34.MacNee W. Is chronic obstructive pulmonary disease an accelerated aging disease? Ann Am Thorac Soc. 2016;13 Suppl 5(Supplement_5):S429–S437. doi: 10.1513/AnnalsATS.201602-124AW [DOI] [PubMed] [Google Scholar]
- 35.Lee SH, Hwang ED, Lim JE, et al. The risk factors and characteristics of COPD among nonsmokers in Korea: an analysis of KNHANES IV and V. Lung. 2016;194(3):353–361. doi: 10.1007/s00408-016-9871-6 [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Wang C, Yao W, et al. COPD in Chinese nonsmokers. Eur Respir J. 2009;33(3):509–518. doi: 10.1183/09031936.00084408 [DOI] [PubMed] [Google Scholar]
- 37.Lamprecht B, McBurnie MA, Vollmer WM, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139(4):752–763. doi: 10.1378/chest.10-1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colak Y, Afzal S, Nordestgaard BG, Vestbo J, Lange P. Young and middle-aged adults with airflow limitation according to lower limit of normal but not fixed ratio have high morbidity and poor survival: a population-based prospective cohort study. Eur Respir J. 2018;51(3):1702681. doi: 10.1183/13993003.02681-2017 [DOI] [PubMed] [Google Scholar]
- 39.Tolep K, Kelsen SG. Effect of aging on respiratory skeletal muscles. Clin Chest Med. 1993;14(3):363–378. [PubMed] [Google Scholar]
- 40.Janssens JP. Aging of the respiratory system: impact on pulmonary function tests and adaptation to exertion. Clin Chest Med. 2005;26(3):469–484, vi-vii. doi: 10.1016/j.ccm.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 41.Lim JU, Yeo CD, Rhee CK, et al. Chronic obstructive pulmonary disease-related non-small-cell lung cancer exhibits a low prevalence of EGFR and ALK driver mutations. PLoS One. 2015;10(11):e0142306. doi: 10.1371/journal.pone.0142306 [DOI] [PMC free article] [PubMed] [Google Scholar]