Abstract
Background
Ovarian carcinomas presenting homologous recombination deficiency (HRD), which is observed in about 50% of cases, are more sensitive to platinum and PARP inhibitor therapies. Although platinum resistant disease has a low chance to be responsive to platinum-based chemotherapy, a set of patients is retreated with platinum and some of them are responsive. In this study, we evaluated copy number alterations, HR gene mutations and HR deficiency scores in ovarian cancer patients with prolonged platinum sensitivity.
Methods
In this retrospective study (2005 to 2014), we selected 31 patients with platinum resistant ovarian cancer retreated with platinum therapy. Copy number alterations and HR scores were evaluated using the OncoScan® FFPE platform in 15 cases. The mutational profile of 24 genes was investigated by targeted-NGS.
Results
The median values of the four HRD scores were higher in responders (LOH = 15, LST = 28, tAI = 33, CS = 84) compared with non-responders (LOH = 7.5, LST = 17.5, tAI = 23, CS = 47). Patients with high LOH, LST, tAI and CS scores had better response rates, although these differences were not statistically significant. Response rate to platinum retreatment was 22% in patients with CCNE1 gains and 83.5% in patients with no CCNE1 gains (p = 0.041). Furthermore, response rate was 54.5% in patients with RB1 loss and 25% in patients without RB1 loss (p = 0.569). Patients with CCNE1 gains showed a worse progression free survival (PFS = 11.1 months vs 3.7 months; p = 0.008) and a shorter overall survival (OS = 39.3 months vs 7.1 months; p = 0.007) in comparison with patients with no CCNE1 gains. Patients with RB1 loss had better PFS (9.0 months vs 2.6 months; p = 0.093) and OS (27.4 months vs 3.6 months; p = 0.025) compared with cases with no RB1 loss. Four tumor samples were BRCA mutated and tumor mutations were not associated with response to treatment.
Conclusions
HR deficiency was found in 60% of our cases and HRD medium values were higher in responders than in non-responders. Despite the small number of patients tested, CCNE1 gain and RB1 loss discriminate patients with tumors extremely sensitive to platinum retreatment.
Electronic supplementary material
The online version of this article (10.1186/s12885-019-5622-4) contains supplementary material, which is available to authorized users.
Keywords: Ovarian cancer, Homologous recombination deficiency, Target-next generation sequencing, Copy number alterations, Treatment response, CCNE1 gains, RB1 loss
Background
Ovarian cancer, the most lethal gynecologic cancer, is expected to account for 14,700 deaths in the USA in 2018 [1]. High grade serous carcinoma (HGSC), the most frequent histological type [2], is molecularly characterized by a few recurrent mutations, including in TP53 (almost all tumors) and BRCA1 or BRC2 genes. HGSCs present high genomic instability with copy number alterations (CNA) affecting a large fraction of the genome [3]. Approximately 50% of these tumors are characterized by homologous recombination (HR) deficiency, which has been associated with BRCA1 or BRCA2 germline or somatic mutations (20 and 5% of cases, respectively), BRCA1 promoter methylation (10% of cases), additional mutations in HR repair pathway genes and CNA in their regulators (PTEN and EMSY) [3].
Patients carrying tumors with BRCA mutation tend to have elevated sensitivity to platinum-based chemotherapy [4] and PARP inhibitors [5–8]. These cases have shown a better medium term prognosis [9] even if the cure rate and long term prognosis is unaltered [10]. Secondary mutations have been associated with resistance to PARP inhibitors [11, 12].
Two strategies have been used to identify tumors with HR deficiency or alterations in genes involved in the DNA repair system other than BRCA1 and BRCA2 mutations. The first approach is to identify mutations in genes related to HR pathway [13], based on next generation sequencing (NGS), which has become feasible with the fast development of sequencing technologies. The second is the identification of “genomic scars”, which are supposed to be a functional consequence of HR deficiency independently of its cause [14–17]. Clinical trials revealed that even patients with no HR deficiency, evaluated by two different HR deficiency scores, can achieve response to PARP inhibitors [8, 13]. These outcomes could be explained by other mechanisms of action than synthetic lethality of the HR pathway deficiency or failure in identifying the HR defect, or both.
In addition to the genomic scars of HR deficiency, gains or losses involving specific genes have also been associated with response to therapy in ovarian cancer [18]. Cyclin E1 and RB1 are cell-cycle proteins associated with the G1-S phase cell-cycle transition. CCNE1 copy number gain is described in about 20% of HGSC and is seemingly rare in BRCA mutated tumors [3]. Tumors with CCNE1 copy number gains are more resistant to platinum therapy [19] while RB1 loss are associated with high sensitivity to platinum therapy [20, 21].
Platinum resistant disease has a low chance to be responsive to platinum-based chemotherapy. The standard treatment is monotherapy using different drugs than platinum salts [2]. In daily clinical practice and despite of the resistant profile, a set of patients is retreated with platinum therapy and some of them are responsive to platinum retreatment [22].
In this study, we sought to evaluate the association of HR pathway mutations, HR deficiency scores and CCNE1 and RB1 CNA with response to platinum retreatment in ovarian cancer patients in the platinum-resistant setting.
Methods
Patients
From 2005 to 2014, 405 patients with ovarian carcinoma were treated at AC Camargo Cancer Center, São Paulo, Brazil. Thirty-five of them presented platinum resistant recurrence and were retreated with platinum therapy. Patients with unavailable data regarding the platinum retreatment were excluded (4 patients) and a retrospective review of the medical records was performed (Additional file 1). Based on the quantity and quality of tumor DNA, 15 of 31 cases were selected for SNP array (OncoScan® FFPE, Thermo Fisher Scientific, Waltham, MA, USA) analyses, and 11 of them were also evaluated by targeted-next generation sequencing. Nine patients had the primary tumor naive of treatment, five patients had the tumor sample collected at platinum resistant recurrence, and one had the tumor sample collected at platinum sensitive recurrence. The study was conducted in accordance with the Declaration of Helsinki ethical guidelines and approved by the institutional Ethics Committee (CEP# 1933/14).
Clinical data
Clinical features were retrieved from the medical records including age at diagnosis of platinum resistant recurrence, tumor histological subtype, family or personal history of ovarian and breast cancer, number of previous treatment lines, platinum free interval (PFI), and type of chemotherapy associated to platinum (Table 1).
Table 1.
Clinical features of 31 patients with ovarian cancer who had previous platinum resistant relapse and were retreated with platinum
| Clinical Characteristics | Number of cases (%) |
|---|---|
| Age (years old) | |
| < 65 | 21 (67.7) |
| > 65 | 10 (32.3) |
| Histology | |
| High grade serous carcinoma | 21 (67.7) |
| Endometrioid | 1 (3.2) |
| Clear cell carcinoma | 1 (3.2) |
| Undifferentiated carcinoma | 3 (9.7) |
| Carcinosarcoma | 1 (3.2) |
| Mixed | 1 (3.2) |
| Family history of ovarian or breast cancer | |
| No | 19 (61.3) |
| Yes | 9 (29.0) |
| Number of previous treatment lines | |
| 2 | 10 (32.3) |
| 3 | 7 (22.6) |
| 4 | 4 (12.9) |
| 5 | 6 (19.4) |
| 6 | 3 (9.7) |
| 8 | 1 (3.2) |
| Platinum free interval (months) | |
| < 12 | 21 (67.7) |
| > 12 | 10 (32.3) |
| Primary platinum resistance* | |
| No | 12 (38.7) |
| Yes | 19 (61.3) |
| Chemotherapy with platinum rechallenge | |
| Platinum + taxane | 15 (48.4) |
| Platinum + gemcitabina | 13 (41.9) |
| Platinum + doxorubicin | 1 (3.2) |
| Platinum + ifosfamide | 1 (3.2) |
| Monotherapy | 1 (3.2) |
*Primary platinum resistant disease was defined as a first recurrence with a platinum free interval less than 6 months. Patients without primary platinum resistant recurrence, presented platinum resistant disease at second or later recurrences
Recurrence was defined according to the GCIG (Gynecological Cancer Intergroup) criteria after the analysis of RECIST (Response Evaluation Criteria in Solid Tumors) [23, 24]. The CA125 levels were extracted from the medical records. The date of the earliest event was considered for progression. The recurrence detected within 6 months after the last platinum infusion was defined as platinum resistant recurrence. All recurrences that followed this first platinum resistant recurrence were also considered platinum resistant. Progression-free survival (PFS) was defined as the interval between the date of the beginning of the platinum retreatment and disease progression or death by any cause. Overall survival (OS) was defined as the interval between the dates of the beginning of the platinum retreatment and death by any cause. The interval between the date of the last platinum compound infusion and the disease progression that preceded platinum retreatment was used to define the platinum-free interval (PFI).
The CA125 expression levels and the response to platinum retreatment data were retrieved from the medical records. The image reports were also collected. GCIG criteria were used to evaluate RECIST and CA125 data [23, 24]. In accordance, each case was categorized as having “response” (complete or partial response) or “no response” (stable disease or disease progression).
DNA extraction
Ten μm paraffin embedded tissue sections were deparaffinized with xylene, washed with descending concentrations of ethanol and water ultra-pure sterile. DNA was extracted using QIAamp DNA FFPE Tissue Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. DNA integrity was evaluated using Agilent Genomic DNA ScreenTape (Agilent Techologies, Santa Clara, USA) and quantified using a Qubit Fluorometer (Life Technologies, Carlsbad, CA).
OncoScan assay
The OncoScan® FFPE platform (Thermo Fisher Scientific) allows the identification of over 200,000 SNPs and the detection of 74 somatic mutations in nine genes (BRAF, KRAS, EGFR, IDH1, IDH2, PTEN, PIK3CA, NRAS and TP53). However, we focused only in CNAs. The SNP array assay was performed according to the recommended protocol using 80 ng of genomic DNA. After the hybridization (18 h), the arrays were stained and washed (GeneChip® Fluidics Station 450) and loaded into the GeneChip® Scanner 3000 7G (Thermo Fisher Scientific/Affymetrix). The CEL files were generated by Affymetrix Gene Chip Comand Console® software (v. 4.0) and processed by OncoScan Console software (v. 1.3) resulting in OSCHP files and QC metrics.
Data generated from the SNP array was used to calculate previously defined scores of homologous recombination deficiency: loss of heterozygosity (LOH) [16], telomeric allelic imbalance (tAI) [14], large scale transition (LST) [15], and Composite Score (CS) (the sum of LOH, tAI and LST scores) [25]. LOH was calculated as the number of LOH regions spanning at least 15 MB but not involving the entire chromosome. The number of regions with allelic imbalance extending to one of the telomeres but not crossing the centromere, after filtering regions shorter than 11 MB or spanning less than 500 probes, was defined as tAI. The LST score was defined as the number of breakpoints between regions spanning at least 10 MB within a distance of maximum 3 MB. HRD was computed as LOH + tAI + LSTm (adjusted LST score). According to Timms et al. [26], the HRD score introduced by Telli et al. [25] increases with ploidy in both intact and deficient samples. The adjusted LST score is calculated as [LSTm = LST-15.5P], in which P is the tumor ploidy. Using logistic regression analysis, the constant 15.5 was derived to provide the best separation between intact and deficient samples. ASCAT [27] was used for inferring tumor ploidy, calculating allele-specific copy numbers and segmentation. The cut-off values were previously established as HRD markers: > 10 for LOH [16], > 42 for CS [25] and > 15 for near-diploid tumors or > 20 for near-tetraploid tumors for LST scores [15]. The tAI median value was used as cut-off to consider it as HRD marker.
CCNE1 and RB1 CNAs were evaluated using the SNP array with a resolution of 1 probe per 50Kb for both genes. The average copy number for all probes covering each gene > 2.0 and < 2.0 was defined as gain or loss, respectively.
Target enrichment next generation sequencing (tNGS)
The HaloPlexHS target enrichment technology (Illumina 100 custom design with a 229,506 bp region of interest) (Agilent Technologies, 2016) was used to investigate mutations in 24 genes (BRCA1, BRCA2, TP53, BRIP1, CDH1, PALB2, RAD51C, RAD51D, XRCC2, MLH1, MSH2, MSH6, PMS2, EPCAM, APC, MUTYH, BMPR1A, SMAD4, STK11, PTEN, POLD1, POLE, NTHL1 and VHL). The fraction of bases in the region of interest covered 98.76% of the target region. HaloPlexHS libraries were constructed according to manufacturer’s protocol v.B0 (Agilent Technologies, June 2015). Indexes were incorporated for each sample during enrichment, allowing samples to be multiplexed before sequencing. A total of 11 HaloPlexHS libraries were validated on a TapeStation using High Sensitivity screentape (Agilent Technologies). After enrichment, HaloPlexHS libraries were diluted to 10 nM, pooled, denatured and subjected to paired-end (2× 150 bp), single index (8 bp) reversible terminator-based DNA sequencing on a NextSeq550 (Illumina) using a mid-output kit and loading 1.8 pM of denatured library pool.
For each sequenced sample, the raw FastQ files were trimmed with TrimGalore (v. 0.4.2), subsequently mapped to the GRCh37/hg19 human reference genome using MOSAIK (v. 2.2.26) and converted to BAM using Sambamba (v. 0.6.3). The BAM file for each sample was preprocessed with Genome Analysis Toolkit (GATK v. 3.6; local realignment around indels and base quality score recalibration), prior to variant calling. General alignment statistics (e.g. number of aligned reads etc.) was generated with BAMtools (v.2.3.0). Target specific alignment statistics (i.e. per base−/region−/gene−/sample-coverage and coverage percentage of ROIs), were obtained using GATK DepthOfCoverage. Variant calling was performed using the variantcallers: GATK HaplotypeCaller in genomic VCF mode, GATK UnifiedGenotyper (GATK v. 3.6), FreeBayes (v. 1.1.0) and finally PLATYPUS (v. 0.8.1). A single multisample VCF file comprising all analyzed samples was generated for each variantcaller. Four multisample VCF files were subsequently merged to produce a single multisample VCF file.
The variants were annotated with Ingenuity Variant Analysis (Qiagen), SnpEff (v 4.1c), ANNOVAR (v July 2017) and VariantTools (v.2.3), using build-in and custom annotation tracks. Additional file 2 summarizes the pipeline used to evaluate the generated variants.
Statistical analysis
Statistical analyzes were performed using the SPSS (v. 21.0; SPSS, Chicago, IL, USA) software, adopting a two-tailed P < 0.05 value as significant. The association among response rate to platinum retreatment in patients with platinum resistant ovarian cancer and the HRD scores, all mutations, CCNE1 CN gains and RB1 CN losses, were investigated using Mann-Whitney and Fischer’s Exact tests. Correlation analysis between the HRD scores was tested using Pearson’s coefficient and linear regression. Overall survival and progression free survival analyses were performed using Kaplan-Meier and log-rank test.
Results
A total of 31 patients with platinum resistant ovarian cancer was retreated with platinum therapy during a period of 10 years (2005 to 2014) at AC Camargo Cancer Center, SP, Brazil. High grade serous ovarian cancer was found in 68% of all cases, half of the patients received four or more prior treatment lines and 61.3% of the cases presented primary platinum resistant disease. All patients except three, were treated with taxane or gemcitabine in combination with platinum. Table 1 summarizes the clinical and pathological features of these 31 patients.
Genomic alterations
Fifteen of 31 ovarian tumors samples were investigated for genomic alterations. Clinical and molecular data are summarized in Table 2. Twelve cases were HGSC, two undifferentiated carcinomas and one high grade endometroid ovarian carcinoma. The median HRD scores were: tAI = 31, LOH = 9.0, LST = 24 and CS = 64 (details in Table 3). Two undifferentiated carcinomas and the high grade endometrioid tumors showed high HRD scores. Seven cases presented high scores for tAI, seven for LOH, nine for LST and nine cases for CS. Seven of 15 cases presented high values for all scores. Overall, a strong to moderate correlation was observed among the HRD scores (Additional file 3).
Table 2.
Clinical features, response to platinum retreatment and molecular findings of 15 ovarian cancer patientsa
| ID | Histology | Response | HRD | TP53 | BRCA1 | BRCA2 | Other Mutations | Family History | CCNE CN | RB1 CN |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | HGSC | CR | No | c.782 + 1G > A | WT | WT | WT | Yes | 2.3 | 1.9 |
| 2 | HGSC | PR | No | WT | WT | WT | WT | No | 2.9 | 1.8 |
| 3 | HGSC | SD | No | c.672 + 1G > T | WT | WT |
XRCC2 c.643C > T APC c136G > T |
Yes | 4.0 | 1.9 |
| 4 | HGSC | SD | Yes | nd | nd | nd | nd | No | 2.1 | 2.0 |
| 5 | HGSC | PR | Yes | c.734G > A | WT | WT | WT | No | 1.9 | 1.1 |
| 6 | HGSC | CR | Yes | WT | c.415C > A | WT | MUTYH c.991G > T | No | 1.5 | 1.5 |
| 7 | UC | PR | Yes | c.245delC | c.5044G > T | c.8350C > T | WT | Yes | 2.0 | 3.0 |
| 8 | UC | DP | No | WT | c.3931_3934delAACA | WT | WT | Yes | 2.3 | 1.9 |
| 9 | HGSC | DP | Yes | nd | nd | nd | nd | Yes | 2.6 | 1.1 |
| 10 | HGSC | PR | Yes | c.536A > G c.4G > T |
WT | WT | POLE c.4111C > T | No | 1.5 | 1.2 |
| 11 | HGSC | PR | Yes | c.920-2A > T | WT | WT |
APC c.5758C > T c.6610C > T |
Yes | 2.0 | 1.5 |
| 12 | HGSC | DP | No | c.718delA | WT | WT | XRCC2 c.110C > A | No | 3.5 | 3.2 |
| 13 | HGSC | SD | No | nd | nd | nd | nd | No | 1.7 | 1.8 |
| 14 | HGSC | DP | Yes | nd | nd | nd | nd | No | 2.4 | 2.0 |
| 15 | HGE | DP | Yes | c.524G > A | c.5096G > A | WT | WT | No | 2.2 | 1.1 |
aTarget-next generation sequencing was performed in 11 of 15 cases. HRD homologous recombination deficiency, CN copy number, HGSC high grade serous carcinoma, UC undifferentiated carcinoma, HGE high grade endometrioid carcinoma, CR complete response, PR partial response, SD stable disease, DP disease progression, nd not determined, WT wild type alleles. Copy number gain was defined as CN > 2.0; copy number loss was defined as CN < 2.0. HRD was considered with CS score ≥ 42
Table 3.
Homologous Recombination Deficiency (HRD) scores evaluated in 15 high grade ovarian cancer samples
| ID | tAI | LOH | LST | CS |
|---|---|---|---|---|
| 1 | 11 | 4 | 15 | 30 |
| 2 | 13 | 5 | 10 | 28 |
| 3 | 15 | 3 | 9 | 27 |
| 4 | 36 | 25 | 22 | 83 |
| 5 | 48 | 28 | 28 | 104 |
| 6a | 39 | 15 | 30 | 84 |
| 7a | 33 | 22 | 32 | 87 |
| 8a | 12 | 5 | 13 | 30 |
| 9 | 35 | 14 | 28 | 77 |
| 10 | 40 | 19 | 26 | 85 |
| 11 | 15 | 0 | 28 | 43 |
| 12 | 15 | 6 | 9 | 30 |
| 13 | 31 | 9 | 24 | 64 |
| 14 | 8 | 4 | 5 | 17 |
| 15a | 33 | 16 | 29 | 78 |
| Median (P25–75) | 31.0 (13–36) | 9.0 (4–19) | 24.0 (10–28) | 64 (30–84) |
tAI elomeric allellic imbalance score, LOH loss of heterozygosity score, LST large scale transition score, CS composite score, P25–75 interquartile range. The cut-off values were the median 30 for tAI, > 10 for LOH, > 42 for CS and > 15 for near-diploid tumors or > 20 for near-tetraploid tumors for LST scores
aBRCA1 mutated
The tNGS performed in 11 ovarian cancer revealed 18 somatic pathogenic variants including eight cases with TP53 mutations, three with BRCA1, and one UC presented BRCA1 and BRCA2 mutations. Detailed information on BRCA1 and BRCA2 mutations are presented in Additional file 4. Pathogenic variants were also identified in XRCC2, MUTYH, POLE and APC genes (Table 2). Moreover, two TP53 variants (c.536A > G and c.524G > A) identified in two tumors were covered by the OncoScan® FFPE platform, confirming the tNGS results. The mutational profiling of all cases of our study was obtained by tNGS performed in tumor samples and the allelic frequency detected for the genes tested is compatible with somatic mutations.
Three of four BRCA1 mutated tumors showed high HRD scores pointing to homologous recombination deficiency. Two of these four BRCA1 mutated tumors also presented gains of CCNE1 (CN > 2.0). In addition, two cases with XRCC2 mutation presented low HRD score and the highest value for CCNE1 gains (CN = 4.0 and 3.5). Eight of 15 patients had CCNE1 copy number gain and 11 of 15 patients presented RB1 copy number loss (Table 2). Five of eight patients with CCNE1 gains presented low HRD scores (Table 2).
Overall response rate (ORR), progression free survival (PFS) and overall survival (OS)
Considering the entire cohort (N = 31), ORR was 51.6%, PFS was 7.6 months and OS was 13.4 months. Among the 15 patients molecularly evaluated (SNP array), two presented complete response, five showed partial response, two stable disease and six disease progression (ORR = 46.7%, PFS = 8.8 months and OS = 10.2 months) (detailed data are presented in Table 4).
Table 4.
Review of the response to platinum retreatment in patients who were tested with tNGS (11 cases) and OncoScan® FFPE platform (15 cases)
| ID | Pre-treatment CA125 | Post-treatment CA125 |
CA125 Confirmation | Largest lesion pre -treatment | Largest lesion post-treatment | Treatment Response |
|---|---|---|---|---|---|---|
| 1 | 102.9 | 32.2 | – | SUV 4.54 in pelvis | No PET-CT contrast enhancement | CR |
| 2 | 115 | 62 | 31 | Pleural (72 mm) | Pleural (70 mm) | PR |
| 3 | 40.9 | 20.1 | 17.1 | Peritoneal (31 mm) | Peritonium (26 mm) | SD |
| 4 | 637 | 1143 | – | Liver (45 mm) | Liver (54 mm) | DP |
| 5 | 92.3 | 79 | 24.2 | Peritoneal (63 mm) | Peritonium (47 mm) |
PR |
| 6 | 35 | 18.6 | 0 | SUV 11.0 in liver, 3.2 in adrenal, 8.0 pre-sacral | No PET-CT contrast enhancement | CR |
| 7 | 1174 | 222,9 | 71.5 | Pleural effusion | Pleural effusion resolution* | PR |
| 8 | 258 | 331 | – | SUV 3.0 in peritoneal lesion, 1.9 in epiplon, 3.0 in gut | SUV 11.6 in peritoneal lesion, 3.7 in epiplon, 9.5 in gut | DP |
| 9 | – | – | – | – | Bowel obstruction | DP |
| 10 | 4430 | 1887 | 2180 | Pelvic (101 mm) | Pelvic (90 mm) | PR |
| 11 | 546 | 81.6 | 60 | Pre-sacral (117 mm) | Pre-sacral (106 mm) |
PR |
| 12 | 2600 | 3004 | – | Peritoneal thickening | New hepatic lesion (24 mm) | DP |
| 13 | 32 | 14.2 | 17.6 | Retroperitoneal lesion (40 mm) | Retroperitoneal lesion (40 mm) | SD |
| 14 | – | – | – | – | Bowel obstruction | DP |
| 15 | 52.2 | 94.6 | 185 | – | New brain lesion | DP |
CA125 confirmation: a second CA125 measurement at least two weeks after the post treatment was performed aiming to confirm the treatment response; PET-CT FDG positron emission tomography – compute tomography, SUV standardized uptake value, CR complete response, PR partial response, SD stable disease, DP disease progression
*No surgical procedure was performed between the evaluations
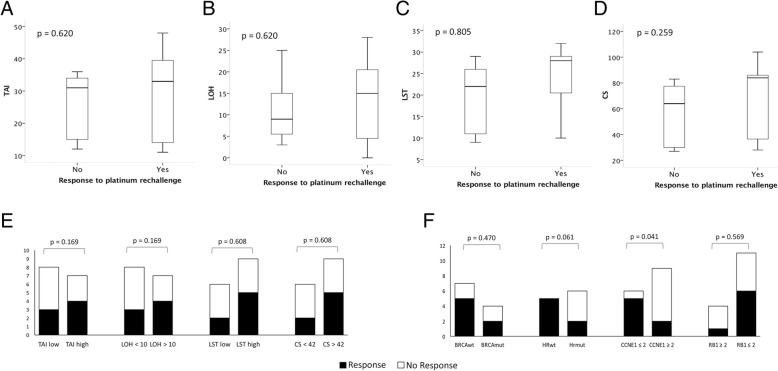
The median values of the four HRD scores were higher in responders (LOH = 15, LST = 28, tAI = 33, CS = 84) compared with non-responders (LOH = 7.5, LST = 17.5, tAI = 23, CS = 47) (Fig. 1). Based on the cutoff values, patients with high LOH, LST, tAI and CS scores had better response rates, although these differences were not statistically significant (Fig. 1). Interestingly, two of four BRCA1 mutated cases presented a response to platinum retreatment while in two patients with XRCC2 mutation, one presented a stable disease and the other tumor progression (Fig. 1).
Fig. 1.
Homologous recombination deficiency scores according to the response to platinum rechallenge. a Telomeric allelic imbalance (tAI); b Loss of heterozygosity score (LOH); c Large scale transition score (LST); d Composite score (CS). Overall response rates according to molecular alterations. e Telomeric allelic imbalance score (tAI) Loss of heterozygosity score (LOH); Large scale transition score (LST) and Composite score (CS); f BRCA mutation; Homologous recombination (HR) gene mutation; CCNE1 copy number gain; CCNE1 copy number gain and RB1 copy number loss
Ovarian cancer samples with no CCNE1 CN gains were associated with higher response rate compared with cases with CCNE1 CN gains (ORR = 83.3 vs 22.2%; p = 0.041). Although not statistically significant, patients with RB1 CN loss had higher response rates compared with patients without RB1 CN loss (ORR 54.5% versus 25.0%). Two patients with BRCA1 mutation showing no response to platinum retreatment also presented CCNE1 CN gain (Fig. 1). CNA of both, RB1 (loss) and CCNE1 (gains) were observed in 6 cases.
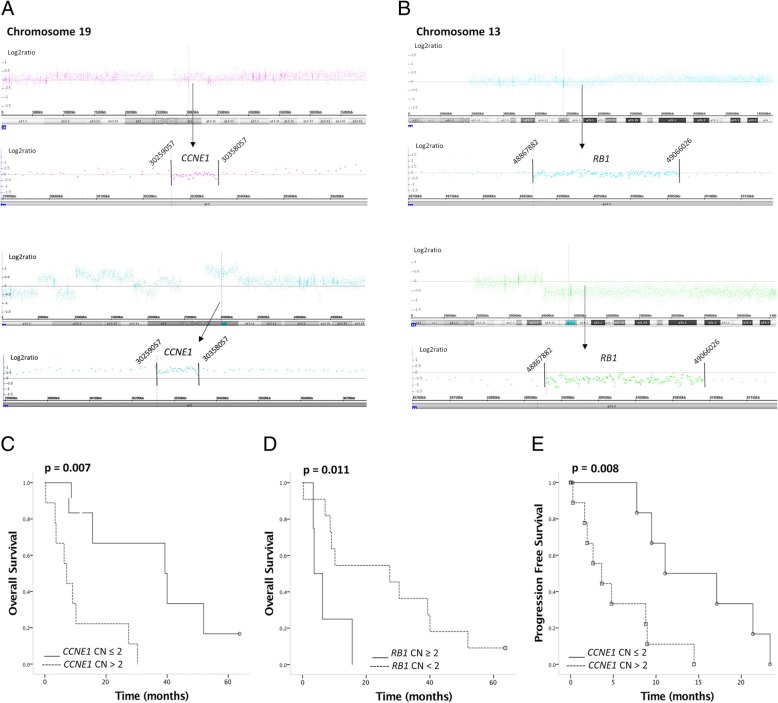
No significant differences were observed in the PFS and OS according to the homologous recombination deficiency scores (Additional files 5 and 6). However, RB and CCNE1 CNAs were significantly associated with these parameters. Patients with CCNE1 gains presented worse PFS compared with patients without CCNE1 gains (PFS = 7.1 months vs 39.3 months; p = 0.007) (Fig. 2). Patients with RB1 losses had better PFS compared with cases with no RB1 losses (PFS = 9.0 months vs 2.6 months; p = 0.093) (Additional file 6).
Fig. 2.
Representative examples of CCNE1 copy number status. a CCNE1 copy number normal was observed in 7 cases. b CCNE1 copy number gain was detected t in 8 cases. c Overall survival according to CCNE1 copy number alteration and d RB1 copy number alteration. e PFS according to CCNE1 copy number alteration
Cases with CCNE1 CN gains presented worse overall survival compared to those with no CCNE1 CN gains (OS = 39.3 months vs 7.1 months; p = 0.007). In contrast, better overall survival was found in cases with RB1 CN loss compared with those with no RB1 CN loss (OS = 27.4 months versus 3.6 months; p = 0.025) (Fig. 2).
Discussion
In this study, we evaluated markers of response to platinum retreatment in a selected group of patients with heavily pretreated platinum resistant ovarian cancer. The rationale was to identify patients that are still sensitive even after a platinum free interval shorter than 6 months and numerous previous treatment lines.
Nine of 15 cases (60%) of our cohort presented HR deficiency, which is in accordance with the 50% described in the TCGA dataset [3]. Patients with CS score higher than 42 had an ORR of 55.6% versus 33.3% observed in those with lower scores. In addition, the HRD median values of each score were higher among responders than in non-responders. However, the differences were not statistically significant probably due to the small number of cases evaluated.
Two scores based on the patterns of CNA and LOH were used in the phase III clinical trials of PARP inhibitors showing their ability to identify patients with BRCA mutation [7, 8, 13]. These studies also described a second group of patients negative for BRCA mutations but with high sensitivity to PARP inhibitors. Our findings give additional evidence that the scores are able to identify patients with high sensitivity to platinum agents. The absence of statistical significance in our study may be due to the small number of cases or the accuracy of these scores. Interestingly, the BRCA non-mutated cases and those with low HRD (based on the scores) benefited from treatment with PARP inhibitors [7, 8, 13]. The definition of all scores, except tAI [14], were based on the BRCA mutation as a gold standard to define HRD [15–17]. Therefore, the CNA and LOH pattern calculated by the scores are similar to the ones promoted by BRCA mutations, which may be true for most but not necessarily all causes of the HRD.
Cyclin E1 overexpression promotes cell cycle progression abrogating DNA repair during the G1 phase. In addition, CCNE1 amplification has been associated with an apparent synthetic lethality in cases with HR deficiency [28]. Nine of our 15 tumors (60%) showed CCNE1 copy number gains while data from TCGA (509 high grade serous ovarian carcinomas) presented CCNE1 gains in 32.4% or focal amplification in 20.8% [3]. We observed that cases showing CCNE1 gains had lower ORR, and shorter PFS and OS compared with those not presenting gains. Previous studies in ovarian cancer patients described increased expression levels of cyclin E1 and an association with worse survival [29]. At the genomic level, two studies described high frequency of CCNE1 gains in patients with primary platinum resistant disease [19, 30]. The worse survival observed in the TCGA cohort for patients with CCNE1 amplification was attributed to its negative association with BRCA mutations [3]. In our study, two of four patients harboring BRCA mutations also presented CCNE1 gains. This finding suggests that the correlation between CCNE1 gain and outcome is not exclusively due to its negative association with BRCA mutations. Ten of our 15 patients had primary platinum resistant disease and 7 of 9 patients with CCNE1 gains presented primary resistant disease. This finding supports the association of CCNE1 aberrations and resistance to platinum therapy and may explain the higher than expected frequency of CCNE1 gains in our study. Previous studies showed CCNE1 gains and BRCA mutation or homologous recombination deficiency as mutually exclusive [29]. However, the authors showed that complete mutually exclusive alterations were not observed between low levels of CCNE1 gains and BRCA mutations. Our two patients with co-occurrence of BRCA mutation and CCNE1 gain presented low CCNE1 copy number gains (2.2 and 2.3).
The RB1 protein is involved in the S-phase checkpoint to repair DNA breaks and in the regulation of DNA replication. The loss of the tumor suppressor RB1 leads to cell cycle progression and replication fork progression leading to replication stress and DNA damage, which could be repaired by HR machinery [31]. We found an association of RB1 loss with PFS and OS. To our knowledge, two previous studies addressed the impact of RB1 loss in ovarian carcinoma. In 2013, Milea et al. showed loss of RB1 protein expression associated with longer OS [20]. Recently, Garsed et al. reported the association of RB1 loss and HR gene mutations with extremely long PFS and OS [21]. Taken together, these findings suggest that RB1 loss is a biomarker with the potential to identify sensitive patients to platinum treatment. In addition, this alteration could be used in the clinical practice and potentially select BRCA mutated patients with higher chance to be PARP inhibitors responsive.
Four of 15 tumors (26%) presented BRCA mutations, an expected frequency in high grade serous carcinomas [32]. No differences in the response of the treatment were found between mutated and non-mutated tumors. Although, BRCA mutations are well known markers of response to platinum and PARP inhibitors therapy, other studies also failed to show higher frequency of BRCA mutations in long term responders [21]. In addition to BRCA, two non-responder patients presented tumors with low HR deficiency scores and XRCC2 mutations. XRCC2 is involved in the repair of DNA double-strand breaks by HR pathway. This finding highlights the value of an investigation using a panel of genes in ovarian cancer. Furthermore, conclusions regarding HR deficiency based solely on the presence of certain mutations may not be precise, as was found in the ARIEL2 trial [13].
Our study has several limitations, mostly due to the small sample size and its retrospective nature. For example, we found the ORR higher than expected for platinum resistant patients. High ORR may be due to patient over-selection including those who have received several previous treatment lines. Despite the limitation of the ORR evaluation, CCNE1 gain and RB1 loss were both associated to OS, which is an objective endpoint even in retrospective studies. No association between HR deficiency scores and response to therapy was found. The small number of patients limits conclusions regarding the low accuracy of these scores, even if previous literature data also pointed out to the limitation of the scores. However, the strength of our study was the evaluation of well selected individuals for whom it would be expected a low response rate with platinum retreatment. Unexpectedly, a high response rate to platinum retreatment was found suggesting that this selected cohort might be enriched for extremely sensitive tumors. HR deficiency scores were not able to show a strong association with therapy response. Interestingly, CCNE1 copy number gain was a negative prediction marker of platinum sensitivity, and RB1 copy number loss identified patients with sensitive disease.
In this study we explored the mutational profile and HR deficiency score in ovarian cancer patients to better understand the platinum resistant recurrence as defined by the platinum free interval. We demonstrated that HR deficiency scores, CCNE1 gains and RB1 losses could be used to distinguish patients who are still sensitive to platinum retreatment from those resistant to platinum therapy. Considering similar mechanisms of sensitivity to platinum salts and PARP inhibitors, these markers could be useful to better select the patients for PARP inhibitors therapy in the platinum resistant relapse.
Conclusions
The prediction of response to platinum retreatment goes beyond HR deficiency. CCNE1 copy number gains revealed a different subtype of ovarian carcinoma and could be used as a negative selection marker for platinum therapy and PARP inhibitors. Furthermore, RB1 losses identified patients with higher chance to be responsive to the treatment. Theses markers add information to precision therapy in the context of the cost-effectivity for PARP inhibitors therapy.
Additional files
Flowchart representative of the inclusion criteria adopted in the study. (JPG 245 kb)
Summary of the bioinformatic pipeline used to classify the variants detected by tNGS. (JPG 509 kb)
Spearman correlation of four different homologous recombination deficiency scores. TAI, Telomeric allelic imbalance; LOH, Loss of heterozygosity score; LST, Large scale transition score; CS, Composite score. (JPG 353 kb)
BRCA1 and BRCA2 variants categorized according to ACMG (American College of Medical Genetics and Genomics). (DOCX 14 kb)
Progression free survival according to the molecular alterations. A. Telomeric allelic imbalance (tAI); B. Loss of heterozygosity score (LOH); C. Large scale transition score (LST); D. Composite score (CS); F. RB1 copy number gain. (JPG 170 kb)
Overall survival according to the genomic imbalances. A. Telomeric allelic imbalance (TAI); B. Loss of heterozygosity score (LOH); C. Large scale transition score (LST); D. Composite score (CS). (JPG 374 kb)
Acknowledgments
The authors thank the Nucleic Acid Bank of A.C.Camargo Cancer Center São Paulo, Brazil for sample processing. We are grateful to Priscila Mayrink de Miranda for her help in conducting the SNP array experiments.
Funding
This study was supported by grants from the National Institute of Science and Technology in Oncogenomics (FAPESP # 2008/57887–9 and CNPq # 573589/08–9). The funding agencies had no role in the design of the study, sample collection, analysis, interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CNA
Copy number alterations
- CS
Composite score
- FFPE
Formalin fixed paraffin embedded
- GCIG
Gynecological Cancer Intergroup
- HR
Homologous recombination
- HRD
Homologous recombination deficiency
- LOH
Loss of heterozigosity
- LST
Large scale transition
- NGS
Next generation sequencing
- OS
Ooverall survival
- PARP
Poly(ADP-ribose) polymerase
- PFI
Platinum-free interval
- PFS
Progression free survival
- RECIST
Response evaluation criteria in solid tumors
- SNP
Single nucleotide polymorphism
- tAI
Telomeric allelic imbalance
Authors’ contributions
AABAC, SRR and MIA conceived and designed the experiments. SRR contributed with reagents and material. AABAC, ARGR, CES, GB and LB contributed with clinical data. LMCA performed the experiments. SJL and JB developed the computational analysis of the HRD scores. AHP and MMAJ evaluated the target-NGS data. MDB performed the histological analysis and macrodissected the samples. AABAC performed the statistical analysis. SRR and MIA supervised the whole study. SRR and AABAC wrote and edited the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki ethical guidelines and approved by the AC Camargo Cancer Center Ethics Committee (CEP# 1933/14). The need for informed consent has been waived by the AC Camargo Cancer Center Ethics Committee.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alexandre A. B. A. da Costa, Email: alexandreandredacosta@gmail.com
Luisa M. do Canto, Email: luisaaamatos@gmail.com
Simon Jonas Larsen, Email: sjlarsen@imada.sdu.dk.
Adriana Regina Gonçalves Ribeiro, Email: argribeiro@gmail.com.
Carlos Eduardo Stecca, Email: carlosstecca@gmail.com.
Annabeth Høgh Petersen, Email: Annabeth.Hogh.Petersen@rsyd.dk.
Mads M. Aagaard, Email: Mads.Jorgensen@rsyd.dk
Louise de Brot, Email: loudebrot@gmail.com.
Jan Baumbach, Email: jan.baumbach@imada.sdu.dk.
Glauco Baiocchi, Email: glbaiocchi@yahoo.com.br.
Maria Isabel Achatz, Email: miachatz@gmail.com.
Silvia Regina Rogatto, Email: silvia.rogatto2@gmail.com.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 3.Network TCGA. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation–positive women with ovarian Cancer: a report from the Australian ovarian Cancer study group. J Clin Oncol. 2012;30:2654–2663. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ledermann JA, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: an updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol. 2016;17:1579–1589. doi: 10.1016/S1470-2045(16)30376-X. [DOI] [PubMed] [Google Scholar]
- 6.Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–1284. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 7.Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949–1961. doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian Cancer. N Engl J Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 9.Bolton K, Chenevix-Trench G, Goh C, Sadetzki S, Ramus SJ, Karlan BY, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian Cancer. JAMA. 2012;307:382–390. doi: 10.1001/jama.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLaughlin JR, Rosen B, Moody J, Pal T, Fan I, Shaw PA, et al. Long-term ovarian Cancer survival associated with mutation in BRCA1 or BRCA2. J Natl Cancer Inst. 2013;105:141–148. doi: 10.1093/jnci/djs494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barber LJ, Sandhu S, Chen L, Campbell J, Kozarewa I, Fenwick K, et al. Secondary mutations in BRCA2associated with clinical resistance to a PARP inhibitor. J Pathol. 2013;229:422–429. doi: 10.1002/path.4140. [DOI] [PubMed] [Google Scholar]
- 12.Christie EL, Fereday S, Doig K, Pattnaik S, Dawson S-J, Bowtell DDL. Reversion of BRCA1/2Germline mutations detected in circulating tumor DNA from patients with high-grade serous ovarian Cancer. J Clin Oncol. 2017;35:1274–1280. doi: 10.1200/JCO.2016.70.4627. [DOI] [PubMed] [Google Scholar]
- 13.Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75–87. doi: 10.1016/S1470-2045(16)30559-9. [DOI] [PubMed] [Google Scholar]
- 14.Birkbak NJ, Wang ZC, Kim J-Y, Eklund AC, Li Q, Tian R, et al. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov. 2012;2:366–375. doi: 10.1158/2159-8290.CD-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popova T, Manié E, Rieunier G, Caux-Moncoutier V, Tirapo C, Dubois T, et al. Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res. 2012;72:5454–5462. doi: 10.1158/0008-5472.CAN-12-1470. [DOI] [PubMed] [Google Scholar]
- 16.Abkevich V, Timms KM, Hennessy BT, Potter J, Carey MS, Meyer LA, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. 2012;107:1776–1782. doi: 10.1038/bjc.2012.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang ZC, Birkbak NJ, Culhane AC, Drapkin R, Fatima A, Tian R, et al. Profiles of genomic instability in high-grade serous ovarian Cancer predict treatment outcome. Clin Cancer Res. 2012;18:5806–5815. doi: 10.1158/1078-0432.CCR-12-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Zhang CP, Zheng C-H, Xia J. Identification of ovarian cancer subtype-specific network modules and candidate drivers through an integrative genomics approach. Oncotarget. 2015;7:4298–4309. doi: 10.18632/oncotarget.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etemadmoghadam D, deFazio A, Beroukhim R, Mermel C, George J, Getz G, et al. Integrated genome-wide DNA copy number and expression analysis identifies distinct mechanisms of primary Chemoresistance in ovarian carcinomas. Clin Cancer Res. 2009;15:1417–1427. doi: 10.1158/1078-0432.CCR-08-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milea A, George SH, Matevski D, Jiang H, Madunic M, Berman HK, et al. Retinoblastoma pathway deregulatory mechanisms determine clinical outcome in high-grade serous ovarian carcinoma. Mod Pathol. 2013;27:991–1001. doi: 10.1038/modpathol.2013.218. [DOI] [PubMed] [Google Scholar]
- 21.Garsed DW, Alsop K, Fereday S, Emmanuel C, Kennedy CJ, Etemadmoghadam D, et al. Homologous recombination DNA repair pathway disruption and retinoblastoma protein loss are associated with exceptional survival in high-grade serous ovarian Cancer. Clin Cancer Res. 2018;24:569–580. doi: 10.1158/1078-0432.CCR-17-1621. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen TT, Wright JD, Powell MA, Gibb RK, Rader JS, Allsworth JE, et al. Prognostic factors associated with response in platinum retreatment of platinum-resistant ovarian cancer. Int J Gynecol Cancer. 2008;18:1194–1199. doi: 10.1111/j.1525-1438.2007.01184.x. [DOI] [PubMed] [Google Scholar]
- 23.Vergote I, Rustin GJS, Eisenhauer EA, Kristensen GB, Pujade-Lauraine E, Parmar MKB, et al. Re: new guidelines to evaluate the response to treatment in solid tumors [ovarian Cancer] J Natl Cancer Inst. 2000;92:1534–1535. doi: 10.1093/jnci/92.18.1534. [DOI] [PubMed] [Google Scholar]
- 24.Rustin GJS, Quinn M, Thigpen T, Bois d A, Pujade-Lauraine E, Jakobsen A, et al. Re: new guidelines to evaluate the response to treatment in solid tumors (ovarian Cancer) J Natl Cancer Inst. 2004;96:487–488. doi: 10.1093/jnci/djh081. [DOI] [PubMed] [Google Scholar]
- 25.Telli ML, Timms KM, Reid J, Hennessy B, Mills GB, Jensen KC, et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast Cancer. Clin Cancer Res. 2016;22:3764–3773. doi: 10.1158/1078-0432.CCR-15-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Timms KM, Abkevich V, Huges E, Neff C, Reid J, Morris B, et al. Association of BRCA1/2 defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res. 2014;16:475–484. doi: 10.1186/s13058-014-0475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Loo P, Nordgard SH, Lingjaerde OC, Russnes HG, Rye IH, Sun W, et al. Allele-specific copy number analysis of tumors. PNAS. 2010;107:16910–16915. doi: 10.1073/pnas.1009843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Etemadmoghadam D, Weir BA, Au-Yeung G, Alsop K, Mitchell G, George J, et al. Synthetic lethality between CCNE1 amplification and loss of BRCA1. PNAS. 2013;110:19489–19494. doi: 10.1073/pnas.1314302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Qi XR, Ming X, Wang L, Wang Y, Zhao X. Prognostic value of cyclin E expression in patients with ovarian cancer: a meta-analysis. J BUON. 2017;22:64–71. [PubMed] [Google Scholar]
- 30.Patch A-M, Christie EL, Etemadmoghadam D, Garsed DW, George J, et al. Whole–genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–494. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 31.Coschi CH, Ishak CA, Gallo D, Marshall A, Talluri S, Wang J, et al. Haploinsufficiency of an RB-E2F1-Condensin II complex leads to aberrant replication and aneuploidy. Cancer Discov. 2014;4:840–853. doi: 10.1158/2159-8290.CD-14-0215. [DOI] [PubMed] [Google Scholar]
- 32.Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D'Andrea AD. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian Cancer. Cancer Discov. 2015;5:1137–1154. doi: 10.1158/2159-8290.CD-15-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flowchart representative of the inclusion criteria adopted in the study. (JPG 245 kb)
Summary of the bioinformatic pipeline used to classify the variants detected by tNGS. (JPG 509 kb)
Spearman correlation of four different homologous recombination deficiency scores. TAI, Telomeric allelic imbalance; LOH, Loss of heterozygosity score; LST, Large scale transition score; CS, Composite score. (JPG 353 kb)
BRCA1 and BRCA2 variants categorized according to ACMG (American College of Medical Genetics and Genomics). (DOCX 14 kb)
Progression free survival according to the molecular alterations. A. Telomeric allelic imbalance (tAI); B. Loss of heterozygosity score (LOH); C. Large scale transition score (LST); D. Composite score (CS); F. RB1 copy number gain. (JPG 170 kb)
Overall survival according to the genomic imbalances. A. Telomeric allelic imbalance (TAI); B. Loss of heterozygosity score (LOH); C. Large scale transition score (LST); D. Composite score (CS). (JPG 374 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.