Abstract
Trans-generational maternal effects have been shown to influence a broad range of offspring phenotypes. However, very little is known about paternal trans-generational effects. Here, we tested the trans-generational effects of maternal and paternal age, and their interaction, on daughter and son reproductive fitness in Drosophila melanogaster. We found significant effects of parent ages on offspring reproductive fitness during a 10 day postfertilization period. In daughters, older (45 days old) mothers conferred lower reproductive fitness compared with younger mothers (3 days old). In sons, father’s age significantly affected reproductive fitness. The effects of 2 old parents were additive in both sexes and reproductive fitness was lowest when the focal individual had 2 old parents. Interestingly, daughter fertility was sensitive to father’s age but son fertility was insensitive to mother’s age, suggesting a sexual asymmetry in trans-generational effects. We found the egg-laying dynamics in daughters dramatically shaped this relationship. Daughters with 2 old parents demonstrated an extreme egg dumping behavior on day 1 and laid >2.35× the number of eggs than the other 3 age class treatments. Our study reveals significant trans-generational maternal and paternal age effects on fertility and an association with a novel egg laying behavioral phenotype in Drosophila.
Keywords: age, carry-over effects, fertility, parental, paternal, trans-generational
Investigations of aging effects on fitness, particularly fertility, have largely focused on the influence of an organism’s age on reproductive senescence within a generation, for example, offspring viability (Kern et al. 2001). For example, studies in Drosophila melanogaster have shown increasing female age to be associated with a range of reproductive phenotypes and behaviors including the numbers of eggs laid and egg to adult viability (reviewed in Miller et al. 2014). Very little attention has been aimed at understanding trans-generational effects of parental age on offspring fitness traits (but see Koch et al. 2018), in spite of a growing body of evidence that trans-generational effects are prevalent throughout model animal study systems (Mousseau and Dingle 1991; Sartorius and Nieschlag 2010; Rando 2012) and can impact human health (D’Onofrio et al. 2014; McGrath et al. 2014).
One of the first examples of trans-generational effects of maternal age on offspring quality was observed in mice (Wang and vom Saal 2000) in which maternal age at first pregnancy was associated with body weight, testes size, and epidydimis size in offspring. The conclusions from that study were that hormone concentrations in utero could have imprinting effects on the offspring and these effects could change with changing female physiology, and therefore age. More recently, investigations have revealed that epigenetic information of parental diet can be inherited through the male germline (mouse: Carone et al. 2010) and that paternal diet per se can influence offspring characteristics (D. melanogaster: Valtonen et al. 2012). Hereafter, we refer to the broad term “epigenetics” as chemical modifications to DNA and/or histones that are stably maintained, and while not changing the DNA sequence, can heritably alter gene expression (reviewed in Feil and Fraga 2012).
Although maternal effects (Mousseau and Dingle 1991) and in utero environment can influence offspring phenotypes, paternal effects are more difficult to detect, since males contribute little more than sperm and seminal fluid in the ejaculate when paternal parental care is absent (Clutton-Brock 1991; Carone et al. 2010). In spite of this limited provisioning to offspring, paternally derived seminal proteins exert a large impact on female reproductive physiology and egg-laying behavior (Avila et al. 2011) and these effects can be detected in the next generation (Priest et al. 2008). For example, paternal tetracycline exposure has been shown to affect offspring sperm viability in pseudoscorpions Cordylochernes scorpioides (Zeh et al. 2012). Also in Drosophila, paternal age has been shown to influence olfactory memory (Burns and Mery 2010); consistent with various psychiatric disorder associations with advanced paternal age in humans (D’Onofrio et al. 2014; McGrath et al. 2014).
An early study in Drosophila serrata revealed maternal age effects could impact offspring fitness, and were cumulative in effect across 2 generations of old mothers (Hercus and Hoffmann 2000). A more recent study found that offspring viability in D. melanogaster is influenced by a complex 3-way interaction between parental relatedness, parental age, and gametic age at successive developmental stages (Tan et al. 2013). A maternal age × paternal age interaction has also been noted in D. melanogaster daughters and the highest reproductive fitness was found in the old mother: young father combination (Nystrand and Dowling 2014). Focusing more specifically on paternal age, a previous comparison between young (2 days old), intermediate (13–14 days old), and old (32–33 days old) paternal ages in D. melanogaster revealed that offspring of old fathers suffered significantly lower larval viability (egg-to-adult survival) than those from young fathers (Price and Hansen 1998). In the same study, there were no significant effects of paternal age class on daughter fecundity, measured as the number of eggs laid, and male fertility was not directly assessed. Instead, son mating ability (presence or absence of copulation) was measured revealing no significant effect of paternal age treatment.
Beyond fertility, other studies have found no consistent evidence across various genotypes that paternal age influences longevity (D. melanogaster: Priest et al. 2002), although there is some evidence that paternal age can affect longevity in humans (Gavrilov et al. 1997). Reproductive potential declines with age in insects (Parsons 1964; David et al. 1975) and also in humans (Tarín et al. 2000). The aim of this investigation was to test whether there was any influence of parental age on offspring fertility in an F1 generation, to understand whether parental age can alter offspring phenotypic traits and carry-over between generations. Here, using the fruit fly D. melanogaster, we tested whether there was an association between the age of a focal fly’s parents and the fitness of the focal fly, measured as the number of eggs laid, the total number of offspring produced and the egg-to-adult viability. We tested fitness in both sexes; with 4 experimental parent age treatments (Supplementary Figure S1). Briefly, the parents of the focal individuals were either 3 days old or 45 days old. The experimental parental classes (4 in total) were: 1) young mother: young father, 2) old mother; young father; 3) young mother; old father; and 4) old mother; old father. This study design allowed us to test whether there is any trans-generational influence of parental age on offspring fertility and disentangle whether there is an effect of sex of the parent that was old or young. As a result, we measured the trans-generational effects of parental age on fitness and not the direct fitness of aged individuals because all the daughter and son (F1) individuals in the fertility assay were the same age (3 days old at the start of the 10 day egg lay).
Materials and Methods
Fly Husbandry
We used one genotype throughout the experiment: an inbred Oregon R strain produced by balancer chromosome replacement whose construction was completed by backcrossing for 3 generations to homogenize the genetic background (Montooth et al. 2010). Flies were maintained throughout the experiment at 25 °C on a 12 h: 12 h light: dark regime, with humidity at 70% ± 5%. Before the experimental procedures, flies were density controlled (25 females and 25 males; 4-day egg lay) for 2 generations on standard lab fly food. The recipe for the food in these holding vials was identical to that described below (see Fertility Assay section), except 2% autolyzed yeast was used instead of 6%, and the volume of food was 10 mL.
Experimental Design
The aim of the experiment was to manipulate the age of parents of the focal daughters and sons that were used in the fertility assays (see design scheme in Supplementary Figure S1). To achieve this, we isolated virgin males and females from the density-controlled stocks and housed them in same sex vials for 45 days. During this same 45-day period, the flies from the stocks were maintained (density-controlled) to then become the parents of young (3-day-old) flies, which were used in the experimental crosses. For the 45-day aging treatment virgin flies were transferred every fourth day onto a fresh food source (2% yeast w/v; as above) with additional sprinkled yeast on the surface. Previous life span studies in the same genotype suggest ~85% of the population is alive at day 45 on a similar food type (Villa-Cuesta et al. 2014).
At the stage of generating the focal experimental flies (below), young and old flies were allocated evenly across all aged and young treatment crosses. This was to ensure there was no bias of vial of origin at the parental generation stage and any inherent (vial) variation in quality was spread across all treatments. The “young” or “old” flies were then mated to produce daughters and sons for the experimental generation in a reciprocal design.
Estimating Genetic Variation in Our Sample Population
We conducted a supplementary analysis using RNA-seq reads from a previous study (Mossman et al. 2016, 2017) to estimate the numbers of SNPs within this genetic stock and estimated low levels of genetic variation throughout the transcriptome. These analyses were based on ~87.1 Mb of exome sequence. For brevity here, we have described this experiment in detail in the Supplementary Materials (section iv) and provide a brief description of the results in the main article.
Generation of Experimental Flies
Parents of the focal (experimental) generation were either young (3 days old) or old (45 days old) when they were mated. When the aged flies were 45 days old and the young flies were 3 days old, they were mated in their mating treatment.
These were:
(i) 3-day-old mother × 3-day-old father
(ii) 45-day-old mother × 3-day-old father
(iii) 3-day-old mother × 45-day-old father
(iv) 45-day-old mother × 45-day-old father
A schematic of the crosses and experimental procedures are shown in Supplementary Figure S1. The offspring eclosing from these crosses were used in the fertility assay. Adult flies eclosed and were immediately collected as virgins for the fertility assay. Males and females were separated as virgins and aged for 3 days before the fertility assay was conducted (Supplementary Figure S1).
Where possible, an individual virgin daughter or son was randomly selected from the vial of eclosing adults. In the treatments in which fewer than 25 of the vials produced offspring, we sampled multiple virgin males and virgin females from the same vial. Vial ID was fit as a random term in models to account for this shared vial provenance of some flies.
To test the effects of parental age treatment on male fertility, we used the same virgin tester female type across alternative male types. For example, 3-day-old virgin sons from the treatments (i–iv (above)) were all mated to a 3-day-old virgin female who emerged from the treatment (i). This young mother: young father treatment was considered a reference group, since 2 young flies (3 days old) mating is the most likely of our 4 scenarios to occur in natural populations, based on reproductive fitness relationships with age (Ashburner 1989) and very short sexual maturation times in D. melanogaster (Miller et al. 2014). For daughter fertility measures, we used 3-day-old virgin daughters that were the products of treatments i–iv (above) and mated these to 3-day-old virgin males from treatment (i) (Supplementary Figure S1).
Fertility Assay
The fertility assay was conducted on 6% yeast w/v food with no additional sprinkled yeast on the food surface. The full food recipe is as follows: 11% sugar, 6% autolyzed yeast, 5.2% cornmeal, agar 0.79% w/v in water, and 0.1% tegosept-methyl 4-hydroxybenzoate, from Sigma (St. Louis, MO). Food (approximately 5 mL) was added to each vial (glass narrow shell).
The aim of the fertility assay was to obtain an estimate of offspring productivity of each treatment for both daughter and son fertility over a 10-day period. We mated the treatment isofemale or isomale to the tester male or female individual, respectively, for 24 h. Each vial contained 1 male and 1 female. After 24 h exposure, the male was removed from the female and the female was transferred into a fresh vial. The female was transferred onto a fresh 6% food vial at the same time of day, daily, for 10 days (Supplementary Figure S1). Daughters and sons that died during the egg lay were excluded from the analysis. A summary of the numbers of flies that were not included in the analysis are included in Supplementary Table S4 (Supplementary Materials). Sample sizes (number of single-fly egg-laying vials) for each treatment were: 1) young mother: young father (daughters n = 18, sons n = 28); young mother: old father (daughters n = 23, sons n = 24); old mother: young father (daughters n = 25, sons n = 28); and old mother; old father (daughters n = 23, sons n = 19) (Supplementary Table S4). The number of eggs laid by each isofemale was counted daily to allow estimates of egg-to-adult viability, in addition to total offspring counts.
Offspring Counts
After 10 days of transfers onto fresh food, each isofemale was discarded from the vial and the offspring were allowed to eclose. After 14 days, all the offspring had eclosed and were counted daily. We counted the number of males and females, to provide resolution to investigate any sex ratio distortion effects over time and between treatments. None were detected (data not shown).
Statistics—MCMCglmm
We fitted zero-inflated poisson error structure to our count data models (egg number and total offspring number) because there were zero values in our data sets, which we wanted to model since they may be of biological nature. Zero values may also indicate that flies had not successfully mated to either male or female tester flies, or could represent random infertility among the age treatments. We fit a binomial error structure (“multinomial2”) in the model of the proportion of flies that survived from egg to adulthood (egg-to-adult survival) and used only female and male samples that were successful in producing at least one offspring. We analyzed egg count data, offspring count data and egg-to-adult viability using the (MCMCglmm) package (v 2.19) (Hadfield 2010). MCMCglmm applies Markov Chain Monte Carlo generalized linear mixed models (MCMCglmm) in a Bayesian framework. Using generalized linear models (glms), we identified there was over-dispersion in our count data (ratio of residual deviance/residual df) >1. To account for this, MCMCglmm applies an observation level random effect to deal with data over-dispersion. In some of the experimental treatments, there were not enough independent vials to source a single focal isomale or isofemale to have a target of ~25 vials per treatment (in the case of young mother: old father and old mother: old father). This was only evident in the focal age-treatment flies and not the standard 3-day-old male or female that was mated to the treatment fly. Each young mother: young father son or daughter fly was sourced from an independent vial. To account for shared vial of origin statistically, we fitted the “vial of origin” of the treatment flies as a random effect. In all models the factors: 1) mother’s age, 2) father’s age and 3) their interaction were fitted as fixed effects. The old age class was 45 days old and the young age class was 3 days old. Vial of origin of the experimental fly was the random effect. Results of zero-inflated poisson models for the egg and offspring count data are reported in the main text. We also conducted an analysis on the same count data using zero-truncated models and these are reported in the Supplementary Materials.
Prior Specification
MCMCglmm uses a Markov Chain Monte Carlo algorithm with an inverse Wishart before fit generalized linear mixed models. We applied the default MCMCglmm prior shape for the zero-inflated poisson models, and an inverse gamma prior (prior = list(R = list(nu = 0.002, V = 1), G = list(G1 = list(nu = 0.002,V = 1)))) for the egg-to-adult viability assay.
Markov Chain Parameters
Posterior estimation of the model fixed and random effects were based on Markov chains of 600 000 iterations, with a 150 000 iteration burn-in and a thinning interval of 50. This resulted in a posterior estimated based on 9000 samples from the chain. We report posterior means, posterior modes, the highest posterior density (95% credible interval [CI]), number of effective samples and the associated pMCMC value for each fixed effect. Fixed effects were considered statistically significant if the 95% CIs excluded zero, with the associated P-value (pMCMC) being <0.05.
Model Convergence Checking
Models were checked for convergence by 2 methods: 1) visual inspection of the model traces, and 2) using the Gelman–Rubin statistic (Gelman and Rubin 1992) implemented in the [coda] R package (Plummer et al. 2006). Visual inspection of traces of the sampled posterior revealed there were no cases of reducible chains (e.g., chains did not get stuck in regions of the parameter space). The Gelman–Rubin statistic was calculated using the [gelman.diag] command in R. We compared the between- and within-chain posterior distribution variance with 3 independent MCMC chains of the same model (600 000 iterations; burn-in: 150 000; thinning interval: 50). Chain convergence is evident when the potential scale reduction factor (PSR) is <1.1 (Gelman and Rubin 1992). The PSR was exactly 1.0 in all analyses and we were therefore confident the chains had converged. In Table 1 and Supplementary Table S1, we report the results of the first of the 3 chains in each analysis.
Table 1.
Trans-generational age effects on offspring (F1) fertility traits
| Focal sex | Trait | Term against the intercept | Posterior mode | Posterior mean | Lower 95% CI | Upper 95% CI | Effective samples | pMCMC |
|---|---|---|---|---|---|---|---|---|
| Daughters | (i) Total egg number | Intercept | 4.843 | 4.862 | 4.639 | 5.091 | 9475 | <0.0001 |
| Mother age (young) | 0.433 | 0.421 | 0.108 | 0.734 | 9000 | 0.01 | ||
| Father age (young) | 0.441 | 0.403 | 0.103 | 0.706 | 9649 | 0.01 | ||
| Mother age (young) × Father age (young) | −0.569 | −0.592 | −1.041 | −0.154 | 9000 | <0.01 | ||
| (ii) Total offspring number | Intercept | 4.158 | 4.172 | 3.943 | 4.395 | 7906 | <0.0001 | |
| Mother age (young) | 0.601 | 0.614 | 0.310 | 0.915 | 8125 | <0.001 | ||
| Father age (young) | 0.554 | 0.604 | 0.322 | 0.904 | 9000 | <0.0001 | ||
| Mother age (young) × Father age (young) | −0.541 | −0.507 | −0.933 | −0.100 | 9651 | 0.02 | ||
| (iii) Egg-to-adult survival | Intercept | −0.343 | −0.348 | −0.667 | −0.009 | 8708 | 0.04 | |
| Mother age (young) | 0.848 | 0.833 | 0.398 | 1.272 | 8508 | <0.001 | ||
| Father age (young) | 0.966 | 0.950 | 0.535 | 1.396 | 9779 | <0.0001 | ||
| Mother age (young) × Father age (young) | −0.153 | −0.294 | −0.895 | 0.310 | 9000 | 0.34 | ||
| Sons | (iv) Total egg number | Intercept | 4.880 | 4.868 | 4.648 | 5.095 | 9000 | <0.0001 |
| Mother age (young) | 0.146 | 0.179 | −0.105 | 0.479 | 9305 | 0.23 | ||
| Father age (young) | 0.294 | 0.246 | −0.046 | 0.517 | 9000 | 0.09 | ||
| Mother age (young) × Father age (young) | −0.001 | −0.038 | −0.426 | 0.333 | 9000 | 0.85 | ||
| (v) Total offspring number | Intercept | 4.450 | 4.434 | 4.160 | 4.707 | 9000 | <0.0001 | |
| Mother age (young) | 0.095 | 0.132 | −0.210 | 0.498 | 9000 | 0.46 | ||
| Father age (young) | 0.368 | 0.375 | 0.054 | 0.731 | 8709 | 0.03 | ||
| Mother age (young) × Father age (young) | −0.084 | −0.064 | −0.517 | 0.377 | 9000 | 0.79 | ||
| (vi) Egg-to-adult survival | Intercept | 0.508 | 0.487 | 0.065 | 0.918 | 9000 | 0.03 | |
| Mother age (young) | 0.148 | 0.161 | −0.374 | 0.709 | 9000 | 0.55 | ||
| Father age (young) | 0.415 | 0.422 | −0.071 | 0.982 | 9000 | 0.11 | ||
| Mother age (young) × Father age (young) | −0.411 | −0.278 | −0.963 | 0.440 | 9000 | 0.43 |
Three traits: (i, iv) total egg number; (ii, v) total offspring number; and (iii, vi) egg-to-adult survival of focal individuals were tested using generalized linear mixed models (glmm) implemented in the [MCMCglmm] R package (Hadfield 2010). Results of the Bayesian analyses are shown for each sex. Error distributions were modeled as “zero-inflated poisson,” “zero-inflated poisson,” and binomial (“multinomial2”), respectively. The random effect was modeled as the vial of origin. Markov chains were run for 600 000 iterations with burn-in: 150 000, and thinning interval: 50. Any reduction in sampling is also shown and significance was judged as 95% CIs excluding zero and pMCMC values <0.05 (bold).
Data Availability
In accordance with the Journal of Heredity data archiving policy (Baker 2013), we have deposited the primary data underlying the analyses as follows: Phenotype data are uploaded as Supplementary Materials.
Results
Numbers of Eggs
In focal daughters, there was a significant interaction between maternal and paternal ages (P < 0.01: Table 1(i)) on egg numbers, suggesting maternal age had statistically different effects when mated to different paternal age classes (and vice versa). Focal daughters with younger mothers produced greater numbers of eggs overall (Figure 1A). These results are largely influenced by a distinct egg laying phenotype in daughters with 2 old parents (see below and Figure 2A).
Figure 1.
Trans-generational fitness effects due to parental age in female and male Drosophila melanogaster. Interaction plots (means ± 1 SEM of raw data) describing daughter effects (A, C, E) and son effects (B, D, F) are shown for total egg number (A, B), total offspring number (C, D), and egg-to-adult survival (E, F). The mother’s aging treatment is on the abscissa and the father’s aging treatments are distinct lines: red = young father, blue = old father. The corresponding statistics for each interaction plot can be found in Table 1.
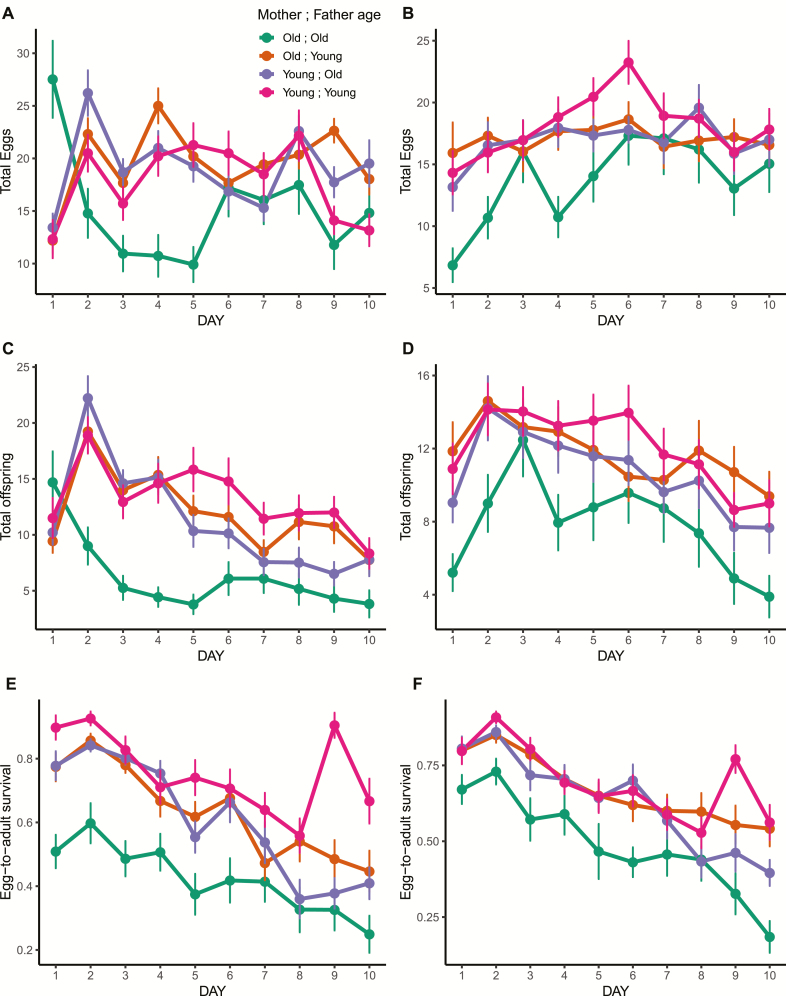
Figure 2.
Phenotypic variation in egg laying, offspring production, and egg-to-adult viability over time. A and B show daughter and son egg numbers, respectively. C and D show daily production of offspring for daughters and sons, respectively. E and F show egg-to-adult survival for offspring of daughters and sons, respectively. The 4 age classes are shown in different colors (legend in A). Means (± 1 SEM) of the raw data are shown.
In focal sons, age of parent either alone or in combination did not influence the number of eggs laid by tester females (Table 1(iv); Figure 1B).
Number of Offspring
There was a significant interaction between mother’s and father’s age on total offspring in focal daughters (Table 1(ii); Figure 1C). The combination of 2 young parents was associated with the greatest offspring numbers, and 2 old parents conferred the lowest offspring numbers, across all 4 age treatments. The effects of father’s age are conditional on the age of the mother (and vice versa).
In focal sons, only father’s age was associated with the number of offspring produced (Table 1(v); Figure 1D). Males with younger fathers produced statistically greater numbers of offspring than males with older fathers. Interestingly, mother’s age and its interaction with father’s age were not associated with total offspring numbers, consistent with no differences in egg numbers.
Egg-to-Adult Survival
In focal daughters, there was a significant large effect of both mother and father age on egg-to-adult survival. Younger mothers and younger fathers conferred increased egg-to-adult viability (Table 1(iii) and Figure 1E). There was no interaction of maternal and paternal age (P = 0.34), suggesting aged parents were influencing egg-to-adult survival in the same way, regardless of the age of the other parent.
In contrast, in focal sons there were no significant effects of maternal (P = 0.55) or paternal age (P = 0.11), or their interaction (P = 0.43) on egg-to-adult survival (Table 1(vi); Figure 1F).
Phenotypic Variation over Time
All 3 phenotypes showed variation over the 10-day experiment. In daughters, we discovered a striking effect of having 2 old parents in egg laying, offspring production, and egg to adult viability. Figure 2 describes these relationships. Using the same experimental protocol for egg laying and offspring production, we routinely observe Drosophila to exhibit an initial increase in egg and offspring production up to days 2–3, then a gradual decrease over time (Supplementary Figure S9 and Camus and Dowling 2018). Daughters with at least 1 young parent showed a typical trend in egg and offspring production over time. By contrast, daughters with 2 old parents demonstrated a unique egg dumping behavior on day 1 and laid, on average, >2.35× the number of eggs than the other 3 age class treatments (Supplementary Table S5). This high number of eggs translated to relatively high numbers of offspring on day 1, then a rapid drop until day 6, when egg and offspring numbers returned to similar levels to the daughters of the other 3 age categories. Egg-to-adult survival was routinely lower in offspring of daughters with 2 old parents across all time points (Figure 2).
In sons, we observed a more subtle pattern of phenotypic changes over time. Two old parents conferred the lowest (although nonsignificant) number of eggs on day 1 in sons; the opposite effect to that observed in daughters. Over time, the number of eggs in the old mother: old father cross increased to similar values to the other age treatments (Figure 2). In general, the egg-to-adult survival decayed at a constant rate in males and there was no appreciable difference between the 4 treatments, either over time, or as a cumulative 10 day total.
Estimates of Genetic Variation in Our Population
Using a trio analysis (Supplementary Materials), we estimate that there are extremely low levels of genetic variation in the flies used in this study. We estimate that between 51 (conservative estimate) and 266 (relaxed thresholds) are found in the RNA-seq libraries of the same genotypes used in the present study, corresponding with approximately 0.51 and 2.66 mutations per generation in the transcriptome. Given the number of nucleotides in the transcriptome that were shared across all 3 libraries, we estimate the mutation rate of our fly line to range from 2.93 × 10−09 and 1.53 × 10−8 mutations per site per generation.
Discussion
We found that fertility of daughters and sons is affected by maternal and paternal age in different ways. Reproductive fitness measures in daughters were influenced to a large degree by mother and father age, which was associated with a unique egg dumping phenomenon. Parental age effects in males were restricted to father’s age, and mother’s age only influenced daughter reproductive fitness.
Another study on trans-generational effects in Drosophila (Nystrand and Dowling 2014) found that daughter reproductive output (number of eclosed offspring over 4 days) was sensitive to a strong interaction between maternal age and paternal ages. This finding was in a different direction to that observed in the current study. There are several possible reasons why the results differ between studies. The young and old age classes in Nystrand and Dowling’s study (Nystrand and Dowling 2014) were 4 and 14 days, respectively, while in the current study these ages were 3 and 45 days. The age classes in the current investigation are more protracted and likely influence different physiological and/or genetic differences in the offspring (particularly in offspring of older parents).
Measures of reproductive fitness across 4 days may not be directly comparable (estimated as R2 = 0.82 in Nystrand and Dowling 2014) with measures over longer durations (10 days in the present study). In the present study, we found a similar strong positive correlation between offspring production in days 1–4, and the total offspring over 10 days (r = 0.91, df = 184, t = 15.31, P < 0.0001). The study design of Nystrand and Dowling (Nystrand and Dowling 2014) also incorporated an immune challenge factor into their experiment, which was included in significant higher order interaction effects, thus influencing the results of mother and father age as first-order effects. Nevertheless, significant age effects have been observed in both studies, suggesting aged Drosophila parents influence offspring fitness, even between young and old flies with only 10 days age difference (Nystrand and Dowling 2014). Interestingly, there are qualitative differences between these studies. Nystrand and Dowling (2014) found that daughters of younger fathers and young mothers had the lowest reproductive fitness overall, an opposite effect to the results we found in the present study; where daughters of young mothers and young fathers produced the highest overall offspring numbers.
Different genetic backgrounds were used between studies and this may explain the trans-generational effect differences. We found low levels of genetic variation in the Oregon R genetic stock used in this experiment based on exome-wide SNP data from a previous study (Mossman et al. 2016, 2017) (Supplementary Materials), however, we cannot rule out the opportunity for cohort selection on age-associated alleles in the old parent treatments. Theoretically, isogenic lines do not exist after one generation of stock creation due to de novo (spontaneous) mutation. The rate of fitness associated mutations (U) is estimated in D. melanogaster to be in the order of 1.2 per generation per diploid genome (Haag-Liautard et al. 2007). The Oregon R stock used in this study was approximately 100 generations old and this estimate suggests 120 fitness-associated mutations may be segregating in our Oregon R stock population. Other estimates of the spontaneous mutation rate in Drosophila are ~2.7 × 10−9 to 3.5 × 10−9 per site per generation (Keightley et al. 2009), suggesting at least ~105 mutations would be present (mutation rate per site per generation × genome size (~1.5 × 108 bases) × 2 ploidy × 100 generations). We estimated the numbers of SNPs that were segregating in the transcriptome of our Oregon R population to be between 51 and 266 (Supplementary Materials). These analyses suggest our initially created stock was isogenic or near isogenic. The mutations that have accumulated since the culture was created are approximately equal to those predicted from spontaneous mutation.
During the 45 day aging of flies, it is a reasonable assumption that any deleterious age-associated alleles in the population would be purged, essentially purifying for neutral or beneficial alleles at those variable sites. However, expression of any allelic variation in crosses between aged flies would depend on the dominance characteristics of those mutations. Daughters and sons of older parents would be expected to harbor less genetic variation since their parents have been subject to age-selection in the previous generation. Parental mating between old and young flies would therefore likely increase the expression of heterosis, when compared with matings between 2 old parents. In contrast, 2 young parents would be expected to produce more genetically variable offspring. We found good evidence that overall reproductive fitness over the 10 days deteriorated with increasing numbers of old parents from 0 old parents (highest fitness: average offspring = 113.8 [females], 106.8 [males]) > 1 old parent (average offspring= 112.3 [females], 99.5 [males]) >2 old parents (lowest fitness: average = 57.7 [females], 77.9 [males]). This overall result is consistent with a cohort selection effect, however, we cannot rule out other nonmutually exclusive mechanisms of parent age-associated offspring fitness. We did not investigate lifespan and we therefore cannot make any assumptions about correlations between reproductive effects and lifespan in these flies (sensu Lansing effects: Lansing 1947).
The differences in fertility of the parental flies were expected, since old flies were in the declining phase of fertility and these produced fewer offspring for the experimental assay. However, the fitness effects we observed in their offspring (the focal experimental generation) are likely to have been influenced by the environment that those larvae were reared in (Santos et al. 1994). Increased density of larvae is typically associated with lower fitness and phenotypic values in those adult flies (Santos et al. 1994; Hoffmann and Loeschcke 2006). We found the opposite effect whereby those offspring from the weakest cross with the lowest larval densities (old mother × old father treatment) also possessed the lowest fertility. This effect suggests larval densities are unlikely to have influenced our main results because we observe the opposite effect to that which would be expected under a conventional density dependent phenotype (Santos et al. 1994; Hoffmann and Loeschcke 2006). Our results may be slightly desensitized estimates of trans-generational age effects because we could not fully control possible larval density effects. We also acknowledge that treatment groups had small sample sizes.
The trans-generational effects of parental age on offspring fitness we found were both maternal and paternal in origin in daughters, while son fitness was sensitive to father’s age alone. We show that paternal age can modify daughter and son fitness. While maternal effects have been suggested to modify the fitness of the adult insects via oviposition behavior [e.g., egg mass provisioning and its influence on the imaginal disc (Labeyrie 1988) and egg size and offspring development time (Vijendravarma et al. 2010)], paternal effects on daughter fitness are more difficult to pinpoint even though they may be large and have consequences for fitness. If paternal age effects are common there may be genetic benefits of females mating to younger males if the sperm haploid genome is higher quality in younger males. This, in turn, may lead to female behaviors to avoid old male sperm (e.g., polyandry; see Radwan 2003 for a detailed review).
Old fathers—poor fertility
There are a number of nonmutually exclusive reasons why flies with old fathers may have poorer reproductive performance. The integrity of the sperm nucleus of an old father may be compromised, either through oxidative stress (Wallace and Melov 1998), pre- and post-meiotic sperm senescence (reviewed in Pizzari et al. 2008), or possibly epigenetic modification (Rando 2012). All 3 possibilities could impair the quality of the embryo (and adult offspring) since half of the diploid genome of the son or daughter focal fly is aged and/or has been exposed to increased germ cell mitotic replication in the previous generation. Furthermore, increased organism age corresponds to increased chance of mutations to accumulate in the germline (Crow 2000), which could also modify the effects of genetic imprinting in the offspring, especially if the organism was to receive a “double dose” of aged haploid genomes.
Old, Old Parents
Forty-five days old is likely to be beyond the physiological extreme of fertility experienced in natural settings, although surprisingly little is known about life history traits in wild fruit fly populations (Lachaise and Silvain 2004; Mansourian et al. 2018). That parental age influences offspring fertility, and this process may be mediated by gamete nucleus quality (Pizzari et al. 2008), it is possible that reproductive senescence may serve as an evolutionary filter to maintain high gamete quality in populations. One consequence of aged gametes in populations may be an influence on mating behavior in females (polyandry), where females mate with >1 male, to ensure high quality gametes from younger males are available for fertilization (Radwan 2003). Old fruit flies, with potentially compromised gamete integrity and increased mutation load (Crow 2000) are not as fit, and their offspring also have compromised fertility to the extent that a double dose of possibly poor quality haploid DNA from parents is associated with significantly reduced fertility.
The egg dumping behavior we discovered in daughters of 2 old parents is a promising future avenue of research and the clear qualitative differences would not have been appreciated if only a total egg production or offspring total was analyzed. Our data suggest their gamete quality was lowest of all 4 age treatments, since egg-to-adult viability was much lower than the treatment with at least 1 young parent. What could explain this egg dumping behavior? We found the egg laying rate in daughters of 2 old parents to resume to normal levels by day 6, however, the egg-to-adult viability remained lowest over all 10 days. Overall, the general pattern of declining egg-to-adult viability over time was observed across all parental age treatments, and is consistent with previous studies (Miller et al. 2014; Koch et al. 2018). In terms of reproductive fitness, the low fidelity of the day 1 embryos was partially compensated by the egg dumping behavior. Paradoxically, the point estimate of fitness on day 1 is highest for the daughters of 2 old parents, yet lowest overall in the 10-day experiment. The opposite is true for sons of 2 old parents, who showed relatively low fitness throughout all 10 days and the lowest numbers of eggs and offspring on day 1, suggesting possible sexually antagonistic pleiotropy of genes modifying reproductive fitness (including female egg laying behavior). In other words, the effects of 2 old parents on egg laying and offspring production were contrasted between daughters and sons, especially during the first day postmating.
Trans-generational Reproductive Plasticity?
Selection for age at reproduction has been shown to affect mating frequency in females (Sgrò et al. 2000), which can have a trans-generational effect on daughter lifetime reproductive success (LRS) (Priest et al. 2008). In the present study, we assumed the flies used in this experiment had been likely selected for early life reproduction, which is known to affect mating frequency; old females have lower mating frequency than young females (Miller et al. 2014). We do not make any assumptions about the quality of the offspring as a function of mating frequency, since this was not manipulated in any way in the experiment.
All daughters, regardless of their parent ages were mated to a standard 3-day-old male whose parents were both young. The effects we detected in daughters of 2 old parents are therefore likely to be the result of a male–female interaction involving components of the male ejaculate. Male Drosophila can plastically alter female reproductive investment and ~22% of this variation is of male genetic origin (Pischedda et al. 2011). Seminal fluid peptides transferred to females during copulation (Gromko et al. 1984) (e.g., sex peptide [SP]) are known to exert large influence over female reproductive behavior in Drosophila (Wolfner 1997; Chapman 2001; Ram and Wolfner 2009; Avila et al. 2011), including suppressing mating receptivity and increasing egg-laying for several days after mating (Rubinstein and Wolfner 2013). Seminal proteins effect other physiological changes in females such as relaxation of oviduct musculature, along with increasing octopamine signaling which increases ovulation rate (Rubinstein and Wolfner 2013). One such seminal protein, ovulin (Acp26Aa), is transferred from males, interacts with the female reproductive tract and increases octopamine signaling thus increasing egg release from the ovary (Rubinstein and Wolfner 2013). Ovulation rate is maximized during the 24 h after mating as a result of ovulin transfer, and octopamine stimulation (Herndon and Wolfner 1995), although these eggs show lower hatchability (Chapman et al. 2001). The amount of ovulin transferred to mated females is significantly lower than the amount transferred to virgin females (Sirot et al. 2011), however the influence of large-scale male age differences on ovulin transfer is unknown.
Egg laying by daughters of 2 old parents phenocopies a maximal ovulation rate, along with low egg-to-adult survival, and is observed to decline rapidly after 24 h, consistent with the effects of ovulin. We therefore hypothesize that transgenerational age effects are influencing the central nervous system of female Drosophila via overstimulation of octopaminergic and/or other neurotransmitter networks (Rodríguez-Valentín et al. 2006) or increasing sensitivity to seminal proteins during the first day postmating. As a result, daughters of 2 old parents dump large quantities of eggs, as observed in the present study. This hypothesis requires testing, but opens a new avenue of research on a potential role of parent age on the neurophysiology of daughter reproductive behavior.
Concluding Remarks
Whether trans-generational age effects are prevalent in human societies is a growing issue, since increasing numbers of humans are delaying the onset of parenthood (Sartorius and Nieschlag 2010). Further investigations in mammalian models are required to investigate whether there is a need for concern of human fertility being impacted by advancing parental ages, and what possibly genetic, epigenetic or environmental mechanisms may underpin such effects. When the consequences of aging are viewed as trans-generational phenomena, many potentially illuminating areas of research emerge.
Funding
J.A.M. and D.M.R. were supported by National Institutes of Health (R01GM067862 and R01AG027849). R.M.M., E.B., and N.M. were supported by a Howard Hughes Medical Institute Summer Studentship to Brown University (HHMI 52006915).
Authors’ Contributions
J.A.M. designed the study and analyzed the data. R.M.S.B., E.B., N.M., and J.A.M. conducted the egg and offspring counts. J.A.M. and D.M.R. wrote the article. All authors gave final approval for publication.
Supplementary Material
Acknowledgments
We thank L. Biancani for experimental food preparation. A.-M. Hernandez and J. Steinhauer gave constructive comments on the article. K. Wharton and M. Bartoletti gave fruitful discussion on stem cell renewal and sources of trans-generational effects. This work was conducted using computational resources and services at the Center for Computation and Visualization, Brown University. L. Alpert Sugden, S. Ramachandran, and J. Palacios gave advice on MCMC analyses. S. Baheti gave tremendous assistance in the RVboost analyses.
Data Accessibility
The datasets supporting this article have been uploaded as part of the Supplementary Materials.
References
- Ashburner M. 1989. Drosophila: a laboratory handbook. New York: Cold Spring Harbor Laboratory Press; p. 141–142. [Google Scholar]
- Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. 2011.Insect Seminal Fluid Proteins: Identification and Function. AnnRevof Entomol. 56: 21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CS. 2013. Journal of heredity adopts joint data archiving policy. J Hered. 104:1. [DOI] [PubMed] [Google Scholar]
- Burns JG, Mery F. 2010. Transgenerational memory effect of ageing in Drosophila. J Evol Biol. 23:678–686. [DOI] [PubMed] [Google Scholar]
- Camus MF, Dowling DK. 2018. Mitochondrial genetic effects on reproductive success: signatures of positive intrasexual, but negative intersexual pleiotropy. Proc Biol Sci. 285: 20180187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, Bock C, Li C, Gu H, Zamore PD, et al.. 2010. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 143:1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T. 2001. Seminal fluid-mediated fitness traits in Drosophila. Heredity (Edinb). 87:511–521. [DOI] [PubMed] [Google Scholar]
- Chapman T, Herndon LA, Heifetz Y, Partridge L, Wolfner MF. 2001. The Acp26Aa seminal fluid protein is a modulator of early egg hatchability in Drosophila melanogaster. Proc Biol Sci. 268:1647–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH. 1991. The evolution of parental care. Princeton (NJ): Princeton University Press. [Google Scholar]
- Crow JF. 2000. The origins, patterns and implications of human spontaneous mutation. Nat Rev Genet. 1:40–47. [DOI] [PubMed] [Google Scholar]
- David J, Cohet Y, Foluillet P. 1975. The variability between individuals as a measure of senescence: a study of the number of eggs laid and the percentage of hatched eggs in the case of Drosophila melanogaster. Exp Gerontol. 10:17–25. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Rickert ME, Frans E, Kuja-Halkola R, Almqvist C, Sjölander A, Larsson H, Lichtenstein P. 2014. Paternal age at childbearing and offspring psychiatric and academic morbidity. JAMA Psychiatry. 71:432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Fraga MF. 2012. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 13:97–109. [DOI] [PubMed] [Google Scholar]
- Gavrilov LA, Gavrilova NS, Kroutko VN, Evdokushkina GN, Semyonova VG, Gavrilova AL, Lapshin EV, Evdokushkina NN, Kushnareva YE. 1997. Mutation load and human longevity. Mutat Res. 377:61–62. [DOI] [PubMed] [Google Scholar]
- Gelman A, Rubin DB. 1992. Inference from iterative simulation using multiple sequences. Stat Sci. 7:457–472. [Google Scholar]
- Gromko MH, Newport MEA, Kortier MG. 1984. Sperm dependence of female receptivity to remating in Drosophila melanogaster. Evolution. 38:1273–1282. [DOI] [PubMed] [Google Scholar]
- Haag-Liautard C, Dorris M, Maside X, Macaskill S, Halligan DL, Charlesworth B, Keightley PD. 2007. Direct estimation of per nucleotide and genomic deleterious mutation rates in Drosophila. Nature. 445:82–85. [DOI] [PubMed] [Google Scholar]
- Hadfield JD. 2010MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Stat Softw. 33:1–22.20808728 [Google Scholar]
- Hercus MJ, Hoffmann AA. 2000Maternal and grandmaternal age influence offspring fitness in Drosophila. Proc Biol Sci. 267:2105–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon LA, Wolfner MF. 1995. A Drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proc Natl Acad Sci U S A. 92:10114–10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA, Loeschcke V. 2006Are fitness effects of density mediated by body size? Evidence from Drosophila field releases. Evol Ecol Res. 8:813–828. [Google Scholar]
- Keightley PD, Trivedi U, Thomson M, Oliver F, Kumar S, Blaxter ML. 2009. Analysis of the genome sequences of three Drosophila melanogaster spontaneous mutation accumulation lines. Genome Res. 19:1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern S, Ackermann M, Stearns SC, Kawecki TJ. 2001. Decline in offspring viability as a manifestation of aging in Drosophila melianogaster. Evolution. 55:1822–1831. [DOI] [PubMed] [Google Scholar]
- Koch RE, Phillips JM, Camus MF, Dowling DK. 2018. Maternal age effects on fecundity and offspring egg-to-adult viability are not affected by mitochondrial haplotype. Ecol Evol. 8:10722–10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labeyrie V. 1988. Maternal influences and the biology of insect populations. Mem Entomol Soc Can. 146:153–169. [Google Scholar]
- Lachaise D, Silvain J-F. 2004How two Afrotropical endemics made two cosmopolitan human commensals: the Drosophila melanogaster–D. simulans palaeogeographic riddle. In: Capy P, Gibert P, Boussy I, editors. Drosophila melanogaster, Drosophila simulans: so similar, so different. Dordrecht: Springer Netherlands; p. 17–39. [DOI] [PubMed] [Google Scholar]
- Lansing AI. 1947. A transmissible, cumulative, and reversible factor in aging. J Gerontol. 2:228–239. [DOI] [PubMed] [Google Scholar]
- Mansourian S, Enjin A, Jirle EV, Ramesh V, Rehermann G, Becher PG, Pool JE, Stensmyr MC. 2018. Wild African Drosophila melanogaster are seasonal specialists on marula fruit. Curr Biol. 28:3960–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JJ, Petersen L, Agerbo E, Mors O, Mortensen PB, Pedersen CB. 2014. A comprehensive assessment of parental age and psychiatric disorders. JAMA Psychiatry. 71:301–309. [DOI] [PubMed] [Google Scholar]
- Miller PB, Obrik-Uloho OT, Phan MH, Medrano CL, Renier JS, Thayer JL, Wiessner G, Bloch Qazi MC. 2014. The song of the old mother: reproductive senescence in female Drosophila. Fly (Austin). 8:127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montooth KL, Meiklejohn CD, Abt DN, Rand DM. 2010. Mitochondrial-nuclear epistasis affects fitness within species but does not contribute to fixed incompatibilities between species of Drosophila. Evolution. 64:3364–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman JA, Tross JG, Jourjine NA, Li N, Wu Z, Rand DM. 2017. Mitonuclear interactions mediate transcriptional responses to hypoxia in Drosophila. Mol Biol Evol. 34:447–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman JA, Tross JG, Li N, Wu Z, Rand DM. 2016. Mitochondrial–nuclear interactions mediate sex-specific transcriptional profiles in Drosophila. Genetics. 204:613–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousseau TA, Dingle H. 1991Maternal effects in insect life histories. Annu Rev Entomol. 36:511–534. [Google Scholar]
- Nystrand M, Dowling DK. 2014. Transgenerational interactions involving parental age and immune status affect female reproductive success in Drosophila melanogaster. Proc Biol Sci. 281:20141242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons PA. 1964. Parental age and the offspring. Q Rev Biol. 39:258–275. [DOI] [PubMed] [Google Scholar]
- Pischedda A, Stewart AD, Little MK, Rice WR. 2011Male genotype influences female reproductive investment in Drosophila melanogaster. Proc Biol Sci. 278:2165–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzari T, Dean R, Pacey A, Moore H, Bonsall MB. 2008. The evolutionary ecology of pre- and post-meiotic sperm senescence. Trends Ecol Evol. 23:131–140. [DOI] [PubMed] [Google Scholar]
- Plummer M, Best N, Cowles K, Vines K. 2006CODA: convergence diagnosis and output analysis for MCMC. R News. 6:1–25. [Google Scholar]
- Price DK, Hansen TF. 1998. How does offspring quality change with age in male Drosophila melanogaster? Behav Genet. 28:395–402. [DOI] [PubMed] [Google Scholar]
- Priest NK, Galloway LF, Roach DA. 2008. Mating frequency and inclusive fitness in Drosophila melanogaster. Am Nat. 171:10–21. [DOI] [PubMed] [Google Scholar]
- Priest NK, Mackowiak B, Promislow DE. 2002. The role of parental age effects on the evolution of aging. Evolution. 56:927–935. [DOI] [PubMed] [Google Scholar]
- Priest NK, Roach DA, Galloway LF. 2008. Cross-generational fitness benefits of mating and male seminal fluid. Biol Lett. 4:6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan J. 2003Male age, germline mutations and the benefits of polyandry. Ecol Lett. 6:581–586. [Google Scholar]
- Ram KR, Wolfner MF. 2009. A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proc Natl Acad Sci USA. 106:15384–15389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando OJ. 2012. Daddy issues: paternal effects on phenotype. Cell. 151:702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Valentín R, López-González I, Jorquera R, Labarca P, Zurita M, Reynaud E. 2006. Oviduct contraction in Drosophila is modulated by a neural network that is both, octopaminergic and glutamatergic. J Cell Physiol. 209:183–198. [DOI] [PubMed] [Google Scholar]
- Rubinstein CD, Wolfner MF. 2013. Drosophila seminal protein ovulin mediates ovulation through female octopamine neuronal signaling. Proc Natl Acad Sci USA. 110:17420–17425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M, Fowler K, Partridge L. 1994. Gene–environment interaction for body size and larval density in Drosophila melanogaster: an investigation of effects on development time, thorax length and adult sex ratio. Heredity (Edinb). 72(Pt 5):515–521. [DOI] [PubMed] [Google Scholar]
- Sartorius GA, Nieschlag E. 2010. Paternal age and reproduction. Hum Reprod Update. 16:65–79. [DOI] [PubMed] [Google Scholar]
- Sgrò CM, Geddes G, Fowler K, Partridge L. 2000. Selection on age at reproduction in Drosophila melanogaster: female mating frequency as a correlated response. Evolution. 54:2152–2155. [DOI] [PubMed] [Google Scholar]
- Sirot LK, Wolfner MF, Wigby S. 2011. Protein-specific manipulation of ejaculate composition in response to female mating status in Drosophila melanogaster. Proc Natl Acad Sci USA. 108:9922–9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CK, Pizzari T, Wigby S. 2013. Parental age, gametic age, and inbreeding interact to modulate offspring viability in Drosophila melanogaster. Evolution. 67:3043–3051. [DOI] [PubMed] [Google Scholar]
- Tarín JJ, Pérez-Albalá S, Cano A. 2000. Consequences on offspring of abnormal function in ageing gametes. Hum Reprod Update. 6:532–549. [DOI] [PubMed] [Google Scholar]
- Valtonen TM, Kangassalo K, Pölkki M, Rantala MJ. 2012. Transgenerational effects of parental larval diet on offspring development time, adult body size and pathogen resistance in Drosophila melanogaster. PLoS One. 7:e31611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijendravarma RK, Narasimha S, Kawecki TJ. 2010. Effects of parental larval diet on egg size and offspring traits in Drosophila. Biol Lett. 6:238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Cuesta E, Fan F, Rand DM. 2014. Rapamycin reduces Drosophila longevity under low nutrition. IOSR J Pharm. 4:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC, Melov S. 1998. Radicals r’aging. Nat Genet. 19:105–106. [DOI] [PubMed] [Google Scholar]
- Wang MH, vom Saal FS. 2000. Maternal age and traits in offspring—the timing of a mouse’s first litter influences the development of her pups. Nature. 407: 469–470. [DOI] [PubMed] [Google Scholar]
- Wolfner MF. 1997Tokens of love: functions and regulation of Drosophila male accessory gland products. Insect Biochem Mol. 27:179–192. [DOI] [PubMed] [Google Scholar]
- Zeh JA, Bonilla MM, Adrian AJ, Mesfin S, Zeh DW. 2012. From father to son: transgenerational effect of tetracycline on sperm viability. Sci Rep. 2:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
In accordance with the Journal of Heredity data archiving policy (Baker 2013), we have deposited the primary data underlying the analyses as follows: Phenotype data are uploaded as Supplementary Materials.
The datasets supporting this article have been uploaded as part of the Supplementary Materials.