Abstract
Aims
The effect of first-line antianginal agents, β-blockers, and calcium antagonists on clinical outcomes in stable coronary artery disease (CAD) remains uncertain.
Methods and results
We analysed the use of β-blockers or calcium antagonists (baseline and annually) and outcomes in 22 006 stable CAD patients (enrolled 2009–2010) followed annually to 5 years, in the CLARIFY registry (45 countries). Primary outcome was all-cause death. Secondary outcomes were cardiovascular death and the composite of cardiovascular death/non-fatal myocardial infarction (MI). After multivariable adjustment, baseline β-blocker use was not associated with lower all-cause death [1345 (7.8%) in users vs. 407 (8.4%) in non-users; hazard ratio (HR) 0.94, 95% confidence interval (CI) 0.84–1.06; P = 0.30]; cardiovascular death [861 (5.0%) vs. 262 (5.4%); HR 0.91, 95% CI 0.79–1.05; P = 0.20]; or cardiovascular death/non-fatal MI [1272 (7.4%) vs. 340 (7.0%); HR 1.03, 95% CI 0.91–1.16; P = 0.66]. Sensitivity analyses according to β-blocker use over time and to prescribed dose produced similar results. Among prior MI patients, for those enrolled in the year following MI, baseline β-blocker use was associated with lower all-cause death [205 (7.0%) vs. 59 (10.3%); HR 0.68, 95% CI 0.50–0.91; P = 0.01]; cardiovascular death [132 (4.5%) vs. 49 (8.5%); HR 0.52, 95% CI 0.37–0.73; P = 0.0001]; and cardiovascular death/non-fatal MI [212 (7.2%) vs. 59 (10.3%); HR 0.69, 95% CI 0.52–0.93; P = 0.01]. Calcium antagonists were not associated with any difference in mortality.
Conclusion
In this contemporary cohort of stable CAD, β-blocker use was associated with lower 5-year mortality only in patients enrolled in the year following MI. Use of calcium antagonists was not associated with superior mortality, regardless of history of MI.
Keywords: Stable coronary artery disease, Beta-blockers, Calcium antagonists, Prognosis, Mortality
See page 1408 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy874)
Introduction
β-blockers and calcium antagonists are recommended for treatment of angina in patients with stable coronary disease (CAD).1,2 They are widely used in stable CAD, including angina free patients3 even though their role in improving outcomes remains uncertain. Large observational series have shown association of β-blocker use with improved outcomes in acute coronary syndromes.4–6
Randomized clinical trials (RCTs) established unequivocal benefits of β-blockers in chronic heart failure with left ventricular (LV) systolic dysfunction7 and in relatively old trials in acute myocardial infarction (MI),8 but no trials assessed their benefit compared with placebo in stable CAD.9,10 Thus, the question was investigated in observational11–14 and post hoc trial analyses.15
Regarding calcium antagonists, there are no data supporting a benefit on mortality and a few trials supporting a benefit on cardiovascular morbidity compared with placebo in stable CAD.16,17 Trials comparing long-acting dihydropyridines18 or non-dihydropyridines19–21 with β-blockers in stable CAD found no difference in clinical outcomes.
In the absence of RCTs, we assessed the association between β-blocker or calcium antagonist use and clinical outcomes, focusing on mortality, in the prospective observational longitudinal registry of patients with stable CAD (CLARIFY; ISRCTN43070564) acknowledging that residual confounding factors from measured or unmeasured variables can persist even after adjustment.
Methods
Design, setting and participants
The present study is a post hoc analysis of the CLARIFY registry. The registry design has been previously described.3 Briefly, 32 703 stable CAD outpatients were enrolled (November 2009–June 2010) in 45 countries and followed annually up to 5 years.
Patients were eligible for enrolment if they fulfilled ≥1 of the following criteria (not mutually exclusive): documented MI >3 months ago; coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) >3 months ago; chest pain with proven myocardial ischaemia; or previous coronary angiography showing ≥1 coronary stenosis >50%. Exclusion criteria were hospitalization for cardiovascular disease within the previous 3 months; planned revascularization; and conditions interfering with life expectancy including severe heart failure. Medical care was at the discretion of each physician. Each year 1% of the sites were randomly selected for on-site audits of 100% of data reporting.
CLARIFY was conducted in accordance with the Declaration of Helsinki and local ethical approval was obtained in each country. All patients gave informed consent.
Study outcomes
The primary outcome was all-cause mortality. Secondary outcomes were cardiovascular mortality and the composite of cardiovascular mortality/non-fatal MI. Exploratory analyses examined non-cardiovascular mortality, MI (fatal/non-fatal), and stroke (fatal/non-fatal).
Cardiovascular mortality was defined as death following MI or stroke, and other cardiovascular mortality (death ascribed to heart failure, cardiac or vascular procedure/surgery, ruptured aneurysm, pulmonary embolism). Unknown causes of death and death that could not be definitely ascribed to non-cardiovascular mortality were classed as cardiovascular for analyses. Outcomes were not adjudicated, but reported by investigators.
Statistical analyses
β-blocker and calcium antagonist use was assessed at baseline and yearly. Patients with a history of MI were categorized according to the delay since prior MI at the time of enrolment in three subgroups: ≤1 year, 1 to ≤3 years, and >3 years post-MI.
To account for the treatment status (β-blockers or calcium antagonists) of the patient prior to the event, sensitivity analyses were performed using as covariates the last available record of the use of β-blocker or calcium antagonist before an event. To assess the impact of β-blocker daily doses, sensitivity analyses based on ≤half dose, half to ≤full dose, or full target dose were performed. Full doses were defined according to usual clinical therapeutic dose for each β-blocker. For the five most frequently used β-blockers with an indication for post-MI secondary prevention, for LV systolic dysfunction, or for angina relief, full target daily doses were defined as 10 mg for bisoprolol,22 200 mg for metoprolol,23 100 mg for atenolol,18 50 mg for carvedilol,24 and 10 mg for nebivolol.25
Hazard ratios (HRs) associated with β-blocker use vs. non-use and calcium antagonist use vs. non-use, and 95% confidence intervals (CIs) were calculated from Cox proportional hazards models. Hazard ratios were estimated after adjustment for cardiovascular risk factors, medical history of cardiovascular disease, treatments, geographical areas, and pulmonary comorbidities: systolic and diastolic blood pressure, LV ejection fraction, history of revascularization by PCI/CABG, asthma or chronic obstructive pulmonary disease (COPD), and the REACH cardiovascular event risk score.26 The REACH score (detailed in Supplementary material online, Table S1) was used to adjust for sex, age, current smoking, diabetes, body mass index <20 kg/m2, number of vascular beds involved (cerebrovascular, peripheral, and coronary artery disease), history of recent MI (≤1 year), heart failure or atrial fibrillation, statin or aspirin therapies, and geographical zones. Multivariable analyses were performed in patients who had a complete dataset for all the variables entered in the models, constituting the study cohort. In order to address missing data, especially regarding LV systolic function, a sensitivity analysis considered the same model with LV ejection fraction as a categorical variable (including a missing category).
The sensitivity analysis pertaining to the relation between β-blocker daily dose and outcomes, used an adjustment model based on the REACH risk score.
Data were managed and analysed by the Robertson Centre for Biostatistics (University of Glasgow, UK). All analyses were conducted using R and the survival package.27
Results
A total of 32 378 participants were available for analysis. At the end of 4 years 5111 (15.8%) and 5108 (15.8%) were censored respectively in the β-blocker analysis and the calcium antagonist analysis (details in Supplementary material online, Tables S2 and S3). Overall a complete baseline dataset (with follow-up and no missing covariates) was available for 22 006 (68.0%) in the β-blocker use analysis, and for 22 004 (68.0%) for the calcium antagonist analysis. The sensitivity analysis including patients in whom LV ejection fraction was missing involved 31 987 patients (98.8% of the total study population).
β-blocker analyses
At baseline, β-blockers were used in 17 135 (77.9%) patients. Among 4871 non-users, 1931 had a prior history of intolerance or contraindication to β-blockers, mainly due to asthma/COPD (n = 558), bradycardia (n = 555), fatigue (n = 551), erectile dysfunction (n = 270), and hypotension (n = 269). For patients in whom dose was available, 45.1% received less than half of the target dose, 41.6% half to less than full dose, and 13.3% the full target dose. The five most frequently used β-blockers were bisoprolol (35.6%), metoprolol (27.2%), carvedilol (12.6%), atenolol (12.3%), and nebivolol (6.5%).
Three quarters of patients were male. Compared with non-users, users were younger, had higher prevalence of cardiovascular risk factors, more frequent history of previous MI or hospitalization for heart failure, and less frequent asthma/COPD. Users had lower resting heart rate, similar blood pressure, and higher prevalence of current heart failure symptoms. Twenty-five percent of users had current angina despite β-blocker therapy. They had lower LV systolic function and received more frequently evidence-based preventive treatments. Despite these differences in baseline characteristics, the REACH cardiovascular risk score was similar between groups (Table 1). Baseline characteristics of all enrolled patients with data pertaining to β-blocker use gave similar results (Supplementary material online, Table S4).
Table 1.
Baseline characteristics among patients analysed in the multivariable adjusted model according to β-blocker use (n = 22 006) or calcium antagonist use (n = 22 004)
| Variables | β-blockers (n = 17 135) | No β-blockers (n = 4871) | P-value | Calcium antagonists (n = 5885) | No calcium antagonists (n = 16 119) | P-value |
|---|---|---|---|---|---|---|
| REACH cardiovascular event risk score | 11.00 (9.00–13.00) | 11.00 (9.00–13.00) | 11.00 (9.00–13.00) | 11.00 (9.00–13.00) | ||
| 11.2 ± 3.1 | 11.1 ± 3.2 | 0.49 | 11.5 ± 3.1 | 11.0 ± 3.2 | ||
| CV risk factors | ||||||
| Gender male | 78.3% | 78.0% | 0.70 | 74.0% | 79.8% | <0.0001 |
| Age (years) | 63.3 ± 10.5 | 65.5 ± 10.7 | <0.0001 | 65.7 ± 10.0 | 63.1 ± 10.7 | <0.0001 |
| Smoking | ||||||
| Current | 12.3% | 11.9% | 10.2% | 13.0% | ||
| Former | 46.5% | 46.8% | 45.3% | 47.0% | ||
| Never | 41.2% | 41.3% | 0.74 | 44.6% | 40.1% | <0.0001 |
| Family history of premature CAD | 29.1% | 28.3% | 0.33 | 29.2% | 28.8% | 0.55 |
| Treated hypertension | 74.0% | 66.1% | <0.0001 | 87.4% | 66.7% | <0.0001 |
| Diabetes | 30.4% | 26.6% | <0.0001 | 35.8% | 27.3% | <0.0001 |
| Dyslipidaemia | 79.0% | 78.0% | 0.12 | 82.9% | 77.3% | <0.0001 |
| Past medical history | ||||||
| Myocardial infarction | 64.8% | 52.5% | <0.0001 | 52.5% | 65.6% | <0.0001 |
| Percutaneous coronary intervention | 58.9% | 56.8% | 0.01 | 55.6% | 59.5% | <0.0001 |
| Peripheral arterial disease | 10.3% | 13.1% | <0.0001 | 14.1% | 9.8% | <0.0001 |
| Carotid disease | 8.9% | 9.0% | 0.82 | 12.0% | 7.8% | <0.0001 |
| Stroke | 3.8% | 4.4% | 0.06 | 5.4% | 3.4% | <0.0001 |
| Hospitalization for CHF | 5.9% | 4.6% | 0.0006 | 5.1% | 5.8% | 0.07 |
| Atrial fibrillation/flutter | 7.8% | 7.5% | 0.53 | 8.3% | 7.5% | 0.04 |
| Asthma/COPD | 5.0% | 5.8% | <0.0001 | 10.0% | 6.4% | <0.0001 |
| Clinical examination | ||||||
| Systolic blood pressure (mmHg) | 131.0 ± 16.8 | 131.1 ± 15.8 | 0.61 | 135.3 ± 16.7 | 129.5 ± 16.2 | <0.0001 |
| Diastolic blood pressure (mmHg) | 77.7 ± 10.0 | 77.2 ± 9.6 | 0.001 | 78.4 ± 10.2 | 77.3 ± 9.8 | <0.0001 |
| Resting heart rate (b.p.m.) | 67.4 ± 10.3 | 69.7 ± 11.3 | <0.0001 | 68.4 ± 10.6 | 67.7 ± 10.6 | <0.0001 |
| Current angina | 25.0% | 21.4% | <0.0001 | 28.1% | 22.8% | <0.0001 |
| Current heart failure symptoms | 20.1% | 13.0% | <0.0001 | 18.9% | 18.4% | 0.43 |
| LVEF measurements | ||||||
| Mean LVEF % | 55.6 ± 11.1 | 58.0 ± 10.4 | <0.0001 | 57.9 ± 10.1 | 55.5 ± 11.2 | <0.0001 |
| LVEF ≤45% | 18.7% | 12.8% | <0.0001 | 12.3% | 19.3% | <0.0001 |
| Medications | ||||||
| Aspirin | 89.1% | 83.8% | <0.0001 | 86.7% | 88.3% | 0.002 |
| Thienopyridine | 28.5% | 29.5% | 0.16 | 28.6% | 28.7% | 0.81 |
| Dual antiplatelet therapy | 28.9% | 27.0% | 0.009 | 26.8% | 28.3% | 0.03 |
| Statins | 85.6% | 79.8% | <0.0001 | 84.1% | 84.4% | 0.49 |
| Angiotensin-converting enzyme inhibitors | 57.2% | 42.7% | <0.0001 | 48.8% | 55.9% | <0.0001 |
| Angiotensin II receptor blockers | 25.5% | 31.5% | <0.0001 | 35.6% | 23.6% | <0.0001 |
| β-blockers | NA | NA | NA | 69.6% | 80.9% | <0.0001 |
| Calcium antagonists | 23.9% | 36.7% | <0.0001 | NA | NA | NA |
Data are % for categorical data, and mean ± standard deviation or median (interquartile range) for continuous data depending on the distribution.
CAD, coronary artery disease; CHF, chronic heart failure; COPD, chronic obstructive pulmonary disease; NA, not available.
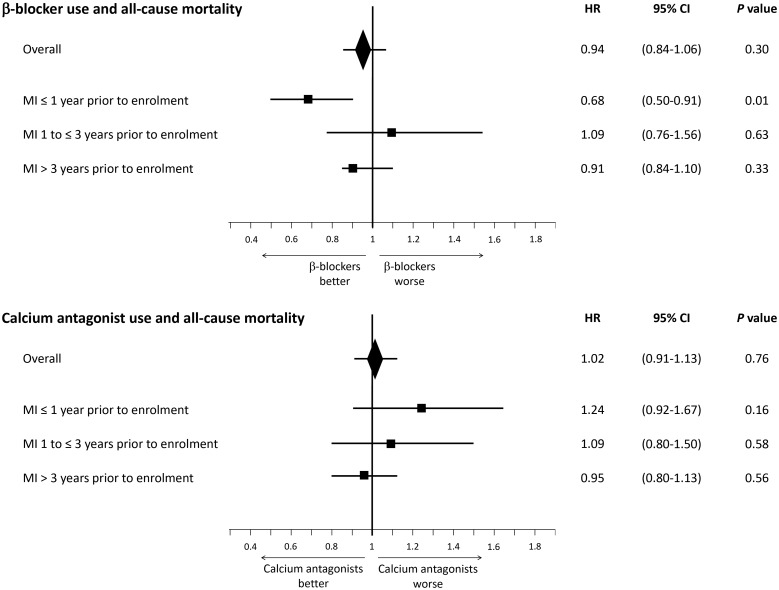
Overall, the rate of death was 1.80 per 100 patient years. Event rates are detailed in Supplementary material online,Table S5. For the primary and secondary outcomes, multivariable adjusted HRs showed no association between β-blocker use at baseline and outcomes. The HR for all-cause mortality was 0.94 (95% CI 0.84–1.06) (Take home figure, Table 2). Hazard ratios for cardiovascular mortality and the composite of cardiovascular mortality/non-fatal MI were 0.91 (95% CI 0.79–1.05) and 1.03 (95% CI 0.91–1.16), respectively. Regarding exploratory analyses, there were no associations between β-blockers and outcomes (Table 2).
Take home figure.
Multivariable adjusted associations with all-cause mortality according to β-blocker use and calcium antagonist use at baseline, overall and after categorization by time since myocardial infarction prior to enrolment. P-values, hazard ratios, and confidence intervals are derived from comparing β-blocker users to non-users and calcium antagonist users to non-users at baseline in a survival analysis from Cox proportional hazards models with multivariable adjustment for the REACH cardiovascular event risk score,26 systolic/blood pressure, left ventricular ejection fraction, history of coronary revascularization, peripheral artery disease, and asthma/chronic obstructive pulmonary disease. CI, confidence interval; HR, hazard ratio; MI, myocardial infarction.
Table 2.
Multivariable adjusted associations according to β-blocker use and calcium antagonist use at baseline
| 5-Year outcomes | β-blockers (n = 17 135) | No β-blockers (n = 4871) | HR (95% CI) | P-value | Calcium antagonists (n = 5885) | No calcium antagonists (n = 16 119) | HR (95% CI) | P-value |
|---|---|---|---|---|---|---|---|---|
| Primary outcome | ||||||||
| All-cause mortality | 1345 (7.8%) | 407 (8.4%) | 0.94 (0.84–1.06) | 0.30 | 493 (8.4%) | 1259 (7.8%) | 1.02 (0.91–1.13) | 0.76 |
| Secondary outcomes | ||||||||
| Cardiovascular mortality | 861 (5.0%) | 262 (5.4%) | 0.91 (0.79–1.05) | 0.20 | 311 (5.3%) | 812 (5.0%) | 1.01 (0.88–1.16) | 0.87 |
| Cardiovascular mortality/non-fatal MI | 1272 (7.4%) | 340 (7.0%) | 1.03 (0.91–1.16) | 0.66 | 457 (7.8%) | 1155 (7.2%) | 1.05 (0.94–1.17) | 0.39 |
| Exploratory analyses | ||||||||
| Non-cardiovascular mortality | 484 (2.8%) | 145 (3.0%) | 1.00 (0.83–1.21) | 0.99 | 182 (3.1%) | 447 (2.8%) | 1.03 (0.86–1.22) | 0.77 |
| MI | 587 (3.4%) | 140 (2.9%) | 1.14 (0.94–1.37) | 0.18 | 214 (3.6%) | 513 (3.2%) | 1.12 (0.95–1.32) | 0.17 |
| Stroke | 375 (2.2%) | 92 (1.9%) | 1.13 (0.89–1.42) | 0.32 | 150 (2.5%) | 317 (2.0%) | 1.18 (0.96–1.43) | 0.11 |
HRs, CIs, and P-values are derived from comparing β-blocker users/non-users and calcium antagonist users/non-users at baseline in a survival analysis using Cox proportional hazards models with multivariable adjustment for the REACH cardiovascular event score,26 systolic/diastolic blood pressure, left ventricular ejection fraction, history of percutaneous coronary artery, coronary artery bypass graft, peripheral artery disease, and asthma/chronic obstructive pulmonary disease.
CI, confidence interval; HR, hazard ratio; MI, myocardial infarction.
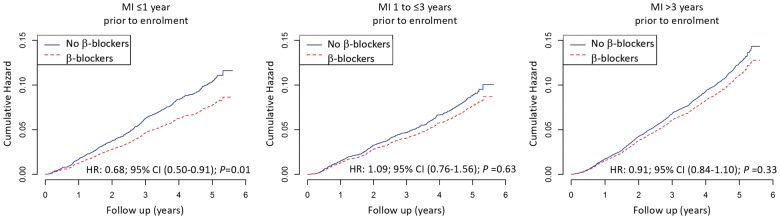
Among patients with MI ≤1 year prior to enrolment, β-blocker use was associated with a lower risk of all-cause mortality [205 (7.0%) for users vs. 59 (10.3%) for non-users, HR 0.68, 95% CI 0.50–0.91; P = 0.01], lower cardiovascular mortality [132 (4.5%) for users vs. 49 (8.5%) for non-users, HR 0.52, 95% CI 0.37–0.73; P = 0.0001], and lower cardiovascular mortality/non-fatal MI [212 (7.2%) for users vs. 59 (10.3%) for non-users, HR 0.69, 95% CI 0.52–0.93; P = 0.01]. In patients with prior MI >1 year, there was no difference in outcomes between groups (Figure 1, Take home figure, and Table 3).
Figure 1.
Cumulative hazard of all-cause mortality according to ß-blocker use at baseline, categorized by the time elapsed since the index myocardial infarction prior to enrolment. P-values, hazard ratios, and confidence intervals are derived from comparing β-blocker users to non-users at baseline in a survival analysis from Cox proportional hazards models with multivariable adjustment for the REACH cardiovascular event risk score,26 systolic/blood pressure, left ventricular ejection fraction, history of coronary revascularization, peripheral artery disease, and asthma/chronic obstructive pulmonary disease. CI, confidence interval; HR, hazard ratio; MI, myocardial infarction.
Table 3.
Multivariable adjusted associations according to β-blocker use at baseline categorized by the time elapsed since the index MI prior to enrolment
| 5-Year outcomes | MI ≤1 year |
1 year < MI ≤ 3 years |
MI >3 years |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 3506) |
(n = 2932) |
(n = 7222) |
|||||||||||
| β-blockers (n = 2931) | No β-blockers (n = 575) | HR (95% CI) | P-value | β-blockers (n = 2426) | No β-blockers (n = 506) | HR (95% CI) | P-value | β-blockers (n = 5746) | No β-blockers (n = 1476) | HR (95% CI) | P-value | ||
| Primary outcome | |||||||||||||
| All-cause mortality | 205 (7.0%) | 59 (10.3%) | 0.68 (0.50–0.91) | 0.01 | 177 (7.3%) | 40 (7.9%) | 1.09 (0.76–1.56) | 0.63 | 551 (9.6%) | 158 (10.7%) | 0.91 (0.84–1.10) | 0.33 | |
| Secondary outcomes | |||||||||||||
| Cardiovascular mortality | 132 (4.5%) | 49 (8.5%) | 0.52 (0.37–0.73) | 0.0001 | 111 (4.6%) | 28 (5.5%) | 0.99 (0.64–1.53) | 0.96 | 366 (6.4%) | 104 (7.0%) | 0.90 (0.72–1.13) | 0.37 | |
| Cardiovascular mortality/non-fatal MI | 212 (7.2%) | 59 (10.3%) | 0.69 (0.52–0.93) | 0.01 | 173 (7.1%) | 37 (7.3%) | 1.05 (0.73–1.52) | 0.78 | 513 (8.9%) | 132 (8.9%) | 1.00 (0.82–1.21) | 0.97 | |
| Exploratory analyses | |||||||||||||
| Non-CV mortality | 73 (2.5%) | 10 (1.7%) | 1.42 (0.73–2.77) | 0.31 | 66 (2.7%) | 12 (2.4%) | 1.32 (0.70–2.51) | 0.39 | 185 (3.2%) | 54 (3.7%) | 0.94 (0.69–1.28) | 0.69 | |
| MI | 122 (4.2%) | 27 (4.7%) | 0.85 (0.56–1.31) | 0.47 | 88 (3.6%) | 15 (3.0%) | 1.25 (0.71–2.18) | 0.44 | 222 (3.9%) | 51 (3.5%) | 1.10 (0.81–1.50) | 0.54 | |
| Stroke | 60 (2.0%) | 8 (1.4%) | 1.45 (0.69–3.06) | 0.33 | 49 (2.0%) | 11 (2.2%) | 1.04 (0.52–2.06) | 0.91 | 150 (2.6%) | 40 (2.7%) | 0.98 (0.69–1.40) | 0.92 | |
HRs, CIs, and P-values are derived from comparing β-blocker users to non-users at baseline, with categorization by the time elapsed since MI prior to enrolment, in a survival analysis using Cox proportional hazards models with multivariable adjustment for the REACH cardiovascular event score,26 systolic/diastolic blood pressure, left ventricular ejection fraction, history of percutaneous coronary artery, coronary artery bypass graft, peripheral artery disease, and asthma/chronic obstructive pulmonary disease.
CI, confidence interval; HR, hazard ratio; MI, myocardial infarction.
In patients with no history of PCI, β-blocker use at baseline was associated with lower cardiovascular death [427 (6.1%) for users vs. 155 (7.4%) for non-users and HR 0.81, 95% CI 0.67–0.98; P = 0.03] (Supplementary material online, Table S6). There was no association between β-blocker use and mortality after categorization by presence of angina (Supplementary material online, Table S7). Sensitivity analyses according to the last assessment of β-blocker use prior to an event showed consistent results with no difference in risk for all outcomes except for an association with stroke (HR 1.39, 95% CI 1.10–1.74; P = 0.005) (Supplementary material online, Table S8).
Sensitivity analyses pertaining to β-blocker doses showed consistent results (Supplementary material online,Table S9).
When considering LV ejection fraction as a categorical variable and including a missing category, analyses performed among 31 987 patients showed consistent results. β-blocker use (n = 24 119) compared with non-use (n = 7868) was not associated with lower all-cause death [1856 (7.7%) and 666 (8.5%), respectively with a HR 0.96, 95% CI 0.87–1.05; P = 0.33] or lower cardiovascular death [1184 (4.9%) and 419 (5.3%), respectively with HR 0.95, 95% CI 0.85–1.07; P = 0.43] (Supplementary material online, Table S10).
Calcium antagonist analyses
At baseline, calcium antagonists were used in 5885 (26.7%) patients. Among users, 4697 (79.8%) were receiving long-acting dihydropyridines, 864 (14.7%) diltiazem and 286 (4.9%) verapamil.
Users included more females, were older, with higher prevalence of cardiovascular risk factors, more frequent history of peripheral artery disease, carotid disease, stroke or asthma/COPD, and less frequent history of prior MI or hospitalization for heart failure. Users had higher resting heart rate and blood pressure. They had higher prevalence of current anginal symptoms, despite calcium antagonist therapy, and higher LV ejection fraction. Users had less antiplatelet therapy, more angiotensin II receptor blockers. Despite these differences, the REACH cardiovascular risk score was similar between groups (Table 1). Baseline characteristics of all enrolled patients with data pertaining to calcium antagonist use found similar results (Supplementary material online, Table S4).
Overall the rate of death was 1.80 per 100 patient years. Event rates are detailed in Supplementary material online, Table S11. Multivariable adjusted HRs for the primary and secondary outcomes, as well as exploratory analyses showed no association between calcium antagonist use at baseline and any outcome (Take home figure, Table 2).
There was no association between calcium antagonist use and mortality, regardless of the history of PCI or presence of angina (Supplementary material online, Tables S12 and S13). In patients with MI in the year preceding enrolment, there was no association between calcium antagonist use and mortality (Take home figure, Table 4).
Table 4.
Multivariable adjusted associations according to calcium antagonists use at baseline categorized by the time elapsed since the index MI prior to enrolment
| 5-Year outcomes | MI ≤1 year |
1 year < MI ≤ 3 years |
MI >3 years |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 3506) |
(n = 2931) |
(n = 7222) |
||||||||||
| Calcium antagonists (n = 594) | No calcium antagonists (n = 2912) | HR (95% CI) | P-value | Calcium antagonists (n = 617) | No calcium antagonists (n = 2314) | HR (95% CI) | P-value | Calcium antagonists (n = 1880) | No calcium antagonists (n = 5342) | HR (95% CI) | P-value | |
| Primary outcome | ||||||||||||
| All-cause mortality | 58 (9.8%) | 206 (7.1%) | 1.24 (0.92–1.67) | 0.16 | 53 (8.6%) | 164 (7.1%) | 1.09 (0.80–1.50) | 0.58 | 183 (9.7%) | 526 (9.8%) | 0.95 (0.80–1.13) | 0.56 |
| Secondary outcomes | ||||||||||||
| Cardiovascular mortality | 36 (6.1%) | 145 (5.0%) | 1.12 (0.77–1.63) | 0.55 | 34 (5.5%) | 105 (4.5%) | 1.09 (0.73–1.62) | 0.68 | 121 (6.4%) | 349 (6.5%) | 0.97 (0.78–1.19) | 0.74 |
| Cardiovascular mortality/non-fatal MI | 52 (8.8%) | 219 (7.5%) | 1.05 (0.77–1.44) | 0.74 | 50 (8.1%) | 160 (6.9%) | 1.05 (0.76–1.45) | 0.77 | 176 (9.4%) | 469 (8.8%) | 1.05 (0.88–1.26) | 0.58 |
| Exploratory analyses | ||||||||||||
| Non-cardiovascular mortality | 22 (3.7%) | 61 (2.1%) | 1.46 (0.88–2.40) | 0.14 | 19 (3.1%) | 59 (2.5%) | 1.10 (0.65–1.87) | 0.72 | 62 (3.3%) | 177 (3.3%) | 0.93 (0.69–1.25) | 0.58 |
| MI | 27 (4.5%) | 122 (4.2%) | 0.97 (0.63–1.49) | 0.89 | 23 (3.7%) | 80 (3.5%) | 0.95 (0.59–1.53) | 0.84 | 83 (4.4%) | 190 (3.6%) | 1.24 (0.96–1.62) | 0.10 |
| Stroke | 16 (2.7%) | 52 (1.8%) | 1.38 (0.78–2.45) | 0.27 | 16 (2.6%) | 44 (1.9%) | 1.19 (0.66–2.13) | 0.57 | 55 (2.9%) | 135 (2.5%) | 1.00 (0.73–1.38) | 0.98 |
HRs, CIs, and P-values are derived from comparing calcium antagonist users to non-users at baseline, with categorization by the time elapsed since MI prior to enrolment in a survival analysis using Cox proportional hazards models with multivariable adjustment for the REACH cardiovascular event score,26 systolic/diastolic blood pressure, left ventricular ejection fraction, history of percutaneous coronary artery, coronary artery bypass graft, peripheral artery disease, and asthma/chronic obstructive pulmonary disease.
CI, confidence interval; HR, hazard ratio; MI, myocardial infarction.
Sensitivity analyses considering the last assessment of calcium antagonist use prior to an event gave consistent results (Supplementary material online, Table S8).
When considering LV ejection fraction as a categorical variable and including a missing category, analyses performed among 31 984 patients showed consistent results. Calcium antagonist use (n = 8735) compared with non-use (n = 23 249) was not associated with lower all-cause death [722 (8.3%) and 1800 (7.7%), respectively with HR 0.97, 95% CI 0.89–1.06; P = 0.56] or lower cardiovascular death [456 (5.2%) and 1147 (4.9%), respectively with HR 0.98, 95% CI 0.87–1.09; P = 0.67] (Supplementary material online, Table S10).
Discussion
In this large international study of contemporary stable CAD with a high rate use of evidence-based secondary prevention therapies, β-blocker use at baseline was associated with lower 5-year all-cause mortality only in patients enrolled in the year following MI.
Results were consistent when considering change of β-blocker use before an event and doses of β-blockers. The use of calcium antagonists was not associated with any differences in outcome. β-blocker use appeared associated with lower cardiovascular mortality in patients with no history of PCI.
No RCT has shown a benefit of β-blockers compared with placebo on hard clinical outcomes in stable CAD without LV systolic dysfunction. Evidence of these agents improving outcome is derived from old trials in acute MI, mostly performed before the advent of secondary prevention and reperfusion therapies; the benefit of β-blockers was mainly driven by their use in the acute phase of MI.10
Observational studies and post hoc RCT analyses have questioned the benefit from β-blockers on mortality, notwithstanding limitations in these studies relating to sample size, geographical scope, or selected patient profile. FAST-MI12 found no association between β-blocker cessation 1 year after MI and all-cause mortality. A French reimbursement database study14 showed no impact on mortality of stopping β-blockers beyond 1 year after MI without heart failure. The Kaiser Permanente study13 showed evidence of lower risk of death with β-blocker use in patients enrolled in the acute phase after MI. The REACH11 registry found no association between β-blocker use and mortality in stable CAD outpatients but suggested benefit in patients with recent prior MI (<1 year); however, LV function and β-blocker type or dose were unknown. The present results are consistent with a post hoc analysis of the CHARISMA trial15 showing no association between β-blocker use and lower mortality in a selected trial population of stable patients with or without prior MI. However, outcomes may differ between the selected patient populations enrolled in clinical trials and patients enrolled using similar criteria in population based observational studies.28,29
In the assessment of calcium antagonists in stable CAD (Supplementary material online, Table S14), the ACTION16 trial failed to demonstrate improved mortality compared with placebo. TIBET18 and APSIS19 trials did not show any improvement in mortality when compared with β-blockers. Other RCTs performed in hypertensive CAD patients failed to demonstrate an improvement in mortality with calcium antagonists compared with placebo17 or compared with β-blocker.20 A meta-analysis21 did not find lower mortality. There are no robust observational studies from which we can draw conclusions.
While RCTs are the gold standard to test the efficacy of medical therapies,30 observational studies may be useful when evaluating the impact of long-term use of drugs in broad patient populations.28
There are several strengths to the current analyses. CLARIFY was a large international study encompassing various profiles within stable CAD (e.g. with or without prior MI or prior revascularization and with or without angina or ischaemia) and capturing LV function, uses of treatments over time, doses of β-blockers, and also detailed information regarding contraindications or intolerance to β-blockers. Multivariable adjustment used the REACH risk score as it correlates well with outcomes.26 Finally, the most robust outcome, all-cause mortality, was the main outcome of the present analysis, and the results were consistent when accounting for β-blocker or calcium antagonist status over time, dose, after categorization by presence of angina or history of PCI or when including patients with missing LV ejection fraction in the adjustment model.
These findings about β-blockers as well as those from previous studies are contrary to the preconceived notion that they are beneficial for all CAD patients. β-blockers demonstrated their benefit in term of clinical outcomes in settings where there was sympathetic neuro-hormonal activation (LV systolic dysfunction, congestive heart failure, and acute MI). The absence of benefit in stable CAD without LV dysfunction or stabilized CAD remote from MI may be explained by the lack of sympathetic activation. Regarding calcium antagonists and mortality in a contemporary cohort, our findings are consistent with the results from available RCTs.16
Limitations
There are several limitations to this analysis. Analyses were not formally prespecified before data collection and the methods of analysis were not prospectively defined. Patients with severe heart failure or life-threatening arrhythmias as a complication to recent MI and in whom the benefit of β-blockers is clear were excluded. Outcomes were not adjudicated. However, the results were consistent for non-adjudicated outcomes and for all-cause mortality. Observational studies have inherent limitations: residual confounding from measured or unmeasured variables cannot be excluded (related to both contraindication and indication bias) and could contribute to the observed differences in mortality between groups. In addition, enrolment and follow-up did not start at the time of treatment initiation or at the time of diagnosis, which can cause bias. A third of patients without β-blocker at baseline were nor treated because of prior symptoms of intolerance or contraindication, which may reflect imbalance in baseline risk between groups related in particular to comorbidities such as asthma or COPD. It is noteworthy, however, that the REACH score at baseline was nearly identical between β-blocker users and non-users.
Conclusions
In this large contemporary cohort of stable CAD after multivariable adjustment including LV ejection fraction, β-blockers were not associated with lower all-cause mortality or improved outcomes, except in patients enrolled in the year following an MI. The use of calcium antagonists was not associated with superior outcomes, regardless of a prior history of MI.
Considering previous studies and this present analysis, β-blockers should be preferentially used in the first year following MI. Beyond 1 year following MI or in stable CAD patients without prior MI, both β-blockers and calcium antagonists may be used for symptom relief but a mortality benefit should not be assumed. A large adequately powered RCT would be required to settle the issue of whether first line anti-ischaemic agents impact prognosis and outcomes in patients with stable CAD. However, it is uncertain whether it would be feasible to mobilize the resources and patient numbers to adequately address this question.
Supplementary Material
Acknowledgements
Servier supported the CLARIFY study. Dr Sorbets was supported by the The French Federation of Cardiology. Editorial support, limited to editing, checking content and language, and formatting, was provided by Sophie Rushton-Smith, PhD (MedLink Healthcare Communications), funded by Servier.
Funding
The CLARIFY registry was supported by Servier. The sponsor had no role in the study design or in data analysis, and interpretation; or in the decision to submit the manuscript for publication, but assisted with the set-up, data collection and management of the study in each country. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict of interest: E.S. reports grants from French Federation of Cardiology, personal fees and non-financial support from Servier, during the conduct of the study; personal fees and non-financial support from Novartis, personal fees and non-financial support from Bayer, personal fees and non-financial support from Astra-Zeneca, personal fees and non-financial support from Merck Sharpe & Dohme, outside the submitted work. P.G.S. reports grants and personal fees from Servier, during the conduct of the study; grants and personal fees from Bayer/Janssen, grants and personal fees from Merck, grants and personal fees from Sanofi, grants and personal fees from Amarin, personal fees from Amgen, personal fees from Bristol Myers Squibb, personal fees from Boehringer-Ingelheim, personal fees from Pfizer, personal fees from Novartis, personal fees from Regeneron, personal fees from Lilly, personal fees from AstraZeneca, outside the submitted work. N.D. reports personal fees from Servier, during the conduct of the study; grants and personal fees from Amgen, grants and personal fees from Bayer, grants, personal fees and non-financial support from Astra-Zeneca, grants and personal fees from Boerhinger Ingelheim, grants from Daiichi Sankyo, grants and personal fees from Eli Lilly, grants and personal fees from MSD, grants from Novartis, personal fees from Novo Nordisk, grants and personal fees from Pfizer, grants and personal fees from Sanofi, outside the submitted work. I.F. reports grants and personal fees from Servier, during the conduct of the study. M.T. reports personal fees from Servier, during the conduct of the study; personal fees from Bayer, personal fees from Celyad, personal fees from Janssen Cilag, personal fees from Kowa, grants from National Center for Research and Development (Poland), personal fees from Perfuse Group, personal fees from Servier, outside the submitted work. R.F. reports grants and personal fees from Servier International, during the conduct of the study; personal fees from Merck Serono, personal fees from Bayer, grants and personal fees from Novartis, outside the submitted work. B.M. reports other from Boehringer Ingelheim, other from MSD, outside the submitted work. J.C.T. reports personal fees from Servier, during the conduct of the study; grants from Amarin, grants from Astra Zeneca, grants, personal fees and other from DalCor, grants from Esperion, grants from Ionis, grants from Merck, grants and personal fees from Pfizer, grants and personal fees from Sanofi, grants and personal fees from Servier, outside the submitted work. K.M.F. reports personal fees and non-financial support from Servier, during the conduct of the study; personal fees from AstraZeneca, personal fees from TaurX, non-financial support from Armgo, personal fees and non-financial support from Broadview Ventures, personal fees from CellAegis, personal fees from Celixir, outside the submitted work; Director of Vesalius Trials Ltd; And minimal stockholder of Armgo and CellAegis. All other authors have declared no conflict of interest.
References
- 1. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Ryden L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL.. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 2. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr, Smith SC Jr, Spertus JA, Williams SVAmerican College of Cardiology Foundation. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012;126:e354–e471. [DOI] [PubMed] [Google Scholar]
- 3. Sorbets E, Greenlaw N, Ferrari R, Ford I, Fox KM, Tardif J-C, Tendera M, Steg PG.. Rationale, design, and baseline characteristics of the CLARIFY registry of outpatients with stable coronary artery disease. Clin Cardiol 2017;40:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D'Ascenzo F, Celentani D, Brustio A, Grosso A, Raposeiras-Roubin S, Abu-Assi E, Henriques JPS, Saucedo J, Gonzalez-Juanatey JR, Wilton SB, Kikkert WJ, Nunez-Gil I, Ariza-Sole A, Song X, Alexopoulos D, Liebetrau C, Kawaji T, Huczek Z, Nie SP, Fujii T, Correia L, Kawashiri MA, Garcia-Acuna JM, Southern D, Alfonso E, Terol B, Garay A, Zhang D, Chen Y, Xanthopoulou I, Osman N, Mollmann H, Shiomi H, Kowara M, Filipiak K, Wang X, Yan Y, Fan JY, Ikari Y, Nakahayshi T, Sakata K, Yamagishi M, Kalpak O, Kedev S, Moretti C, D'Amico M, Gaita F.. Association of beta-blockers with survival on patients presenting with ACS treated with PCI: a propensity score analysis from the BleeMACS registry. Am J Cardiovasc Drugs 2018;18:299–309. [DOI] [PubMed] [Google Scholar]
- 5. Dondo TB, Hall M, West RM, Jernberg T, Lindahl B, Bueno H, Danchin N, Deanfield JE, Hemingway H, Fox KAA, Timmis AD, Gale CP.. Beta-blockers and mortality after acute myocardial infarction in patients without heart failure or ventricular dysfunction. J Am Coll Cardiol 2017;69:2710–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldberger JJ, Bonow RO, Cuffe M, Liu L, Rosenberg Y, Shah PK, Smith SC Jr, Subacius H OBTAIN Investigators. Effect of beta-blocker dose on survival after acute myocardial infarction. J Am Coll Cardiol 2015;66:1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH.. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996;334:1349–1355. [DOI] [PubMed] [Google Scholar]
- 8.ISIS-1 investigators. Randomised trial of intravenous atenolol among 16 027 cases of suspected acute myocardial infarction. First International Study of Infarct Survival Collaborative Group. Lancet 1986;2:57–66. [PubMed] [Google Scholar]
- 9. Freemantle N, Cleland J, Young P, Mason J, Harrison J. β Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ 1999;318:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bangalore S, Makani H, Radford M, Thakur K, Toklu B, Katz SD, DiNicolantonio JJ, Devereaux PJ, Alexander KP, Wetterslev J, Messerli FH.. Clinical outcomes with beta-blockers for myocardial infarction: a meta-analysis of randomized trials. Am J Med 2014;127:939–953. [DOI] [PubMed] [Google Scholar]
- 11. Bangalore S, Steg G, Deedwania P, Crowley K, Eagle KA, Goto S, Ohman EM, Cannon CP, Smith SC, Zeymer U, Hoffman EB, Messerli FH, Bhatt DL. β -Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA 2012;308:1340–1349. [DOI] [PubMed] [Google Scholar]
- 12. Puymirat E, Riant E, Aissaoui N, Soria A, Ducrocq G, Coste P, Cottin Y, Aupetit JF, Bonnefoy E, Blanchard D, Cattan S, Steg G, Schiele F, Ferrières J, Juillière Y, Simon T, Danchin N.. Beta-blockers and mortality after myocardial infarction in patients without heart failure: multicentre prospective cohort study. BMJ 2016;354:i4801.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andersson C, Shilane D, Go AS, Chang TI, Kazi D, Solomon MD, Boothroyd DB, Hlatky MA.. Beta-blocker therapy and cardiac events among patients with newly diagnosed coronary heart disease. J Am Coll Cardiol 2014;64:247–252. [DOI] [PubMed] [Google Scholar]
- 14. Neumann A, Maura G, Weill A, Alla F, Danchin N.. Clinical events after discontinuation of beta-blockers in patients without heart failure optimally treated after acute myocardial infarction: a cohort study on the French Healthcare Databases. Circ Cardiovasc Qual Outcomes 2018;11:e004356. [DOI] [PubMed] [Google Scholar]
- 15. Bangalore S, Bhatt DL, Steg PG, Weber MA, Boden WE, Hamm CW, Montalescot G, Hsu A, Fox KA, Lincoff AM.. Beta-blockers and cardiovascular events in patients with and without myocardial infarction: post-hoc analysis from the CHARISMA trial. Circ Cardiovasc Qual Outcomes 2014;7:872–881. [DOI] [PubMed] [Google Scholar]
- 16. Poole-Wilson PA, Lubsen J, Kirwan BA, van Dalen FJ, Wagener G, Danchin N, Just H, Fox KA, Pocock SJ, Clayton TC, Motro M, Parker JD, Bourassa MG, Dart AM, Hildebrandt P, Hjalmarson A, Kragten JA, Molhoek GP, Otterstad JE, Seabra-Gomes R, Soler-Soler J, Weber S Coronary disease Trial Investigating Outcome with Nifedipine gastrointestinal therapeutic system investigators. . Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet 2004;364:849–857. [DOI] [PubMed] [Google Scholar]
- 17. Nissen SE, Tuzcu EM, Libby P, Thompson PD, Ghali M, Garza D, Berman L, Shi H, Buebendorf E, Topol EJ, Investigators C.. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA 2004;292:2217–2225. [DOI] [PubMed] [Google Scholar]
- 18. Dargie HJ, Ford I, Fox KM.. Total ischaemic burden European Trial (TIBET). Effects of ischaemia and treatment with atenolol, nifedipine SR and their combination on outcome in patients with chronic stable angina. The TIBET Study Group. Eur Heart J 1996;17:104–112. [DOI] [PubMed] [Google Scholar]
- 19. Rehnqvist N, Hjemdahl P, Billing E, Bjorkander I, Eriksson SV, Forslund L, Held C, Nasman P, Wallen NH.. Effects of metoprolol vs verapamil in patients with stable angina pectoris. The Angina Prognosis Study in Stockholm (APSIS). Eur Heart J 1996;17:76–81. [DOI] [PubMed] [Google Scholar]
- 20. Pepine CJ, Handberg EM, Cooper-DeHoff RM, Marks RG, Kowey P, Messerli FH, Mancia G, Cangiano JL, Garcia-Barreto D, Keltai M, Erdine S, Bristol HA, Kolb HR, Bakris GL, Cohen JD, Parmley WW, Investigators I.. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003;290:2805–2816. [DOI] [PubMed] [Google Scholar]
- 21. Bangalore S, Parkar S, Messerli FH.. Long-acting calcium antagonists in patients with coronary artery disease: a meta-analysis. Am J Med 2009;122:356–365. [DOI] [PubMed] [Google Scholar]
- 22.CIBIS-II investigators. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9–13. [PubMed] [Google Scholar]
- 23. Chen ZM, Pan HC, Chen YP, Peto R, Collins R, Jiang LX, Xie JX, Liu LS.. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366:1622–1632. [DOI] [PubMed] [Google Scholar]
- 24. Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001;357:1385–1390. [DOI] [PubMed] [Google Scholar]
- 25. Flather MD, Shibata MC, Coats AJS, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, Soler-Soler J, Tavazzi L, Spinarova L, Toman J, BöHm M, Anker SD, Thompson SG, Poole-Wilson PA; SENIOR Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005;26:215–225. [DOI] [PubMed] [Google Scholar]
- 26. Wilson PW, D'Agostino R Sr, Bhatt DL, Eagle K, Pencina MJ, Smith SC, Alberts MJ, Dallongeville J, Goto S, Hirsch AT, Liau CS, Ohman EM, Rother J, Reid C, Mas JL, Steg PG REACH Registry. An international model to predict recurrent cardiovascular disease. Am J Med 2012;125:695–703.e1. [DOI] [PubMed] [Google Scholar]
- 27. Therneau TM. A package for survival analysis in S. version 2.38. 2015. https://CRAN.R-project.org/package=survival/.
- 28. Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet 2005;365:82–93. [DOI] [PubMed] [Google Scholar]
- 29. Steg PG, Lopez-Sendon J, Lopez de Sa E, Goodman SG, Gore JM, Anderson FA Jr, Himbert D, Allegrone J, Van de WF.. External validity of clinical trials in acute myocardial infarction. Arch Intern Med 2007;167:68–73. [DOI] [PubMed] [Google Scholar]
- 30. Mauri L. Why we still need randomized trials to compare effectiveness. N Engl J Med 2012;366:1538–1540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.