Key Points
Question
Have survival rates improved for infants 1 year or younger who received allogeneic hematopoietic cell transplantation (HCT)?
Findings
This cohort study evaluated 2498 infants who received HCT. For infants with nonmalignant diseases, survival rates improved from 2000 through 2004 to 2005 through 2009 and stabilized after this time; for infants with malignant conditions, survival did not improve from 2000 through 2014.
Meaning
Although children and adults who receive HCT are surviving longer than before, infants who need HCT face unique challenges; research to enhance survival should focus on optimizing donor selection, refining conditioning regimens, reducing toxicity, and improving supportive care.
This cohort study analyzes infants who received allogeneic hematopoietic cell transplants at age 12 months or younger to determine changes over time in complications and outcomes.
Abstract
Importance
Studies demonstrating improved survival after allogeneic hematopoietic cell transplant generally exclude infants.
Objective
To analyze overall survival trends and other outcomes among infants who undergo allogeneic hematopoietic cell transplant.
Design, Setting, and Participants
In this cohort study, we used time-trend analysis to evaluate 3 periods: 2000 through 2004, 2005 through 2009, and 2010 through 2014. The study was conducted in a multicenter setting through the Center for International Blood and Marrow Transplant Research, which is made up of a voluntary working group of more than 450 transplant centers worldwide. Two groups of infants aged 1 year or younger in 2 cohorts were included: those with malignant conditions, such as leukemia, and those with nonmalignant disorders, including immunodeficiencies. Data analysis was conducted from July 2017 to December 2018.
Exposures
Allogeneic hematopoietic cell transplant.
Main Outcomes and Measures
Survival trends, disease relapse, and toxicity.
Results
A total of 2498 infants with a median age of 7 months (range, <1-12 months) were included. In the nonmalignant cohort (n = 472), survival rates improved from the first to the second period (hazard ratio, 0.77 [95% CI, 0.63-0.93]; P = .007) but did not change after 2004. Compared with infants with nonmalignant diseases (n = 2026; 3-year overall survival: 2000-2004, 375/577 [65.0%]; 2005-2009, 503/699 [72.0%]; and 2010-2014, 555/750 [74.0%]), those with malignant conditions had poorer survival rates, without improvement over time (3-year overall survival: 2000-2004, 109/199 [54.8%]; 2005-2009, 104/161 [64.6%]; and 2010-2014, 66/112 [58.9%]). From 2000 through 2014, relapse rates increased in infants with malignant conditions (3-year relapse rate: 2000-2004, 19% [95% CI, 14%-25%]; 2005-2009, 23% [95% CI, 17%-30%]; 2010-2014, 36% [95% CI, 27%-46%]; P = .01). Sinusoidal obstruction syndrome was frequent, occurring with a cumulative incidence of 13% (95% CI, 11%-16%) of infants with nonmalignant diseases and 32% (95% CI, 22%-42%) of those with malignant diseases. Generally, recipients of human leukocyte antigen–identical sibling bone marrow grafts had the best outcomes.
Conclusions and Relevance
Survival rates have not improved for infants with malignant diseases over the 15-year study period. Infants with nonmalignant diseases had improved survival rates in the earlier but not the later study period. Higher relapses for the malignant cohort and toxicities for all infants remain significant challenges. Strategies to reduce relapse and toxicity and optimize donor and graft selection may improve outcomes in the future.
Introduction
Allogeneic hematopoietic cell transplant (allo-HCT) is a potentially curative therapy for malignant and nonmalignant diseases, but it is associated with considerable morbidity and mortality. Overall survival outcomes after allo-HCT have improved over time: patients who received transplants in 2003 through 2007 experienced a 41% reduction in the hazard of death from any cause and a 52% reduction in transplant-related mortality compared with those diagnosed in 1993 through 1997.1 A similar decrease in transplant-related mortality was noted in patients with acute myeloid leukemia who were younger than 50 years.2 However, these studies did not focus on infants, who receive a small portion of overall transplants performed annually. In infants, unlike in adults, nonmalignant disorders are frequent indications for allo-HCT. Infants may be at higher risk for toxicities than adults. Although children are considered to have better tolerance to high-intensity or myeloablative conditioning regimens and perhaps better immune reconstitution owing to a functional thymus,3 infants may be at higher risk of transplant-associated complications.4 To explore if outcomes have improved in this vulnerable population, this study analyzes trends in overall survival among infants receiving allo-HCT. Furthermore, this study analyzes factors contributing to these trends, as well as 2 major organ toxicities: idiopathic pneumonia syndrome (IPS) and sinusoidal obstruction syndrome (SOS).
Methods
Data Source
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a research collaboration between the National Marrow Donor Program/Be the Match and the Medical College of Wisconsin. It is made up of a voluntary working group of more than 450 transplant centers worldwide that contribute detailed data on hematopoietic cell transplants (HCT) to a statistical center at the Medical College of Wisconsin.
Participating centers are required to report all transplants consecutively; patients are followed up longitudinally, and compliance is monitored by onsite audits. Computerized checks for discrepancies, physician reviews of submitted data, and onsite audits of participating centers ensure data quality.
The CIBMTR collects data at 2 levels: transplant essential data on all patients and a comprehensive report form on a subset of patients selected through a weighted randomization scheme. The comprehensive form captures additional data about the patient, disease, and treatment. For this study, both levels of data were used to maximize the information on the study participants.
Ethical Review of Study and Informed Consent of Participants
All transplant centers must have local institutional review board approval for CIBMTR’s research database research protocol and informed consent for data sharing. The institutional review board of the National Marrow Donor Program approved and provided oversight for this study.
Reporting Race/Ethnicity
In this study, the parents or guardians of each infant reported the infant’s race and ethnicity, as summarized in the Results section and detailed in eTable 1 and eTable 2 in the Supplement. The standard forms of CIBMTR collect information on race and ethnicity.
Patients
We included infants, defined as children up to 1 year of age, with malignant or nonmalignant diseases undergoing first allo-HCT between 2000 through 2014. Exclusions included receiving multiple graft types, receiving a transplant from an identical twin, lacking parental consent, and lacking sufficient follow-up data.
This study had 4 main objectives. First, it aimed to describe 1-year, 3-year, and 5-year overall survival of infants receiving allo-HCT using time-trend analysis, with the time divided into 3 periods: 2000 through 2004, 2005 through 2009, and 2010 through 2014. Infants with malignant and nonmalignant diseases were evaluated separately. Second, the study sought to identify characteristics associated with overall survival for each group. Third, we aimed to analyze other transplant-associated end points, such as disease relapse, transplant-related mortality, and disease-free survival (DFS) in infants with malignant diseases. Finally, this analysis describes the incidence of and risk factors for IPS and SOS, using a subset of data from comprehensive report forms for transplants after 2008.
Definition of End Points
For overall survival, an event was defined as death from any cause. Surviving infants were censored at the last date of follow-up. Disease-free survival was defined as patients surviving without death or relapse. Transplant-related mortality was defined as death that occurred in the absence of disease relapse or progression or within 28 days of transplant. Relapse was the competing risk. Idiopathic pneumonia syndrome was reported based on the judgement of the treating physician, and it was defined as the presence of hypoxia and diffuse interstitial infiltrates not caused by fluid overload or infection. Similarly, SOS was reported by the treating physician, and the definition includes clinical or pathological evidence of SOS.
Statistical Analyses
Patient-associated and transplant-associated factors were compared between period-of-transplant groups using median and range for continuous variables and percentages for categorical variables. Transplant-associated mortality, relapse, IPS, and SOS rates were calculated using the cumulative-incidence function to account for competing risks. (Death before IPS and SOS is the competing risk for IPS and SOS, respectively.) Overall survival and DFS probabilities were calculated using the Kaplan-Meier estimator.
Multivariable regression analysis for each outcome used Cox proportional hazards regression for overall survival and DFS, and the cause-specific hazards model for transplant-related mortality, relapse, IPS, and SOS to assess the trend over time. The models were built using forward stepwise selection based on a cutoff for inclusion of P < .01. The final model was confirmed using backward elimination and forward selection. The proportional hazards assumption and interaction between the 3 periods, recipients’ age, and significant covariates were tested for each outcome. If the proportional hazards assumption was violated for a given covariable, it was analyzed as a time-dependent variable. Center effect was examined using the score test.5 If there was a significant center effect, the marginal proportional hazards model6 was used to account for center effect. The main effect was period of transplant, with the cohort divided into 3 groups (2000-2004, 2005-2009, and 2010-2014). Variables tested included age (<6 months vs 6 to 12 months), sex, disease type (malignant vs nonmalignant), disease subtype (including leukemia subtype or a specific nonmalignant diagnosis), disease status for malignant conditions only (with early status defined as acute leukemia in first complete remission or myelodysplasia without excess of myeloblasts, intermediate status defined as acute leukemia in any complete remission beyond the first, and advanced status defined as acute leukemia not in remission or myelodysplasia with an excess of myeloblasts), graft source (bone marrow, peripheral blood, umbilical cord blood); donor type and human leukocyte antigen (HLA) match; conditioning regimen intensity (myeloablative or reduced intensity/nonmyeloablative); patient region (US vs non-US); and use of total body irradiation.
In the multivariable model for overall mortality (the inverse of overall survival) in the cohort with nonmalignant diseases, donor type and graft source were identified as significant and confounding covariates. Inclusion of an interaction term in this model would have resulted in some groups with very small numbers. Therefore, 2 multivariable models were built: 1 including all variables plus donor type (eTable 5 in the Supplement) and another including all variables plus graft source (eTable 6 in the Supplement). Effect sizes for the other variables were similar between these 2 models; data from the donor type model are described here.
A subset analysis was done on the cohort of infants who received transplants during 2008 through 2014 to identify risk factors associated with the outcomes overall survival, IPS, and SOS (eTables 2 and 3 in the Supplement). Covariates included in these multivariable models included age, sex, donor type, graft source, conditioning regimen, and disease type (malignant vs nonmalignant conditions). Adjusted survival probabilities were calculated based on the final Cox model for overall survival.7
All analyses were performed from July 2017 to December 2018 using SAS version 9.4 (SAS Institute). Two-sided P values less than .05 were considered statistically significant.
Results
We identified 2498 infants from 181 centers who were eligible for analysis. Baseline and demographic characteristics are shown in Table 1 and eTable 1 in the Supplement, with variables stratified by period-of-transplant groups and disease groups. The population was racially and ethnically diverse: 1630 (65.3%) were white, 181 (7.2%) were African American, and 130 (5.2%) were Asian; Hispanic ethnicity was reported by 303 (12.1%) of this population.
Table 1. Summary of Baseline, Demographic, Patient-Associated, and Transplant-Associated Characteristics of Infants Receiving First Allogeneic Hematopoietic Cell Transplant.
| Characteristics | Patients, No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| Malignant Conditions | Nonmalignant Conditions | All | |||||
| 2000-2004 | 2005-2009 | 2010-2014 | 2000-2004 | 2005-2009 | 2010-2014 | ||
| Patients, No. | 199 | 161 | 112 | 577 | 699 | 750 | 2498 |
| Centers, No. | 88 | 77 | 61 | 118 | 121 | 102 | 181 |
| Age, median (range), mo | 9 (<1-12) | 9 (2-12) | 9 (<1-12) | 6 (1-12) | 7 (<1-12) | 6 (<1-12) | 7 (<1-12) |
| Male | 99 (49.7) | 86 (53.4) | 55 (49.1) | 340 (58.9) | 437 (62.5) | 477 (63.6) | 1494 (59.8) |
| Malignant disease | |||||||
| Acute myelogenous leukemia | 73 (36.7) | 55 (34.2) | 37 (33.0) | NA | NA | NA | 165 (6.6) |
| Acute lymphoblastic leukemia and other acute leukemia | 81 (40.7) | 58 (36.0) | 43 (38.4) | NA | NA | NA | 182 (7.2) |
| Myelodysplastic syndrome/myeloproliferative disorder | 40 (20.1) | 35 (21.7) | 31 (27.7) | NA | NA | NA | 106 (4.2) |
| Other leukemia, chronic myelogenous leukemia, other malignant condition | 5 (2.5) | 13 (8.1) | 1 (0.9) | NA | NA | NA | 19 (0.7) |
| Nonmalignant disease | |||||||
| Inherited bone marrow failure | NA | NA | NA | 21 (3.6) | 12 (1.7) | 22 (2.9) | 55 (2.2) |
| Hemoglobinopathies | NA | NA | NA | 5 (0.9) | 14 (2.0) | 6 (0.8) | 25 (1.0) |
| SCID | NA | NA | NA | 221 (38.3) | 290 (41.5) | 323 (43.0) | 834 (33.4) |
| Non-SCID | NA | NA | NA | 103 (17.8) | 126 (18.0) | 149 (19.8) | 378 (15.1) |
| Osteopetrosis | NA | NA | NA | 62 (10.7) | 45 (6.4) | 36 (4.8) | 143 (5.7) |
| Mucopolysaccharidosis/other storage disorder | NA | NA | NA | 81 (14.0) | 77 (11.0) | 54 (7.2) | 212 (8.4) |
| Histiocytic disorder | NA | NA | NA | 82 (14.2) | 132 (18.9) | 146 (19.5) | 360 (14.4) |
| Other nonmalignant condition | NA | NA | NA | 2 (0.3) | 3 (0.4) | 14 (1.8) | 19 (0.8) |
| Donor type | |||||||
| UCB (HLA 6/6) | 10 (5.0) | 13 (8.1) | 6 (5.3) | 18 (3.1) | 66 (9.4) | 71 (9.4) | 184 (7.4) |
| UCB (HLA 5/6) | 9 (4.5) | 37 (22.9) | 23 (20.5) | 50 (8.6) | 96 (13.7) | 137 (18.3) | 352 (14.1) |
| UCB (HLA ≤4/6) | 12 (6.0) | 11 (6.8) | 4 (3.6) | 25 (4.3) | 54 (7.7) | 39 (5.2) | 145 (5.8) |
| UCB (HLA match not reported) | 33 (16.6) | 13 (8.1) | 7 (6.2) | 102 (17.6) | 89 (12.7) | 36 (4.8) | 280 (11.2) |
| HLA-identical sibling | 64 (32.1) | 44 (27.3) | 29 (25.9) | 136 (23.6) | 138 (19.7) | 170 (22.7) | 581 (23.2) |
| Other relativesa | 12 (6.0) | 10 (6.2) | 7 (6.2) | 126 (21.8) | 119 (17.0) | 104 (13.9) | 378 (15.1) |
| 8/8 HLA–matched, unrelated | 17 (8.5) | 22 (13.6) | 20 (17.8) | 36 (6.2) | 76 (10.8) | 135 (18.0) | 306 (12.2) |
| 7/8 HLA–partially matched, unrelated | 15 (7.5) | 6 (3.7) | 8 (7.1) | 24 (4.1) | 31 (4.4) | 38 (5.1) | 122 (4.8) |
| ≤6/8 HLA–mismatched, unrelated | 3 (1.5) | 1 (0.6) | 0 | 12 (2.1) | 7 (1.0) | 3 (0.4) | 26 (1.0) |
| Unrelated, HLA match not reported | 24 (12.0) | 4 (2.5) | 8 (7.1) | 44 (7.6) | 22 (3.1) | 17 (2.3) | 119 (4.7) |
| Not reported | 0 | 0 | 0 | 4 (0.7) | 1 (0.14) | 0 | 5 (0.2) |
| Graft source | |||||||
| Bone marrow | 106 (53.3) | 62 (38.5) | 59 (52.7) | 269 (46.6) | 309 (44.2) | 407 (54.3) | 1212 (48.5) |
| Peripheral blood | 29 (14.6) | 25 (15.5) | 13 (11.6) | 113 (19.6) | 85 (12.1) | 60 (8.0) | 325 (13.0) |
| Umbilical cord blood | 64 (32.2) | 74 (45.9) | 40 (35.7) | 195 (33.8) | 305 (43.6) | 283 (37.7) | 961 (38.5) |
| Conditioning regimen intensity | |||||||
| Myeloablative conditioning | 163 (81.9) | 153 (95.0) | 107 (95.5) | 431 (74.7) | 492 (70.4) | 454 (60.5) | 1800 (72.0) |
| Reduced-intensity/nonmyeloablative conditioning | 2 (1.0) | 2 (1.2) | 5 (4.5) | 76 (13.2) | 164 (23.5) | 190 (25.3) | 439 (17.6) |
| No conditioning | 0 | 0 | 0 | 70 (12.1) | 43 (6.1) | 106 (14.1) | 219 (8.7) |
| Not reported | 34 (17.1) | 6 (3.7) | 0 | 0 | 0 | 0 | 40 (1.6) |
| Total body irradiation and conditioning intensity | |||||||
| Myeloablative conditioning with total body irradiation | 55 (27.6) | 41 (25.5) | 20 (17.8) | 24 (4.1) | 15 (2.1) | 20 (2.6) | 175 (7.0) |
| Myeloablative conditioning without total body irradiation | 108 (54.3) | 112 (69.6) | 87 (77.7) | 407 (70.5) | 477 (68.2) | 434 (57.9) | 1625 (65.0) |
| Reduced-intensity conditioning without total body irradiation | 2 (1.0) | 2 (1.2) | 5 (4.5) | 76 (13.2) | 164 (23.5) | 190 (25.3) | 439 (17.6) |
| Not reported or no conditioning | 34 (17.1) | 6 (3.7) | 0 | 70 (12.1) | 43 (6.1) | 106 (14.1) | 259 (10.4) |
| Follow-up, median (range), mo | 122 (3-173) | 74 (3-125) | 35 (6-63) | 119 (3-193) | 73 (3-127) | 34 (3-76) | 60 (3-193) |
Abbreviations: HLA, human leukocyte antigen; NA, not applicable; SCID, severe combined immunodeficiency; UCB, umbilical cord blood.
Other relative includes haploidentical donors.
Of the total infants, 472 infants (18.9%) had a malignant diagnosis (of whom 182 [38.6%] had acute lymphoblastic leukemia). For infants with diseases in this category, the group skewed toward older infants (411 [87.0%] were aged 6-12 months). Over time, the distributions of diagnoses and disease statuses were similar. Umbilical cord blood (UCB) was the most frequent donor type (178 [37.7%]) followed by HLA-identical siblings (137 [29.0%]). In addition, in recipients of transplants from sources other than UCB, bone marrow was the preferred graft type (227 [48.0%]). Virtually all transplants in this group were myeloablative (432 [91.5%]). Of these, one-third included total body irradiation, and most used calcineurin inhibitors for graft-vs-host disease prophylaxis (406 [86.0%]).
Of 2026 infants with nonmalignant diseases (of whom 1254 [61.9%] were male), severe combined immunodeficiency (SCID) was the most frequent indication (834 [41.2%]), and the distribution of diagnoses was similar across periods. Umbilical cord blood was most common donor type (783 [38.6%]), followed by HLA-identical siblings (444 [21.9%]). Mismatched UCB and 8/8 HLA-matched unrelated donors increased over time, while use of HLA-identical siblings remained about the same, and HLA-matched/mismatched other relatives decreased over time. The use of bone marrow also predominated in the recipients who received transplants other than UCB (985 [78.5%]); the use of peripheral blood decreased substantially over time. Use of reduced-intensity/nonmyeloablative conditioning regimens increased in frequency over time, and very few patients received total body irradiation (59 [2.9%]). A total of 219 patients (10.8%) with nonmalignant diseases received no conditioning regimen prior to transplant. Subset analysis population of infants who received a transplant from 2008 to 2014 are shown in eTable 2 in the Supplement.
Patients With Malignant Diseases
Overall Survival
Overall survival was nonsignificantly lower for infants with malignant diseases than those with nonmalignant diseases, and overall survival did not improve over time (Table 2). Diagnosis was the only significant factor influencing overall survival in the multivariate analysis; compared with patients with acute myeloid leukemia, patients with acute lymphoblastic leukemia had a higher mortality (hazard ratio [HR], 1.45 [95% CI, 1.06-1.98]; P = .02; eTable 4 in the Supplement). Neither the main effect nor age was significant in this model. Additional covariates are shown in eTable 4 in the Supplement.
Table 2. Univariable Trend Analysis of Outcomes for Entire Cohort.
| Outcomes | 2000-2004 (n = 776) | 2005-2009 (n = 860) | 2010-2014 (n = 862) | P Value | Overall P Value | |||
|---|---|---|---|---|---|---|---|---|
| No.a | Probability (95% CI) | No.a | Probability (95% CI) | No.a | Probability (95% CI) | |||
| Overall Survivalb | ||||||||
| Malignant disease, y | ||||||||
| 1 | 199 | 63 (56-70) | 161 | 69 (62-76) | 112 | 69 (61-78) | .35 | .31 |
| 3 | 55 (48-62) | 65 (57-72) | 59 (49-68) | .20 | ||||
| Nonmalignant disease, y | ||||||||
| 1 | 577 | 70 (66-74) | 699 | 75 (72-78) | 750 | 79 (76-82) | .002 | <.001 |
| 3 | 65 (60-68) | 72 (68-75) | 74 (71-77) | .001 | ||||
| SCID, y | ||||||||
| 1 | 221 | 70 (63-76) | 290 | 81 (77-86) | 323 | 80 (76-85) | .005 | .001 |
| 3 | 64 (58-71) | 78 (73-82) | 75 (69-80) | .005 | ||||
| Non-SCID, y | ||||||||
| 1 | 103 | 83 (76-90) | 126 | 89 (84-94) | 149 | 86 (80-91) | .38 | .12 |
| 3 | 76 (67-84) | 87 (80-92) | 83 (77-89) | .12 | ||||
| Histiocytic disorder, y | ||||||||
| 1 | 82 | 63 (53-73) | 132 | 61 (53-69) | 146 | 79 (72-85) | .002 | .03 |
| 3 | 58 (47-68) | 60 (51-68) | 72 (64-79) | .04 | ||||
| Acute myeloid leukemia, y | ||||||||
| 1 | 73 | 64 (53-75) | 55 | 76 (64-86) | 37 | 62 (46-77) | .24 | .29 |
| 3 | 56 (45-68) | 70 (57-82) | NE | .21 | ||||
| Acute lymphoblastic leukemia/other acute leukemia, y | ||||||||
| 1 | 81 | 57 (46-67) | 58 | 53 (41-66) | 43 | 70 (55-82) | .20 | .91 |
| 3 | 50 (39-61) | 48 (35-61) | NE | .93 | ||||
| Myelodysplastic syndrome/myeloproliferative disorder, y | ||||||||
| 1 | 40 | 75 (60-87) | 35 | 77 (62-89) | 31 | 77 (61-90) | .96 | .78 |
| 3 | 65 (50-80) | 74 (59-87) | NE | .54 | ||||
| Cohort With Malignant Disease | ||||||||
| Transplant-associated mortality, yc | ||||||||
| 1 | 176 | 22 (16-28) | 157 | 17 (11-23) | 112 | 13 (8-20) | .18 | .08 |
| 3 | 25 (19-32) | 17 (12-24) | 15 (9-22) | .08 | ||||
| Relapse, yc | ||||||||
| 1 | 176 | 17 (11-22) | 157 | 19 (13-26) | 112 | 32 (24-41) | .01 | .003 |
| 3 | 19 (14-25) | 23 (17-30) | 36 (27-46) | .01 | ||||
| Disease-free survival, yb | ||||||||
| 1 | 176 | 62 (54-69) | 157 | 64 (56-71) | 112 | 54 (45-63) | .24 | .28 |
| 3 | 56 (48-63) | 59 (52-67) | 49 (40-59) | .26 | ||||
Abbreviation: SCID, severe combined immunodeficiency syndrome.
Number evaluated.
Calculated via Kaplan Meier method.
Calculated via cumulative incidence function.
Transplant-Related Mortality
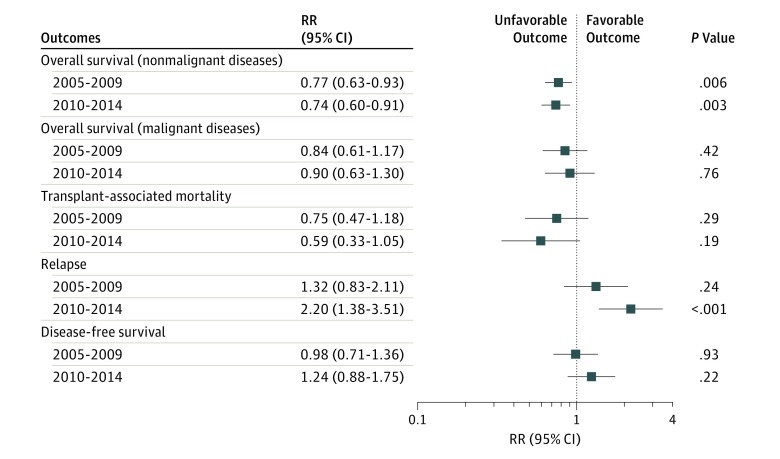
In univariable analysis, transplant-related mortality did not change significantly over time. In the multivariable model, only disease subtype retained significance; compared with patients with acute myeloid leukemia, patients with acute lymphoblastic leukemia (HR, 3.08 [95% CI, 1.77-5.34]; P < .001) and myelodysplastic syndrome (HR, 2.29 [95% CI, 1.24-4.26]; P = .008) experienced higher transplant-related morality. The main effect, period of transplant, was not associated with transplant-related mortality (eTable 7 in the Supplement).
Relapse
Relapse rates increased over the study period (cumulative incidence at 1 year: 2000-2004, 17% [95% CI, 11-22]; 2005-2009, 19% [95% CI, 13%-26%]; 2010-2014, 32% [95% CI, 24%-41%; P = .01; at 3 years: 2000-2004, 19% [95% CI, 14%-25%]; 2005-2009, 23% [95% CI, 17%-30%]; 2010-2014, 36% [95% CI, 27%-46%; P = .01; Table 2). In the multivariable model, period of transplant, disease type, and use of total body irradiation in the conditioning were all associated with disease relapse. Compared with 2000 through 2004, relapse was higher in the later period (HR, 2.34 [95% CI, 1.47-3.75]; P < .001). Relapse was also lower for patients with myelodysplastic syndrome than patients with acute myeloid leukemia (HR, 0.36 [95% CI, 0.20-0.67]; P = .001). Lastly, use of total body irradiation was associated with higher rates of disease relapse (HR, 1.90 [95% CI, 1.24-2.92; P = .03; eTable 8 in the Supplement).
Disease-Free Survival
In univariable analysis, DFS was not significantly different across the time periods of the study (Table 2). In multivariable models, disease type was the only variable contributing to this outcome; compared with patients with acute myeloid leukemia, patients with acute lymphoblastic leukemia had a higher rate of treatment failure (ie, disease relapse or death; HR, 1.51 [95% CI, 1.10-2.06]; P = .01). The main effect, period of transplant, was not associated with DFS (eTable 9 in the Supplement).
Patients With Nonmalignant Diseases
Overall survival improved over time for patients with nonmalignant diseases (cumulative survival at 1 year: 2000-2004, 70% [95% CI, 66%-74%]; 2005-2009, 75% [95% CI, 72%-78%); 2010-2014: 79% [95% CI, 76%-82%; P = .002; at 3 years: 2000-2004, 375/577; 65.0% [95% CI, 60%-68%]; 2005-2009, 503/699; 72.0% [95% CI, 68%-75%]; 2010-2014, 555/750; 74.0% [95% CI, 71%-77%; P = .001; Table 2) and was mainly observed among infants with SCID (cumulative survival at 1 year: 2000-2004, 70% [95% CI, 63%-76%]; 2005-2009, 81% [95% CI, 77%-86%]; 2010-2014, 80% [95% CI, 76%-85%]; P = .005; at 3 years: 2000-2004, 64% [95% CI, 58%-71%]; 2005-2009, 78% [95% CI, 73%-82%]; 2010-2014: 75% [95% CI, 69%-80%]; P = .005) and histiocytic disorders (cumulative survival at 1 year: 2000-2004, 63% [95% CI, 53%-73%]; 2005-2009, 61% [95% CI, 53%-69%]; 2010-2014, 79% [95% CI, 72%-85%; P = .002; at 3 years: 2000-2004, 58% [95% CI, 47%-68%]; 2005-2009, 60% [95% CI, 51%-68%]; 2010-2014, 72% [95% CI, 64%-79%]; P = .04).
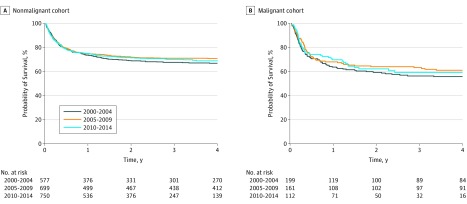
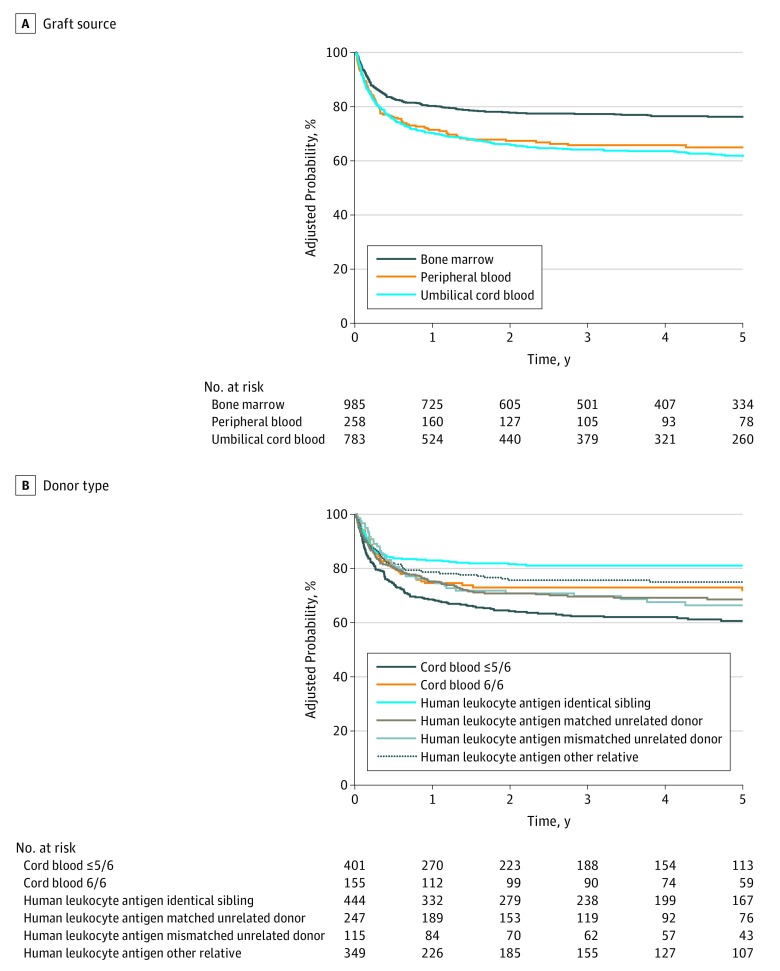
Improvement in overall survival was observed in the second and third periods of the study compared with the first (2005-2009: HR, 0.77 [95% CI, 0.63-0.93]; P = .007; 2010-2014: HR, 0.74 [95% CI, 0.60-0.91]; P = .004). However, there were no further improvements in overall survival between the second period and the third period (HR, 1.04 [95% CI, 0.85-.127]; P = .71; Figures 1 and 2). In addition to transplant period and disease, donor type and graft source also were associated with survival (Figure 3; eTable 5 and eTable 6 in the Supplement).
Figure 1. Forest Plot of Multivariable Models Showing Time Trend.
RR indicates relative risk.
Figure 2. Adjusted Survival Curves by Period and Transplant Indication.
Figure 3. Adjusted Survival Curves for the Nonmalignant Cohort by Graft Source and Donor Type.
Cause of Death
Of the infants with malignant diseases, 199 (42.2%) died, as did 621 infants (30.7%) with nonmalignant diseases. Causes of death are shown in eTables 10 through 14 in the Supplement.
Subset Analysis of Toxicity
Cumulative incidence of SOS at 100 days was 16% (95% CI, 13%-19%) among patients treated from 2008 to 2014. Cumulative incidence of IPS at 100 days and 1 year posttransplant were 5% (95% CI, 4%-7%) and 7% (95% CI, 5%-9%), respectively.
Sinusoidal Obstruction Syndrome
Sinusoidal obstruction syndrome occurred in 32% (95% CI, 22%-42%) of patients with malignant diseases and 13% (95% CI, 11%-16%; P < .001) of patients with nonmalignant diseases. In multivariable analysis of the 641 evaluable patients, those with a malignant condition had an increased risk (relative risk, 2.55 [95% CI, 1.66-3.94]; P < .001) of developing SOS, and the incidence of SOS increased over time (HR, 2.68 [95% CI, 1.40-5.11]; P = .003 for the period 2010-2014 [reference, 2000-2004]). In addition, infants aged 6 to 12 months had a lower risk of developing SOS than those younger than 6 months old (HR, 0.50 [95% CI, 0.26-0.99]; P = .049). In the nonmalignant disease cohort, there was no change in the incidence of SOS over time. Age, donor type, and graft source did not influence the risk of developing SOS. Compared with patients receiving myeloablative therapy, recipients of reduced intensity conditioning had lower rates of SOS (HR, 0.11 [95% CI, 0.04-0.26]; P < .001), as did those with no conditioning (HR, 0.06 [95% CI, 0.01-0.43]; P = .005). Patients with osteopetrosis (HR, 2.45 [95% CI, 1.36-4.40]; P = .003) or histiocytic disorders (HR, 1.68 [95% CI, 1.05-2.68]; P = .03) compared with those with SCID, experienced higher rates of SOS (eTables 15 and 16 in the Supplement).
Interstitial Pneumonia Syndrome
Interstitial pneumonia syndrome occurred in 5% of the entire cohort by 100 days posttransplant. In infants with malignant diseases, the incidence of IPS did not change over time and infants aged 6 to 12 months had a lower risk of developing IPS compared with those younger than 6 months old (HR, 0.36 [95% CI, 0.14-0.92]; P = .03; eTable 17 in the Supplement). In the nonmalignant disease cohort, there was no change in the incidence of IPS, and no other variables were significantly associated with this outcome (eTable 18 in the Supplement).
Discussion
This is a study of allo-HCT outcomes in almost 2500 ethnically and racially diverse infants from 181 centers, one-third of which were outside of the United States. We observed that among infants who received allo-HCT, most were treated for nonmalignant conditions. For these patients, overall survival improved significantly between the first period (2000-2004) and the second period (2005-2009), with essentially no change between the second period and the third (2010-2014) period. Outcomes were best for patients with immunodeficiencies; this group likely drove the overall improvement in survival.
The time-trend analysis suggests that key changes in practice led to improved survival, which plateaued after 2005. There are several possible contributors to this observation.
Improvements in Supportive Care
Better monitoring, prophylaxis, and management of infections and organ toxicities may all have resulted in better transplant outcomes. These improvements may have included better monitoring for viral illnesses, such as cytomegalovirus, with implementation of a preemptive therapy strategy and more effective and safer antifungal therapies.8 The association of better outcomes with these improvements is supported by the reduction in graft-vs-host disease and infection as the cause of death over time (eTable 10 in the Supplement).
Improved Donor/Graft Selection
Wider use of 8/8 HLA-matched unrelated donors with high resolution HLA typing9 may have resulted in better outcomes. While allele-level matching of UCB donors was not evaluated in this study, this has been shown to alter outcomes in patients with malignant and nonmalignant diseases10,11 and may have contributed to better survival.
The Increasing Use of Reduced-Intensity/Nonmyeloablative Conditioning Regimens and Less Use of Total Body Irradiation
The use of reduced-intensity/nonmyeloablative conditioning increased from 76 cases (13.2%) to 164 cases (23.5%; P < .001) between the first and second period. This change mirrors the trend in survival, suggesting that this modification contributed with the observed improved survival in this group. The use of total body irradiation in HCT for infants reflects the overall practice in transplantation for all patients, with availability of more nonirradiation options for conditioning regimen. The use of total body irradiation was evaluated only among recipients of HCT for malignant conditions, because this practice is rare in patients with nonmalignant diseases. Total body irradiation was associated with an increased risk of disease relapse (eTable 7 in the Supplement), which was a surprising finding and raises potential issues of dosimetry in this population that need to be further explored.
Changes in Practice in Disease Subgroups
Improvement in outcomes of patients with SCID could reflect early diagnosis, increasing awareness of infection prevention practices, early detection and treatment of opportunistic infections, and better supportive care.12 These improvements were a result from the development of newborn-screening programs for diagnosis of SCID, the first such program being established in 2008.13,14
Over time, fewer infants with malignant diseases underwent allo-HCT, and they fared less well compared with those with nonmalignant diseases. Although relapse rates increased over time, DFS and overall survival remained stable. Poor outcomes seen in infants with acute lymphoblastic leukemia may reflect the well-established aggressive nature of cases of this disease in infants, which include mixed-lineage leukemia gene rearrangements and mixed-phenotype acute leukemia.15,16,17 The increase in rates of relapse could be explained by changes in practice in selection of transplant candidates.
The strategy of upfront transplants declined over time. With improvements in nontransplant therapies and better risk stratification in acute leukemia, practice may have changed such that, in more recent years, only the highest-risk patients were referred for transplant.18,19,20,21 An international study, Interfant-99,22 demonstrated that only a subset of patients with mixed-lineage leukemia gene rearranged acute lymphoblastic leukemia benefit from allo-HCT in first complete remission. Although the frequency of patients with high-risk disease in each cohort appeared stable over time (eTable 1 in the Supplement), these definitions are based on clinical assessments (such as remission status), which may not reflect other biologically defined, high-risk disease independent of the response from prior therapy.
In the toxicity analyses, patients with malignant disease and recipients of myeloablative conditioning had a higher risk for development of SOS. This population is likely more susceptible to prior chemotherapy exposure, a known risk factor for SOS.
Among the nonmalignant cohort, those with osteopetrosis had an increased risk of SOS. Patients with osteopetrosis and metabolic disorders require myeloablative regimens for donor-cell engraftment.23,24 Patients with these disorders are more likely to have received cytotoxic therapy, similarly to that taken by patients with malignant conditions, which may contribute for the development of this complication.
Older infants (aged 6-12 months) experienced both less SOS and IPS than the younger ones. This is possibly associated with organ immaturity among younger infants, which might predispose them to excess toxicity.
Conclusions
In conclusion, survival in infants with nonmalignant diseases improved in the study period, although after 2004, survival remains unchanged. Overall survival in infants with malignant diseases has not improved over the last 15 years, per this analysis. Relapse risk appears to be substantial in this population, which could represent changes in practice over time. Transplant toxicities such as SOS and IPS are high, particularly in younger infants and those with malignant conditions or those receiving myeloablative regimens. Understanding these trends is important to identify potential approaches to reduce mortality among infants who are candidates for allo-HCT in the future.
eTable 1. Detailed baseline, demographic, patient-related and transplant-related characteristics of infants receiving first allogeneic hematopoietic cell transplant.
eTable 2. Baseline demographics of infants who received allogeneic transplants reported to the CIBMTR on Comprehensive Report Form between 2008-2014.
eTable 3. Univariable analysis of outcomes by year of transplant group in Comprehensive Report Form patients between 2008-2014.
eTable 4. Multivariable analysis of overall survival among malignant cohort.
eTable 5. Multivariable analysis of overall survival among non-malignant cohort, including donor type.
eTable 6. Multivariable analysis of overall survival among non-malignant cohort, including graft source.
eTable 7. Multivariable analysis of transplant-related mortality among malignant cohort.
eTable 8. Multivariable analysis of relapse among malignant cohort.
eTable 9. Multivariable analysis of disease-free survival among malignant cohort.
eTable 10. Causes of death.
eTable 11. Breakdown of primary disease as cause of death.
eTable 12. Breakdown of organ failure as cause of death.
eTable 13. Breakdown of “other” as cause of death.
eTable 14. Cause of death in malignant vs. non-malignant split by year of transplant.
eTable 15. Multivariable analysis of sinusoidal obstruction syndrome in non-malignant cohort (including donor type).
eTable 16. Multivariable analysis of sinusoidal obstruction syndrome in non-malignant cohort (including graft source).
eTable 17. Multivariable analysis of idiopathic pneumonia syndrome in malignant cohort.
eTable 18. Multivariable analysis of idiopathic pneumonia syndrome in non-malignant cohort.
References
- 1.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):-. doi: 10.1056/NEJMoa1004383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horan JT, Logan BR, Agovi-Johnson MA, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol. 2011;29(7):805-813. doi: 10.1200/JCO.2010.32.5001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinberg K, Blazar BR, Wagner JE, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001;97(5):1458-1466. doi: 10.1182/blood.V97.5.1458 [DOI] [PubMed] [Google Scholar]
- 4.Savic RM, Cowan MJ, Dvorak CC, et al. Effect of weight and maturation on busulfan clearance in infants and small children undergoing hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19(11):1608-1614. doi: 10.1016/j.bbmt.2013.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Commenges D, Andersen PK. Score test of homogeneity for survival data. Lifetime Data Anal. 1995;1(2):145-156. doi: 10.1007/BF00985764 [DOI] [PubMed] [Google Scholar]
- 6.Lee EW, Wei LJ, Amato DA, Leurgans S. Cox-type regression analysis for large numbers of small groups of correlated failure time observations In: Klein JP, Goel PK, eds. Survival Analysis: State of the Art. Dordrecht, the Netherlands: Springer Netherlands; 1992:237-247. doi: 10.1007/978-94-015-7983-4_14 [DOI] [Google Scholar]
- 7.Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88(2):95-101. doi: 10.1016/j.cmpb.2007.07.010 [DOI] [PubMed] [Google Scholar]
- 8.Spees LP, Martin PL, Kurtzberg J, et al. Reduction in mortality after umbilical cord blood transplantation in children over a 20-year period (1995-2014). Biol Blood Marrow Transplant. 2018;S1083-8791(18)30751-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grunebaum E, Mazzolari E, Porta F, et al. Bone marrow transplantation for severe combined immune deficiency. JAMA. 2006;295(5):508-518. doi: 10.1001/jama.295.5.508 [DOI] [PubMed] [Google Scholar]
- 10.Eapen M, Klein JP, Ruggeri A, et al. ; Center for International Blood and Marrow Transplant Research, Netcord, Eurocord, and the European Group for Blood and Marrow Transplantation . Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood. 2014;123(1):133-140. doi: 10.1182/blood-2013-05-506253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eapen M, Wang T, Veys PA, et al. Allele-level HLA matching for umbilical cord blood transplantation for non-malignant diseases in children: a retrospective analysis. Lancet Haematol. 2017;4(7):e325-e333. doi: 10.1016/S2352-3026(17)30104-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorsey MJ, Dvorak CC, Cowan MJ, Puck JM. Treatment of infants identified as having severe combined immunodeficiency by means of newborn screening. J Allergy Clin Immunol. 2017;139(3):733-742. doi: 10.1016/j.jaci.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan K, Puck JM. Development of population-based newborn screening for severe combined immunodeficiency. J Allergy Clin Immunol. 2005;115(2):391-398. doi: 10.1016/j.jaci.2004.10.012 [DOI] [PubMed] [Google Scholar]
- 14.Routes JM, Grossman WJ, Verbsky J, et al. Statewide newborn screening for severe T-cell lymphopenia. JAMA. 2009;302(22):2465-2470. doi: 10.1001/jama.2009.1806 [DOI] [PubMed] [Google Scholar]
- 15.Brown P. Treatment of infant leukemias: challenge and promise. Hematology Am Soc Hematol Educ Program. 2013;2013:596-600. doi: 10.1182/asheducation-2013.1.596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunger SP, Loh KM, Baker KS, Schultz KR. Controversies of and unique issues in hematopoietic cell transplantation for infant leukemia. Biol Blood Marrow Transplant. 2009;15(1)(suppl):79-83. doi: 10.1016/j.bbmt.2008.10.015 [DOI] [PubMed] [Google Scholar]
- 17.Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. 2011;29(5):551-565. doi: 10.1200/JCO.2010.30.7405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dreyer ZE, Dinndorf PA, Camitta B, et al. Analysis of the role of hematopoietic stem-cell transplantation in infants with acute lymphoblastic leukemia in first remission and MLL gene rearrangements: a report from the Children’s Oncology Group. J Clin Oncol. 2011;29(2):214-222. doi: 10.1200/JCO.2009.26.8938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh K, Tomizawa D, Moriya Saito A, et al. Early use of allogeneic hematopoietic stem cell transplantation for infants with MLL gene-rearrangement-positive acute lymphoblastic leukemia. Leukemia. 2015;29(2):290-296. doi: 10.1038/leu.2014.172 [DOI] [PubMed] [Google Scholar]
- 20.Kosaka Y, Koh K, Kinukawa N, et al. Infant acute lymphoblastic leukemia with MLL gene rearrangements: outcome following intensive chemotherapy and hematopoietic stem cell transplantation. Blood. 2004;104(12):3527-3534. doi: 10.1182/blood-2004-04-1390 [DOI] [PubMed] [Google Scholar]
- 21.Pieters R, Schrappe M, De Lorenzo P, et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet. 2007;370(9583):240-250. doi: 10.1016/S0140-6736(07)61126-X [DOI] [PubMed] [Google Scholar]
- 22.Mann G, Attarbaschi A, Schrappe M, et al. ; Interfant-99 Study Group . Improved outcome with hematopoietic stem cell transplantation in a poor prognostic subgroup of infants with mixed-lineage-leukemia (MLL)-rearranged acute lymphoblastic leukemia: results from the Interfant-99 Study. Blood. 2010;116(15):2644-2650. doi: 10.1182/blood-2010-03-273532 [DOI] [PubMed] [Google Scholar]
- 23.Boelens JJ, Rocha V, Aldenhoven M, et al. ; EUROCORD, Inborn error Working Party of EBMT and Duke University . Risk factor analysis of outcomes after unrelated cord blood transplantation in patients with hurler syndrome. Biol Blood Marrow Transplant. 2009;15(5):618-625. doi: 10.1016/j.bbmt.2009.01.020 [DOI] [PubMed] [Google Scholar]
- 24.Orchard PJ, Fasth AL, Le Rademacher J, et al. Hematopoietic stem cell transplantation for infantile osteopetrosis. Blood. 2015;126(2):270-276. doi: 10.1182/blood-2015-01-625541 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Detailed baseline, demographic, patient-related and transplant-related characteristics of infants receiving first allogeneic hematopoietic cell transplant.
eTable 2. Baseline demographics of infants who received allogeneic transplants reported to the CIBMTR on Comprehensive Report Form between 2008-2014.
eTable 3. Univariable analysis of outcomes by year of transplant group in Comprehensive Report Form patients between 2008-2014.
eTable 4. Multivariable analysis of overall survival among malignant cohort.
eTable 5. Multivariable analysis of overall survival among non-malignant cohort, including donor type.
eTable 6. Multivariable analysis of overall survival among non-malignant cohort, including graft source.
eTable 7. Multivariable analysis of transplant-related mortality among malignant cohort.
eTable 8. Multivariable analysis of relapse among malignant cohort.
eTable 9. Multivariable analysis of disease-free survival among malignant cohort.
eTable 10. Causes of death.
eTable 11. Breakdown of primary disease as cause of death.
eTable 12. Breakdown of organ failure as cause of death.
eTable 13. Breakdown of “other” as cause of death.
eTable 14. Cause of death in malignant vs. non-malignant split by year of transplant.
eTable 15. Multivariable analysis of sinusoidal obstruction syndrome in non-malignant cohort (including donor type).
eTable 16. Multivariable analysis of sinusoidal obstruction syndrome in non-malignant cohort (including graft source).
eTable 17. Multivariable analysis of idiopathic pneumonia syndrome in malignant cohort.
eTable 18. Multivariable analysis of idiopathic pneumonia syndrome in non-malignant cohort.