Abstract
Background
Despite its high worldwide morbidity and mortality, there is yet no licensed vaccine for shigellosis. We reported the safety and immunogenicity of Shigella O-specific polysaccharide-protein conjugates in adults and young children and efficacy of Shigella sonnei conjugate in young adults.
Methods
A double-blinded, randomized and vaccine-controlled Phase 3 evaluation of S. sonnei and S. flexneri 2a O-SP–rEPA conjugates, 25 μg, injected IM twice, 6 weeks apart, into healthy 1 to 4 year-olds, is reported. The children were followed for 2 years by telephone every other week and stool cultures were obtained for each episode of acute diarrhea (≥3 loose stools/day or a bloody/mucous stool). Sera were taken randomly from 10% of the participants for IgG anti-LPS and anti-carrier levels.
Results
Of the 2799 enrollees, 1433 received S. sonnei and 1366 S. flexneri 2a conjugates; 2699 (96.4%) completed the two-year follow up. Local reactions occurred in ~5% and ~4% had temperatures ≥38.0°C lasting 1-2 days. There were no serious adverse events attributable to the vaccines. Of the 3,295 stool cultures obtained, 125 yielded S. sonnei and 21 S. flexneri 2a. Immunogenicity and efficacy were age-related. The overall efficacy of the S. sonnei conjugate was 27.5%; 71.1% (P=0.043) in the 3-4 year-olds. The numbers for S. flexneri 2a were too few for meaningful analysis. Cross protection by S. flexneri 2a for non-vaccine S. flexneri types was found, but the numbers were too few for statistical significance. There was an age-related rise of vaccine-specific IgG anti-LPS in both groups, peaking at about 10 weeks and declining thereafter, but remaining ≥4 fold higher than in the controls 2 years after the second dose.
Conclusions
Shigella conjugates are safe and immunogenic in 1 to 4 year-olds. The S. sonnei conjugate elicited 71.1% efficacy in the 3 to 4 year-olds and can be predicted to be efficacious in individuals older than 3 years of age. These results urge studies with our improved conjugates.
INTRODUCTION
Shigellosis continues to be an important cause of dysentery and diarrhea worldwide. In the United States, about 18,000 cases/year are reported to the CDC, and it is estimated that about 180 million cases with 660,000 deaths occur annually in developing countries [1, 2]. It is unlikely that improvement in drinking water and sanitary conditions will occur in the foreseeable future in most developing areas of the world. Further, resistance to the most commonly used, cheap antibiotics has made treatment unavailable to many afflicted communities.
Despite its discovery over a century ago and the efforts of many laboratories, there is yet no vaccine for Shigella [3]. We proposed that a critical level of serum IgG, specific for the O-SP domain of the LPS of this pathogen, would confer immunity to shigellosis by inducing complement-mediated lysis of the inoculum on the epithelial surface of the small intestine [4-6]. Because the O-SP is not immunogenic, probably due to its comparatively low molecular weight, methods were developed to bind it covalently to carrier proteins [7, 8]. These conjugates were safe and immunogenic in adults and in young children [7-11]. Further, our S. sonnei conjugate conferred immunity to Israeli soldiers at high risk for shigellosis during their training [11]. Because the highest incidence, morbidity and mortality caused by Shigella occur in young children, we conducted a Phase 3 trial (safety, immunogenicity, and efficacy) in 1 to 4 year-olds of our S. sonnei and S. flexneri 2a conjugates at 15 sites in Israel.
METHODS AND MATERIALS
Study Protocol
The study was approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (OH-CH-N003), the US FDA (BB IND 7443), the Ethics Committee of the Sheba Medical Center (2633) and by the National Ethics Committee of the Israeli Ministry of Health, and assigned a Single Project Assurance Number by the Office of Human Research Protection of the US Department Health and Human Services.
Participants were healthy 1 to 4 year-olds recruited from 15 clinics throughout Israel. The parents/guardians of the participants were given the information sheet, discussed the proposed study with the clinic directors and signed the consent form. Excluded were children with a chronic disease receiving medication, those who received systemic steroids during the month preceding vaccination, those who had severe side effects following vaccinations and those not available for follow-up. Vaccination was delayed for those who had a respiratory or enteric infection the previous week, those who were vaccinated the preceding month or who had planned to have a vaccination during the month following the administration of the investigational vaccine, or if the child had a temperature (>38.0°C) at the time of vaccination.
Randomization to vaccine “A” or “B” was done using the last digit of the National Identification Card number (given to all Israeli children at birth). Five of the numbers from 0-9 were randomized to vaccine A and the other 5 to vaccine B. This vaccine assignment was recorded both on the Physician’s Examination and Vaccination form and on the volunteer’s chart. Both vaccines were clear aqueous solutions in the same type of vial and label. The randomization scheme was kept by the Pharmacy Development Service, NIH, and given only to the members of the Data and Safety Monitoring Board. Vials that were opened were discarded at the end of the week without exception. The enrollment period was May 1, 2003 to January 31, 2006.
The vaccines were administered IM in 0.5 mL at each community clinic by the research nurse. Local and systemic reactions were sought at 30 minutes, 6, 24 and 48 hours after vaccination by a structured questionaire. Adverse reactions that occurred were sought for 48 hours after they were no longer detectable. Serious adverse events (SAEs) were recorded throughout the study period. Adverse reactions were recorded at each site by the research staff and transferred to the study headquarters.
Vaccines
Two lots of conjugates prepared by PDMI, similar to those used in the Phase 2 studies of 4-7 year-olds and in 1-4 year-olds [9, 12] were used. The investigational vaccines were composed of the O-SP of S. sonnei or of S. flexneri 2a covalently bound to recombinant exoprotein A of Pseudomonas aeruginosa (rEPA). To increase binding to the S. flexneri 2a O-SP, the rEPA was succinylated prior to conjugation [12-14]. Both conjugates were dissolved in saline to a final concentration of 50 μg/mL, 0.01% thimerosal added, dispensed in 5 dose vials and stored at 4-7°C. S. sonnei and S. flexneri 2a were chosen to serve as controls for each other because: 1. their O-SPs are structurally and antigenicity unrelated [15-17]; 2. infection with one does not confer immunity to the other [18].
Surveillance
Parents were contacted by telephone every other week. In addition, they were asked to report each episode of diarrhea to the clinic. Stool specimens were obtained for each episode of diarrhea (≥3 loose stools/day or a single bloody/mucous stool), cultured for bacterial pathogens (Shigella Spp., Salmonella Spp. and Campylobacter Spp) and examined for viral pathogens (rotavirus and adenovirus) by antigen-detection assays at the Maccabi Healthcare Services Laboratories. All Shigella isolates were sent for confirmation and typing to the Department of Clinical Microbiology of the Sheba Medical Center and to the Reference Center for Shigella of the Israeli Ministry of Health. Blood cultures were obtained from children with acute diarrhea and fever of ≥38.5°C. The per-protocol follow-up period was 2 years; however, all vaccinees were followed until the last one completed the 2-year follow-up (January 31, 2008).
Antibody assay
Serum IgG anti O-SP of S. sonnei and of S. flexneri 2a and IgG anti P. aeruginosa exotoxin A (ETA) were measured by ELISA using standard reference sera. Levels less than the sensitivity of the ELISA were assigned one half of that value [7, 8].
Statistics
Statistical analysis was performed using SAS software version 9.1 (SAS institute Inc., Cary, NC USA). IgG anti-LPS concentrations were expressed as the geometric means (G.M.) and compared by the Wilcoxon rank sum test. P<0.05 was considered statistically significant. For multiple comparisons, P<0.01 was considered statistically significant.
Efficacy was calculated by the formula:
Comparison of rates between the study groups used χ2 and Fisher’s Exact tests with P<0.05 considered statistically significant. All tests were 2 tailed.
RESULTS
A total of 2,799 children, including Jews, Arabs, Druze, and Bedouins, were enrolled at 15 sites throughout Israel (Table 1). There were 1,455 males (52%) and 1,344 females (48%), 1,029 (36.8 %) were >1 to 2 years-old, 1,013 (36. 2 %) were >2 to 3 years-old, and 757 (27.0 %) were >3 years old. All enrolled children received the first dose of an investigational vaccine, 2,748 (98.2%) received 2 doses. Ten were excluded because of protocol violation (4 due to age >4 years, 3 due to more than 2 immunizations, 3 due to different vaccines in the same recruit), and 39 additional participants dropped out due to loss of contact, relocation or death; 2,699 (96.4%) completed the 2-year follow-up.
Table 1.
Enrollment by gender, age and vaccine type
| Vaccine | Total | Male | Female | 1-2 yr | >2-3 yr | >3-4 yr | >4 yr |
|---|---|---|---|---|---|---|---|
| S. sonnei | |||||||
| N= | 1433 | 732 | 701 | 533 | 514 | 385 | 1 |
| % | 51.2 | 51.1 | 48.9 | 37.2 | 35.9 | 26.8 | 0.1 |
| S. flexneri 2a | |||||||
| N= | 1366 | 723 | 643 | 496 | 499 | 368 | 3 |
| % | 48.8 | 52.9 | 47.1 | 36.3 | 36.5 | 26.9 | 0.2 |
| Total | |||||||
| N= | 2799 | 1455 | 1344 | 1029 | 1013 | 753 | 4 |
| % | 100 | 52.0 | 48.0 | 36.8 | 36.2 | 26.9 | 0.1 |
Safety
The acute adverse events to the investigational vaccines by vaccine type and dose are shown in Table 2. Local pain was noted in approximately 5% of the vaccinees; other reactions were less common. Fever was noted after each injection in approximately 4% of the vaccines (Table 2). None of the 309 SAEs, including 4 deaths, reported during the study, was considered related to the vaccines. Causes of death were drowning, electrical injury, murder and thrombocytopenia with brain hemorrhage.
Table 2.
Adverse events per vaccine type and dose
| Adverse event |
S. sonnei
|
S. flexneri 2a |
||||||
|---|---|---|---|---|---|---|---|---|
| Dose 1 | Dose 2 | Dose 1 | Dose 2 | |||||
| N=1433 | N=1405 | N=1366 | N=1343 | |||||
| N | % | N | % | N | % | N | % | |
| Local pain | 82 | 5.72 | 79 | 5.62 | 61 | 4.47 | 64 | 4.77 |
| Swelling | 11 | 0.77 | 19 | 1.35 | 11 | 0.81 | 9 | 0.67 |
| Redness | 7 | 0.49 | 15 | 1.07 | 6 | 0.44 | 6 | 0.45 |
| Fever | 56 | 3.91 | 36 | 2.56 | 72 | 5.27 | 51 | 3.80 |
| Nausea | 22 | 1.53 | 9 | 0.64 | 10 | 0.73 | 11 | 0.82 |
| Vomiting | 28 | 1.95 | 8 | 0.57 | 14 | 1.03 | 13 | 0.97 |
IgG LPS antibodies (Tables 3 & 4)
Table 3.
Age-related IgG anti-LPS levels of sera drawn randomly >2 weeks after the second vaccine dose
| G.M. IgG anti-LPS (EU)* | ||||
|---|---|---|---|---|
| Vaccine | Age (yr) | N= | S. sonnei | S. flexneri 2a |
| S. sonnei | 1-2 | 38 | 1.40 | 3.43 |
| >2-3 | 44 | 3.71 | 7.53 | |
| >3-4 | 29 | 6.38 | 9.51 | |
| S. flexneri 2a | 1-2 | 43 | 0.25 | 18.98 |
| >2-3 | 53 | 0.42 | 26.96 | |
| >3-4 | 30 | 0.76 | 43.86 | |
1.40 vs. 3.71 P=0.01; 3.71 vs. 6.38 P=0.25; 1.40 vs. 6.38 P=0.002; P for trend =0.001
0.25 vs. 0.42 P=0.12; 0.42 vs. 0.76 P=0.05; 0.25 vs. 0.76 P=0.001; P for trend =0.002
3.43 vs. 7.53 P=0.02; 7.53 vs. 9.51 P=0.28; 3.43 vs. 9.51 P=0.0002; P for trend =0.001
18.98 vs 26.96 P=0.13; 26.96 vs, 43.86 P=0.09; 18.98 vs. 41.68 P=0.007; P for trend =0.005
6.38 vs. 0.76 P=0.0001
3.71 vs. 0.42 P<0.0001
1.40 vs. 0.25 P<0.0001
Table 4.
IgG anti-LPS of sera drawn randomly by time from the second vaccine dose
| G.M. IgG anti-LPS (EU)* | ||||
|---|---|---|---|---|
| Vaccine | Week | N= | S. sonnei | S. flexneri 2a |
| S. sonnei | 2-10 | 24 | 12.93 | 6.67 |
| >10-30 | 29 | 3.98 | 5.52 | |
| >30 | 58 | 1.48 | 6.20 | |
| S. flexneri 2a | 2-10 | 34 | 0.74 | 52.89 |
| >10-30 | 40 | 0.25 | 28.62 | |
| >30 | 52 | 0.38 | 16.42 | |
12.93 vs. 3.98 P=0.02; 3.98 vs. 1.48 P=0.007; 12.93 vs. 1.48 P<0.0001; P for trend =0.0007 52.89 vs. 28.62 P=0.02; 28.62 vs. 16.42 P=0.02; 52.89 vs. 16.42 P<0.0001; P for trend =0.005
As observed for surface bacterial polysaccharides, including those of Shigella, there was a “natural” age-related development of IgG LPS antibodies in the control groups (about a 2.5-fold increase between 1-2 and 3-4 year olds) for both LPSs, likely independent of interaction with the homologous bacteria [19-21]. Overall both vaccines induced similar antibody levels to those of the Phase 2 study [9, 12]; an age related increase in vaccine-induced antibody levels was found when vaccinees’ ages were stratified by year (Table 3.) Among the S. sonnei vaccinees, there was a 4.5-fold difference in the level of IgG S. sonnei antibodies between the ages of 1-2 and 3-4 (6.38 vs 1.40, P=0.002), and a 2.7 fold difference for the S. flexneri 2a antibodies in S. flexneri 2a vaccines (9.51 vs 3.43, p=0.0002). Significant age related rises to both O-SPs were also found in the controls (development of “natural immunity”, reviewed in ref 5); S.sonnei antibodies in S.flexneri 2a recipients, p for trend=0.002 and S. flexneri 2a antibodies in S.sonnei recipients,P for trend=0.001, but the levels in the vaccinees were 9-fold (S.sonnei) and 4.5-fold (S.flexneri 2a) higher. Compared to the controls, both vaccines elicited statistically significant responses (each P<0.001).
Both vaccine-induced antibodies were short lived, peaking at around 10 weeks after the second injection and declining thereafter (Table 4).
IgG anti-ETA
A recombinant non toxic variant of P. aeruginosa exotoxin A was the carrier, but the antibodies were measured against the exotoxin A. This carrier has also been used successfully in the efficacy study of Vi-rEPA in 2-5 year olds and in infants [22, 23]. A rise in IgG anti-ETA was detected in almost all vaccinees, similar to that observed in the Phase 2 study [9, 12].
Stool cultures
The overall rate of diarrhea in the study population was 0.6 episodes/child/year, similarly distributed between the S. sonnei and S. flexneri 2a conjugate groups (0.61 and 0.59 respectively). Rates of diarrhea were significantly affected by age: 0.79, 0.56 and 0.35/child/year in the 1-2, 2-3 and 3-4 year olds, respectively (p<0.001).
Of 3295 stool cultures obtained, 716 were positive for pathogens. Shigella was the most common bacterial isolate, 202, followed by Campylobacter Spp, 140. Of the Shigella, there were 125 S. sonnei and 65 S. flexneri, of which 29 isolates were of type 6, 21 type 2a, 5 type 1b, 3 type 1a, 1 each of types 2b and 3a and 5 not identified.
There were 8 S. boydii, 3 S. dysenteriae and 1 Shigella Spp (not identified). There were no significant differences in isolation rates of the other pathogens between the vaccine groups.
Efficacy (Tables 5a & b)
Table 5.
Efficacy of 2 doses of Shigella conjugate vaccines by age, per-protocol
| a. Shigella sonnei | |||||||
|---|---|---|---|---|---|---|---|
| Vaccine administered | |||||||
| S. sonnei | S. flexneri 2a | ||||||
| Age | N= | Cases | N= | Cases | Efficacy | (95% CI) | P |
| 1-2 yr | 516 | 18 | 476 | 16 | 3.8% | (101.1, 46.5) | 0.91 |
| >2-3 yr | 497 | 8 | 481 | 12 | 35.5% | (−56.4, 73.4) | 0.33 |
| >3-4 yr | 371 | 3 | 358 | 10 | 71.1% | (−4.43, 92.0) | 0.04 |
| All ages | 1384 | 29 | 1315 | 38 | 27.5% | (−16.9, 54.0) | 0.18 |
| b. Shigella flexneri 2a | |||||||
|---|---|---|---|---|---|---|---|
| Vaccine administered | |||||||
| S. sonnei | S. flexneri 2a | ||||||
| Age | N= | Cases | N= | Cases | Efficacy | (95% CI) | P |
| 1-2 yr | 516 | 3 | 476 | 3 | −8.4% | (−434.5, 78.0) | 0.99 |
| >2-3 yr | 497 | 4 | 481 | 3 | 22.5% | (−244.4, 82.6) | 0.99 |
| >3-4 yr | 371 | 1 | 358 | 1 | −3.6% | (−1550, 93.5) | 0.99 |
| All ages | 1384 | 8 | 1315 | 7 | 7.9% | (−153.2, 66.5) | 0.87 |
The per-protocol efficacy analysis was based on 2699 children who received 2 doses of one of the investigational vaccines and were followed for 2 years.. The overall attack rate for S. sonnei was 1.34%/yr and for S. flexneri type 2a 0.83%/yr. Most S. sonnei cases occurred in the 1-2 year-olds, declining in the 2-3 year-olds and further in the 3-4 year-olds. Two clusters of S. sonnei shigellosis occurred in 2 communities in June-July 2006; 8 cases occurred within 3 weeks, 7 of which in S. flexnei 2a vaccine receipients and 17 cases occurred within 5 weeks, 7 of which in S. flexnei 2a receipients, 22/25 were >3years old. Other than one positive culture for Salmonella enterica, there were no positive blood cultures among vaccinees that had fever in addition to diarrhea.
There was an age-related efficacy for recipients of the S. sonnei conjugate: 3.8% for the 1-2 year-olds, 35.5% for the 2–3 year-olds and 71.1% (P=0.043) for the 3-4-years old. Because of the small number of isolates during the first 10 weeks after the second injection when antibody levels were at their highest, no efficacy could be assessed for that time.
There were too few cases of the S. flexneri 2a infection for statistical significance. A reason for the small number of shigella isolates is that the study started at the descending part of the bi-annual incidence curve of shigellosis in Israel. As observed with other communicable diseases transmitted by human contact, there is a cyclic pattern to shigellosis in Israel with peaks every 2-3 years. A new epidemic occurs when the time limited herd immunity provided by recovery from disease wanes and a new, naïve cohort of infants and children develops[24]. Protection from non-vaccine types of S. flexneri, in S. flexneri 2a conjugate recipients, was noticed. These types included type 6, the most common S. flexneri isolate during the study. The overall efficacy of S. flexneri 2a vaccine against all S. flexneri non-type 2a was 44.9%, and against type 6 alone - 51.7%; both these values were not statistically significant. Intent to treat analysis of all enrolled children yielded almost identical results (not shown).
DISCUSSION
Protection was conferred by the S. sonnei O-SP-rEPA conjugate in 3-4 year-olds, 71.1% efficacy (P=0.04), 35.5% in the 2-3 year-olds (P not significant) but there was no efficacy in the 1-2 years-old group. Efficacy paralleled the age-related immunogenicity of the S. sonnei conjugate, demonstrated during the 2 year follow up, at the time when the peak antibody levels have declined (still significantly higher than in the controls, P <0.01). Homologous efficacy was not significant in recipients of the S. flexneri 2a conjugate likely due to the small number of cases. No serious adverse reactions related to the immunization were observed. These results extend our efficacy data in adults and provide a vaccine for S. sonnei shigellosis in individuals older than 3 years of age. Moreover, the data confirm our proposal that a critical (protective) level of serum IgG anti-O-SP antibodies confers immunity to shigellosis [5, 6]. Importantly, this information will allow a more precise prediction of the efficacy of our improved O-SP conjugates, including in those less than 3 years of age [25].
In developing countries, S. flexneri is the major cause of shigellosis. The structure and antigenicity of Group B Shigellae O-SPs are related [17]. All, except type 6, are composed of the tetrasaccharide repeat unit:
Addition of glucose and OAc moieties to this tetrasaccharide backbone, under the control of phage infections, confers the fine antigenic specificities of the Group B Shigella O-SPs [15, 17].
The repeat unit of S. flexneri type 6 O-SP is:
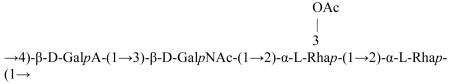 [26].
[26].
It is likely that the disaccharide Rhap-(1→2)-Rhap (O-acetylated in the same position in the type 2a O-SP) accounts for the cross-reactivity of the types 2a and the other S. flexneri types, particularly type 6. The data, showing efficacy of the S. flexneri 2a conjugate against the cross-reactive types of Group B shigellae, although not statistically significant, is consistent with previous studies in animals [27, 28].
This is the first Shigella vaccine candidate to demonstrate efficacy in young children older than 3 years of age. A vaccine of improved immunogenicity is considered for evaluation in infants. Orally-administered streptomycin-dependent strains of S. flexneri types 1, 2a, and S. sonnei induced type-specific immunity in 80% of 2 to 7 year-olds in hyperendemic regions of Yugoslavia [29]. Four doses of about 1010 viable organisms were administered at 3-day intervals. There were no reported serologic assays performed on the participants. The virulence of these strains was not attenuated and they were not used because of the high rate of severe adverse reactions they elicited.
Bacteriologists have concluded that Shigella and Escherichia coli should be considered as one Genus [30-32]. As an example, the virulent E. coli O157 and S. dysenteriae type 1 excrete the same exotoxin denoted as shigella toxin and cause similar diseases, including the hemolytic-uremic syndrome. Immunity to infection with both of these pathogens has also been proposed to be related to LPS-specific serum IgG [33]. On the basis of published data and the results of our clinical trials, we predict that a critical (protective) level of serum IgG anti O157-O-SP will confer immunity to this pathogen.
Acknowledgement
We thank the members of the Data and Safety Monitoring Board for their participation: Brian Reichman, Zvi Spirer and Amos Etzioni.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Gupta A, Polyak CS, Bishop RD, Sobel J, Mintz ED. Laboratory-confirmed shigellosis in the United States, 1989-2002: epidemiologic trends and patterns. Clin Infect Dis. 2004 May 15;38(10):1372–7. doi: 10.1086/386326. [DOI] [PubMed] [Google Scholar]
- [2].Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999;77(8):651–66. [PMC free article] [PubMed] [Google Scholar]
- [3].Shiga K. The trend of prevention therapy and epidemiology of dysentery since the discovery of its causative organism. N Engl J Med. 1936;215:1205–11. [Google Scholar]
- [4].Cohen D, Green MS, Block C, Slepon R, Ofek I. Prospective study of the association between serum antibodies to lipopolysaccharide O antigen and the attack rate of shigellosis. J Clin Microbiol. 1991 Feb;29(2):386–9. doi: 10.1128/jcm.29.2.386-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Robbins JB, Chu C, Schneerson R. Hypothesis for vaccine development: protective immunity to enteric diseases caused by nontyphoidal salmonellae and shigellae may be conferred by serum IgG antibodies to the O-specific polysaccharide of their lipopolysaccharides. Clin Infect Dis. 1992 Aug;15(2):346–61. doi: 10.1093/clinids/15.2.346. [DOI] [PubMed] [Google Scholar]
- [6].Robbins JB, Schneerson R, Szu SC. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995 Jun;171(6):1387–98. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- [7].Chu CY, Liu BK, Watson D, Szu SS, Bryla D, Shiloach J, et al. Preparation, characterization, and immunogenicity of conjugates composed of the O-specific polysaccharide of Shigella dysenteriae type 1 (Shiga’s bacillus) bound to tetanus toxoid. Infect Immun. 1991 Dec;59(12):4450–8. doi: 10.1128/iai.59.12.4450-4458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Taylor DN, Trofa AC, Sadoff J, Chu C, Bryla D, Shiloach J, et al. Synthesis, characterization, and clinical evaluation of conjugate vaccines composed of the O-specific polysaccharides of Shigella dysenteriae type 1, Shigella flexneri type 2a, and Shigella sonnei (Plesiomonas shigelloides) bound to bacterial toxoids. Infect Immun. 1993 Sep;61(9):3678–87. doi: 10.1128/iai.61.9.3678-3687.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ashkenazi S, Passwell JH, Harlev E, Miron D, Dagan R, Farzan N, et al. Safety and immunogenicity of Shigella sonnei and Shigella flexneri 2a O-specific polysaccharide conjugates in children. J Infect Dis. 1999 Jun;179(6):1565–8. doi: 10.1086/314759. [DOI] [PubMed] [Google Scholar]
- [10].Cohen D, Ashkenazi S, Green M, Lerman Y, Slepon R, Robin G, et al. Safety and immunogenicity of investigational Shigella conjugate vaccines in Israeli volunteers. Infect Immun. 1996 Oct;64(10):4074–7. doi: 10.1128/iai.64.10.4074-4077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cohen D, Ashkenazi S, Green MS, Gdalevich M, Robin G, Slepon R, et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997 Jan 18;349(9046):155–9. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- [12].Passwell JH, Ashkenazi S, Harlev E, Miron D, Ramon R, Farzam N, et al. Safety and immunogenicity of Shigella sonnei-CRM9 and Shigella flexneri type 2a-rEPAsucc conjugate vaccines in one- to four-year-old children. Pediatr Infect Dis J. 2003 Aug;22(8):701–6. doi: 10.1097/01.inf.0000078156.03697.a5. [DOI] [PubMed] [Google Scholar]
- [13].Passwell JH, Harlev E, Ashkenazi S, Chu C, Miron D, Ramon R, et al. Safety and immunogenicity of improved Shigella O-specific polysaccharide-protein conjugate vaccines in adults in Israel. Infect Immun. 2001 Mar;69(3):1351–7. doi: 10.1128/IAI.69.3.1351-1357.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pavliakova D, Chu C, Bystricky S, Tolson NW, Shiloach J, Kaufman JB, et al. Treatment with succinic anhydride improves the immunogenicity of Shigella flexneri type 2a O-specific polysaccharide-protein conjugates in mice. Infect Immun. 1999 Oct;67(10):5526–9. doi: 10.1128/iai.67.10.5526-5529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Carlin NI, Lindberg AA, Bock K, Bundle DR. The Shigella flexneri O-antigenic polysaccharide chain. Nature of the biological repeating unit. Eur J Biochem. 1984 Feb 15;139(1):189–94. doi: 10.1111/j.1432-1033.1984.tb07993.x. [DOI] [PubMed] [Google Scholar]
- [16].Kenne L, Lindberg B, Peterson K, Katzenelienbogen E, Romanowska E. Structural studies of the O-specific side-chains of the Shigella sonnei Phase I lipopolysaccharide. Carbohydrate Research. 1980;78:119–26. [Google Scholar]
- [17].Kenne L, Lindberg B, Petersson K, Katzenellenbogen E, Romanowska E. Structural studies of Shigella flexneri O-antigens. Eur J Biochem. 1978 Nov 2;91(1):279–84. doi: 10.1111/j.1432-1033.1978.tb20963.x. [DOI] [PubMed] [Google Scholar]
- [18].Formal SB, Oaks EV, Olsen RE, Wingfield-Eggleston M, Snoy PJ, Cogan JP. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J Infect Dis. 1991 Sep;164(3):533–7. doi: 10.1093/infdis/164.3.533. [DOI] [PubMed] [Google Scholar]
- [19].Fothergill LaW J. Influenzal meningitis: The relation of age incidence to the bactericidalo power of blood against the causal organism. j Immunol. 1933;24:273–9. [Google Scholar]
- [20].Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–26. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Passwell JH, Freier S, Shor R, Farzam N, Block C, Lison M, et al. Shigella lipopolysaccharide antibodies in pediatric populations. Pediatr Infect Dis J. 1995 Oct;14(10):859–65. doi: 10.1097/00006454-199510000-00008. [DOI] [PubMed] [Google Scholar]
- [22].Lin FY, Ho VA, Khiem HB, Trach DD, Bay PV, Thanh TC, et al. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med. 2001 Apr 26;344(17):1263–9. doi: 10.1056/NEJM200104263441701. [DOI] [PubMed] [Google Scholar]
- [23].Thiem VD, Lin F-Y, Cahn DG, et al. Manuscript in prepration. Vi-rEPA conjugate vaccine is safe and immunogenic in infants and compatible with routine immunization. [Google Scholar]
- [24].Cohen D, Bassal R, Valinski L, Vasilev V, Green MS, Shigella Surveillance Network . Cyclic Occurrence of Epidemics of Shigella sonnei Shigellosis in Israel. Vaccines for Enteric Diseases; Malaga: Sep 9-11, 2009. at. [Google Scholar]
- [25].Robbins JB, Kubler-Kielb J, Vinogradov E, Mocca C, Pozsgay V, Shiloach J, et al. Synthesis, characterization, and immunogenicity in mice of Shigella sonnei O-specific oligosaccharide-core-protein conjugates. Proc Natl Acad Sci U S A. 2009 May 12;106(19):7974–8. doi: 10.1073/pnas.0900891106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dmitriev BA, Knirel YA, Sheremet OK, Shashkov AA, Kochetkov NK, Hofman IL. Somatic antigens of Shigella. The structure of the specific polysaccharide of Shigella newcastle (Sh. flexneri type 6) lipopolysaccharide. Eur J Biochem. 1979 Jul;98(1):309–16. doi: 10.1111/j.1432-1033.1979.tb13190.x. [DOI] [PubMed] [Google Scholar]
- [27].Noriega FR, Liao FM, Maneval DR, Ren S, Formal SB, Levine MM. Strategy for cross-protection among Shigella flexneri serotypes. Infect Immun. 1999 Feb;67(2):782–8. doi: 10.1128/iai.67.2.782-788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Van De Verg LL, Bendiuk NO, Kotloff K, Marsh MM, Ruckert JL, Puryear JL, et al. Cross-reactivity of Shigella flexneri serotype 2a O antigen antibodies following immunization or infection. Vaccine. 1996 Aug;14(11):1062–8. doi: 10.1016/0264-410x(96)00006-0. [DOI] [PubMed] [Google Scholar]
- [29].Mel D, Gangarosa EJ, Radovanovic ML, Arsic BL, Litvinjenko S. Studies on vaccination against bacillary dysentery. 6 Protection of children by oral immunization with streptomycin-dependent Shigella strains. Bull World Health Organ. 1971;45(4):457–64. [PMC free article] [PubMed] [Google Scholar]
- [30].Huan PT, Bastin DA, Whittle BL, Lindberg AA, Verma NK. Molecular characterization of the genes involved in O-antigen modification, attachment, integration and excision in Shigella flexneri bacteriophage SfV. Gene. 1997 Aug 22;195(2):217–27. doi: 10.1016/s0378-1119(97)00143-1. [DOI] [PubMed] [Google Scholar]
- [31].Liu B, Knirel YA, Feng L, Perepelov AV, Senchenkova SN, Wang Q, et al. Structure and genetics of Shigella O antigens. FEMS Microbiol Rev. 2008 Jul;32(4):627–53. doi: 10.1111/j.1574-6976.2008.00114.x. [DOI] [PubMed] [Google Scholar]
- [32].Orskov I, Orskov F, Jann B, Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ahmed A, Li J, Shiloach Y, Robbins JB, Szu SC. Safety and immunogenicity of Escherichia coli O157 O-specific polysaccharide conjugate vaccine in 2-5-year-old children. J Infect Dis. 2006 Feb 15;193(4):515–21. doi: 10.1086/499821. [DOI] [PubMed] [Google Scholar]


