Abstract
Objective:
Evaluate postpartum HIV care outcomes.
Design:
Prospective clinical cohort of women with HIV and a live birth at the University of North Carolina, 1996–2014.
Methods:
We estimated two stages of the HIV care continuum in the first 24 months postpartum: care retention (≥2 visits per year, ≥90 days apart) and viral suppression (HIV RNA <400 copies/mL). Multivariable models were fit using logistic regression.
Results:
Among 1416 women, 141 experienced a live birth at a median age of 28 years, with 74% virally suppressed at delivery. Among all women, 48% were retained in care and 25% maintained viral suppression for the first 24 months postpartum. Among women with available HIV RNA measures, 42% were suppressed at 24 months. HIV care retention estimates were stable across calendar years, but viral suppression rates at 24 months postpartum, among women with available HIV RNA measures, increased from 33% to 67% from 1996–2001 to 2009–2014 (P=0.04). Being ≥30 years old was positively, and receiving <12 weeks of antenatal ART was negatively, associated with HIV care retention at 24 months postpartum (adjusted odds ratio [AOR]: 2.41, 95% CI: 1.09–5.29 and AOR: 0.27, 95% CI: 0.08–0.86). Older maternal age and viral suppression at delivery were both positively associated with virologic suppression at 24 months postpartum (AOR: 2.52, CI: 1.02–6.22, and AOR: 6.42 CI: 1.29–31.97, respectively).
Conclusion:
HIV care continuum outcomes decrease substantially postpartum, with younger women and those with less antenatal HIV care less likely to successfully remain engaged in HIV care following childbirth.
Keywords: HIV, postpartum care, retention in care, antiretroviral therapy, highly active
INTRODUCTION
Antiretroviral therapy (ART) is highly effective in preventing mother to child transmission (MTCT) of HIV.(1–4) With routine HIV testing, ART provision, and other MTCT prevention interventions, the number of perinatally infected infants born annually in the US has decreased dramatically. An MTCT elimination goal of <1 perinatal HIV infection per 100,000 live births has been set by the US Centers for Disease Control and Prevention, and though this goal has not yet been achieved, recent rates have fallen from 1.8 to 1.1 perinatal infections per 100,000 live births between 2010 and 2014.(5, 6)
Recommendations for ART during pregnancy, and among women of childbearing age, have undergone marked changes over time.(1, 3, 4) Pregnancy is a critical time for HIV care, and protecting the health of the fetus is a clear incentive for women to engage in care.(7) The postpartum period is also an important time for maternal engagement in HIV care, but following childbirth, women may face substantial challenges attending clinical appointments and adhering to ART. Although limited, the studies conducted in the United States to date suggest that women may not receive optimal HIV care postpartum.(8–12) Furthermore, postpartum ART adherence may also be lower than ART adherence during pregnancy leading to lower virologic suppression rates following childbirth.(13–17)
The HIV care continuum model describes the stages from HIV testing and diagnosis through HIV RNA suppression, with recent United States estimates suggesting that as few as 30% of people living with HIV (PLWH), and 26% of women living with HIV (WLWH), have undetectable HIV RNA levels.(18–20) To date, little information is available on the HIV care experiences of pregnant WLWH in the United States, including postpartum HIV care engagement. In this study, we evaluated the HIV care continuum for 24 months postpartum and assessed the effect of demographic and clinical characteristics on postpartum HIV care engagement and HIV RNA suppression, within a large HIV clinical cohort in North Carolina.
METHODS
Study Design and Study Population
We identified all pregnancies resulting in a live birth among participants in the University of North Carolina CFAR HIV Clinical Cohort (UCHCC). The UCHCC has been previously described, and includes over 5,200 patients with HIV, who have received HIV primary care at a large tertiary care center in North Carolina since 1996.(21) UCHCC data collection includes data from institutionally available electronic health records and standardized semi-annual medical record reviews, including all laboratory data, clinical diagnoses, antiretroviral therapy provision, and clinical appointment data. For this study, all pregnancies recorded after HIV diagnosis underwent further medical record review for collection of pregnancy-specific information.
For these analyses, we included all pregnancies with a live birth from January 1, 1996 through December 31, 2014, to allow for at least 2 years of postpartum follow-up, and only included pregnancies that occurred after women initiated HIV care at our institution. We excluded two women who died within 2 years of delivery (one with disseminated Mycobacterium avium complex, and the other with dilated cardiomyopathy), and one woman who had a subsequent pregnancy during the 24-month follow-up. Pregnancies were included if the women were at least 18 years old and HIV positive at the time of delivery. Among women with more than one eligible pregnancy during the time period (18%), we included only the most recent pregnancy in these analyses, and considered multiple births as a single observation. UCHCC participants provided written informed consent, and the UCHCC and this specific study were approved by the UNC Institutional Review Board.
Measures
Our primary outcome of interest was engagement in care, defined using the Institute of Medicine’s (IOM) definition of attending at least 2 HIV care visits in a given year, at least 90 days apart.(22, 23) We only considered attended scheduled visits with an HIV care provider, and excluded walk-in and emergency care visits. To account for childbirth hospital stays, we excluded visits and laboratory tests within 7 days of the delivery date. Our secondary outcome was HIV RNA suppression, defined as an HIV RNA level less than 400 copies/mL in a given year. We excluded HIV RNA measures within 3 months of delivery to provide a washout period, and used the measure closest to the midpoint of each year. Calendar years of delivery were categorized into three intervals (1996–2001, 2002–2008 and 2009–2014), based on changes in ART use and sample size considerations.
Statistical Analysis
We used Kaplan-Meier curves to examine the distribution of time from delivery to first postpartum HIV care visit. To test for differences between groups we used Fisher’s exact, Chi-square, and Mann-Whitney U tests, and Cochran-Armitage test for trend, as appropriate. To identify factors associated with our outcomes of interest we fit multivariable logistic regression models, including demographic, clinical and pregnancy related patient characteristics. Only factors that were associated with our outcome of interest with a P<0.20 in bivariable analyses were included in our final multivariable model. For all estimates, 95% confidence intervals were calculated, P values were two-sided, and <0.05 was considered statistically significant. All analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC).
RESULTS
Among 1416 women participating in the UCHCC, 141 had a live birth between 1996 and 2014 and met study inclusion criteria. The median maternal age at delivery was 28 years (interquartile range [IQR], 24–32). Most women were either non-Hispanic Black (62%) or non-Hispanic White (21%), with 18% of other race/ethnicity including 13% Hispanic (Table 1). The median year of delivery was 2004 (IQR, 2000–2008). Most women had a prior pregnancy, with gravidity of 1, 2, 3, 4 and ≥5 among 10%, 27%, 27%, 20% and 15%, respectively, and an overall median gravidity of 3 (IQR, 2–4). The median number of prior live births was 1 (IRQ, 1–2).
TABLE 1.
Demographic and Clinical Characteristics of Women Living with HIV at Delivery, UCHCC 1996–2014
| Characteristic* | N=141 |
|---|---|
| Age <30 years, n (%) | 84 (60) |
| Race, n (%) | |
| White, non-Hispanic | 29 (21) |
| Black, non-Hispanic | 87 (62) |
| Other/Hispanic | 25 (18) |
| Antenatal HIV diagnosis, n (%) | 40 (28) |
| HIV RNA <400 copies/mL, n (%) | 90 (74) |
| CD4 count, median cells/mm3 (IQR) | 491 (339, 677) |
| Antenatal ART use, n (%) | |
| <12 weeks | 24 (17) |
| 12–38 weeks | 88 (62) |
| ≥38 weeks | 29 (21) |
| Calendar year of delivery, n (%) | |
| 1996–2001 | 47 (33) |
| 2002–2008 | 61 (43) |
| 2009–2014 | 33 (23) |
| Gravidity, n (%) | |
| 1 | 13 (10) |
| ≥2 | 111 (90) |
| Prior live births, n (%) | |
| 0 | 29 (22) |
| 1 | 45 (35) |
| ≥2 | 55 (43) |
Abbreviations. IQR, interquartile range; ART, antiretroviral therapy.
All characteristics measured at delivery. Missing data for HIV RNA (n=19), CD4 cell count (n=19), gravidity (n=17), and prior live births (n=12).
Over one-quarter of the women were diagnosed with HIV during the index pregnancy (28%) (Supplemental Figure). The median number of HIV care visits during pregnancy was 6 among all women (IQR, 4–8), and did not differ by timing of HIV diagnosis (P=0.61). Overall, women received a median of 25 weeks of antenatal ART (IQR, 16–37), with 2 women receiving no antenatal ART (1%), and 24 women receiving <12 weeks of antenatal ART (17%). Across calendar time 11%, 21%, and 18% received <12 weeks of antenatal ART in years 1996–2001, 2002–2008 and 2009–2014 (P=0.34). Of the women with <12 weeks of ART, 54% were diagnosed with HIV during the index pregnancy, in comparison to 30% of women receiving 12–38 weeks of ART. At delivery, the median CD4 cell count was 491 cells/mm3 (IQR, 339–677), with 12 women having a CD4 cell count <200 cells/mm3, and 74% were virologically suppressed (among women with CD4 cell count and HIV RNA levels available at delivery, respectively). Among women who were not virologically suppressed at delivery (n=32), the median HIV RNA level was 3.6 log10 copies/mL (IQR, 3.0–4.2). Virologic suppression at delivery differed across calendar years with 45%, 78% and 94% suppressed during 1996–2001, 2002–2008 and 2009–2014 (P<0.001). Maternal age was also associated with virologic suppression at delivery, with 67% and 83% of <30 and ≥30 year old women virologically suppressed at delivery, respectively (P=0.04).
During follow-up, 36%, 58% and 82% had their first HIV care visit within 30, 60 and 180 days of delivery, with sixteen women (11%) attending no HIV care visits in 24 months of follow-up. The median time to first HIV care visit among women that had at least one visit was 41 days after delivery (IQR, 22–75). Among all women, the median number of HIV care visits in the first 12 months postpartum was 3 visits (IQR, 1–6), and 2 visits (IQR, 0–4) during 12–24 months postpartum (P=0.001) (Figure 1).
FIGURE 1.
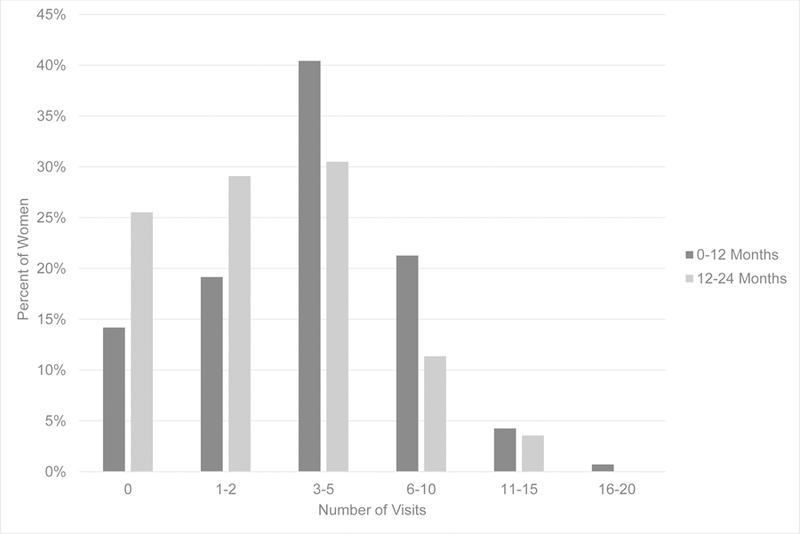
Postpartum HIV care visit distribution stratified by time from delivery among women living with HIV, UCHCC 1996–2014. Visits include attended scheduled visits with an HIV care provider, and exclude walk-in and emergency care visits.
Using the IOM criteria as a measure of HIV care retention, 70% and 48% of women were retained in care at 12 and 24 months postpartum, respectively. Overall, 38% were virologically suppressed during the first year postpartum and 25% in both years postpartum. Among those with available HIV RNA measures, 52% and 42% were suppressed at 12 and 24 months postpartum, respectively. Postpartum retention in care did not vary across calendar years (Figure 2), with little difference across the three calendar year intervals covering 1996 through 2014 (12 month retention P=0.42; 24 month retention P=0.24). The proportion of women virologically suppressed increased across years 1996–2001 to 2009–2014, with 12 month postpartum virologic suppression increasing from 26% to 61% (P=0.002), and 24 month virologic suppression increasing from 19% to 36% (P=0.09). Among women with available HIV RNA measures, virologic suppression increased comparing years 1996–2001 to 2009–2014 from 33% to 80% in the first 12 months postpartum (P<0.001), and 33% to 67% in 24 months postpartum (P=0.04).
FIGURE 2.
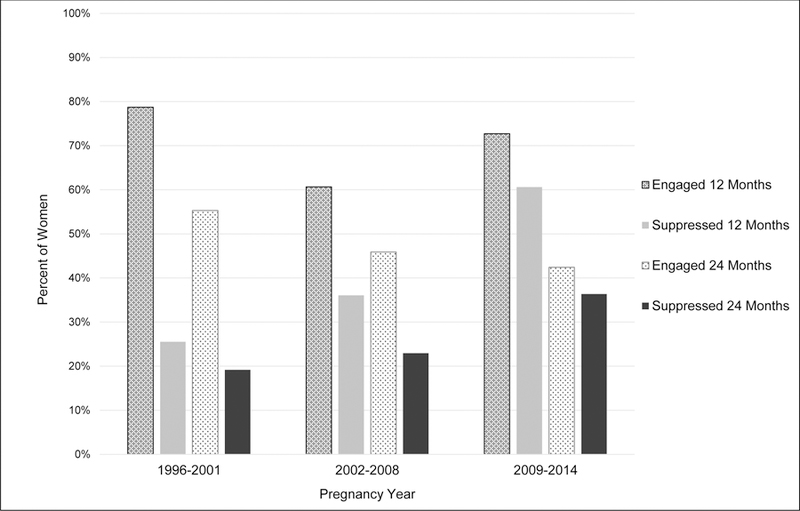
Postpartum HIV care continuum among women living with HIV, UCHCC 1996–2014. HIV care retention at 12 and 24 months postpartum defined according to the Institute of Medicine care retention criteria, and virologic suppression as HIV RNA <400 copies/mL.
In multivariable analyses mothers ≥30 years of age were more likely to be retained in HIV care based on the IOM criteria at both 12 and 24 months postpartum (adjusted odds ratio [AOR]: 2.44, 95% CI: 1.00–5.94 and AOR: 2.41, 95% CI: 1.09–5.29, respectively) (Table 2). Conversely, receiving <12 weeks of ART during pregnancy, in comparison to 12–38 weeks of ART, was negatively associated with receiving adequate HIV care at both 12 and 24 months postpartum (AOR: 0.24, 95% CI: 0.08–0.68, and AOR: 0.27, 95% CI: 0.08–0.86, respectively).
TABLE 2.
Characteristics Associated With 12 and 24 Month Postpartum HIV Care Retention Among Women Living with HIV in the UCHCC, 1996–2014 (N=141)
| 12 Month Retention | 24 Month Retention | |||
|---|---|---|---|---|
| Characteristic* | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
| Age ≥30 years | 2.19 (1.01, 4.76) | 2.44 (1.00, 5.94) | 2.46 (1.23, 4.90) | 2.41 (1.09, 5.29) |
| Race | ||||
| White, non-Hispanic | 1.12 (0.44, 2.85) | -- | 1.32 (0.57, 3.07) | -- |
| Black, non-Hispanic | ref | -- | ref | -- |
| Other | 0.76 (0.30, 1.93) | -- | 0.71 (0.29, 1.76) | -- |
| Antenatal HIV diagnosis | 0.88 (0.40, 1.93) | -- | 0.54 (0.26, 1.15) | 0.91 (0.36, 2.31) |
| HIV RNA <400 copies/mL | 1.36 (0.57, 3.23) | -- | 1.29 (0.57, 2.89) | -- |
| CD4 count, per 100 cell/mm3 increase | 1.01 (0.87, 1.17) | -- | 1.00 (0.88, 1.14) | -- |
| Antenatal ART use | ||||
| <12 weeks | 0.18 (0.07, 0.46) | 0.24 (0.08, 0.68) | 0.24 (0.08, 0.70) | 0.27 (0.08, 0.86) |
| 12–38 weeks | ref | ref | ref | ref |
| ≥38 weeks | 0.77 (0.30, 2.01) | 0.62 (0.21, 1.89) | 1.29 (0.55, 3.02) | 0.96 (0.35, 2.60) |
| Calendar year of delivery | ||||
| 1996−2001 | 2.40 (1.01, 5.71) | 2.31 (0.78, 6.82) | 1.46 (0.68, 3.13) | -- |
| 2002−2008 | ref | ref | ref | -- |
| 2009–2014 | 1.73 (0.69, 4.35) | 1.84 (0.68, 4.93) | 0.87 (0.37, 2.04) | -- |
| Gravidity | ||||
| 1 | 2.86 (0.60, 13.58) | 3.24 (0.62, 16.86) | 2.85 (0.83, 9.80) | 3.65 (0.97, 13.73) |
| ≥2 | ref | ref | ref | ref |
Abbreviations. OR, odds ratio; CI, confidence interval; ART, antiretroviral therapy.
All characteristics measured at delivery except HIV care visit within 60 days. HIV care retention based on the Institute of Medicine criteria.
Older maternal age was also associated with better virological postpartum outcomes in bivariable and multivariable analyses (Table 3). Specifically, HIV RNA suppression at 12 and 24 months postpartum was more likely among women ≥30 years of age at delivery (AOR: 2.74, 95% CI: 1.17–6.41 and AOR: 2.52, 95% CI: 1.02–6.22, respectively). Additionally, virologic suppression at 12 and 24 months postpartum was also strongly associated with virologic suppression at delivery although the estimates were imprecise (AOR: 6.12, 95% CI: 1.53–24.45 and AOR: 6.42, 95% CI: 1.29–31.97, respectively).
TABLE 3.
Characteristics Associated With 12 and 24 Month Postpartum HIV Viral Suppression Among Women Living with HIV in the UCHCC, 1996–2014 (N=141)
| 12 Month Suppression | 24 Month Suppression | |||
|---|---|---|---|---|
| Characteristic* | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
| Age ≥30 years | 3.16 (1.56, 6.42) | 2.74 (1.17, 6.41) | 2.92 (1.33, 6.41) | 2.52 (1.02, 6.22) |
| Race | ||||
| White, non-Hispanic | 1.28 (0.54, 3.01) | -- | 2.34 (0.91, 5.97) | 2.27 (0.75, 6.88) |
| Black, non-Hispanic | ref | -- | ref | ref |
| Other | 1.42 (0.58, 3.50) | -- | 2.50 (0.94, 6.65) | 2.55 (0.83, 7.82) |
| Antenatal HIV diagnosis | 1.28 (0.61, 2.70) | -- | 1.01 (0.43, 2.36) | -- |
| HIV RNA <400 copies/mL | 10.11 (2.87, 35.57) | 6.12 (1.53, 24.45) | 7.50 (1.68, 33.51) | 6.42 (1.29, 31.97) |
| CD4 count, per 100 cell/mm3 increase | 1.07 (0.93, 1.22) | -- | 1.08 (0.93, 1.25) | -- |
| Antenatal ART use | ||||
| <12 weeks | 0.36 (0.12,1.06) | 0.67 (0.18, 2.48) | 0.53 (0.17, 1.72) | -- |
| 12–38 weeks | ref | ref | ref | -- |
| ≥38 weeks | 0.97 (0.42, 2.28) | 0.86 (0.28, 2.60) | 0.85 (0.32, 2.24) | -- |
| Calendar year of delivery | ||||
| 1996–2001 | 0.61 (0.26, 1.41) | 0.72 (0.22, 2.33) | 0.80 (0.31, 2.04) | 1.25 (0.36, 4.33) |
| 2002–2008 | ref | ref | ref | ref |
| 2009–2014 | 2.73 (1.14, 6.52) | 2.16 (0.83, 5.58) | 1.92 (0.76, 4.85) | 1.45 (0.54, 3.94) |
| Gravidity | ||||
| 1 | 0.99 (0.30, 3.22) | -- | 0.89 (0.23, 3.46) | -- |
| ≥2 | ref | -- | ref | -- |
Abbreviations. OR, odds ratio; CI, confidence interval; ART, antiretroviral therapy.
All characteristics measured at delivery except HIV care visit within 60 days. HIV care retention based on the Institute of Medicine criteria.
DISCUSSION
In this study of WLWH participating in a large Southeastern US clinical cohort with a live birth between 1996 and 2014, we observed dramatic decreases in HIV care retention and virologic suppression across 24 months postpartum. Nearly all women received some ART during pregnancy, and almost three out of four were virologically suppressed at delivery with this percentage increasing over time. However, in the first 12 months postpartum only 70% were retained in HIV care, decreasing further during 12 to 24 months postpartum with less than half of the women remaining in HIV clinical care.
Others have observed the drop in HIV care continuum postpartum in the United States. In reports from Philadelphia and Mississippi 12-month postpartum retention was estimated to be just under 40% in both studies, and 20% in Atlanta among women with perinatal HIV infection.(8, 9, 11) Higher rates were observed in New York State with three-quarters of WLWH with a live birth remaining in HIV care through one year postpartum.(10, 24)
A number of factors influence the postpartum retention estimates available including how engagement in care was defined, data sources and differences in study population. In the Philadelphia and New York State studies, data were obtained by linking HIV and perinatal surveillance system records.(8, 10, 24) In contrast, other studies, including this one, were based on institutionally available health records.(11, 32) Relying on surveillance system data may allow for improved capture of HIV care visits, in comparison to using single site institutional health records, if women transfer HIV care postpartum. However, our institution-based results were comparable to surveillance-based estimates from New York State (10, 24) and higher than those from Philadelphia (8), as well as, higher than estimates from the two other studies conducted at single institutions in Houston (32) and Atlanta (11). Additional regional differences in public health policies and infrastructure available to support pregnant and postpartum women and people living with HIV care may affect care retention. Policies including health care coverage through Medicaid and safety net programs for those without health insurance may be relevant to observed differences, including the relatively higher retention rates observed in New York State and in our cohort.(10, 12, 24)
This study included WLWH living in North Carolina which is disproportionately affected by the HIV epidemic, with high HIV-related morbidity and mortality rates in comparison to other US regions.(25) In response, the North Carolina Division of Public Health has long supported innovative public health programs including early HIV detection and linkage to HIV care.(26–28) Moreover, at our center a coordinated health care system approach to providing care to pregnant WLWH exists, including coordination between HIV, obstetric and pediatric providers and co-location of services including prenatal, labor and delivery, pediatric and HIV care. Our coordination of care approach extends from pre-pregnancy, through pregnancy and into the postpartum period, with consistency and coordination in HIV and gynecologic clinical care providers, and co-location of services, with reproductive health services provided during HIV care visits. Postpartum HIV and obstetric visits were offered through the same clinical care providers that provided care during pregnancy, with ART provision based on clinical care guidelines. An institutional system to contact patients who miss appointments exists, including follow-up letters, phone calls and in more recent years text messages. Notwithstanding, North Carolina WLWH face ongoing structural barriers to HIV care engagement.(29) Medicaid covers pregnancy related services for low income women, but only through 60 days postpartum.(30) Other programs designed to provide affordable HIV treatment exist, however, in a recent study conducted in our source study population, patients reported ongoing financial barriers preventing them from accessing HIV care and ART.(31)
In this study, WLWH with longer time on prenatal ART were more likely to receive recommended postpartum HIV care. A number of studies have observed that care received prenatally is associated with postpartum care outcomes.(8–10, 24, 32) In part, establishing consistent care engagement during pregnancy translates to better postpartum HIV care and clinical outcomes by promoting patient-level behaviors and addressing existing barriers to receiving HIV care.(12) Additionally receiving less than recommended HIV prenatal care may help identify individuals at high risk of future inconsistent HIV care access. Previous work has identified prior care patterns as a critical factor predicting future HIV care engagement.(33, 34) This suggests that irrespective of the underlying reasons, identifying individuals early who may be at high risk of inconsistently accessing HIV care and tailoring interventions to this population will be critical to achieving postpartum and longer-term retention in care goals.
Consistent with prior studies, older maternal age was associated with better postpartum HIV care outcomes.(8, 9, 24, 32) These results are further supported by prior work in the UCHCC, in Birmingham, Alabama, and a large North American HIV cohort collaboration showing that older patients are more likely to receive consistent HIV care.(35–37) Ensuring adequate retention in care among younger patients may be especially critical given that retention in care may be more important to virologic suppression among younger individuals.(38)
HIV RNA suppression rates also dropped precipitously in the 24 months following childbirth, consistent with our findings on postpartum HIV care retention, and with prior literature.(8, 10, 17) Not surprisingly, virologic suppression rates increased in more recent calendar years, consistent with changes in available ART and with virologic suppression rates observed in the UCHCC.(3, 39) Older mothers were more likely to be virologically suppressed postpartum possibly reflecting better ART adherence among older individuals.(17) Additionally, women virologically suppressed at delivery were more likely to remain virologically suppressed following childbirth, lending further support to the central role of prenatal HIV care access on postpartum HIV care outcomes.
There were several limitations to our study. We were unable to assess a number of factors likely relevant to prenatal and postpartum HIV care retention. For example, qualitative studies have identified stigma, fear, social support, mental health, intimate partner violence and logistical factors such as transportation and time constraints as affecting prenatal and postpartum HIV care.(32, 40, 41) Additionally, the specific ART regimen received may further affect HIV care retention, including the availability of single-tablet regimens. Our study was nested in an ongoing HIV clinical cohort, which enabled us to identify pregnant WLWH with available HIV, prenatal and labor and delivery records. However, it is possible women transferred care to another facility postpartum introducing misclassification and overestimating the proportion of women lost to care. Since we relied on data from a single tertiary care center, our sample size was limited and the generalizability of our findings may be limited geographically. Finally, we restricted our analyses to 24 months following delivery, and longer follow-up is needed to estimate effects on HIV clinical outcomes including morbidity and mortality.
In summary, this study underscores the importance of adequate prenatal care for retention in HIV care and improved clinical outcomes in the immediate postpartum period and beyond. Young women may be at especially high risk for poor postpartum HIV outcomes, and interventions tailored to this group are especially needed. Ensuring access to, and retention in, comprehensive HIV care is central to individual HIV clinical outcomes, as well as, secondary and perinatal HIV transmission.(23, 42, 43) A number of strategies to improve retention in HIV care postpartum have been proposed, including improved care coordination, perinatal case management, peer support interventions and use of technology-based interventions.(12) New recommendations by the American College of Obstetricians and Gynecologists for a “fourth trimester” of care for mothers following childbirth may also support improved postpartum outcomes among WLWH.(44) However, to date few clinical studies support evidence based interventions, and increased attention to improving women’s prenatal and postpartum HIV health care access is needed.
Supplementary Material
ACKNOWLEDGEMENTS
Funding/Support:
This study was funded by the University of North Carolina Center for AIDS Research, an NIH-funded program (Grant Award Number P30 AI50410); Traineeship support for JSC was provided by NIAID/NIH (Grant Award Number T32 AI070114).
REFERENCES
- 1.Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O’Sullivan MJ, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 1994;331(18):1173–1180. [DOI] [PubMed] [Google Scholar]
- 2.Sturt AS, Dokubo EK, Sint TT. Antiretroviral therapy (ART) for treating HIV infection in ART-eligible pregnant women. Cochrane Database Syst Rev 2010(3):Cd008440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States Available at http://aidsinfo.nih.gov/contentfiles/Ivguidelines/PerinatalGL.pdf. Accessed June 11, 2018.
- 4.U.S. Public Health Service Task Force. Recommendations for the use of zidovudine to reduce perinatal transmission of human immunodeficiency virus. MMWR Recomm Rep 1994;43(RR-11):1–20. [PubMed] [Google Scholar]
- 5.Nesheim S, Taylor A, Lampe MA, Kilmarx PH, Fitz Harris L, Whitmore S, et al. A framework for elimination of perinatal transmission of HIV in the United States. Pediatrics 2012;130(4):738–744. [DOI] [PubMed] [Google Scholar]
- 6.Center for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2015. HIV Surveillance Supplemental Report 2017;22(2). Available at https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-22-2.pdf. Accessed June 11, 2018. [Google Scholar]
- 7.Napravnik S, Royce R, Walter E, Lim W. HIV-1 infected women and prenatal care utilization: barriers and facilitators. AIDS Patient Care STDS 2000;14(8):411–420. [DOI] [PubMed] [Google Scholar]
- 8.Adams JW, Brady KA, Michael YL, Yehia BR, Momplaisir FM. Postpartum engagement in HIV care: an important predictor of long-term retention in care and viral suppression. Clin Infect Dis 2015;61(12):1880–1887. [DOI] [PubMed] [Google Scholar]
- 9.Rana AI, Gillani FS, Flanigan TP, Nash BT, Beckwith CG. Follow-up care among HIV-infected pregnant women in Mississippi. J Womens Health 2010;19(10):1863–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swain CA, Smith LC, Nash D, et al. Postpartum loss to HIV care and HIV viral suppression among previously diagnosed HIV-infected women with a live birth in New York State. PLoS One 2016;11(8):e0160775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meade CM, Hussen SA, Momplaisir F, Badell M, Hackett S, Sheth AN. Long term engagement in HIV care among postpartum women with perinatal HIV infection in the United States. AIDS Care 2018;30(4):488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Momplaisir FM, Storm DS, Nkwihoreze H, Jayeola O, Jemmott JB. Improving postpartum retention in care for women living with HIV in the United States. AIDS 2018;32(2):133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mellins CA, Chu C, Malee K, Allison S, Smith R, Harris L, et al. Adherence to antiretroviral treatment among pregnant and postpartum HIV-infected women. AIDS Care 2008;20(8):958–968. [DOI] [PubMed] [Google Scholar]
- 14.Bardeguez AD, Lindsey JC, Shannon M, Tuomala RE, Cohn SE, Smith E, et al. Adherence to antiretrovirals among US women during and after pregnancy. J Acquir Immune Defic Syndr 2008;48(4):408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaz MJ, Barros SM, Palacios R, Senise JF, Lunardi L, Amed AM, et al. HIV-infected pregnant women have greater adherence with antiretroviral drugs than non-pregnant women. Int J STD AIDS 2007;18(1):28–32. [DOI] [PubMed] [Google Scholar]
- 16.Nachega JB, Uthman OA, Anderson J, Peltzer K, Wampold S, Cotton MF, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS 2012;26(16):2039–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel M, Tedaldi E, Armon C, Nesheim S, Lampe M, Palella F Jr., et al. HIV RNA suppression during and after pregnancy among women in the HIV Outpatient Study, 1996 to 2015. J Int Assoc Provid AIDS Care 2018;17:2325957417752259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg AE, Purcell DW, Gordon CM, Barasky RJ, del Rio C. Addressing the challenges of the HIV continuum of care in high-prevalence cities in the United States. J Acquir Immune Defic Syndr 2015;69 Suppl 1:S1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradley H, Hall HI, Wolitski RJ, Van Handel MM, Stone AE, LaFlam M, et al. Vital Signs: HIV diagnosis, care, and treatment among persons living with HIV--United States, 2011. MMWR Morb Mort Wkly Rep 2014;63(47):1113–1117. [PMC free article] [PubMed] [Google Scholar]
- 20.Stein R, Xu S, Marano M, Williams W, Cheng Q, Eke A, et al. HIV testing, linkage to HIV medical care, and interviews for partner services among women - 61 Health Department Jurisdictions, United States, Puerto Rico, and the U.S. Virgin Islands, 2015. MMWR Morb Mort Wkly Rep 2017;66(41):1100–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Napravnik S, Eron JJ Jr., McKaig RG, Heine AD, Menezes P, Quinlivan E. Factors associated with fewer visits for HIV primary care at a tertiary care center in the Southeastern U.S. AIDS Care 2006;18 Suppl 1:S45–50. [DOI] [PubMed] [Google Scholar]
- 22.Institute of Medicine. Monitoring HIV Care in the United States: indicators and data systems Available at 10.17226/13225. Accessed June 11, 2018. [DOI] [PubMed]
- 23.Mugavero MJ, Westfall AO, Cole SR, Geng EH, Crane HM, Kitahata MM, et al. Beyond core indicators of retention in HIV care: missed clinic visits are independently associated with all-cause mortality. Clin Infect Dis 2014;59(10):1471–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swain CA, Smith LC, Nash D, Pulver WP, Gordon D, Bian F, et al. Postpartum human immunodeficiency virus care among women diagnosed during pregnancy. Obstet Gynecol 2016;128(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reif SS, Whetten K, Wilson ER, McAllaster C, Pence BW, Legrand S, et al. HIV/AIDS in the Southern USA: a disproportionate epidemic. AIDS Care 2014;26(3):351–359. [DOI] [PubMed] [Google Scholar]
- 26.Patterson KB, Leone PA, Fiscus SA, Kuruc J, McCoy SI, Wolf L, et al. Frequent detection of acute HIV infection in pregnant women. AIDS 2007;21(17):2303–2308. [DOI] [PubMed] [Google Scholar]
- 27.Kuruc JD, Cope AB, Sampson LA, Gay CL, Ashby RM, Foust EM, et al. Ten years of screening and testing for acute HIV infection in North Carolina. J Acquir Immune Defic Syndr 2016;71(1):111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sena AC, Donovan J, Swygard H, Clymore J, Mobley V, Sullivan K, et al. The North Carolina HIV bridge counselor program: outcomes from a statewide level intervention to link and reengage HIV-infected persons in care in the South. J Acquir Immune Defic Syndr 2017;76(1):e7–e14. [DOI] [PubMed] [Google Scholar]
- 29.Rebeiro PF, Gange SJ, Horberg MA, Abraham AG, Napravnik S, Samji H, et al. Geographic variations in retention in care among HIV-infected adults in the United States. PloS One 2016;11(1):e0146119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gifford K, Walls J, Ranji U, Salganicoff A, Gomez I, Henry J Kaiser Family Foundation. Medicaid coverage of pregnancy and perinatal benefits: results from a state survey Available at https://www.kff.org/womens-health-policy/report/medicaid-coverage-of-pregnancy-and-perinatal-benefits-results-from-a-state-survey/. Accessed June 11, 2018.
- 31.Wohl DA, Kuwahara RK, Javadi K, Kirby C, Rosen DL, Napravnik S, et al. Financial barriers and lapses in treatment and care of HIV-infected adults in a southern state in the United States. AIDS Patient Care STDS 2017;31(11):463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siddiqui R, Bell T, Sangi-Haghpeykar H, Minard C, Levison J. Predictive factors for loss to postpartum follow-up among low income HIV-infected women in Texas. AIDS Patient Care STDS 2014;28(5):248–253. [DOI] [PubMed] [Google Scholar]
- 33.Donovan J, Sullivan K, Wilkin A, et al. Past care predicts future care in out-of-care people living with HIV: results of a clinic-based retention-in-care intervention in North Carolina. AIDS Behav 2018:s10461–018-2106–5. [DOI] [PubMed]
- 34.Lubelchek RJ, Fritz ML, Finnegan KJ, Trick WE. Use of a real-time alert system to identify and re-engage lost-to-care HIV patients. J Acquir Immune Defic Syndr 2016;72(2):e52–55. [DOI] [PubMed] [Google Scholar]
- 35.Mugavero MJ, Lin HY, Willig JH, Westfall AO, Ulett KB, Routman JS, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis 2009;48(2):248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howe CJ, Cole SR, Napravnik S, Eron JJ. Enrollment, retention, and visit attendance in the University of North Carolina Center for AIDS Research HIV clinical cohort, 2001–2007. AIDS Res Hum Retroviruses 2010;26(8):875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rebeiro P, Althoff KN, Buchacz K, Gill J, Horberg M, Krentz H, et al. Retention among North American HIV-infected persons in clinical care, 2000–2008. J Acquir Immune Defic Syndr 2013;62(3):356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yehia BR, Rebeiro P, Althoff KN, Agwu AL, Horberg MA, Samji H, et al. Impact of age on retention in care and viral suppression. J Acquir Immune Defic Syndr 2015;68(4):413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davy-Mendez T, Eron JJ, Zakharova O, Wohl DA, Napravnik S. Increased Persistence of Initial Treatment for HIV Infection With Modern Antiretroviral Therapy. J Acquir Immune Defic Syndr 2017;76(2):111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boehme AK, Davies SL, Moneyham L, Shrestha S, Schumacher J, Kempf MC. A qualitative study on factors impacting HIV care adherence among postpartum HIV-infected women in the rural southeastern USA. AIDS Care 2014;26(5):574–581. [DOI] [PubMed] [Google Scholar]
- 41.Buchberg MK, Fletcher FE, Vidrine DJ, Levison J, Peters MY, Hardwicke R, et al. A mixed-methods approach to understanding barriers to postpartum retention in care among low-income, HIV-infected women. AIDS Patient Care STDS 2015;29(3):126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016;375(9):830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mandelbrot L, Tubiana R, Le Chenadec J, Dollfus C, Faye A, Pannier E, et al. No perinatal HIV-1 transmission from women with effective antiretroviral therapy starting before conception. Clin Infect Dis 2015;61(11):1715–1725. [DOI] [PubMed] [Google Scholar]
- 44.Presidential Task Force on Redefining the Postpartum Visit Committee on Obstetric Practice. Optimizing postpartum care. Obstet Gynecol 2018;131(5):949–951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


