Abstract
Introduction.
Depression increases during menopause, and subclinical depressive symptoms increase risk for major depression. Insomnia is common among postmenopausal women and increases depression-risk in this already-vulnerable population. Recent evidence supports the efficacy of cognitive-behavioral therapy for insomnia (CBTI) to treat menopausal insomnia, but it remains unclear whether treating insomnia also alleviates co-occurring depressive symptoms and depressogenic features. This trial tested whether CBTI improves depressive symptoms, maladaptive thinking, and somatic hyperarousal in postmenopausal women with insomnia, and whether sleep restriction therapy (SRT)—a single component of CBTI—is equally efficacious. Materials and methods. Single-site, randomized controlled trial. 117 postmenopausal women (56.34±5.41 years) with peri-or-postmenopausal onset of chronic insomnia were randomized to 3 treatment conditions: sleep hygiene education control (SHE), SRT; and CBTI. Blinded assessments were performed at baseline, posttreatment, and 6-month follow-up.
Results.
CBTI produced moderate-to-large reductions in depressive symptoms, whereas SRT produced moderate reductions but not until 6 months posttreatment. Treatment effects on maladaptive thinking were mixed. CBTI and SRT both produced large improvements in dysfunctional beliefs about sleep, but weaker influences on presleep cognitive arousal, rumination, and worry. Presleep somatic arousal greatly improved in the CBTI group and moderately improved in the SRT group. Improvements in depression, maladaptive thinking, and hyperarousal were linked to improved sleep. SHE produced no durable treatment effects.
Conclusions.
CBTI and SRT reduce depressive symptoms, dysfunctional beliefs about sleep, and presleep somatic hyperarousal in postmenopausal women, with CBTI producing superior results. Despite its cognitive emphasis, cognitive arousal did not respond strongly or durably to CBTI.
Name: Behavioral Treatment of Menopausal Insomnia: Sleep and Daytime Outcomes
URL: clinicaltrials.gov
Registration: NCT01933295
Keywords: Sleep, Menopause, Worry, Rumination, Cognitive arousal, Depression, Dysfunctional beliefs about sleep
Introduction
Risk for depression onset increases during menopause,1,2 and women experiencing menopausal symptoms report elevated levels of depressive symptoms.3 Insomnia symptoms are among the most common complaints during and after the menopause transition4–6 such that nearly half of postmenopausal women (43–48%) suffer from insomnia symptoms.4 Critically, insomnia (both the disorder and subclinical symptoms) often triggers depression7–9 such that insomnia precedes >40% of incident depression cases in the general adult population.10 By extension, not only is menopause transition a window of vulnerability for depression onset,2 but women with menopausal insomnia are likely at even greater risk for depression owing to the added burden of poor sleep. It is therefore imperative to identify safe and efficacious treatments for menopause-related insomnia disorder that also alleviate co-occurring depressive symptoms and associated features, such as maladaptive thinking and somatic hyperarousal.
Menopause itself—via hormonal changes and related symptoms—disrupts sleep and increases risk for insomnia disorder.5,11 Recent evidence from randomized clinical trials (RCTs)—including the MSFlash trials and our own—show that nonpharmacological insomnia treatments substantially reduce insomnia symptoms in peri and postmenopausal women.12–14 Importantly, cognitive-behavioral therapy for insomnia (CBTI) and sleep restriction therapy (SRT; a brief nonpharmacological insomnia treatment comprised of a single component of CBTI15) delivered via face-to-face13 or telemedicine12 boast much larger reductions in menopause-related insomnia symptoms than hormone replacement therapy, antidepressant medication, yoga, and exercise.16 These data support CBTI and SRT as first-line treatments for menopausal insomnia.
Yet given the high comorbidity10,17 and even shared etiology18,19 between insomnia and depression, gold standard treatments for insomnia disorder related to menopause should also ideally alleviate co-occurring depressive symptoms and depressogenic behaviors in peri- and postmenopausal women at risk for depression. Indeed, antidepressant effects are touted as a unique strength of nonpharmacological insomnia treatments as CBTI reduces concurrent depressive symptoms in insomnia patients.20–22 However, it remains unclear whether CBTI and SRT reduce depressive symptoms associated with menopause and menopause-related insomnia. Furthermore, to maximally target co-occurring depression and reduce risk for future depression, insomnia interventions would ideally also target depressogenic behaviors and features, like maladaptive thinking and somatic hyperarousal.
Individuals with insomnia are more burdened by faulty cognitive processing than good sleepers, as evidenced by greater cognitive arousal.23–27 Notably, multiple forms of cognitive arousal exist (e.g., rumination, worry), which growing data show to be similarly associated with depression.28–30 Importantly, we have shown that insomniacs who ruminate on stress (i.e., they are cognitively aroused) are at especially high risk for developing depression, even compared to individuals with insomnia or elevated cognitive arousal alone.25 Further compounding disease-risk, insomniacs harbor dysfunctional beliefs about sleep (e.g., catastrophizing negative fallout of a poor night’s sleep).31 These dysfunctional beliefs are not only tied to insomnia,32,33 but also to depression and suicidal ideation.34,35 These unrealistic and harmful beliefs reflect negative cognitive content. Cognitive catalyst models of depression etiology show that faulty cognitive processing (cognitive arousal, often in the forms of worry and rumination) intensifies the impact of already-existing negative cognitive content (e.g., dysfunctional beliefs) to fuel depression.36–38 To illustrate: an insomniac who harbors negative cognitive schema about sleep and other areas and is prone to ruminating on these negative thoughts (e.g., ruminating on one’s inability to sleep or on trouble at work) is at high risk for developing depression. Thus, to maximally treat co-occurring depression and prevent future depression in peri- and postmenopausal women with insomnia disorder, cognitive-behavioral insomnia treatments should defuse depressogenic cognitive arousal and alter negative cognitive content. And, critically, as both cognitive arousal and dysfunctional sleep beliefs also fuel insomnia,23,32,33 targeting these cognitive phenomena may reduce risk for insomnia disorder relapse among remitters.
Other areas of hyperarousal also interfere with sleep. Nocturnal somatic hyperarousal (e.g., muscle tension) in bed presages poor sleep and is tied to reduced sleep efficiency and quality.39 Thus, insomniacs endorse more somatic hyperarousal than good sleepers, particularly in bed while trying to fall asleep.40 Indeed, hyperarousal is at the center of most prevailing etiological models of insomnia.41–44 Much like cognitive arousal, somatic arousal, even when considering only the presleep period, is also linked to depression thereby representing an independent but common factor linking insomnia and depression.45,46 By extension, insomnia treatments that decrease presleep somatic hyperarousal in alleviating insomnia may also produce improved immediate and long-term depression outcomes.
The primary goal of this RCT was to compare CBTI, SRT, and sleep hygiene education (SHE) minimal intervention control for the treatment of menopause-related sleep and daytime impairment outcomes.47 The insomnia findings of this RCT have been reported previously.13 The present study sought to determine whether CBTI and SRT reduce depressive symptoms, maladaptive thinking, and somatic hyperarousal in postmenopausal women with chronic insomnia as compared to SHE control. In clinical practice, postmenopausal women who present with major depression would likely receive a referral for mental health treatment directly targeting depression. Thus, we investigated postmenopausal insomniacs with subclinical depressive symptoms in this RCT. This is crucial because patients with insomnia disorder and subclinical depressive symptoms may be less likely to receive depression-focused treatment, even though subclinical depressive symptoms substantially increase risk for developing future major depression.48 As insomniacs present with elevated depression symptoms, it is crucial for insomnia interventions to further reduce these depressive symptoms in addition to altering depressogenic cognitive processing and content and somatic hyperarousal to minimize future disease-risk. In this RCT, we specifically look at treatment effects on depressive symptoms, negative cognitive content (dysfunctional beliefs about sleep), cognitive arousal (presleep cognitive arousal, stress-related rumination, and worry), and presleep somatic hyperarousal. We hypothesized that patients receiving CBTI or SRT would report reductions in all outcomes as compared to patients receiving SHE after completing treatment and at 6-month follow-up. In addition, we anticipated that the additional components of CBTI (i.e., cognitive therapy, progressive muscle relaxation, stimulus control, and sleep hygiene) would add substantial value to treatment and produce larger and more durable effects than SRT for depressive symptoms and features.
Methods
Participants and Procedure
This study was conducted in a 6-hospital health system in the state of Michigan. Women were recruited from primary care and a sleep clinic, the community via newspaper advertisements, and from a database of prior sleep center studies. To be eligible, women must have been postmenopausal (12 consecutive months without menses), reported wake after sleep onset (wakefulness in the middle of the night after falling asleep) of an hour or more on ≥ 3 nights per week, and met criteria for DSM-5 insomnia disorder with onset or exacerbation during the peri- or postmenopausal period per clinical interview with a registered nurse with specialty training in behavioral sleep medicine. And as the sleep maintenance phenotype has been most closely tied to the menopause transition and symptoms,49,50 objective sleep disturbance had to be evident per mean wake after sleep onset of 45 minutes or more on 2 overnight polysomnography (PSG) studies (adaptation night + baseline night, neither of which could have wake after sleep onset < 30 minutes). Exclusionary criteria included DSM-5 major depression per diagnostic interview, severe psychiatric illness (e.g., schizophrenia, bipolar) per patient report, untreated or uncontrolled major medical illness (e.g., hypertension, diabetes) per patient report, sleep-wake disorders other than insomnia (examined on PSG adaptation night and per patient report [obstructive sleep apnea defined as apnea-hypopnea index ≥ 15, periodic limb movements defined as arousal frequency ≥ 15]). We also excluded women taking medications influencing sleep (prescription and non-prescription sleep aids, herbal supplements, and any antidepressants taken at night), although women receiving hormone therapy were permitted to participate. Women having hot flashes were eligible to participate in this RCT.
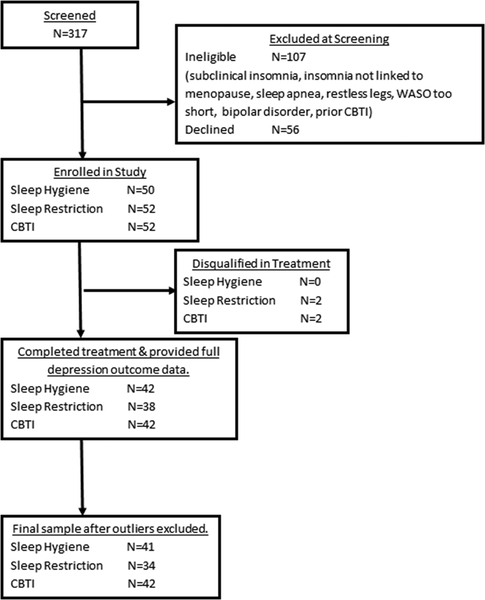
Refer to Figure 1 flow chart of study enrollment and participation. A total of 317 postmenopausal women were screened for eligibility. Of these individuals, 107 women were ineligible and another 56 declined to participate or had scheduling conflicts. Thus, 154 postmenopausal women were randomized to 1 of 3 treatment conditions: Sleep hygiene education treatment as usual (SHE: N=50), (2) Sleep restriction therapy (SRT: N=52), or Cognitive-behavioral therapy for insomnia (CBTI: N=52). Two subjects in both the SRT and CBTI conditions were disqualified during treatment for changes in medication or new onset comorbid sleep disorder. These two allocations were replaced in random order by a research staff member not involved with this study, and recruitment included two more individuals to replace those who were disqualified. This resulted in 50 subjects completing treatment in each of the 3 conditions. While double-blind could not be achieved given the nature of the behavioral interventions, subjects were not informed which treatments were considered control versus active, or of the specific hypotheses. Assessments of sleep, depression, maladaptive thinking, and hyperarousal were collected prior to treatment, at posttreatment (within 2 weeks of completing treatment), and 6 months after treatment completion. Of the 150 treatment completers, 122 women provided data for depressive symptoms at both posttreatment and 6-month follow-up (Figure 1).
Figure 1.
Flow chart of study enrollment, participation, and analysis inclusion.
Sleep hygiene education (SHE), i.e., minimal intervention control condition.
Women randomized to the online sleep hygiene education condition received 6 weekly emails including general, non-personalized information on the following topics: the basics of endogenous sleep regulation; the impact of sleep on health problems such as obesity, diabetes, and hypertension; the effects of stimulants and other sleep-disruptive substances; the relationship between sleep, diet, and exercise; and tips on creating a sleep-conducive bedroom environment. Sleep hygiene is neither the primary cause nor a sufficient therapeutic target in insomnia disorder and therefore served as an ideal minimal intervention control condition and real-world comparator.47,51
Cognitive-behavioral therapy for insomnia (CBTI).
Women randomized to CBTI completed 6 face-to-face sleep therapy sessions with a registered nurse who specializes in behavioral sleep medicine. CBTI is a structured, multi-modal treatment that targets sleep-disruptive behaviors and beliefs (see Perlis et al. 200652). Data from clinical trials consistently show that CBTI is as efficacious as pharmacological treatment in the short-term, but produces superior treatment response in the long-term.53,54 CBTI patients received 6 weekly sessions, which covered behavioral (sleep restriction and stimulus control) and cognitive (e.g., cognitive restructuring) components, as well as relaxation strategies (e.g., progressive muscle relaxation and autogenic training) and sleep hygiene education. Fidelity monitoring for the nurse therapist included weekly supervision meetings with one of two licensed PhD clinical psychologists, both of whom are certified in behavioral sleep medicine. Supervision meetings included discussions of cases, problem-solving, and listening to and providing feedback based on recorded therapy session.
Sleep restriction therapy (SRT) is an effective standalone behavioral treatment for insomnia disorder.55 Although SRT actually predates CBTI, SRT is now commonly packaged as part of CBTI and is typically considered one of CBTI’s main active components. As CBTI consists of SRT plus multiple other components, SRT is the briefer of the two interventions. Here, SRT was delivered as a 2-week intervention. Specifically, the initial face-to-face session consisted of reviewing patient sleep history, education and rationale for sleep restriction practices, and behavioral homework. Four follow-up sessions (3 phone contacts, each 3–4 days apart, followed by a 2nd face-to-face session) were then delivered across the following 2 weeks and were used to titrate sleep schedules based on sleep diary data. Fidelity monitoring for the SRT condition was the same as described in the CBTI section above.
All study procedures were approved by the institutional review board.
Measures
Depressive symptoms were measured using the Beck Depression Inventory, 2nd edition (BDI-II),56 a 21-item self-report measure of depressive symptoms. BDI-II scores range from 0 to 63, with higher scores indicating greater severity. BDI-II scores > 20 indicate moderately severe depression. Maladaptive thinking was measured across four surveys. The Dysfunctional Beliefs and Attitudes about Sleep scale, 16-item version (DBAS)31 is a self-report measure of dysfunctional beliefs about sleep (e.g., ‘I have little ability to manage the negative consequences of disturbed sleep’). Scores represent average item scores across the measure with scores above 4 representing irrational or dysfunctional beliefs about sleep. The Presleep Arousal Scale Cognitive factor (PSAS Cognitive)40 was used to measure cognitive arousal during the presleep period, i.e., when individuals are in bed attempting to fall asleep. The PSAS Cognitive scale consists of 8 items (e.g., ‘review or ponder events of the day’ and ‘can’t shut off your thoughts’) and possible scores range from 8 to 40 with higher scores indicating greater presleep cognitive arousal. The 20-item Event Related Rumination Inventory (ERRI)57 measures intrusive and deliberate rumination in response to life stress. Each item is on a 0 to 3 scale with higher ratings reflecting higher levels of rumination. The inventory score is represented by the mean item level rating. The 16-item Penn State Worry Questionnaire (PSWQ)58 measures trait tendency to engage in worry. Higher scores represent higher levels of trait worry with PSWQ scores ≥ 40 representing moderate worry or worse. Somatic hyperarousal was measured using the Presleep Arousal Scale Somatic factor (PSAS Somatic).40 The PSAS Somatic scale consists of 8 items (e.g., ‘heart racing, pounding, or beating irregularly’ and ‘a tight, tense feeling in your muscles’) and possible scores range from 8 to 40 with higher scores indicating greater presleep cognitive arousal. Lastly, insomnia symptoms were assessed using the Insomnia Severity Index (ISI).59 ISI scores ≥ 15 represent clinical insomnia, and ISI scores ≤ 7 indicate no significant insomnia symptoms. In the present study, insomnia remission is operationalized as ISI ≤ 7 after treatment.
Analysis Plan
Analyses were conducted using SPSS version 25. A total of 122 women provided outcome data at both posttreatment assessments. We excluded from analysis 5 statistical outliers on the BDI-II (scores > 3 SDs above the mean), resulting in a total sample of 117 for our analyses. In addition, we also ran analyses in patients who provided only partial posttreatment data (presented in supplementary materials). Overall demographics and pretreatment characteristics were first presented and compared across the 3 treatment conditions using oneway ANOVA to identify group differences before treatment. To test treatment effects, we first ran 3×2 repeated measures ANOVAs to examine Treatment x Time interactions for changes in depressive symptoms, maladaptive thinking, and hyperarousal from pretreatment to immediate posttreatment. After testing for Treatment x Time interaction effects, paired samples t-tests were conducted within each condition to test for potential simple effects; significant results and statistical trends were then followed-up with Cohen’s d estimation of effect size specifically designed for paired samples t-tests, which accounts for the correlation between the pre- and posttreatment values.60 In addition, cross-sectional one-way ANOVAs with LSD posthoc comparisons were used to compare mean levels for each treatment outcome to determine differences in symptom levels across groups. These analyses were then repeated for 6-month follow-up data. We then ran exploratory bivariate correlations between changes in insomnia symptoms (pre- to posttreatment, and then pre- to 6-month follow-up) and changes in each of our primary outcome variables. These results showed whether changes in depressed mood, maladaptive thinking, and hyperarousal were associated with improvements in insomnia symptoms, irrespective of treatment condition. Lastly, we compared insomnia remitters (ISI ≤ 7) vs non-remitters at posttreatment and 6-month follow-up to compare levels of depressive symptoms, maladaptive thinking, and somatic hyperarousal.
Results
Sample characteristics
Refer to Table 1 for full sample characteristics. Our sample was largely comprised of non-Hispanic White (54.7%) and non-Hispanic Black (38.5%) women. Prior to treatment, mean ISI scores were in the clinical range (ISI: 15.03±3.97) and 4.3% of the sample endorsed moderately severe depression. Across the four cognition surveys, high levels of maladaptive thinking were observed. DBAS mean scores were in the elevated range, reflecting high levels of dysfunctional beliefs about sleep before treatment. Unexpectedly, presleep cognitive arousal levels were lower than what has been reported in other studies of poor sleepers, insomniacs, and individuals with psychiatric illness and chronic pain.39,61,62 Tendency to worry was in the moderate range and consistent with levels reported by other insomniacs,63 whereas pretreatment ERRI scores were slightly lower than what is reported by individuals after experiencing major life stress.57 Self-reported somatic hyperarousal was high and consistent with or higher than other clinical samples.39,61,62 Importantly, no factors differed across groups prior to treatment.
Table 1.
Sample characteristics prior to treatment.
| All subjects | SHE | SRT | CBTI | ||
|---|---|---|---|---|---|
| Sample size | 117 | 41 | 34 | 42 | |
| Age | 56.34 ± 5.41 | 57.34 ± 5.97 | 56.62 ± 4.95 | 55.14 ± 5.06 | F(2,114)=1.80, p=.17 |
| Race | |||||
| White | 64; 54.7% | 24; 58.5% | 19; 55.9% | 21; 50.0% | |
| Black | 45; 38.5% | 16; 39.0% | 11; 32.4% | 18; 42.9% | |
| Hispanic or Latinx | 1; 0.9% | -- | 1; 2.9% | -- | |
| Other | 1; 0.9% | -- | -- | 1; 2.4% | |
| Did not answer | 6; 5.1% | 1; 2.4% | 3; 8.8% | 2; 4.8% | |
| Hormone replacement Therapy | 4; 3.4% | 3; 7.3% | 1; 2.9% | 0; 0.0% | |
| Medical Menopause | 27; 23.1% | 7; 17.1% | 7; 20.6% | 13; 31.0% | χ2(2)=2.42, p=.30 |
| Years since last menstruation | 7.18±7.32 | 7.46±8.56 | 6.34±6.87 | 6.42±6.72 | F(2,105)=.26, p=.77 |
| Employed | 86; 74.4% | 29; 70.7% | 23; 67.6% | 34.0%; 81.0% | χ2(2)=1.96, p=.38 |
| BMI (self-reported) | 25.42±3.58 | 26.87±4.96 | 26.87±4.08 | F(2,88)=1.20, p=.31 | |
| Pre-treatment | |||||
| BDI-II | 8.26 ± 5.00 | 8.80 ± 5.62 | 6.77 ± 4.15 | 8.93 ± 4.85 | F(2,114)=2.18, p=.12 |
| DBAS | 4.37 ± 1.45 | 4.51 ± 1.67 | 4.32 ± 1.33 | 4.28 ± 1.32 | F(2,114)=0.30, p=.74 |
| PSAS Cognitive | 12.38 ±3.76 | 12.59 ± 3.00 | 11.55 ± 4.02 | 12.85 ± 4.18 | F(2,112)=1.20, p=.30 |
| ERRI | 1.17 ± .71 | 1.22 ± .68 | 1.05 ± .72 | 1.21 ± .73 | F(2,112)=0.70, p=.50 |
| PSWQ | 45.39 ± 12.64 | 47.44 ± 13.57 | 42.76 ± 11.01 | 45.51 ± 12.85 | F(2,113)=1.28, p=.28 |
| PSAS Somatic | 19.90 ± 7.20 | 21.46 ± 7.87 | 18.91 ± 6.62 | 19.15 ± 6.87 | F(2,112)=1.51, p=.23 |
Note: BDI-II = Beck depression inventory, 2nd edition. DBAS = dysfunctional beliefs and attitudes about sleep scale, 16-item version. PSAS Cognitive = presleep arousal scale, cognitive factor. PSAS Somatic = presleep arousal scale, somatic factor. ERRI = event-related rumination inventory. PSWQ = Penn State worry questionnaire. F-statistic and p-value represents results from one-way ANOVAs comparing scores across the 3 groups.
Treatment effects on depressive symptoms
See Table 2 for full results and Figure 2 as a visual aid for these findings. Please refer to Supplementary Table 1 for replication of these findings in all patients who provided complete or partial posttreatment outcomes.
Table 2.
Comparing CBTI vs SRT vs SHE on depressive symptoms and maladaptive thinking.
| Posttreatment | Δ Pre- to posttreatment | 6-month Follow-up | Δ Pre- to 6-month follow-up | |
|---|---|---|---|---|
| BDI-II | F(2,114)=2.18, p=. 12 | F(2,114)=2.05, p=. 13 | F(2,114)=3.11, p<.05 | F(2,114)=3.97, p=.02 |
| SHE | 7.95 ± 5.93 | t(40)=−1.58, p=.15 | 7.56 ± 5.42bc | t(40)=−1.68, p=.10 |
| SRT | 5.65 ± 4.84 | t(33)=−1.78, p=.08 | 5.24 ± 3.81a | t(33)=−2.91, p<.01, d=.50 |
| CBTI | 6.38 ± 4.76 | t(41)=−3.53, p<.01, d=.55 | 5.24 ± 4.88a | t(41)=−5.14, p<.001, d=.79 |
| DBAS | F(2,114)=9.88, p<.001 | F(2,114)=11.25, p<.001 | F(2,112)=11.58, p<.001 | F(2,112)=9.04, p<.001 |
| SHE | 4.39 ± 1.78bc | t(40)=−0.62, p=.54 | 4.33 ± 1.35bc | t(40)=−0.93, p=.36 |
| SRT | 3.68 ± 1.21ac | t(33)=−4.06, p<.001, d=.70 | 3.51 ± 1.24ac | t(33)=−4.85, p<.001, d=.84 |
| CBTI | 2.90 ± 1.48ab | t(41)=−6.74, p<.001, d=1.05 | 2.87 ± 1.48ab | t(39)=−6.10, p<.001, d=.97 |
| PSAS Cognitive | F(2,72)=0.41, p=.69 | F(2,68)=0.75, p=.48 | F(2,54)=0.13, p=.88 | F(2,54)=0.18, p−.84 |
| SHE | 11.40 ± 3.42 | t(19)=−1.81, p=.09 | 11.93 ± 3.69 | t(13)=−0.33, p=.74 |
| SRT | 11.70 ± 4.12 | t(21)=−2.26, p=.03, d=.50 | 11.50 ± 2.93 | t(18)=−1.06, p=.31 |
| CBTI | 10.75 ± 4.21 | t(30)=−2.85, p<.01, d=.51 | 11.26 ± 4.87 | t(25)=−1.35, p=.19 |
| ERRI | F(2,72)=0.07, p=.93 | F(2,68)=0.49, p=.62 | F=(2,54)=0.41, p=.66 | F(2,54)=0.76, p=.47 |
| SHE | .88 ± .59 | t(19)=−3.05, p<.01, d=.67 | .96 ± .76 | t(13)=−.96, p=.35 |
| SRT | .93 ± .61 | t(21)=−1.83, p=.08 | 1.01 ± .55 | t(18)=−0.51, p=.62 |
| CBTI | .95 ± .81 | t(30)=−3.04, p=.01, d=.55 | .84 ± .68 | t(25)=−3.23, p<.01, d=.65 |
| PSWQ | F(2,113)=3.05, p=.05 | F(2,113)=3.94, p=.05 | F(2,111)=2.81, p=.07 | F(2,111)=0.70, p=.50 |
| SHE | 47.32 ± 13.45 | t(40)=−0.09, p=.93 | 45.49 ± 11.76 | t(40)=−1.49, p=.14 |
| SRT | 40.65 ± 10.41 | t(33)=−1.73, p=.09 | 39.79 ± 9.78 | t(33)=−2.30, p=.03, d=.40 |
| CBTI | 42.88 ± 11.73 | t(40)=−1.77, p=.09 | 41.15 ± 11.24 | t(38)=−3.04, p<.01, d=.53 |
| PSAS Somatic | F(2,72)=2.21, p=.12 | F(2,68)=1.54, p=.22 | F(2,54),4.55, p=.02 | F(2,54)=2.55, p=.09 |
| SHE | 17.50 ± 7.34 | t(19)=−1.70, p=.11 | 18.36 ± 7.26c | t(13)=−0.74, p=.47 |
| SRT | 16.65 ± 5.60 | t(21)=−2.81, p=.01, d=.61 | 18.80 ± 6.34c | t(18)=−0.54, p=.60 |
| CBTI | 14.19 ± 5.21 | t(30)=−4.71, p<.001, d=.89 | 14.00 ± 4.83ab | t(25)=−5.02, p<.001, d=1.06 |
BDI-II = Beck depression inventory, 2nd edition. DBAS = dysfunctional beliefs and attitudes about sleep scale, 16-item version. PSAS Cognitive = presleep arousal scale, cognitive factor. PSAS Somatic = presleep arousal scale, somatic factor. ERRI = event-related rumination inventory. PSWQ = Penn State worry questionnaire. F-statistics in the Posttreatment and 6-month Follow-up columns represent one-way ANOVAs comparing scores across groups.
= significantly different from SHE.
= significantly different from SRT.
= significantly different from CBTI. F-statistics in the Δ Pre- to posttreatment and Δ Pre- to 6-month follow-up columns represent Treatment x Time interactions in a 3×2 repeated measures ANOVA. t-statistics represent results from paired samples t-tests.
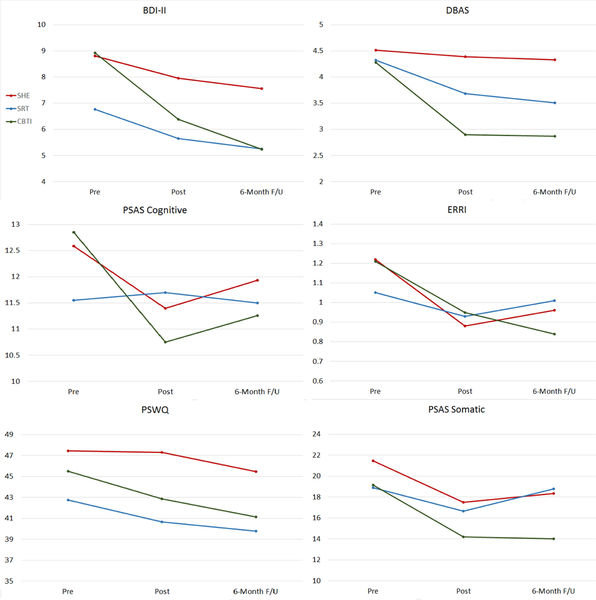
Figure 2.
Changes in depressive symptoms, maladaptive thinking, and somatic hyperarousal by treatment condition.
We first evaluated changes in depressive symptoms. A 3×2 repeated measures ANOVA testing changes in depressive symptoms from pretreatment to posttreatment showed a non-significant Treatment x Time interaction (p=.12). Follow-up paired samples t-tests revealed a small decrease in depressive symptoms in the SRT group (d=.31) and a medium decrease in the CBTI group (d=.55), but no change in the SHE group (p=.15).
We then ran a repeated measures ANOVA evaluating changes in depression scores from pretreatment to 6-month follow-up (Table 2). A significant Treatment x Time interaction was observed (p<.05), and patients receiving SRT or CBTI both reported lower depressive symptoms 6 months after completing treatment. Indeed, SRT patients reported a medium decrease in depressive symptoms (d=.50), whereas CBTI patients reported a large decrease in symptoms (d=.79). Notably, no changes were reported by patients receiving SHE, who reported higher levels of depression at 6-month follow-up than both CBTI and SRT patients. Depressive symptoms at 6-month follow-up did not differ between SRT and CBTI conditions.
Treatment effects on maladaptive thinking
We next tested effects on maladaptive thinking (Table 2). A repeated measures ANOVA evaluating changes in dysfunction sleep beliefs revealed a significant Treatment x Time interaction such that CBTI patients reported very large decreases in dysfunctional beliefs about sleep (d=1.05), SRT patients reported medium-large decreases (d=.70), and SHE patients had no change (p=.54). Posttreatment, CBTI patients reported the healthiest sleep beliefs, whereas SHE patients reported the most dysfunctional beliefs. SRT patients reported healthier beliefs than SHE patients, but more dysfunctional than CBTI patients. These patterns were maintained 6 months later (Table 2).
Presleep cognitive arousal moderately decreased during treatment in the CBTI and SRT groups (CBTI d=.51, SRT d=.50; Table 2). Even so, we observed no group differences in presleep cognitive arousal levels at posttreatment (p=.69). By 6-month follow-up, presleep cognitive arousal levels were no longer significantly different from pretreatment levels in any group (p=.88), indicating that presleep cognitive arousal returns to pretreatment levels in the months after completing insomnia treatment.
Stress-related rumination moderately decreased during treatment in the CBTI and SHE groups (CBTI d=.55, SHE d=.67; Table 2). No Treatment x Time interaction was observed (p=.62), and groups did not differ on rumination levels at posttreatment (p=.93). By 6-month follow-up, only CBTI patients reported lower levels of stress-related rumination compared to their pretreatment levels (d=.65), whereas patients in the SHE condition no longer exhibited any improvement from pretreatment baseline (p=.35).
Repeated measures ANOVA models revealed Treatment x Time interactions that approached significance for worry at posttreatment (p=.05) and was non-significant at 6-month follow-up (p=.50). Worry levels were slightly lower at posttreatment for SRT (d=.30) and CBTI patients (d=.30) compared to pretreatment levels, whereas these reductions became moderately sized 6 months later (SRT d=.40, CBTI d=.53; Table 2). Even so, CBTI and SRT patients did not differ from SHE or SRT patients on worry at either time-point.
Treatment effects on somatic hyperarousal
CBTI patients reported large decreases in somatic hyperarousal by the end of treatment (d=.89) and SRT patients reported a medium decrease (d=.61), whereas no change was observed in SHE patients (p=.12; Table 2). Despite differential rates of change, groups did not differ significantly on somatic arousal immediately after treatment. Six months later, a Treatment x Time interaction was observed (p=.02), and only CBTI patients reported a decrease in somatic hyperarousal from pretreatment baseline, which was large (d=1.06). Neither the SHE or SRT group reported long-term decreases in hyperarousal, indicating that somatic arousal did not improve in the SHE group, and that arousal returned to baseline in the months after treatment in the SRT group. Accordingly, hyperarousal was lowest in the CBTI group at 6-month follow-up. Improved sleep is linked to decreases in depression, maladaptive thinking, and hyperarousal.
Finally, we explored associations between reductions in insomnia symptoms (i.e., change scores in ISI; see Drake et al for full insomnia outcomes for this RCT13) and changes in our primary outcomes; see Table 3 for full results. Improvements in insomnia were strongly correlated with both improvements in depression and dysfunctional beliefs about sleep at both posttreatment and 6-month follow-up. Indeed, insomnia remitters reported substantially lower depression scores than non-remitters at posttreatment (t[115]=−3.66, p<.001, d=.74) and 6-month follow-up (t[110]=−3.16, p<.01, d=.60), see Table 4. Similarly, insomnia remitters reported more adaptive beliefs about sleep compared to non-remitters at both posttreatment (t[115]=−5.18, p<.001, d=1.02) and 6-month follow-up (t[110]=−4.72, p<.001, d=.90).
Table 3.
Correlations between changes in insomnia symptoms and changes in depressive symptoms, maladaptive thinking, and hyperarousal.
| Δ ISI | ||
|---|---|---|
| Pre to Posttreatment | Pre to Follow-up | |
| Δ BDI-II | .38*** | .28** |
| Δ DBAS | .53*** | .41*** |
| Δ PSAS Cognitive | .15 | −.05 |
| Δ ERRI | .25* | .18 |
| Δ PSWQ | .29** | .14 |
| Δ PSAS Somatic | .27* | .21 |
Note:
p<.05.
p<.01.
p<.001.
All correlations represent associations between changes in ISI and the dependent variable.
Table 4.
Comparing depressive symptoms, maladaptive thinking, and hyperarousal between insomnia remitters and non-remitters.
| Insomnia Remission Status | ||||||
|---|---|---|---|---|---|---|
| Posttreatment | 6-month follow-up | |||||
| Remitters | Non-Remitters | Remitters | Non-Remitters | |||
| BDI-II | 4.41±4.07 | 7.96±5.44 | Cohen’s d=.74 | 4.57±4.13 | 7.31±4.95 | Cohen’s d=.60 |
| DBAS | 2.68±1.42 | 4.17±1.51 | Cohen’s d=1.02 | 2.92±1.29 | 4.15±1.43 | Cohen’s d=.90 |
| PSAS Cognitive | 9.79±2.41 | 12.33±4.56 | Cohen’s d=.70 | 10.00±2.15 | 12.50±3.74 | Cohen’s d=.82 |
| ERRI | .89±.76 | .95±.63 | -- | .87±.62 | .94±.68 | -- |
| PSWQ | 39.61±10.17 | 46.04±12.70 | Cohen’s d=.56 | 38.25±10.35 | 45.78±10.55 | Cohen’s d=.72 |
| PSAS Somatic | 13.00±4.29 | 18.05±6.35 | Cohen’s d=.93 | 13.88±4.20 | 19.58±7.10 | Cohen’s d=.98 |
Note: Insomnia severity index score of 7 or below indicates remission. BDI-II = Beck depression inventory, 2nd edition. DBAS = dysfunctional beliefs and attitudes about sleep scale, 16-item version. PSAS Cognitive = presleep arousal scale, cognitive factor. PSAS Somatic = presleep arousal scale, somatic factor. ERRI = event-related rumination inventory. PSWQ = Penn State worry questionnaire.
Insomnia symptom improvement was not associated with any changes in presleep cognitive arousal (Table 3). Yet, insomnia remitters reported substantially lower presleep cognitive arousal than non-remitters at posttreatment (t[73]=−2.90, p<.01, d=.70) and at 6-month follow-up (t[57]=−3.22, p<.01, d=.82; see Table 4). To identify the cause of this, we used logistic regression to test whether presleep cognitive arousal prior to treatment predicted odds of remitting from insomnia at posttreatment and 6-month follow-up. Pretreatment presleep cognitive arousal did not predict insomnia remission at posttreatment (OR=.93, 95% CI=.83–1.04, p=.19), but did predict insomnia remission at 6-month follow-up (OR=.87, 95% CI=.78-.98, p=.02) such that each 1-point increases on the PSAS Cognitive scale corresponded with 15% decrease in odds of insomnia remission (OR−1=1.15). Thus, while reductions in insomnia symptoms may not correlate with changes in presleep cognitive arousal, it is possible that insomnia remitters have lower presleep cognitive arousal after treatment than nonremitters because those with less presleep cognitive arousal to begin with are more likely to remit.
Reductions in stress-related rumination and worry were both associated with improved insomnia symptoms at posttreatment, but by the time patients reached 6-month follow-up, changes in stress-related rumination or worry were no longer related to improvements in insomnia symptoms (Table 3). Interestingly, insomnia remitters did not differ on rumination at either posttreatment (t[73]=−0.37, p=.72) or follow-up (t[71]=−0.39, p=.70), whereas remitters reported less worry at both time-points (posttreatment: t[115]t=−2.79, p<.01; 6-month follow-up: t[110]=−3.56, p<.01).
Lastly, a pattern was observed for somatic hyperarousal such that improvements in insomnia were associated with reduced presleep somatic arousal after completing treatment, but were no longer correlated 6 months later. Insomnia remitters reported lower levels of presleep somatic hyperarousal compared to non-remitters at both posttreatment (t[73]=−3.92, p<.001) and 6-month follow-up (t[71]=−3.28, p<.01).
Discussion
In a sample of 117 postmenopausal women with chronic insomnia disorder, we evaluated the efficacy of CBTI and SRT in comparison to sleep hygiene education to alleviate subclinical depressive symptoms and to reduce maladaptive thinking and somatic hyperarousal. Both CBTI and SRT outperformed sleep hygiene education and resulted in moderate reductions in depressive symptoms, which were maintained 6 months later. Treatment effects on maladaptive thinking were mixed, with the most robust effects observed for CBTI and SRT reducing dysfunctional beliefs about sleep. CBTI produced the largest and most durable improvements in presleep somatic hyperarousal. Overall, the findings indicated that nonpharmacological insomnia interventions reduce depressive symptoms, maladaptive thinking, and somatic hyperarousal in women with menopause-related chronic insomnia disorder.
Prior reports, including from this trial, have confirmed the efficacy of CBTI and SRT for menopause-related insomnia.12,13 Findings from the present study add to the literature by showing that CBTI and SRT reduce subclinical depressive symptoms in women with menopause-related insomnia, and that these reductions in depressive symptoms are directly related to improvements in sleep. Indeed, CBTI and SRT patients reported lower depressive symptoms 6 months after treatment than patients who received SHE control, suggesting that successful treatment of insomnia may reduce risk for future depression in postmenopausal women. These findings are consistent with recent evidence showing that treating insomnia with CBTI prevents future depression.64 Further, our findings suggest that postmenopausal women with insomnia disorder and comorbid major depression may experience reductions in both sleep and mood symptoms via cognitive-behavioral insomnia interventions. These results are consistent with prior findings showing that CBTI improves symptoms of insomnia anddepression in patients with both disorders.20,21,65,66
Beyond targeting disease symptomatology, we examined whether CBTI and SRT alter some of the very same insomniogenic and depressogenic factors implicated in insomnia and depression development in the first place including negative cognitive content, faulty cognitive processing, and somatic hyperarousal. Consistent with extant evidence showing that nonpharmacological insomnia treatments improve attitudes toward sleep,67–69 both CBTI and SRT substantially reduced dysfunctional beliefs about sleep. However, these findings also add to the literature by showing that CBTI patients develop more adaptive attitudes toward sleep than patients treated with SRT, thereby showing that CBTI is superior to SRT and sleep education in altering negative cognitive content endemic to insomniacs. Improving sleep-focused beliefs is critical, not only because they are common among insomniacs and depressed individuals,32 but also because these beliefs fuel future insomnia,33 depression,34 and even depressive behavior like suicidal ideation.35 By extension, CBTI patients may be at lower risk for insomnia relapse or depression incidence than patients receiving SRT, SHE, or no treatment.
CBTI and SRT were less successful at producing robust and durable improvements to cognitive arousal (in other words, faulty cognitive processing). We specifically looked at three forms of cognitive arousal: presleep cognitive arousal, stress-related rumination, and worry. Improvements in presleep cognitive arousal were short-lived such that presleep cognitive arousal improved upon completing treatment but then returned to baseline by 6-month follow-up. Counter-intuitively, treatment-related improvements in insomnia symptoms did not correspond to changes in cognitive arousal, yet patients who remitted from insomnia after treatment reported less presleep cognitive arousal. Taken together, it is clear that individuals who no longer have insomnia engage in less nocturnal rumination, but there are no clear durable treatment effects in this sample, which suggests that CBTI and SRT may not be adequate to address presleep cognitive arousal. Notably, CBTI treatment effects on PSG-defined objective insomnia ratings are at times more modest than effects on self-reported insomnia ratings,67 therefore it is possible that reductions in nocturnal rumination may be attenuated by lesser treatment gains in objective nighttime wakefulness.
Similarly, treatment effects on stress-related rumination and worry were mixed and ultimately unconvincing. Acute reductions in rumination and worry were linked to treatment-related improvements in insomnia symptoms. And only CBTI produced significant long-term reductions in stress-related rumination and worry, but at no point after treatment did CBTI patients report lower levels of rumination than SRT or SHE patients. These findings indicate that these reductions may not be clinically meaningful. By extension, these results suggest that standard 6-session CBTI, despite its ostensible cognitive emphasis (via one session of cognitive therapy), does not adequately address cognitive arousal. However, emerging evidence supports mindfulness-based strategies to effectively reduce cognitive arousal such as rumination, worry, and presleep cognitive arousal.70–73 To produce clinically meaningful reductions in insomnia-related faulty cognitive processing (i.e., cognitive arousal, rumination, worry), insomnia patients with high cognitive arousal may benefit from an augmentation strategy that enhances CBTI with mindfulness.70,74
Presleep somatic hyperarousal was highly elevated in this clinical sample. CBTI produced the strongest and most durable effects on somatic hyperarousal with large reductions observed at both posttreatment and 6-month follow-up. SRT produced only acute reductions, whereas hyperarousal returned to pretreatment baseline 6 months after completing treatment. Accordingly, reductions in hyperarousal were related to improvements in insomnia symptoms, and remitters reported substantially lower levels of somatic arousal in the presleep period after treatment.
These findings taken together show that CBTI and—to a lesser extent—SRT reduce depressive symptoms, negative cognitive content, and presleep somatic hyperarousal in women with menopause-related chronic insomnia. Despite these benefits, our data also show there is considerable room for improvement for CBTI and SRT pertaining to cognitive-emotional arousal in particular. Indeed, reductions in cognitive-emotional arousal are not considered among primary targets in cognitive-focused insomnia interventions as evidenced by most insomnia RCTs not reporting changes (or lack thereof) in cognitive arousal related to CBTI or other deliveries. Considering that worry and rumination are central to cognitive models of insomnia23,44,75 and of course depression76 (i.e., the most common comorbidity in insomnia), future studies should monitor changes in these cognitive factors as primary outcomes in insomnia treatment. The present study showed that current protocols produce some—albeit non-robust—movement in these over-practiced cognitive processes; it may take just a few more sessions of cognitive therapy or mindfulness to produce larger and more robust outcomes. How much better can patients be served if CBTI and other insomnia interventions not only improved sleep efficiency, but also meaningfully improved emotion regulation through the reduction of worry and rumination, particularly during the presleep period?
CBTI in particular is well-equipped to improve sleep-related cognitive content in postmenopausal insomniacs as evidenced by more rationale attitudes and beliefs about sleep after treatment. Despite its cognitive emphasis, CBTI’s effects on cognitive processing marked by high arousal (i.e., an unrelenting tendency to worry and ruminate particularly when trying to sleep when in the context of insomnia) can be improved. Indeed, cognitive content vs processing is an important distinction with both serving important etiological roles in depression development after stress exposure.37 And given that insomniacs and individuals at risk for insomnia disorder show cognitive arousal profiles similar to individuals with anxiety and depression,18,23,77–79 it is perhaps unsurprising that a single session of cognitive therapy (as part of a standard six-session CBTI regimen) may be insufficient to substantially alter the manner in which affected individuals process stress and other negative information. And while some CBTI patients may indicate significant improvement in insomniogenic and depressogenic thinking, other patients—perhaps with more complex etiology, greater psychiatric comorbidity, or under severe stress—may benefit from CBTI treatment regimens augmented with techniques specifically targeting metacognitive processes. Given that mindfulness-based treatments reduce depression and cognitive arousal,73 these non-insomnia focused treatments may serve as critical add-ons or adjunctive therapies for patients requiring higher levels of care. Indeed, growing evidence shows that mindfulness meditation can be combined with CBTI components to improve insomnia and presleep arousal,70,80 yet more research is needed to more fully characterize the added value of mindfulness to CBTI for improved cognitive arousal outcomes.
Limitations and future directions
The present study should be interpreted in light of certain limitations. Our primary limitation centers on our inability to test treatment effects on clinical depression comorbid with insomnia disorder. As women with DSM-5 major depression were excluded from this trial, we focused on examining whether these treatments alter subclinical levels of depressive symptoms. Future trials should include postmenopausal women with comorbid insomnia and depression to examine how well these interventions treat these highly comorbid disorders. By extension, as the present study only examined women with subclinical depression levels, we likely experienced statistical restriction of range, which may have produced type II errors and/or underestimated treatment effect sizes on outcomes. In addition, the 3 conditions had different treatment delivery modalities and dosing (SHE = 6 weekly emails; SRT = 2 in-person sessions and 3 phone calls over 2 weeks; CBTI = 6 weekly face-to-face sessions), which may have contributed to differences in treatment effects. Relatedly, adherence in the control condition was not monitored, thus we cannot verify the extent to which subjects in this condition engaged in their sleep hygiene materials. We also did not assess for treatment expectations, which may have influenced outcomes. Another limitation concerns a lack of follow-up assessments beyond 6 months after treatment. Longer-term prospective data would improve our understanding of the durability of these antidepressant effects in postmenopausal women and whether CBTI and SRT prevent future depression onset.
Conclusions
Women with menopausal insomnia disorder report lower levels of depression symptoms, maladaptive thinking, and somatic hyperarousal after successful insomnia remission. Although CBTI and SRT both produce improvements in these areas, CBTI was the superior treatment option based on more immediate, durable, and larger reductions in depression, dysfunctional beliefs about sleep, and presleep somatic hyperarousal. Even so, treatment effects on overall cognitive arousal—including worry, rumination, and presleep perseverative cognitions—were less robust. This suggests that postmenopausal women with insomnia would likely benefit from better equipping CBTI and SRT to target cognitive arousal and stress dysregulation, which may improve treatment effects for both insomnia and depression, as well as reduce risk for future relapse of these disorders. Future research should explore whether CBTI can be tailored to meet the clinical needs for patients presenting with high levels of stress and emotion dysregulation.
Supplementary Material
Highlights for Treating Chronic Insomnia Improves Depressive Symptoms, Maladaptive Thinking, and Hyperarousal in Postmenopausal Women: A Randomized Clinical Trial of Cognitive-Behavioral Therapy for Insomnia, Sleep Restriction Therapy, and Sleep Hygiene Education.
-CBTI and SRT reduce depressive symptoms in postmenopausal women with insomnia
-CBTI and SRT reduce dysfunctional sleep beliefs and presleep somatic arousal
-Treatment effects on presleep cognitive arousal, rumination, and worry are mixed
-Sleep hygiene does not reduce depression, maladaptive thinking, or somatic arousal
-Treatment outcomes may be enhanced by better targeting cognitive arousal
Acknowledgements:
This study was funded by the National Institute of Nursing Research (R01 NR013959, PI: Drake). Dr. Cheng’s effort was funded by the National Heart, Lung, & Blood Institute (K23 HL13866, PI: Cheng).
Disclosure statement: Dr. Kalmbach has received research support from Merck & Co. Dr. Cheng has received research support from Harmony Biosciences. Dr. Roth. has received research support from Aventis, Cephalon, Glaxo Smith Kline, Neurocrine, Pfizer, Sanofi, Schering-Plough, Sepracor, Somaxon, Syrex, Takeda, TransOral, Wyeth and Xenoport and has acted as a consultant for Abbott, Acadia, Acoglix, Actelion, Alchemers, Alza, Ancil, Arena, Astra Zeneca, Aventis, AVER, BMS, BTG, Cephalon, Cypress, Dove, Elan, Eli Lilly, Evotec, Forest, Glaxo Smith Kline, Hypnion, Impax, Intec, Intra-Cellular, Jazz, Johnson & Johnson, King, Lundbeck, McNeil, Medici Nova, Merck & Co., Neurim, Neurocrine, Neurogen, Novartis, Orexo, Organon, Prestwick, Procter-Gamble, Pfizer, Purdue, Resteva, Roche, Sanofi, Schering-Plough, Sepracor, Servier, Shire, Somaxon, Syrex, Takeda, TransOral, Vanda, Vivometrics, Wyeth, Yamanuchi, and Xenoport. Dr. Drake has received research support from Merck & Co., Eisai Co., Aladdin Dreamer, Jazz, Actelion, and Teva, and has served on speakers bureau for Merck & Co. No other financial or non-financial interests exist.
Abbreviations list.
- ANOVA
analysis of variance
- BDI-II
Beck depression inventory, 2nd edition
- CBTI
cognitive-behavioral therapy for insomnia
- DBAS
dysfunctional beliefs about sleep scale
- DSM-5
diagnostic and statistical manual of mental disorders, 5th edition
- ERRI
event-related rumination inventory
- HRT
hormone replacement therapy
- ISI
insomnia severity index
- PSAS
presleep arousal scale
- PSG
polysomnography
- PSWQ
Penn State worry questionnaire
- RCT
randomized controlled trial
- SRT
sleep restriction therapy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Institution where work was performed: Henry Ford Health System.
References
- 1.Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in womenin transition to menopause. Archives of general psychiatry. 2004;61(1):62–70. [DOI] [PubMed] [Google Scholar]
- 2.Freeman EW. Associations of depression with the transition to menopause. Menopause. 2010;17(4):823–827. [DOI] [PubMed] [Google Scholar]
- 3.Avis NE, Crawford S, Stellato R, Longcope C. Longitudinal study of hormone levels and depression among women transitioning through menopause. Climacteric. 2001;4(3):243–249. [PubMed] [Google Scholar]
- 4.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10(1):19–28. [DOI] [PubMed] [Google Scholar]
- 5.Eichling PS, Sahni J. Menopause related sleep disorders. Journal of Clinical Sleep Medicine. 2005;1(03):291–300. [PubMed] [Google Scholar]
- 6.Attarian H, Hachul H, Guttuso T, Phillips B. Treatment of chronic insomnia disorder in menopause: evaluation of literature. Menopause. 2015;22(6):674–684. [DOI] [PubMed] [Google Scholar]
- 7.Taylor DJ. Insomnia and depression. Sleep. 2008;31(4):447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. Journal of affective disorders. 2011;135(1):10–19. [DOI] [PubMed] [Google Scholar]
- 9.Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rössler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31(4):473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. Journal of psychiatric research. 2003;37(1):9–15. [DOI] [PubMed] [Google Scholar]
- 11.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep medicine reviews. 2002;6(2):97–111. [DOI] [PubMed] [Google Scholar]
- 12.McCurry SM, Guthrie KA, Morin CM, et al. Telephone-based cognitive behavioral therapy for insomnia in perimenopausal and postmenopausal women with vasomotor symptoms: a MsFLASH randomized clinical trial. JAMA internal medicine. 2016;176(7):913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drake CL, Kalmbach DA, Arnedt JT, et al. Treating Chronic Insomnia in Postmenopausal Women: A Randomized Clinical Trial Comparing Cognitive-Behavioral Therapy for Insomnia (CBTI), Sleep Restriction Therapy, and Sleep Hygiene Education. SLEEP. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nowakowski S, Heimbach EK, Manber R, et al. Effects of Cognitie Behavioral Therapy for Menopausal Insomnia on Depressive Symptoms. The North American Menopause Society Annua Meeting; 2017; Philadelphia. [Google Scholar]
- 15.Vallières A, Ceklic T, Bastien CH, Espie CA. A preliminary evaluation of the physiological mechanisms of action for sleep restriction therapy. Sleep disorders. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guthrie KA, Larson JC, Ensrud KE, et al. Effects of pharmacologic and nonpharmacologic interventions on insomnia symptoms and self-reported sleep quality in women with hot flashes: a pooled analysis of individual participant data from four MSFLASH trials. Sleep. 2018;41(1):zsx190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalmbach DA, Pillai V, Arnedt JT, Drake CL. DSM-5 Insomnia and Short Sleep: Comorbidity Landscape and Racial Disparities. Sleep. 2016;39(12):2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez-Mendoza J, Vela-Bueno A, Vgontzas AN, et al. Cognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosom Med. 2010;72(4):397–403. [DOI] [PubMed] [Google Scholar]
- 19.Hammerschlag AR, Stringer S, de Leeuw CA, et al. Genome-wide association analysis of insomnia complaints identifies risk genes and genetic overlap with psychiatric and metabolic traits. Nature genetics. 2017;49(11):1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manber R, Edinger JD, Gress JL, Pedro-Salcedo MGS, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manber R, Bernert RA, Suh S, Nowakowski S, Siebern AT, Ong JC. CBT for insomnia in patients with high and low depressive symptom severity: adherence and clinical outcomes. FOCUS. 2014;12(1):90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor DJ, Pruiksma KE. Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: a systematic review. International Review of Psychiatry. 2014;26(2):205–213. [DOI] [PubMed] [Google Scholar]
- 23.Pillai V, Drake CL. Sleep and repetitive thought: the role of rumination and worry in sleep disturbance In: Sleep and affect. Elsevier; 2015:201–225. [Google Scholar]
- 24.Harvey AG. Unwanted Intrusive Thoughts in Insomnia. 2005.
- 25.Kalmbach DA, Pillai V, Drake CL. Nocturnal insomnia symptoms and stress-induced cognitive intrusions in risk for depression: A 2-year prospective study. PloS one. 2018;13(2):e0192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey AG. Pre-sleep cognitive activity: A comparison of sleep-onset insomniacs and good sleepers. British Journal of Clinical Psychology. 2000;39(3):275–286. [DOI] [PubMed] [Google Scholar]
- 27.Harvey AG, Greenall E. Catastrophic worry in primary insomnia. Journal of behavior therapy and experimental psychiatry. 2003;34(1):11–23. [DOI] [PubMed] [Google Scholar]
- 28.McEvoy PM, Watson H, Watkins ER, Nathan P. The relationship between worry, rumination, and comorbidity: Evidence for repetitive negative thinking as a transdiagnostic construct. Journal of Affective Disorders. 2013;151(1):313–320. [DOI] [PubMed] [Google Scholar]
- 29.McEvoy PM, Brans S. Common versus unique variance across measures of worry and rumination: Predictive utility and mediational models for anxiety and depression. Cognitive Therapy and Research. 2013;37(1):183–196. [Google Scholar]
- 30.Kalmbach DA, Pillai V, Ciesla JA. The correspondence of changes in depressive rumination and worry to weekly variations in affective symptoms: A test of the tripartite model of anxiety and depression in women. Australian Journal of Psychology. 2016;68(1):52–60. [Google Scholar]
- 31.Morin CM, Vallières A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16). Sleep. 2007;30(11):1547–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carney CE, Edinger JD, Manber R, Garson C, Segal ZV. Beliefs about sleep in disorders characterized by sleep and mood disturbance. Journal of psychosomatic research. 2007;62(2):179–188. [DOI] [PubMed] [Google Scholar]
- 33.Jansson M, Linton SJ. Psychological mechanisms in the maintenance of insomnia: arousal, distress, and sleep-related beliefs. Behaviour research and therapy. 2007;45(3):511–521. [DOI] [PubMed] [Google Scholar]
- 34.Sadler P, McLaren S, Jenkins M. A psychological pathway from insomnia to depression among older adults. International psychogeriatrics. 2013;25(8):1375–1383. [DOI] [PubMed] [Google Scholar]
- 35.McCall WV, Batson N, Webster M, et al. Nightmares and dysfunctional beliefs about sleep mediate the effect of insomnia symptoms on suicidal ideation. Journal of Clinical Sleep Medicine. 2013;9(02):135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciesla JA, Roberts JE. Rumination, negative cognition, and their interactive effects on depressed mood. Emotion. 2007;7(3):555. [DOI] [PubMed] [Google Scholar]
- 37.Ciesla JA, Felton JW, Roberts JE. Testing the cognitive catalyst model of depression: Does rumination amplify the impact of cognitive diatheses in response to stress? Cognition & emotion. 2011;25(8):1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciesla JA, Roberts JE. Self-directed thought and response to treatment for depression: A preliminary investigation. Journal of Cognitive Psychotherapy. 2002;16(4):435. [Google Scholar]
- 39.Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosomatic medicine. 2003;65(2):259–267. [DOI] [PubMed] [Google Scholar]
- 40.Nicassio PM, Mendlowitz DR, Fussell JJ, Petras L. The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behaviour research and therapy. 1985;23(3):263–271. [DOI] [PubMed] [Google Scholar]
- 41.Riemann D “Hyperarousal and insomnia: state of the science”. Sleep Med Rev. 2010;14(1):17. [DOI] [PubMed] [Google Scholar]
- 42.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep medicine reviews. 2010;14(1):9–15. [DOI] [PubMed] [Google Scholar]
- 43.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep medicine reviews. 2013;17(4):241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalmbach DA, Cuamatzi-Castelan AS, Tonnu CV, et al. Hyperarousal and sleep reactivity in insomnia: current insights. Nature and science of sleep. 2018;10:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greaves-Lord K, Ferdinand RF, Sondeijker FE, et al. Testing the tripartite model in young adolescents: Is hyperarousal specific for anxiety and not depression? Journal of Affective Disorders. 2007;102(1–3):55–63. [DOI] [PubMed] [Google Scholar]
- 46.Jansson-Fröjmark M, Norell-Clarke A. Psychometric properties of the Pre-Sleep Arousal Scale in a large community sample. Journal of Psychosomatic Research. 2012;72(2):103–110. [DOI] [PubMed] [Google Scholar]
- 47.Irish LA, Kline CE, Gunn HE, Buysse DJ, Hall MH. The role of sleep hygiene in promoting public health: A review of empirical evidence. Sleep medicine reviews. 2015;22:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horwath E, Johnson J, Klerman GL, Weissman MM. Depressive symptoms as relative and attributable risk factors for first-onset major depression. Archives of general psychiatry. 1992;49(10):817–823. [DOI] [PubMed] [Google Scholar]
- 49.Ohayon MM. Severe hot flashes are associated with chronic insomnia. Archives of Internal Medicine. 2006;166(12):1262–1268. [DOI] [PubMed] [Google Scholar]
- 50.Bolge SC, Balkrishnan R, Kannan H, Seal B, Drake CL. Burden associated with chronic sleep maintenance insomnia characterized by nighttime awakenings among women with menopausal symptoms. Menopause. 2010;17(1):80–86. [DOI] [PubMed] [Google Scholar]
- 51.Stepanski EJ, Wyatt JK. Use of sleep hygiene in the treatment of insomnia. Sleep medicine reviews. 2003;7(3):215–225. [DOI] [PubMed] [Google Scholar]
- 52.Perlis ML, Jungquist C, Smith MT, Posner D. Cognitive behavioral treatment of insomnia: A session-by-session guide. Vol 1: Springer Science & Business Media; 2006. [Google Scholar]
- 53.Riemann D, Perlis ML. The treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep medicine reviews. 2009;13(3):205–214. [DOI] [PubMed] [Google Scholar]
- 54.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Annals of internal medicine. 2016;165(2):125–133. [DOI] [PubMed] [Google Scholar]
- 55.Miller CB, Espie CA, Epstein DR, et al. The evidence base of sleep restriction therapy for treating insomnia disorder. Sleep medicine reviews. 2014;18(5):415–424. [DOI] [PubMed] [Google Scholar]
- 56.Beck AT, Steer RA, Brown GK. Beck Depression Inventory-ii (bdi-ii). San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 57.Cann A, Calhoun LG, Tedeschi RG, Triplett KN, Vishnevsky T, Lindstrom CM. Assessing posttraumatic cognitive processes: The event related rumination inventory. Anxiety, Stress, & Coping. 2011;24(2):137–156. [DOI] [PubMed] [Google Scholar]
- 58.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the penn state worry questionnaire. Behaviour research and therapy. 1990;28(6):487–495. [DOI] [PubMed] [Google Scholar]
- 59.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep medicine. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 60.Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychological methods. 2002;7(1):105. [DOI] [PubMed] [Google Scholar]
- 61.Smith M, Perlis M, Smith M, Giles D, Carmody T. Sleep quality and presleep arousal in chronic pain. Journal of behavioral medicine. 2000;23(1):1–13. [DOI] [PubMed] [Google Scholar]
- 62.Alfano CA, Pina AA, Zerr AA, Villalta IK. Pre-sleep arousal and sleep problems of anxiety-disordered youth. Child Psychiatry & Human Development. 2010;41(2):156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Espie CA, Inglis SJ, Harvey L, Tessier S. Insomniacs’ attributions: psychometric properties of the Dysfunctional Beliefs and Attitudes about Sleep Scale and the Sleep Disturbance Questionnaire. Journal of Psychosomatic Research. 2000;48(2):141–148. [DOI] [PubMed] [Google Scholar]
- 64.Christensen H, Batterham PJ, Gosling JA, et al. Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): a randomised controlled trial. The Lancet Psychiatry. 2016;3(4):333–341. [DOI] [PubMed] [Google Scholar]
- 65.Lancee J, van den Bout J, van Straten A, Spoormaker VI. Baseline depression levels do not affect efficacy of cognitive-behavioral self-help treatment for insomnia. Depression and anxiety. 2013;30(2):149–156. [DOI] [PubMed] [Google Scholar]
- 66.Manber R, Buysse DJ, Edinger J, et al. Efficacy of Cognitive-Behavioral Therapy for Insomnia Combined With Antidepressant Pharmacotherapy in Patients With Comorbid Depression and Insomnia: A Randomized Controlled Trial. The Journal of clinical psychiatry. 2016;77(10):e1316–e1323. [DOI] [PubMed] [Google Scholar]
- 67.Okajima I, Komada Y, Inoue Y. A meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia. Sleep and Biological Rhythms. 2011;9(1):24–34. [Google Scholar]
- 68.Wu R, Bao J, Zhang C, Deng J, Long C. Comparison of sleep condition and sleep-related psychological activity after cognitive-behavior and pharmacological therapy for chronic insomnia. Psychotherapy and psychosomatics. 2006;75(4):220–228. [DOI] [PubMed] [Google Scholar]
- 69.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Does cognitive-behavioral insomnia therapy alter dysfunctional beliefs about sleep? Sleep. 2001;24(5):591–599. [DOI] [PubMed] [Google Scholar]
- 70.Ong JC, Shapiro SL, Manber R. Combining mindfulness meditation with cognitive-behavior therapy for insomnia: a treatment-development study. Behavior therapy. 2008;39(2):171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ong JC, Manber R, Segal Z, Xia Y, Shapiro S, Wyatt JK. A randomized controlled trial of mindfulness meditation for chronic insomnia. Sleep. 2014;37(9):1553–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campbell TS, Labelle LE, Bacon SL, Faris P, Carlson LE. Impact of mindfulness-based stress reduction (MBSR) on attention, rumination and resting blood pressure in women with cancer: a waitlist-controlled study. Journal of behavioral medicine. 2012;35(3):262–271. [DOI] [PubMed] [Google Scholar]
- 73.Deyo M, Wilson KA, Ong J, Koopman C. Mindfulness and rumination: does mindfulness training lead to reductions in the ruminative thinking associated with depression? EXPLORE: The Journal of Science and Healing. 2009;5(5):265–271. [DOI] [PubMed] [Google Scholar]
- 74.Ong JC. Mindfulness-based therapy for insomnia.
- 75.Harvey AG. A cognitive theory and therapy for chronic insomnia. Journal of Cognitive Psychotherapy. 2005;19(1):41. [Google Scholar]
- 76.Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on psychological science. 2008;3(5):400–424. [DOI] [PubMed] [Google Scholar]
- 77.Baglioni C, Spiegelhalder K, Lombardo C, Riemann D. Sleep and emotions: a focus on insomnia. Sleep medicine reviews. 2010;14(4):227–238. [DOI] [PubMed] [Google Scholar]
- 78.Fernandez-Mendoza J, Calhoun SL, Bixler EO, et al. Sleep misperception and chronic insomnia in the general population: the role of objective sleep duration and psychological profiles. Psychosomatic medicine. 2011;73(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fernandez-Mendoza J, Shaffer ML, Olavarrieta-Bernardino S, et al. Cognitive-emotional hyperarousal in the offspring of parents vulnerable to insomnia: a nuclear family study. J Sleep Res. 2014;23(5):489–498. [DOI] [PubMed] [Google Scholar]
- 80.Ong JC, Shapiro SL, Manber R. Mindfulness meditation and cognitive behavioral therapy for insomnia: a naturalistic 12-month follow-up. Explore: The Journal of Science and Healing. 2009;5(1):30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.