Key Points
Question
Is daily aspirin therapy associated with risk of hepatocellular carcinoma in patients with chronic hepatitis B?
Findings
In this Taiwan nationwide cohort study of 10 615 patients with chronic hepatitis B, there was a statistically significant 29% risk reduction of hepatocellular carcinoma in 2123 patients who received daily aspirin compared with the matched 8492 controls.
Meaning
Daily aspirin therapy may be of help to further improve the chemoprevention of hepatitis B virus–related hepatocellular carcinoma.
Abstract
Importance
Antiviral therapy cannot erase hepatocellular carcinoma (HCC) risk in patients with chronic hepatitis B, and it is not indicated for most hepatitis B virus (HBV) carriers. Another effective way of reducing HCC risk needs to be developed. Aspirin may prevent cancer development, but clinical evidence in patients with HBV-related HCC remains limited.
Objective
To investigate the association of daily aspirin therapy with HBV-related HCC risk.
Design, Setting, and Participants
In this Taiwan nationwide cohort study, we screened 204 507 patients with chronic hepatitis B for the period January 1, 1997, to December 31, 2012. After excluding patients with confounding conditions, 2123 patients who continuously received daily aspirin for 90 or more days (treated group) were randomly matched 1:4 with 8492 patients who had never received antiplatelet therapy (untreated group) by means of propensity scores, consisting of the follow-up index date, baseline characteristics, and potentially chemopreventive drug use during follow-up. Data were analyzed from August 1 to November 30, 2018.
Exposures
Daily aspirin therapy during the study period.
Main Outcomes and Measures
Both cumulative incidence of and hazard ratios (HRs) for HCC development were analyzed after adjusting patient mortality as a competing risk event.
Results
Of the 10 615 patients included in the analysis, 7690 (72.4%) were men; mean (SD) age was 58.8 (11.8) years. The cumulative incidence of HCC in the treated group was significantly lower than that in the untreated group in 5 years (5.20%; 95% CI, 4.11%-6.29% vs 7.87%; 95% CI, 7.15%-8.60%; P < .001). In the multivariable regression analysis, aspirin therapy was independently associated with a reduced HCC risk (HR, 0.71; 95% CI, 0.58-0.86; P < .001). Sensitivity subgroup analyses also verified this association (all HRs <1.0). In addition, older age (HR, 1.01 per year; 95% CI, 1.00-1.02), male sex (HR, 1.75; 95% CI, 1.43-2.14), and cirrhosis (HR, 2.89; 95% CI, 2.45-3.40) were independently associated with an increased HCC risk, but nucleos(t)ide analogue (HR, 0.54; 95% CI, 0.41-0.71) or statin (HR, 0.62; 95% CI, 0.42-0.90) use was correlated with a decreased HCC risk.
Conclusions and Relevance
Daily aspirin therapy may be associated with a reduced risk of HBV-related HCC.
This nationwide cohort study examines the risk of hepatocellular carcinoma in Taiwanese patients with chronic hepatitis B who are receiving daily aspirin therapy.
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of cancer death worldwide, and an effective therapy for the prevention of HCC development is needed.1 Hepatitis B virus (HBV) is one of the most important HCC risk factors, and the prevalence of HCC is high in epidemic areas of chronic hepatitis B (CHB).2 However, although current antiviral medicines, such as nucleos(t)ide analogue (NA) therapy, can effectively inhibit the replication of HBV, the possibility of developing a cure for CHB remains elusive.3 Therapy with NAs is associated with reductions in HCC risk, but the risk is not erased.4 Therefore, using only NA therapy may not be enough for HCC prevention. Antiviral therapy is not indicated in most HBV carriers,5,6,7 so another effective way of reducing HCC risk needs to be developed.
Aspirin has been investigated to explore associations between its chemopreventive effect and cancers that are related to chronic inflammation.8,9,10,11 Clinical research has demonstrated that aspirin can reduce cancer risk, particularly in the prevention of colorectal cancer.12,13 In a study using an HBV transgenic mouse model, antiplatelet therapy diminished immune-mediated necroinflammatory reactions, the severity of liver fibrosis, and the development of HCC.14 However, clinical evidence supporting associations between chemopreventive effect and aspirin therapy on HCC remains limited. Because randomized clinical trials of aspirin therapy were mainly conducted in areas with low HCC annual incidence rates (the United States or Europe), the case number might be too low to show a statistically significant effect on HCC risk reduction.15 Thus, analyses using large databases should be encouraged.
Clinical studies particularly designed for HCC chemoprevention by aspirin therapy are relatively sparse. In 2 studies based on large databases, aspirin use was found to be associated with reduced HCC risk, but information regarding some confounding factors, such as viral hepatitis, cirrhosis, and other potentially chemopreventive drug use, was lacking.16,17 In a recent hospital-based study, antiplatelet therapy was associated with a reduced HCC risk in patients with CHB after effective NA therapy.18 However, the drug therapy regimen (eg, frequency and duration) was not defined in that study. In addition, the chemopreventive effect of antiplatelet therapy in patients who did not receive NA therapy remains unclear. Therefore, we conducted a nationwide cohort study to evaluate the association of daily aspirin therapy with HBV-related HCC.
Methods
Study Design
The data used in this nationwide cohort study were obtained from the National Health Insurance Research Database (NHIRD) in Taiwan for the period January 1, 1997, to December 31, 2012. The NHIRD holds the claim data for more than 99% of Taiwan’s population of 23.38 million residents.19 As outlined in previous studies,4,20,21,22,23,24 the database contains comprehensive data, including patients’ demographic data, dates of clinic visits and hospitalizations, disease diagnosis codes, procedure codes, details of prescriptions, and costs. The date, drug name, dosage, administration route, frequency, and duration of prescriptions for each patient can be identified. The NHIRD had been updated every 6 months and was released after completion of the final update. Diseases were defined according to International Classification of Diseases, 9th Revision (ICD-9) codes. The quality of the NHIRD in terms of accuracy involving medications and related diseases has been well validated.25,26 The ICD-9 codes used in this study are listed in the eMethods in the Supplement, and diseases must have been diagnosed at least 3 times in outpatient clinics or once during a hospitalization. The research ethics committee of the National Health Research Institutes in Taiwan approved the present study and waived informed consent.
Study Population
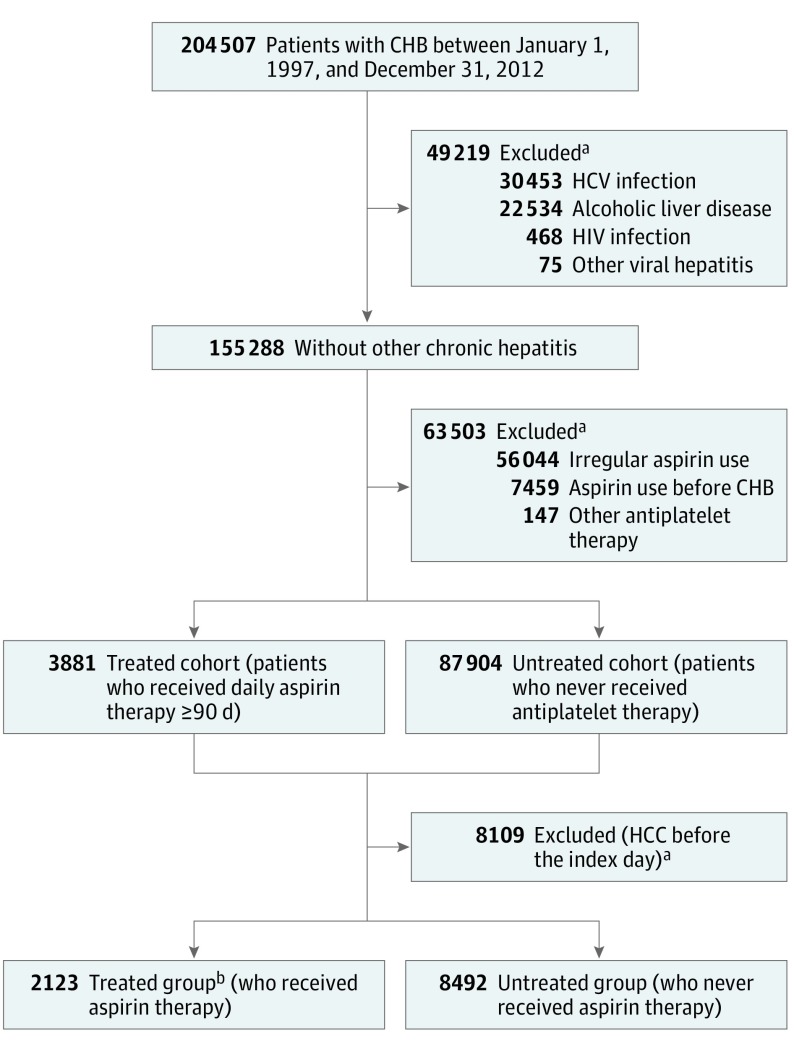
The patient selection process is presented in Figure 1. We screened 204 507 patients with CHB between January 1, 1997, and December 31, 2012. Patients with hepatitis C virus (HCV) infection, other viral hepatitis, HIV infection, and alcoholic liver disease were excluded. Because the effective duration of aspirin therapy for HCC prevention remains unclear, a selection criterion of 90 consecutive days was used for enrolling regular aspirin users. Patients who started to receive daily aspirin therapy and continued for 90 or more days were identified as the aspirin-treated cohort, and patients who never received antiplatelet therapy were assigned to the untreated cohort. A total of 2123 patients who received continuous aspirin therapy (treated group) were randomly matched 1:4 with 8492 patients who had never received aspirin (untreated group) by means of propensity scores, which consisted of index date to the start of follow-up, baseline characteristics (age, sex, cirrhosis, liver decompensation, diabetes, hyperlipidemia, and hypertension), and the use of potentially chemopreventive medicines (NAs, metformin, and statins) during the follow-up period.
Figure 1. Selection of Participants .
CHB indicates chronic hepatitis B; HCC, hepatocellular carcinoma; and HCV, hepatitis C virus.
aSome patients were excluded for more than 1 reason.
bPatients in the treated cohort (received aspirin therapy for ≥90 days) were matched 1:4 with those in the untreated cohort (never received antiplatelet therapy) by means of propensity scores.
Main Outcome Measurement
The occurrence of HCC was the main measured outcome. For avoiding immortal time bias, patients in the aspirin-treated and untreated groups were followed up from the 180th day of initiating aspirin therapy (the index date) and the matched dates of starting follow-up, respectively. Patients who developed HCC before the follow-up index dates were excluded as the data washing period. Study participants were followed up until the dates of HCC diagnosis, death, or the end of the study period (December 31, 2012). All patients who were admitted with a primary diagnosis of HCC were identified, and the diagnosis of HCC was further validated by the registration of patients in the Registry for Catastrophic Illness Patient Database.21,22,23,24 This database is an official subsystem related to insurance reimbursement within the NHIRD, in which histopathologic confirmation or typical imaging characteristics are required for the diagnosis of HCC.
Risk Factor Assessment
Several major coexisting diseases that might be associated with the risk of HCC development were evaluated at the index date of outcome follow-up, including cirrhosis, liver decompensation, diabetes, hyperlipidemia, and hypertension. The use of potentially chemopreventive medications, including NAs, metformin, and statins, was also analyzed. As mentioned in previous studies,4,21,22 NA reimbursement criteria require evidence of active viral hepatitis, such as blood alanine aminotransferase levels 2 times or more the upper limit of the reference range and HBV DNA titers 2000 IU/mL or more. The users of these drugs were defined as patients who took the medicines for more than 1 day per week during the outcome follow-up period.
Statistical Analysis
Propensity analysis was performed to examine the comparability of the 2 study groups.27 A propensity score was calculated through the use of logistic regression to estimate the probabilities of assigning a patient to the treated group. Cumulative incidence rates of HCC development during the follow-up period were calculated, and the estimated rates along with 95% CIs are presented. To avoid risk overestimation, patient mortality before HCC development was treated as a competing risk event. After adjustment for competing mortality, cumulative incidence rates were calculated and compared using a modified Gray method and the Kaplan-Meier method, and the differences in the full time-to-event distributions between the aspirin-treated and untreated groups were compared by a modified log-rank test.21 Furthermore, univariable regression analysis was used to identify potential risk factors, and multivariable regression analyses were further conducted to determine independent risk factors for HCC development. Hazard ratios (HRs) were determined by Cox proportional hazards regression models. Moreover, multivariable stratified analysis was conducted as a sensitivity analysis to evaluate the outcomes of aspirin therapy in the patient subgroups. All analyses were 2-tailed and unpaired, with significance set at P < .05. All data were managed using SAS, version 9.3 software (SAS Institute Inc), and the Cox proportional hazard regression models were constructed using the cmprsk package for R to estimate cumulative incidence under competing mortality events.28
Results
Participants
Of the 10 615 patients included in the analysis, 7690 (72.4%) were men; mean (SD) age was 58.8 (11.8) years. Further demographic characteristics are reported in Table 1. Most patients started aspirin therapy in middle age with a median age of 58.7 years (interquartile range, 50.4-67.7), and 1538 (72.4%) of the patients were men. The median duration of aspirin therapy was 3.1 years (interquartile range, 1.2-6.0). Regarding the daily dosage of aspirin therapy, 2079 (98.0%) of patients took 100 mg/d or less (≤325 mg for 99.9% of patients). Low-dose aspirin was used as an antiplatelet therapy for cardiovascular diseases. Moreover, 1744 patients (82.2%) displayed high-risk factors for cardiovascular diseases (ie, diabetes, hyperlipidemia, or hypertension), 875 patients (41.2%) had coronary arterial disease, and 798 (37.6%) had cerebral vascular disease. Less than 10% of patients had diagnoses other than cardiovascular disease such as cardiac dysrhythmia (147 [6.9%]) and peripheral vascular disease (16 [0.8%]). The HCC-related risk factors were not significantly different between the 2 groups. Small proportions of patients were diagnosed with cirrhosis (362 [17.1%]) or liver decompensation (94 [4.4%]).
Table 1. Demographic Characteristics of the Participantsa.
| Characteristics | Treated (n = 2123) | Untreated (n = 8492) | P Value |
|---|---|---|---|
| Age, y | |||
| Mean (SD) | 58.9 (11.8) | 58.8 (11.8) | .82 |
| Median (IQR) | 58.7 (50.4-67.7) | 58.6 (50.4-67.7) | .83 |
| Sex, No. (%) | |||
| Male | 1538 (72.4) | 6152 (72.4) | >.99 |
| Female | 585 (27.6) | 2340 (27.6) | |
| Aspirin therapy duration, y | |||
| Mean (SD) | 4.0 (3.4) | ||
| Median (IQR) | 3.1 (1.2-6.0) | ||
| Daily aspirin dosage, No. (%), mg | NA | ||
| ≤100 | 2079 (98.0) | ||
| 101-325 | 42 (1.9) | ||
| >325 | 2 (0.1) | ||
| Possible reasons for the use of aspirin therapy, No. (%) | NA | ||
| Risk factors for cardiovascular diseasesb | 1744 (82.2) | ||
| Coronary arterial disease | 875 (41.2) | ||
| Cerebral vascular disease | 798 (37.6) | ||
| Cardiac dysrhythmias | 147 (6.9) | ||
| Peripheral vascular disease | 16 (0.8) | ||
| Major liver-related diseases, No. (%) | |||
| Liver cirrhosis | 362 (17.1) | 1448 (17.1) | >.99 |
| Liver decompensation | 94 (4.4) | 380 (4.5) | .97 |
| Diabetes | 620 (29.2) | 2450 (28.9) | .77 |
| Hyperlipidemia | 664 (31.3) | 2656 (31.3) | >.99 |
| Hypertension | 1386 (65.3) | 5544 (65.3) | >.99 |
| Drug use, No. (%) | |||
| Nucleos(t)ide analogues | 336 (15.8) | 1340 (15.8) | .98 |
| Metformin | 347 (16.3) | 1414 (16.7) | .76 |
| Statins | 215 (10.1) | 860 (10.1) | >.99 |
| Propensity score | |||
| Mean (SD) | 0.15 (0.14) | 0.15 (0.14) | >.99 |
| Median (IQR) | 0.15 (0.03-0.21) | 0.15 (0.03-0.21) | >.99 |
Abbreviations: IQR, interquartile range; NA, not applicable.
Treated patients were those who received aspirin for 90 days or more; untreated, had never received antiplatelet therapy.
Diabetes, hyperlipidemia, hypertension.
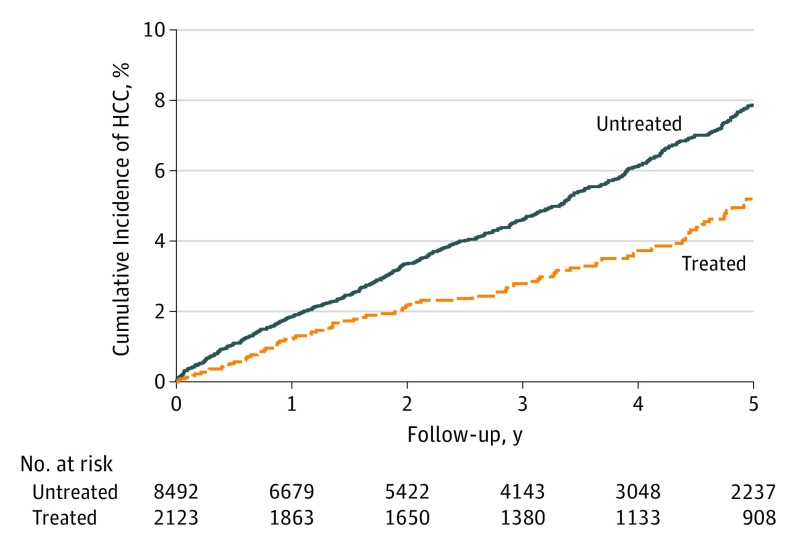
Cumulative Incidence of HCC
The cumulative incidence of HCC is shown in Figure 2. After adjusting for competing mortality risk, the cumulative incidence of HCC in the aspirin-treated group was significantly lower than that in the untreated group in 5 years (5.20%; 95% CI, 4.11%-6.29% vs 7.87%; 95% CI, 7.15%-8.60%; P < .001). As shown in eFigure 1 and eFigure 2 in the Supplement, we redefined the aspirin user selection cutoff of continuous aspirin use for at least 1 or 2 years (median aspirin therapy duration, 4.4 or 5.6 years). The 5-year cumulative incidences of HCC in the aspirin-treated group were significantly lower than those in the untreated groups (cutoff of 1 year: 4.48%; 95% CI, 3.18%-5.79% vs 7.29%; 95% CI, 6.35%-8.24%; cutoff of 2 years: 3.99%; 95% CI, 2.54%-5.44% vs 7.52%; 95% CI, 6.38%-8.66%).
Figure 2. Cumulative Incidence of Hepatocellular Carcinoma Development in the Aspirin-Treated or Untreated Groups.
Follow-up from 180 days after aspirin therapy in the treated (received aspirin therapy for ≥90 days) and untreated (never received antiplatelet therapy).
Multivariable Analysis of Risk Factors
As reported in Table 2, in the multivariable regression analysis that was adjusted for age, male sex, liver cirrhosis, diabetes, hyperlipidemia, hypertension, statin use, metformin use, and NA use, aspirin therapy remained an independent factor that was associated with a 29% risk reduction of HCC development (HR, 0.71; 95% CI, 0.58-0.86; P < .001). Older age (HR, 1.01; 95% CI, 1.00-1.02; P = .001), male sex (HR, 1.75; 95% CI, 1.43-2.14; P < .001), and liver cirrhosis (HR, 2.89; 95% CI, 2.45-3.40; P < .001) were still associated with a higher HCC risk, but the use of NAs (HR, 0.54; 95% CI, 0.41-0.71; P < .001) or statins (HR, 0.62; 95% CI, 0.42-0.90; P = .01) was associated with a lower HCC risk.
Table 2. Cox Proportional Hazards Regression Model Analysis for Risk of Hepatocellular Carcinoma.
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Treated vs untreateda | 0.68 (0.56-0.83) | <.001 | 0.71 (0.58-0.86) | <.001 |
| Age per year | 1.02 (1.01-1.03) | <.001 | 1.01 (1.00-1.02) | .001 |
| Male sex | 1.86 (1.52-2.27) | <.001 | 1.75 (1.43-2.14) | <.001 |
| Liver cirrhosis | 3.20 (2.75-3.72) | <.001 | 2.89 (2.45-3.40) | <.001 |
| Diabetes | 1.18 (1.00-1.38) | .048 | 1.17 (0.95-1.44) | .14 |
| Hyperlipidemia | 0.65 (0.54-0.78) | <.001 | 0.92 (0.75-1.11) | .38 |
| Hypertension | 1.17 (1.00-1.37) | .045 | 1.10 (0.94-1.30) | .22 |
| Nucleos(t)ide analogues | 0.62 (0.48-0.81) | <.001 | 0.54 (0.41-0.71) | <.001 |
| Metformin | 1.01 (0.83-1.23) | .93 | 1.07 (0.83-1.39) | .58 |
| Statin | 0.42 (0.29-0.61) | <.001 | 0.62 (0.42-0.90) | .01 |
Abbreviations: HR, hazard ratio.
Treated patients were those who received aspirin for 90 days or more; untreated, had never received antiplatelet therapy.
Multivariable Stratified Analysis for Aspirin Therapy
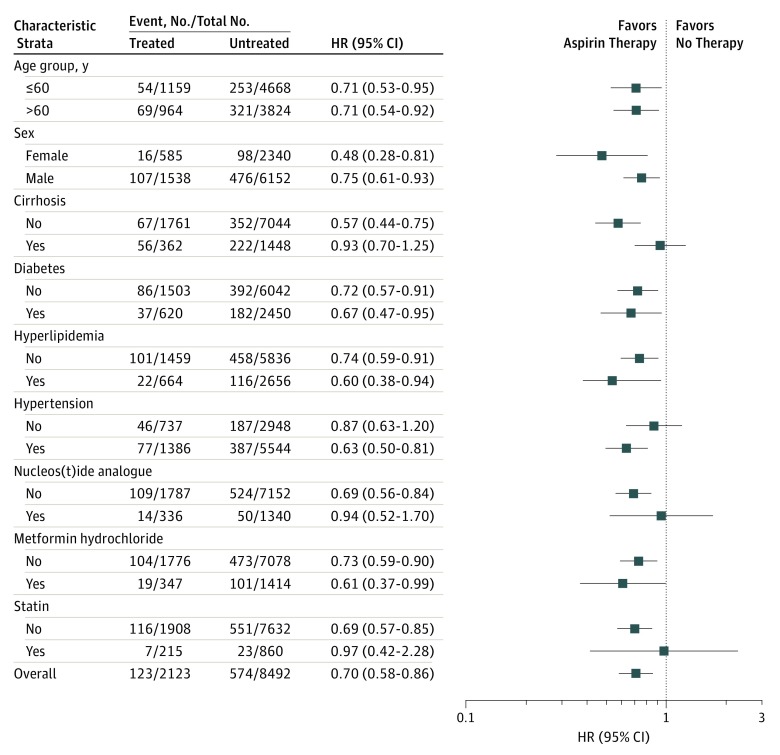
As shown in Figure 3, multivariable stratified analysis was performed for subgroups of patients. Multivariable stratified analyses verified the association of aspirin therapy with reduced HCC risk, including in patients 60 years or younger (HR, 0.71; 95% CI, 0.53-0.95) or older than 60 years (HR, 0.71; 95% CI, 0.54-0.92), female sex (HR, 0.48; 95% CI, 0.28-0.81), male sex (HR, 0.75; 95% CI, 0.61-0.93), those without underlying cirrhosis (HR, 0.57; 95% CI, 0.44-0.75), those with (HR, 0.67; 95% CI, 0.47-0.95) or without (HR, 0.72; 95% CI, 0.57-0.91) coexisting diabetes, those with (HR, 0.71; 95% CI, 0.53-0.95) or without (HR, 0.71; 95% CI, 0.53-0.95) coexisting hyperlipidemia, those with coexisting hypertension (HR, 0.63; 95% CI, 0.50-0.81), non-NA users (HR, 0.69; 95% CI, 0.56-0.84), metformin users (HR, 0.61; 95% CI, 0.37-0.99) or nonusers (HR, 0.73; 95% CI, 0.59-0.90), and non-statin users (HR, 0.69; 95% CI, 0.57-0.85). However, although the HR for all findings was less than 1.0, statistical significance was not reached in several patient subgroups, including those with underlying cirrhosis (HR, 0.93; 95% CI, 0.70-1.25), without coexisting hypertension (HR, 0.87; 95% CI, 0.63-1.20), NA users (HR, 0.94; 95% CI, 0.52-1.70), and statin users (HR, 0.97; 95% CI, 0.42-2.28).
Figure 3. Multivariable Stratified Analyses of the Association Between Aspirin Therapy and Hepatocellular Carcinoma Development.
Comparison of the treated (received aspirin therapy for ≥90 days) and untreated (never received antiplatelet therapy) cohorts. HR indicates hazard ratio.
Peptic Ulcer Bleeding
Patients who were admitted with a diagnosis of peptic ulcer bleeding (PUB) were evaluated. As shown in eFigure 3 and eFigure 4 in the Supplement, the 5-year cumulative incidence of PUB in the aspirin-treated group was not significantly higher than those in the untreated group (6.13%; 95% CI, 5.05%-7.21% vs 5.52%; 95% CI, 4.99%-6.06%; P = .09). The PUB risk in patients receiving aspirin was not significantly higher in those with cirrhosis compared with those without cirrhosis (7.49%; 95% CI, 4.65%-10.32% vs 5.85%; 95% CI, 4.68%-7.01%; P = .41). However, patients selected in the treated group could tolerate aspirin therapy for at least 90 days; therefore, their PUB risk might be underestimated.
As shown in eFigure 5 and the eTable in the Supplement, for the purpose of comparing PUB risk for patients taking daily aspirin therapy for cardiovascular diseases (ie, coronary arterial disease, cerebrovascular disease, or diabetes), we selected risk factor–matched aspirin users who were free from common liver diseases (ie, HBV, HCV, alcoholic liver disease, and other viral hepatitis) from the database as the controls. The PUB risk in the CHB aspirin users was not significantly different compared with that in the aspirin users without liver disease (6.30%; 95% CI, 5.06%-7.55% vs 6.77%; 95% CI, 6.14%-7.39%).
Negative Control Study
As shown in eFigure 6 and eFigure 7 in the Supplement, the 5-year cumulative incidence of suicide or hip fracture in the aspirin-treated group was not significantly different from those in the untreated group.
Discussion
Owing to the limitations of databases in previous surveys of aspirin therapy, certain important information, such as the presence of HBV or HCV infection, was largely unknown.15,29 Although a recent hospital-based study reported that antiplatelet therapy was associated with reduced HCC risk in patients with CHB after effective NA therapy,18 the drug regimen (eg, frequency and duration) should be further defined. In the present study, we report that daily aspirin therapy was associated with a reduced incidence of HCC in patients with CHB. In patients who did not receive NA therapy, aspirin therapy was also independently associated with a decreased HCC risk. Our findings may be of help in future efforts to further improve the chemoprevention of HBV-related HCC, and a proof-of-concept study is thus warranted.
The findings of experimental investigations also support the conclusion of this study. Platelets play an important role in the pathogenesis of HBV-related liver disease by sustaining inflammation.30 Aspirin can block thromboxane A2 production and inhibit the pathways of platelet activation.31,32 In an HBV transgenic mouse model of chronic immune-mediated necroinflammatory liver disease, antiplatelet therapy diminished the number of intrahepatic HBV-specific CD8 T cells, HBV-nonspecific inflammatory cells, the severity of liver fibrosis, and the development of HCC.14 In addition, aspirin could induce apoptosis of HCC cells in vitro8,11 and inhibited HCC tumor growth in a nude mouse xenograft model.10 Furthermore, in a rat model of liver cirrhosis, aspirin also showed beneficial effects in terms of improved fibrosis grade and hepatic regenerative activity.33 Hepatitis B virus–related HCC is generally a consequence of chronic inflammation due to hepatitis, fibrosis, dysplasia, and tumor growth34; sustained suppression of inflammation may contribute to HCC chemoprevention.
Although NA therapy remains the mainstay for the prevention of HBV-related HCC, most HBV carriers do not receive NA therapy. In the subgroup analysis of this study, aspirin therapy could be associated with reductions in HCC risk for patients with CHB who did not receive NA therapy. However, statistical significance was not reached for patients with CHB who received NA therapy, and this study result might be explained by the high proportion of liver cirrhosis among NA users. Cirrhosis was the strongest independent risk factor for HCC development.35 In the subgroup analysis, aspirin therapy was not associated with reduced HCC risk in patients with cirrhosis. Cirrhosis is a precancer stage, and aspirin therapy alone may not be enough to prevent HBV-related HCC. Although recent hospital-based research reported that antiplatelet therapy might reduce HCC risk after effective NA therapy, only a small proportion of patients with cirrhosis were recruited for analysis.18 Further well-designed studies are important for confirming the chemopreventive effect of aspirin therapy in NA users, especially for those with cirrhosis.
Several important confounding variables were adjusted for in this study, including the use of other potentially chemopreventive agents, including NAs, metformin, and statins.15,36 In this study, NA use remained the strongest factor in lowering the risk for HBV-related HCC, and this finding is compatible with those of previous studies.3,5,7 In patients for whom NA therapy is indicated, antiviral therapy should not be delayed. Consistent with findings reported in previous studies,4,37,38 we also found that the use of statins was associated with a reduced HCC risk in the multivariable analysis. However, randomized clinical trials are required to confirm the chemopreventive effect of statins.
The concern regarding the risk of aspirin-induced bleeding in long-term users has been well studied.39 Although the findings of this study suggest that the PUB risk may not be significantly increased in CHB aspirin users compared with that in typical aspirin users, the benefits and harms of aspirin therapy should be weighed as recommended in practice guidelines.40 In addition, aspirin use in patients with cirrhosis should be monitored carefully, because stopping bleeding in them can be more difficult than in patients without cirrhosis. Before aspirin therapy is broadly adopted for HCC prevention in practice, a prospective trial should be conducted to assess its efficacy and safety.
Limitations
Several limitations of this study should be acknowledged. First, although we conducted a quasi-experimental design that took all potential confounders into account, a causal relationship between aspirin therapy and HCC risk could not be directly inferred owing to the observational nature of this study. A prospective proof-of-concept study is mandatory. Second, most patients were middle-aged or older, and therefore our results may not be generalizable to younger patients. However, the median age of patients with HCC was also not young, with most patients in their 60s,22,41 and aspirin therapy in mid-life could still be beneficial for most patients with CHB. Third, detailed laboratory data, such as HBV viral load, could not be obtained from our database. However, NA therapy could be prescribed for patients with high viral loads according to the above-mentioned National Health Insurance reimbursement criteria, and the proportions of patients receiving NA therapy were not different between the 2 groups. Furthermore, the proportions of patients with cirrhosis or liver decompensation were also not different between the 2 groups. The severity of viral hepatitis was therefore likely the same in the 2 groups. To clarify this concern, a prospective study is needed.
Fourth, although most patients in the treated group continuously used aspirin, a proportion of patients stopped aspirin therapy during the study period (eFigure 8 in the Supplement). However, this study design is conservative in evaluating the effect of aspirin therapy, implying that HCC risk for the treated group could be overestimated. As shown in eFigure 2 in the Supplement, with longer median aspirin therapy duration of 5.6 years, the 5-year cumulative incidence of HCC became lower in patients receiving aspirin for at least 2 years. Therefore, the difference in HCC risk between the treated and the untreated groups could have been underestimated, and the conclusion of this study remains unchanged. Fifth, the NHIRD is limited by its insurance claim nature. For improving the accuracy of diagnostic variables in our studies, we used a double-checking system to confirm the diagnosis (ie, Registry for Catastrophic Illness Patient Database) of HCC. In addition, although all data descriptions regarding medicine are detailed, the accuracy may be limited by the patient’s adherence to the prescribed therapy. Furthermore, while some variations in drug use may exist in the real world, this problem among long-term aspirin users can be minimal.
Conclusions
The results of this long-term cohort study suggest that daily aspirin therapy may be associated with a reduced risk of HCC development in patients with CHB.
eMethods. The ICD Codes Used in This Study
eFigure 1. Cumulative Incidence of Hepatocellular Carcinoma Development in Aspirin-Treated (Continuous Aspirin Use for at Least 1 Year) or Untreated Groups
eFigure 2. Cumulative Incidence of Hepatocellular Carcinoma Development in Aspirin-Treated (Continuous Aspirin Use for at Least 2 Years) or Untreated Groups
eFigure 3. Cumulative Incidence of Peptic Ulcer Bleeding in the Aspirin-Treated or Untreated Groups
eFigure 4. Cumulative incidence of Peptic Ulcer Bleeding Among Cirrhotic or Non-Cirrhotic Patients in the Aspirin-Treated Group
eFigure 5. Cumulative Incidence of Peptic Ulcer Bleeding in Typical Aspirin Users With Liver Disease (Chronic Hepatitis B) or Without Liver Disease
eTable. Demographic Characteristics of Typical Patients Who Received Daily Aspirin Therapy for Cardiovascular Diseases
eFigure 6. Cumulative Incidence of Suicide in the Aspirin-Treated or Untreated Groups
eFigure 7. Cumulative Incidence of Hip Fracture in the Aspirin-Treated or Untreated Groups
eFigure 8. The Proportion of Patients Continuing Aspirin Therapy During the Study Period
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127. doi: 10.1056/NEJMra1001683 [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370-398. doi: 10.1016/j.jhep.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 4.Wu CY, Lin JT, Ho HJ, et al. Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B: a nationwide cohort study. Gastroenterology. 2014;147(1):143-151.e5. doi: 10.1053/j.gastro.2014.03.048 [DOI] [PubMed] [Google Scholar]
- 5.Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1-98. doi: 10.1007/s12072-015-9675-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57(1):167-185. doi: 10.1016/j.jhep.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 7.Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases . AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283. doi: 10.1002/hep.28156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li T, Dong ZR, Guo ZY, et al. Aspirin enhances IFN-α-induced growth inhibition and apoptosis of hepatocellular carcinoma via JAK1/STAT1 pathway. Cancer Gene Ther. 2013;20(6):366-374. doi: 10.1038/cgt.2013.29 [DOI] [PubMed] [Google Scholar]
- 9.Li G, Zhang S, Fang H, et al. Aspirin overcomes navitoclax-resistance in hepatocellular carcinoma cells through suppression of Mcl-1. Biochem Biophys Res Commun. 2013;434(4):809-814. doi: 10.1016/j.bbrc.2013.04.018 [DOI] [PubMed] [Google Scholar]
- 10.Hossain MA, Kim DH, Jang JY, et al. Aspirin induces apoptosis in vitro and inhibits tumor growth of human hepatocellular carcinoma cells in a nude mouse xenograft model. Int J Oncol. 2012;40(4):1298-1304. doi: 10.3892/ijo.2011.1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raza H, John A, Benedict S. Acetylsalicylic acid-induced oxidative stress, cell cycle arrest, apoptosis and mitochondrial dysfunction in human hepatoma HepG2 cells. Eur J Pharmacol. 2011;668(1-2):15-24. doi: 10.1016/j.ejphar.2011.06.016 [DOI] [PubMed] [Google Scholar]
- 12.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9(5):259-267. doi: 10.1038/nrclinonc.2011.199 [DOI] [PubMed] [Google Scholar]
- 13.Rothwell PM, Price JF, Fowkes FG, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379(9826):1602-1612. doi: 10.1016/S0140-6736(11)61720-0 [DOI] [PubMed] [Google Scholar]
- 14.Sitia G, Aiolfi R, Di Lucia P, et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci U S A. 2012;109(32):E2165-E2172. doi: 10.1073/pnas.1209182109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrat F. Statin and aspirin for prevention of hepatocellular carcinoma: what are the levels of evidence? Clin Res Hepatol Gastroenterol. 2014;38(1):9-11. doi: 10.1016/j.clinre.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 16.Sahasrabuddhe VV, Gunja MZ, Graubard BI, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2012;104(23):1808-1814. doi: 10.1093/jnci/djs452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrick JL, Sahasrabuddhe VV, Chan AT, et al. NSAID use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the Liver Cancer Pooling Project. Cancer Prev Res (Phila). 2015;8(12):1156-1162. doi: 10.1158/1940-6207.CAPR-15-0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee M, Chung GE, Lee JH, et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology. 2017;66(5):1556-1569. doi: 10.1002/hep.29318 [DOI] [PubMed] [Google Scholar]
- 19.Rachel Lu JF, Chiang TL. Evolution of Taiwan’s health care system. Health Econ Policy Law. 2011;6(1):85-107. doi: 10.1017/S1744133109990351 [DOI] [PubMed] [Google Scholar]
- 20.Chen HP, Shieh JJ, Chang CC, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62(4):606-615. doi: 10.1136/gutjnl-2011-301708 [DOI] [PubMed] [Google Scholar]
- 21.Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308(18):1906-1914. doi: 10.1001/2012.jama.11975 [DOI] [PubMed] [Google Scholar]
- 22.Lee TY, Lin JT, Zeng YS, Chen YJ, Wu MS, Wu CY. Association between nucleos(t)ide analog and tumor recurrence in hepatitis B virus-related hepatocellular carcinoma after radiofrequency ablation. Hepatology. 2016;63(5):1517-1527. doi: 10.1002/hep.28266 [DOI] [PubMed] [Google Scholar]
- 23.Lee TY, Lin JT, Ho HJ, Wu MS, Wu CY. Evaluation of the effect of cumulative operator experience on hepatocellular carcinoma recurrence after primary treatment with radiofrequency ablation. Radiology. 2015;276(1):294-301. doi: 10.1148/radiol.15141864 [DOI] [PubMed] [Google Scholar]
- 24.Lee TY, Wu JC, Yu SH, Lin JT, Wu MS, Wu CY. The occurrence of hepatocellular carcinoma in different risk stratifications of clinically noncirrhotic nonalcoholic fatty liver disease. Int J Cancer. 2017;141(7):1307-1314. doi: 10.1002/ijc.30784 [DOI] [PubMed] [Google Scholar]
- 25.Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(3):236-242. doi: 10.1002/pds.2087 [DOI] [PubMed] [Google Scholar]
- 26.Cheng CL, Lee CH, Chen PS, Li YH, Lin SJ, Yang YH. Validation of acute myocardial infarction cases in the national health insurance research database in Taiwan. J Epidemiol. 2014;24(6):500-507. doi: 10.2188/jea.JE20140076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenbaum PR, Rubin RD. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79(387):516-524. doi: 10.1080/01621459.1984.10478078 [DOI] [Google Scholar]
- 28.cmprsk: Subdistribution analysis of competing risks. http://cran.r-project.org/web/packages/cmprsk/index.html. Accessed November 11, 2018.
- 29.Singh P, Singh S. Re: nonsteroidal antiinflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2013;105(9):666-667. doi: 10.1093/jnci/djt062 [DOI] [PubMed] [Google Scholar]
- 30.Iannacone M, Sitia G, Isogawa M, et al. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med. 2005;11(11):1167-1169. doi: 10.1038/nm1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cattaneo M. Aspirin and clopidogrel: efficacy, safety, and the issue of drug resistance. Arterioscler Thromb Vasc Biol. 2004;24(11):1980-1987. doi: 10.1161/01.ATV.0000145980.39477.a9 [DOI] [PubMed] [Google Scholar]
- 32.Aiolfi R, Sitia G. Chronic hepatitis B: role of anti-platelet therapy in inflammation control. Cell Mol Immunol. 2015;12(3):264-268. doi: 10.1038/cmi.2014.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Assy N, Hussein O, Khalil A, et al. The beneficial effect of aspirin and enoxaparin on fibrosis progression and regenerative activity in a rat model of cirrhosis. Dig Dis Sci. 2007;52(5):1187-1193. doi: 10.1007/s10620-006-9595-1 [DOI] [PubMed] [Google Scholar]
- 34.Fattovich G. Natural history and prognosis of hepatitis B. Semin Liver Dis. 2003;23(1):47-58. doi: 10.1055/s-2003-37590 [DOI] [PubMed] [Google Scholar]
- 35.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5)(suppl 1):S35-S50. doi: 10.1053/j.gastro.2004.09.014 [DOI] [PubMed] [Google Scholar]
- 36.Orman ES, Hayashi PH. Re: nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2013;105(9):667. doi: 10.1093/jnci/djt061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim G, Jang SY, Han E, et al. Effect of statin on hepatocellular carcinoma in patients with type 2 diabetes: a nationwide nested case-control study. Int J Cancer. 2017;140(4):798-806. doi: 10.1002/ijc.30506 [DOI] [PubMed] [Google Scholar]
- 38.Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol. 2012;30(6):623-630. doi: 10.1200/JCO.2011.36.0917 [DOI] [PubMed] [Google Scholar]
- 39.Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312(23):2510-2520. doi: 10.1001/jama.2014.15690 [DOI] [PubMed] [Google Scholar]
- 40.Bibbins-Domingo K; US Preventive Services Task Force . Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164(12):836-845. doi: 10.7326/M16-0577 [DOI] [PubMed] [Google Scholar]
- 41.Liu PH, Hsu CY, Hsia CY, et al. Prognosis of hepatocellular carcinoma: assessment of eleven staging systems. J Hepatol. 2016;64(3):601-608. doi: 10.1016/j.jhep.2015.10.029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. The ICD Codes Used in This Study
eFigure 1. Cumulative Incidence of Hepatocellular Carcinoma Development in Aspirin-Treated (Continuous Aspirin Use for at Least 1 Year) or Untreated Groups
eFigure 2. Cumulative Incidence of Hepatocellular Carcinoma Development in Aspirin-Treated (Continuous Aspirin Use for at Least 2 Years) or Untreated Groups
eFigure 3. Cumulative Incidence of Peptic Ulcer Bleeding in the Aspirin-Treated or Untreated Groups
eFigure 4. Cumulative incidence of Peptic Ulcer Bleeding Among Cirrhotic or Non-Cirrhotic Patients in the Aspirin-Treated Group
eFigure 5. Cumulative Incidence of Peptic Ulcer Bleeding in Typical Aspirin Users With Liver Disease (Chronic Hepatitis B) or Without Liver Disease
eTable. Demographic Characteristics of Typical Patients Who Received Daily Aspirin Therapy for Cardiovascular Diseases
eFigure 6. Cumulative Incidence of Suicide in the Aspirin-Treated or Untreated Groups
eFigure 7. Cumulative Incidence of Hip Fracture in the Aspirin-Treated or Untreated Groups
eFigure 8. The Proportion of Patients Continuing Aspirin Therapy During the Study Period