Abstract
Self-incompatibility (SI) is a genetic mechanism that restricts inbreeding in flowering plants. In the nightshade family (Solanaceae) SI is controlled by a single multiallelic S locus. Pollen rejection in this system requires the interaction of two S locus products: a stylar (S)-RNase and its pollen counterpart (pollen S). pollen S has not yet been cloned. Our understanding of how this gene functions comes from studies of plants with mutations that affect the pollen but not the stylar SI response (pollen-part mutations). These mutations are frequently associated with duplicated S alleles, but the absence of an obvious additional allele in some plants suggests pollen S can also be deleted. We studied Nicotiana alata plants with an additional S allele and show that duplication causes a pollen-part mutation in several different genetic backgrounds. Inheritance of the duplication was consistent with a competitive interaction model in which any two nonmatching S alleles cause a breakdown of SI when present in the same pollen grain. We also examined plants with presumed deletions of pollen S and found that they instead have duplications that included pollen S but not the S-RNase gene. This finding is consistent with a bipartite structure for the S locus. The absence of pollen S deletions in this study and perhaps other studies suggests that pollen S might be required for pollen viability, possibly because its product acts as an S-RNase inhibitor.
Self-incompatibility (SI) in many plant families is controlled by a multiallelic S locus that enables a style to reject any pollen expressing the same S allelic specificity as itself (1). In the Solanaceae, the family that includes tobacco, tomato, and petunia, SI is described as gametophytic because the allelic specificity of each pollen grain is determined by its own haploid genotype. The S locus in this family encodes a secreted extracellular RNase [stylar (S)-RNase] that accumulates in the style (2). Recognizing which S allele each pollen grain expresses is thought to require an interaction between the S-RNase and an unknown product(s) of a second S locus gene called pollen S (3, 4).
As part of a strategy to identify pollen S, we isolated Nicotiana alata plants with gamma ray induced mutations that specifically affect the SI phenotype of pollen but not the SI phenotype of the style (5). Such plants are called pollen-part mutants (PPMs). Because ionizing radiation can cause either the deletion of part of a chromosome or chromosomal aberrations such as translocations, inversions, and fragments (6), the mutations in PPMs are likely to be complex because they can arise through one of a few different types of lesion.
Among the PPMs described so far, the most frequent types of lesion are either translocations or small “centric” fragments (short extra chromosomes) that carry a duplicated copy of an S allele (5, 7–10). Breakdown of the pollen SI response in these plants occurs because of a “competitive interaction” that enables pollen with two different S alleles (but not two identical S alleles) to grow through an incompatible style (7). Apart from PPMs, competitive interaction is also the reason why in some families tetraploids that are derived from self-incompatible diploids are self-fertile (11–14).
Several studies have identified PPMs with apparent deletions of pollen S (“true” PPMs), as well as PPMs with centric fragments that apparently lack an S allele (5, 8, 15). Consistent with this interpretation, molecular analyses of these plants, where done, have found no evidence of extra S-RNase genes in the genome (5, 16).
We previously characterized pollen-part mutations in seven N. alata plants by crosses and by probing DNA blots with S-RNase cDNAs (5). Four plants (named M1-1, M1-6, M1-7, and M1-11) carried duplicated S3 (dS3) alleles, which indicated competitive interaction as the likely reason for the breakdown of SI. The dS3 allele was on a centric fragment in M1-1, M1-6, and M1-11, and was part of a translocation in M1-7. No duplicated S-RNase genes were present in the other three plants (M1-2, M1-5, and M1-10), although a centric fragment was associated with the pollen-part mutation in the M1-2. Because there is no evidence of duplication in M1-5 and M1-10, it is possible that they carry lesions in pollen S. Here we extend our description of the role of competitive interaction in the pollen-part mutation phenotype. We also define the genomic region required for competitive interaction by using cDNAs for genes linked to the S locus (17). When these cDNAs were used to examine the size of the likely S locus deletion in the three true PPMs, part of a duplicated S allele was found in each plant. Our findings are discussed in light of current models of the molecular basis of SI in solanaceous plants and of the potential uses of duplicated S alleles in cloning pollen S.
Materials and Methods
Plant Materials and DNA Blot Analysis.
N. alata lines homozygous for the S3 and S6 alleles and the Melbourne collection of N. alata PPMs (M1-1 to M1-18) have been described (5, 18). Plants were maintained in glasshouses as described (18). Pollen-part mutations were moved into an S6S6 or an S3S6 background by backcrossing M1 plants to S3S6 self-incompatible plants and selecting progeny with the appropriate combination of S alleles. These backcrossed progeny are indicated in the text by the suffix “b.” DNA blot analysis was performed as described (5) and the blots were probed with either S-RNase cDNAs or cDNAs for the indicated S-linked genes (17).
Breeding Analysis.
Tri-allelic PPM plants (i.e., PPMs that express three S allele specificities in their styles) were obtained by crossing M1-1 (genotype S6S6dS3) to S1S1, S2S2, and S7S7 plants as described (5). Pollen collected from an SxS6dS3 plant was used to pollinate styles of an SxS6 plant (where x = 1, 2, or 7). The resulting progeny were self-pollinated by spreading pollen from a dehiscent anther over the stigmas of four or more flowers. Pollinations were compatible if a large capsule developed and incompatible if the flower abscised in the week following pollination. To determine the stylar SI phenotype of a plant, immature floral buds were emasculated and pollinated with pollen from a plant of known S genotype soon after petal opening. Four such pollinations were usually done for each plant. The reciprocal cross was used to determine the SI phenotype of a plant's pollen.
Cytology and Fluorescent in Situ Hybridization.
For cytology, root tips harvested from hydroponically grown plants were fixed and stained with orcein as described (5). For fluorescent in situ hybridization (FISH), freshly harvested root tips were incubated in saturated α-bromonaphthalene for 2 h, washed, fixed in ethanol:acetic acid (3:1), and then transferred to 70% ethanol for storage at 4°C. Stored root tips were washed in water, incubated with a mixture containing 2% cellulase (“Onazuka” RS, Yakult Pharmaceutical, Tokyo) and 20% pectinase (Sigma), then fixed on a glass slide and gently macerated with 26-gauge hypodermic needles. Slides were dehydrated in an ethanol series and incubated with 100 ng of a 48A gene probe that had been labeled by random priming (Boehringer Mannheim) with digoxigenin (DIG). The 48A gene probe was a 4-Kb EcoRI genomic fragment identified by screening a N. alata genomic library (S6S6 genotype) with a 48A cDNA probe (17). The sequence of this fragment has been deposited in the GenBank database (accession no. AJ277643). After hybridization, slides were washed in 50% formamide/1× SSC (1× SSC = 0.15 sodium chloride/0.015 M sodium citrate, pH 7) and blocked in 1% BSA/4× SSC. Root tips were incubated in a 1/10 dilution (in 4× SSC) of fluorescein-labeled sheep anti-DIG Fab fragment (Boehringer Mannheim), washed in 4× SSC/0.05% Tween 20, covered with Vectashield (Vector Laboratories), and stained with propidium iodide. Root tips were examined under a confocal laser beam (Leica, Deerfield, IL) using a ×100 lens attached to a Leica microscope (Leitz DM RBE). Images were captured by using 4D scanner (Leica) and digitally processed by using SCANWARE (Version 4.2 beta) software.
Results
Competitive Interaction Occurs Between a Duplicated S Allele and Other S Alleles.
We previously showed that dS3 can competitively interact with an S6 allele in pollen (5). To exclude the possibility that competitive interaction only occurs between these alleles, we moved dS3 into genetic backgrounds where it would interact with other S alleles. A series of crosses was performed to bring the dS3 allele in M1-1 and the S1, S2, and S7 alleles together. PPM plants of genotype SxS6dS3 (where x = 1, 2, 7) were identified in families produced by crossing M1-1 (S6S6dS3) and a homozygous plant of the appropriate S genotype (e.g., S1S1). These plants were then crossed to SxS6 plants and the resulting families characterized by pollination and by DNA blot analysis with S-RNase cDNA probes. Table 1 shows the classes of progeny present in each family. For instance, S1S3 PPM, S3S6 PPM, and S1S3S6 PPM plants were present in the family obtained by crossing an S1S6dS3 plant and an S1S6 plant. Presence of S1- and S3-RNase genes in S1S3 PPM plants is consistent with these plants having an S1S1dS3 genotype. Pollen-part mutations presumably arise in these plants because the S1 allele and dS3 can competitively interact. Because similar classes of PPM plant were seen in the other two families, dS3 can clearly competitively interact with the S1, S2, and S7 alleles in pollen. Self-incompatible plants in these families may have arisen because the centric fragment containing dS3 was present in a pollen tube's vegetative cell but not in either or both of its sperm cells, or because the centric fragment failed to be passed on to all cells during mitosis in the early embryo.
Table 1.
Summary of breeding and DNA blot analysis for families derived from M1-1
| Cross* | S phenotype of
progeny
|
No. of progeny | S-RNase genes† | |
|---|---|---|---|---|
| Pollen | Style | |||
| S1S6 × S1S6dS3 | PPM | S1S3 | 5 | S1 + S3 (4) |
| PPM | S3S6 | 9 | S3 + S6 (3) | |
| PPM | S1S3S6 | 5 | S1 + S3 + S6 (5) | |
| INC | S1S6 | 1 | S1 + S6 (1) | |
| S2S6 × S2S6dS3 | PPM | S2S3 | 6 | S2 + S3 (4) |
| PPM | S3S6 | 6 | S3 + S6 (4) | |
| PPM | S2S3S6 | 7 | S2 + S3 + S6 (4) | |
| S6S7 × S6S7dS3 | PPM | S3S7 | 4 | S3 + S7 (4) |
| PPM | S3S6 | 5 | S3 + S6 (4) | |
| PPM | S3S6S7 | 6 | S3 + S6 + S7 (4) | |
| INC | S6S7 | 1 | S6 + S7 (1) | |
INC, pollen incompatibility response was the same as a wild-type plant.
In each case the PPM was the staminate parent in a cross to the indicated pistilate parent.
The number of plants examined by DNA blot hybridization is indicated in parentheses.
Markers Define Sizes and Locations of Duplicated S Alleles.
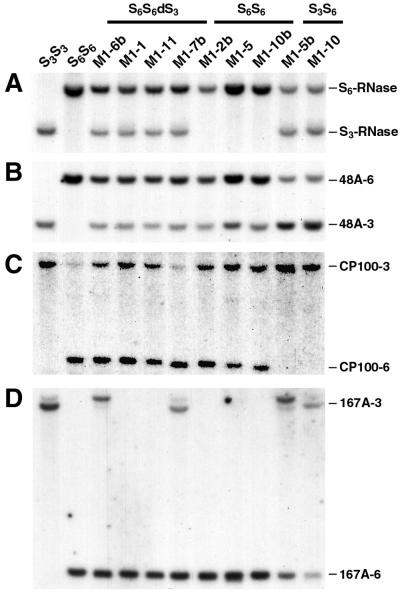
To identify the minimum region of the S locus required for competitive interaction, DNA blot analyses with three S-linked genes were used to examine the extent of the duplications in M1-1, M1-6, M1-7, and M1-11. The pollen-expressed genes 48A and 167A are separated from the S locus by no more than 0.7 cM and by 0.6–1.3 cM, respectively (17). CP100 is a leaf-expressed gene from potato (Solanum tuberosum) (19) that detects a N. alata homologue closely linked to 48A (17). Fig. 1 shows Southern blots of genomic DNA from the PPMs or their progeny. M1-1, M1-6b, M1-7b, and M1-11 were genetically S6S6dS3 and, as expected, carried both the S3- and S6-RNase genes (Fig. 1A).
Figure 1.
DNA blot analysis of SI and M1 plants. DNA blot analyses used 10-μg genomic DNA from the indicated homozygous SI lines and representative M1 plants. DNA was digest with either HindIII (A, C, and D) or SacI (B). Filters were probed with 32P-labeled S3-RNase and S6-RNase cDNAs (A), 48A (B), CP100 (C), or 167A (D). The identity of S-RNase, 48A, CP100, and 167A RFLPs are indicated at the right of the figure.
Hybridization of M1-1 (S6S6dS3) genomic DNA to the 48A probe detected the restriction fragment-length polymorphisms (RFLPs) associated with S3 (48A-3) and S6 (48A-6), indicating that the dS3 in M1-1 extended to 48A (Fig. 1B). Three RFLPs (48A-3 and 48A-6 from M1-1 and the 48A RFLP associated with S1, S2, or S7) were also present in the PPM progeny from the M1-1 outcross families described above (data not shown). M1-1 contained the CP100 RFLP associated with S3 (Fig. 1C) but not the 167A RFLP from S3 (Fig. 1D), indicating that the dS3 in this plant included CP100 but not 167A.
Duplications of 48A-3 were also found in M1-6b, M1-7b, and M1-11 (all S6S6dS3), demonstrating that 48A was present on the dS3s from these plants as well (Fig. 1B). Similarly, CP100-3 was present in M1-6b and M1-11, but not in M1-7b (Fig. 1C). 167A-3 was found in M1-6b and M1-7b, but not in M1-1 or M1-11 (Fig. 1D). This interpretation of Fig. 1D is not affected by the slight variation seen in the mobility of 167A-3, which is of unknown origin. As 167A is genetically further from the S locus than either 48A or CP100 (17), the presence of 167A-3 on the dS3 in M1-6b is consistent with the centric fragment in this plant being significantly larger than the centric fragments in either M1-1 or M1-11 (5).
Locating Duplicated S Alleles by FISH.
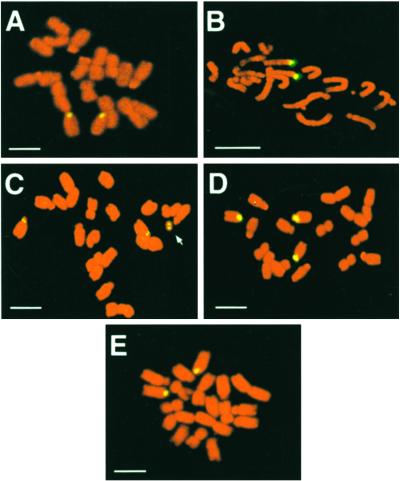
FISH with a 48A gene probe was performed on mitotic root-tip cells to confirm the chromosomal locations of dS3 indicated from genetic analyses. This gene was chosen because it enabled all of the S alleles in a cell to be detected simultaneously. In control experiments with S6S6 and S3S3 cells the 48A probe hybridized with equal intensity to one end of a pair of chromosomes (Fig. 2 A and B). 48A, and hence the S locus, was thus close to the centromere of a telocentric or subtelocentric chromosome. This is consistent with previous cytological analysis of the N. alata S locus (9) and with the chromosomal location of the S locus in tomato and Petunia (20–22). 48A labeling of the centric fragment was observed in M1-1 root-tip cells (Fig. 2C), showing that 48A-3, and therefore dS3, was on the centric fragment in this plant. 48A labeling was found on three metaphase chromosomes of M1-7 (S3S6dS3 genotype, Fig. 2D), consistent with dS3 being part of a translocation in this plant.
Figure 2.
Fluorescent in situ hybridization of a 48A genomic probe to metaphase chromosomes of SI and M1 PPM plants. Fluorescent detection of digoxigenin (DIG)-labeled probes hybridized to S6S6 chromosomes (A), S3S3 chromosomes (B), M1-1 chromosomes (C), M1-7 chromosomes (D), and M1-5 chromosomes (E). The probe used was a 4-Kb EcoRI fragment containing 48A. Yellow spots show hybridization of the DIG-labeled probe. (Scale bars, 10 μm.)
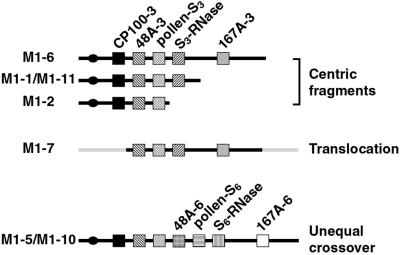
Fig. 3 shows an order for the genes on the S-bearing chromosome that is consistent with the RFLP and FISH analyses. Centric fragments arise by radiation-induced breakage of the long arm of the chromosome, with the size of the fragment being determined by the position of the break. M1-6 has the longest centric fragment and hence has the distal 167A gene. The M1-1 and M1-11 fragments lack this gene because they arose from more proximal breaks. Loss of CP100-3 from the M1-7 translocation places CP100 between the S locus and the centromere.
Figure 3.
Figure showing proposed order of S-linked genes in seven PPM lines. Each line represents a duplicated S-bearing fragment in a PPM plant and each gene is indicated by a box. The name of the PPM is shown at the left and the type of duplication (centric fragment, translocation or unequal exchange) is shown at the right. Spacing between genes is arbitrary. A circle marks the presumed centromere at one end of the S-bearing chromosome.
48A Detects Partially Duplicated S Alleles in “True” PPMs.
It is possible that M1-2, M1-5, and M1-10 have lesions in pollen S because they are genetically S6S6 and only carry the S6-RNase gene (Fig. 1A). To see if this lesion was due to a deletion, we assessed the integrity of the S locus in these plants by DNA blot analysis with the S-linked genes. 48A-3 and CP100-3, but not 167A-3 RFLPs were found in genomic DNA from M1-2b, M1-5 and M1-10b (S6S6) indicating part of an S3-bearing chromosome was present in all three plants (Fig. 1 B–D). This opened up the possibility that the pollen-part of an S3 allele (but not of the S3-RNase gene) had been duplicated in these plants, and that the mutations were hence caused by competitive interaction. Absence of the S3-RNase gene means the duplications can cause pollen-part mutations without changing the plant's stylar S phenotype. The partial S3 alleles that are presumed to be in M1-2, M1-5, and M1-10 will be referred to as dS3p (for duplicated S3 pollen-part only).
We investigated whether the putative dS3p could cause the pollen-part mutations in these plants by analyzing segregation in outcrossed (to plants homozygous for the S1 or S2 alleles) and backcrossed (to S3S6 plants) families. Presence of dS3p in a plant was inferred from the 48A-3 RFLP.
Table 2 shows the data for M1-2, which we concluded was S3S6 with dS3p on a centric fragment (S3S6dS3p). Competitive interaction between S6 and dS3p accounted for the presence of S3S6 PPMs and S6S6 PPMs, and absence of S3S3 PPMs, in the backcrossed family. Presence of the centric fragment in all of the backcross PPM progeny was consistent with dS3p being part of the fragment in M1-2. Competitive interaction also accounted for the breakdown of SI in the S1S6 and S2S6 plants that inherited dS3p and the centric fragment. The alternative hypothesis, that pollen S has been deleted from the S6 allele, cannot account for the presence of self-incompatible S1S6 and S2S6 plants. Curiously, cytology indicated that some self-incompatible S1S3 and S2S3 plants inherited the centric fragment (and 48A-3) but not the pollen-part mutation. This finding is difficult to explain if competitive interaction can occur between dS3p and the S1 and S2 alleles. However, as this was only seen when dS3p and the S3 allele were inherited together through pollen, we hypothesize that dS3p may have become a target for gene silencing.
Table 2.
Summary of breeding and DNA blot analysis for families derived from M1-2
| Cross* | S phenotype of
progeny
|
No. of progeny | Centric fragment† | 48A RFLPs‡ | |
|---|---|---|---|---|---|
| Pollen | Style | ||||
S3S6 ×
S3S6dS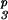
|
PPM | S3S6 | 8 | 1 (1) | 3 + 6 (4) |
| PPM | S6S6 | 10 | 1 (1) | 3 + 6 (4) | |
S1S1 ×
S3S6dS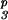
|
INC | S1S3 | 11 | 1 (2) | 1 + 3 (5) |
| INC | S1S6 | 9 | 0 (2) | 1 + 6 (3) | |
| PPM | S1S6 | 2 | 2 (2) | 1 + 3 + 6 (2) | |
S2S2 ×
S3S6dS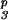
|
INC | S2S3 | 5 | 1 (2) | 2 + 3 (5) |
| INC | S2S6 | 3 | 0 (1) | 2 + 6 (3) | |
| PPM | S2S6 | 1 | 2 (2) | 2 + 3 + 6 (1) | |
INC, pollen incompatibility response was the same as a wild-type plant.
In each case the PPM was the staminate parent in a cross to the indicated pistilate parent.
The number of progeny with a centric fragment (number of plants examined) is indicated.
The number of plants examined by DNA blot hybridization is indicated in parentheses.
Our analysis of M1-5 was complicated by the fact that this plant is an S6S6 PPM with a mutation that lowers stylar S-RNase expression by one of its S6 alleles (5). When not complemented by a wild-type S6 allele, the level of S6-RNase made by this allele (S6spm) is insufficient to reject S6 pollen. M1-5 thus is both a pollen-part and a style-part mutant (SPM) and genetically is S6S6spm. The S6spm allele is, however, incompletely penetrant and reverts at high frequency to a functional S6 allele (5). Because M1-5 also has a putative dS3p, its genotype can be written as S6S6spmdS3p. An outcross between M1-5 and an S2S2 plant would therefore result in four phenotypic classes, assuming the S locus and the pollen-part mutation segregate independently: self-incompatible S2S6 plants, S2S6 PPMs (S2S6dS3p), S2S2 PPM/SPM plants (S2S6spmdS3p), and S2S2 SPM plants (S2S6spm). An analysis of 22 outcrossed plants (10 S2S6, 11 S2S6 PPM, and 1 S2S2 PPM/SPM) found 48A-2 and 48A-6 in all plants. Significantly, 48A-3 was found in all of the PPM plants but in none of the self-incompatible plants (data not shown). The absence of S2S2 SPM plants in the outcross family suggested dS3p might be linked to the S6spm allele. Thus, as with M1-2, it appeared that the breakdown of SI in M1-5 was caused by competitive interaction involving a dS3p.
The nature of the linkage between dS3p and the S6 allele in M1-5 was also investigated. One possibility was that dS3p and the S6 allele were linked following an unequal exchange between an S3- and an S6-bearing chromosome during meiosis. A second possibility was that dS3p had been translocated onto the end of an S6-bearing chromosome. To distinguish between these possibilities, the presence of RFLPs linked to the S6 chromosome in M1-5b (S3S6dS3p) was examined by DNA blot hybridization. 48A-6 and 167A-6 were present in M1-5, but CP100-6 was not (Fig. 1). The loss of this marker is consistent with an unequal crossover generating a recombinant S6 chromosome. FISH with the 48A probe detected only one chromosomal location in M1-5, confirming that 48A-3 and 48A-6 must be close to each other on a recombinant chromosome (Fig. 2E).
Similar genetic and molecular analyses of M1-10 showed that dS3p was linked to an S6 allele (data not shown) and that CP100-6 was not on the recombinant chromosome (Fig. 1). It was therefore likely that an unequal exchange between the S3- and S6-bearing chromosomes can account for this situation as well. The genotype of this plant was therefore S3S6dS3p.
The available evidence indicates the centric fragment in M1-2 arose following a breakage of the S-bearing chromosome between pollen S and the S-RNase gene (Fig. 3). This finding agrees with models that propose the S locus is bipartite with separate genes encoding the SI functions of style and pollen. An unequal exchange that placed CP100-3 and 48A-3 next to an S6 allele in M1-5 and M1-10 would remove CP100-6 if this gene were closer to the centromere than 48A-6, a gene order that agrees with analysis of the dS3 translocation in M1-7. Because we have not yet been able to separate 48A and pollen S, the orientation of these genes with respect to each other cannot be determined. Fig. 3 shows one of the two possible arrangements.
Discussion
The pollen-part mutations examined here all arose through competitive interaction brought about by a duplicated S3 allele. Using markers that flank the S locus, we found duplications in all PPMs that either spanned an entire S3 allele (dS3) or included part of an S3 allele but lacked the S3-RNase gene (dS3p). The failure to detect any deletions of pollen S among the progeny produced by using the ≈5 million irradiated pollen grains examined in this experiment indicates a frequency of less than 0.00002% for pollen S deletions. This rate is many orders of magnitude lower than the deletion rate seen when pollen from other solanaceous species is treated with similar doses of radiation—for example 0.025–0.05% at several different loci of tomato (23). The finding that duplicated S alleles may lack the S-RNase gene raises the possibility that earlier investigators misidentified plants with these duplications as “true” pollen S mutants. This misidentification could easily have occurred because these studies relied entirely on stylar phenotypes to determine whether an additional S allele was present. Indeed, the presence of centric fragments in some true PPMs and some of the plants examined here makes this scenario seem quite plausible (8, 9). Thus the evidence from this and previous studies indicates that deletions in pollen S are probably not tolerated and that pollen S is essential for pollen viability.
This single cause of pollen-part mutations allows us to distinguish between the two models currently used to describe SI in the Solanaceae. Although each model presents an adequate explanation of what is known about allelic recognition, they differ in their predictions about whether pollen S can be deleted from the genome. One model predicts that the product of pollen S encodes a receptor that allows the extracellular S-RNase to enter pollen tubes in an allele-specific manner. According to this model, a deletion of pollen S is tolerated because S-RNases cannot enter a pollen tube lacking a receptor, and hence mutant pollen is able grow through an incompatible style. The other model predicts that S-RNases enter pollen tubes nonspecifically where they encounter pollen S, an inhibitor that can inactivate all S-RNases except those encoded by a matching S allele. This model predicts that pollen S cannot be deleted because pollen tubes lacking pollen S are unable to detoxify S-RNases and hence will be rejected by any style. The inhibitor model is currently favored, because it is consistent with the genetic analysis of PPMs presented here and with the localization of S-RNases within both compatible and incompatible pollen tubes of Solanum chacoense (24).
A variation on the inhibitor model was recently proposed (24, 25). In this version, S-RNase inhibition requires two gene products: a general S-RNase inhibitor and an additional allele-specificity protein encoded by pollen S that prevents the inhibitor from binding to a matching S-RNase. This model predicts that deletions in pollen S are tolerated, as mutant pollen will inhibit all S-RNases entering the pollen tube. Because there is currently no evidence of pollen S deletions, we favor the simpler model where pollen S itself encodes the S-RNase inhibitor.
It was noteworthy that all pollen-part mutations were associated with the duplication of an S3 allele and contained 48A-3. Because the pollen-part mutations were derived from the pollen of irradiated S3S6 plants, it is reasonable to ask why none of the PPMs had a duplication of an S6 allele. We assume the reason lies in part with the particular sensitivity of the chromosome near the S3 allele to fragmentation by ionizing radiation, and in part with the way the chromosomal fragments thus generated were repaired and passed into gametes after meiosis. Earlier SI workers also noted that the frequency at which PPMs arose depended on the S genotypes being mutated (8, 10). Centric fragments derived from an S6-bearing chromosome were, however, found in two self-incompatible “revertant” plants (5). These plants (M1-8 and M1-17) are self-incompatible and are described as revertants because they were derived from pollen tubes that grew through incompatible styles. Presumably the mutation that overcame pollen rejection “reverted” to the unmutated state after fertilization. Both M1-8 and M1-17 have centric fragments that contain 48A-6 but not the S6-RNase gene (J.F.G., V.S., and E.N., unpublished results). Although M1-8 and M1-17 have duplications of an S-linked marker, the plants are self-incompatible presumably because the duplications lack pollen S. The presence of a duplicated 48A in these plants may thus be evidence that this gene is not pollen S.
Several groups are currently attempting to clone pollen S from solanaceous plants or from plants in other families with the same SI system (17, 26, 27). However, low rates of recombination around the S locus have hindered this research by making the ordering of genes and markers in this region of the genome nearly impossible. For instance, an analysis of more than 150 N. alata plants identified no recombinants that separated 48A, CP100, and the S locus (17). The problem is particularly acute for genes that are expressed in pollen and are hence candidates for pollen S. Several have been identified, but the low recombination rates mean that almost all satisfy the principal criterion for pollen S of being tightly linked to the S-RNase gene (17, 26). Here we have shown how duplicated S alleles can overcome these problems by allowing the order of markers and genes to be quickly determined (Fig. 3). This finding suggests it is more useful to identify pollen S by its association with duplicated S alleles that cause pollen-part mutations than by linkage to the S-RNase gene. Whereas genes such as 48A still satisfy this criterion, genes that are genetically close but physically distant from the S locus do not. The small dS3p duplications are especially useful in this regard and furthermore can also be used in experiments aimed at determining the physical size of the S locus, which has been estimated to be greater than 1 Mbp (28). Our analysis of the N. alata PPMs has thus identified material suited to large-scale molecular characterization of the S locus.
Acknowledgments
We thank Bruce McGinness for assistance in the glasshouse, Ingrid Bönig for help with the FISH protocol, and an earlier generation of SI investigators for stimulating our interest in the N. alata PPMs. We thank Drs. Tim Robbins and Andrew Hudson for commenting on earlier drafts of this paper, and Dr. Christiane Gebhardt for the marker CP100. This investigation was supported by grants from the Australian Research Council. J.F.G. was supported by an Overseas Postgraduate Research Scholarship from the Australian Government and M.K. by an International Research and Developing Technology Fellowship from the Japanese Agency for Science and Technology.
Abbreviations
- SI
self-incompatibility
- PPM
pollen-part mutant
- SPM
style-part mutant
- RFLP
restriction fragment-length polymorphism
- FISH
fluorescent in situ hybridization
- S-RNase
stylar RNase
- dS
duplicated S
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AJ277643).
References
- 1.de Nettancourt D. Incompatibility in Angiosperms: Monographs on Theoretical and Applied Genetics 3. Berlin: Springer; 1977. [Google Scholar]
- 2.McClure B A, Haring V, Ebert P R, Anderson M A, Simpson R J, Sakiyama F, Clarke A E. Nature (London) 1989;342:955–957. doi: 10.1038/342955a0. [DOI] [PubMed] [Google Scholar]
- 3.Lee H S, Huang S, Kao T-h. Nature (London) 1994;367:560–563. doi: 10.1038/367560a0. [DOI] [PubMed] [Google Scholar]
- 4.Dodds P N, Ferguson C, Clarke A E, Newbigin E. Sex Plant Reprod. 1999;12:76–87. [Google Scholar]
- 5.Golz J F, Su V, Clarke A E, Newbigin E. Genetics. 1999;152:1123–1135. doi: 10.1093/genetics/152.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Atomic Energy Agency. Manual on Mutation Breeding. Vienna: IAEA; 1977. [Google Scholar]
- 7.Brewbaker J L, Natarajan A T. Genetics. 1960;45:699–704. doi: 10.1093/genetics/45.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandey K K. Nature (London) 1965;206:792–795. [Google Scholar]
- 9.Pandey K K. Heredity. 1967;22:255–284. [Google Scholar]
- 10.Van Gastel A J G, de Nettancourt D. Heredity. 1975;34:381–392. [Google Scholar]
- 11.Stout A B, Chandler C. Science. 1942;96:257. doi: 10.1126/science.96.2489.257-a. [DOI] [PubMed] [Google Scholar]
- 12.Crane M B, Lewis D. J Genet. 1942;43:31–49. [Google Scholar]
- 13.Pandey K K. Genetica. 1968;39:257–271. [Google Scholar]
- 14.Chawla B, Bernatzky R, Liang W, Marcotrigiano M. Theor Appl Genet. 1997;95:992–996. [Google Scholar]
- 15.Olsder J, Hermsen J G T. Euphytica. 1976;25:597–607. [Google Scholar]
- 16.Thompson R D, Uhrig H, Hermsen J G T, Salamini F, Kaufmann H. Mol Gen Genet. 1991;226:283–288. doi: 10.1007/BF00273614. [DOI] [PubMed] [Google Scholar]
- 17.Li J-H, Nass N, Kusaba M, Dodds P, Treloar N, Clarke A E, Newbigin E. Theor Appl Genet. 2000;100:956–964. [Google Scholar]
- 18.Anderson M A, Cornish E C, Mau S-L, Williams E G, Hoggart R, Atkinson A, Bönig I, Greg B, Simpson R, Roche P J, et al. Nature (London) 1986;321:38–44. [Google Scholar]
- 19.Gebhardt C, Ritter E, Barone A, Debner T, Walkemeier B, Schachtschabel U, Kaufmann H, Thompson R D, Bonierbale M B, Ganal M W, et al. Theor Appl Genet. 1991;83:49–57. doi: 10.1007/BF00229225. [DOI] [PubMed] [Google Scholar]
- 20.Tanksley S D, Loaiza-Figueroa F. Proc Natl Acad Sci USA. 1985;82:5093–5096. doi: 10.1073/pnas.82.15.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ten Hoopen R, Harbord R M, Maes T, Nanninga N, Robbins T P. Plant J. 1998;16:729–734. [Google Scholar]
- 22.Entani T, Iwano M, Shiba H, Takayama S, Fukui K, Isogai A. Theor Appl Genet. 1999;99:391–397. doi: 10.1007/s001220051249. [DOI] [PubMed] [Google Scholar]
- 23.Khush G S, Rick C M. Chromosoma. 1968;23:452–484. [Google Scholar]
- 24.Luu D-T, Qin X, Morse D, Cappadocia M. Nature (London) 2000;407:649–651. doi: 10.1038/35036623. [DOI] [PubMed] [Google Scholar]
- 25.Luu D-T, Qin X, Laublin G, Yang Q, Morse D, Cappadocia M. Genetics. 2001;159:329–335. doi: 10.1093/genetics/159.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCubbin A G, Wang X, Kao T-h. Genome. 2000;43:619–627. [PubMed] [Google Scholar]
- 27.Ushijima K, Sassa S, Tamura M, Kusaba M, Tao R, Gradziel T M, Dandekar A M, Hirano H. Genetics. 2001;158:379–386. doi: 10.1093/genetics/158.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCubbin A G, Kao T-h. Sex Plant Reprod. 1999;12:1–5. [Google Scholar]