Abstract
The unique synthesis and reactivity of [(RPNP*)NiH] complexes (1a,b), based on metal–ligand cooperation (MLC), are presented (RPNP* = deprotonated PNP ligand, R = iPr, tBu). Unexpectedly, the dearomatized complexes 1a,b were obtained by reduction of the dicationic complexes [(RPNP)Ni(MeCN)](BF4)2 with sodium amalgam or by reaction of the free ligand with Ni0(COD)2. Complex 1b reacts with CO via MLC, to give a rare case of a distorted-octahedral PNP-based pincer complex, the Ni(0) complex 3b. Complexes 1a,b also react with CO2 via MLC to form a rare example of η1 binding of CO2 to nickel, complexes 4a,b. An unusual CO2 cleavage process by complex 4b, involving C–O and C–P cleavage and C–C bond formation, led to the Ni–CO complex 3b and to the new complex [(PiPr2NC2O2)Ni(P(O)iPr2)] (5b). All complexes have been fully characterized by NMR and X-ray crystallography.
Late-transition-metal complexes of electron-donating and bulky “pincer” ligands have found important applications in synthesis, bond activation, and catalysis.1−6 The lutidine-based pincer ligands (Scheme 1) are highly electron donating ligands with a relatively low trans influence of the pyridinic nitrogen and benzylic “arms” amenable to deprotonation.
Scheme 1. H–X Bond Activation by Aromatization/Dearomatization MLC Reactivity.
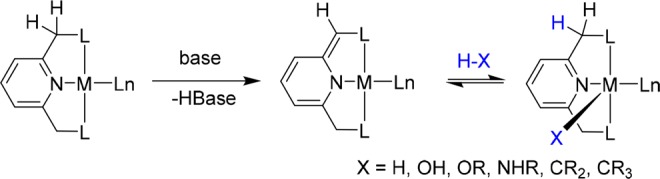
In recent years our group has developed a novel mode of metal–ligand cooperation (MLC), involving aromatization/dearomatization of lutidine-based pincer complexes (Scheme 1).7−12 This mode of reactivity enabled the activation of various substrates such as alcohols,13−17 amines,18−21 nitriles,22,23 boranes,24 dihydrogen,25−27 and dioxygen,28 as well as activation of Csp2–H29 and Csp3–H30 bonds, and it is a key step in the design of several environmentally benign catalytic reactions.7−11
Due to the importance of CO2 as a potential C1 building block,31,32 we have explored the reactivity of dearomatized pincer complexes toward CO2. It was found that MLC is also involved in the activation of CO2 by dearomatized lutidine-based complexes of Fe,33 Ru,34,35 Re,36 Ni,37 and Ir,38 reversibly forming a new C–C bond between the ligand backbone and CO2. Recently, we reported the reductive cleavage of CO2 by dearomatized (tBu-PNP)Ir–H and (tBu-PNP)Rh–H complexes via MLC (PNP = 2,6-bis(di-tert-butylphosphinomethyl)pyridine),38,39 leading to the design of a cycle of photocarbonylation of benzene.39
Herein we report a rare case of Ni(η1-CO2-κC) complexes, obtained by direct coordination of free CO2 to lutidine-based Ni–hydride complexes [(R-PNP*)NiH] (R-PNP* = dearomatized PNP ligand, R = tBu, iPr) involving metal–ligand cooperation.
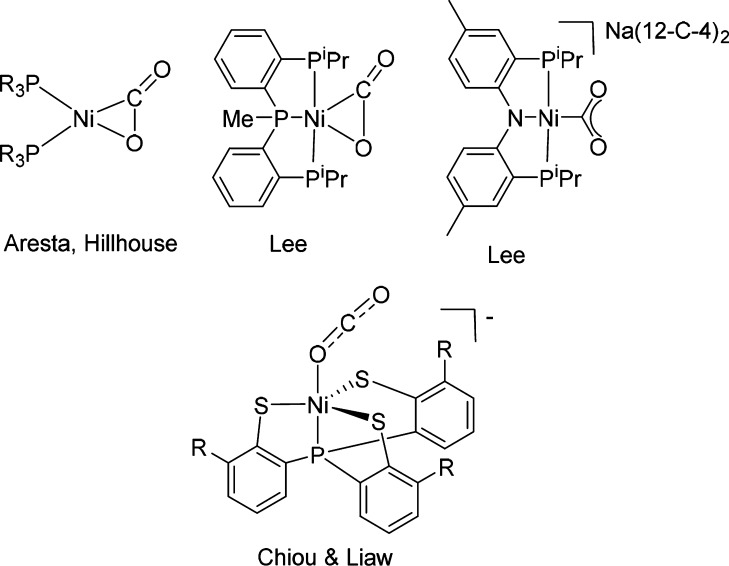
CO2 coordination to Ni complexes is well known,40 and Ni complexes serve as efficient catalysts in CO2 activation and utilization as a C1 building block,31,41 mainly in CO2 hydrogenation42−45 and carboxylation.46−56 The first structurally characterized metal–CO2 complex was (PCy3)2Ni(η2-CO2), reported by Aresta et al. in 1975 (Scheme 2).57 Since then, several similar Ni(η2-CO2) complexes have been reported,58,59 including a unique five-coordinated Ni(η2-CO2) complex.60 A unique coordination mode of μ-η2,η2-CO2 in a dinuclear Ni complex was reported by Sadighi and co-workers,61 and a rare case of NiIII(η1-(CO2)•-κO) was reported by Chiou, Liaw, and co-workers.62 Recently a rare case of NiII(η1-CO2-κC) was reported by Lee63 (Scheme 2).
Scheme 2. Mononuclear Ni CO2 Complexes.
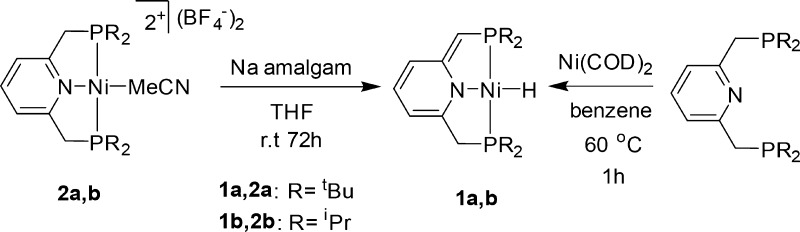
The dearomatized complex [(tBu-PNP*)NiH] (1a, Scheme 3), reported by van der Vlugt et al., was obtained by reaction of LiAlH4 with the dearomatized complex [(tBu-PNP*)NiCl].64 Surprisingly, we obtained the Ni(II) complex 1a by attempting to reduce the dicationic Ni(II) complex [(tBu-PNP)Ni(MeCN)](BF4)264 with sodium amalgam (Scheme 3). Single crystals of complex 1a were obtained by slow evaporation of its benzene solution. The unreported X-ray structure of 1a is shown in Figure 1. The new complex [(iPr-PNP)Ni(MeCN)](BF4)2 was also prepared, and upon similar treatment with sodium amalgam the corresponding dearomatized hydrido complex 1b was obtained (Scheme 3). Crystals suitable for X-ray diffraction of 1b (Figure 1) were obtained by evaporation of its pentane solution. The X-ray structures of 1a,b reveal the expected square-planar geometry.
Scheme 3. Synthesis of Complexes 1a,b.
Figure 1.
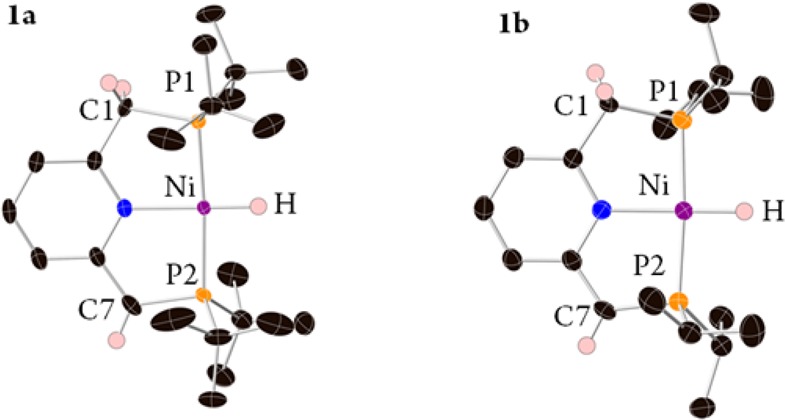
Single-crystal X-ray structures with thermal ellipsoids shown at the 50% probability level of complex 1a (left) and complex 1b (right). Hydrogen atoms, except Ni–H and side arm protons, are omitted for clarity. See the Supporting Information for a full description of the structures.
Complex 1b exhibits in the 1H NMR spectrum a hydride signal at −18.13 ppm (2JPH = 65 Hz), and the 31P{1H} NMR spectrum exhibits an AB pattern centered at 54.72 ppm (2JPP = 225.1 Hz), similar to the spectra reported for 1a.64
We believe that the reduction of complexes 2a,b leads initially to Ni(0) intermediates forced into a square-planar geometry by the relatively rigid PNP pincer ligand. As a d10 ML4 complex, Ni(0) complexes prefer to adopt a tetrahedral geometry, and Ni(0) square-planar complexes are as yet unknown. Only one d10 square-planar complex is known, a Pt(0) complex.65 The postulated unstable Ni(0) intermediates rearrange by metal–ligand cooperation, in which proton transfer from the benzylic position of the pincer ligand to the metal center takes place, yielding the Ni(II) dearomatized hydride complexes 1a,b. In support of this mechanism, reaction of the Ni0(COD)2 complex with the R-PNP ligands (R = tBu, iPr) resulted in the dearomatized complexes 1a,b exclusively (Scheme 3).
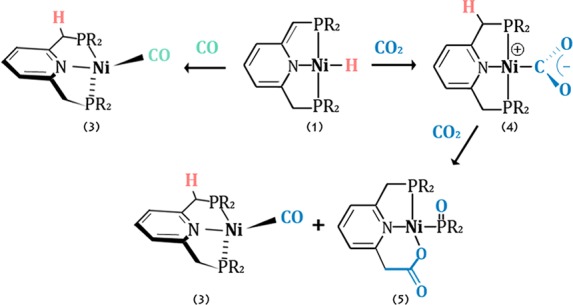
In order to explore the possibility of reverting the metal–ligand cooperative proton transfer from the arm to the metal center, thus gaining a Ni(0) complex, complexes 1a,b were reacted with CO. Upon treatment of complex 1a with 1 equiv of CO no reaction was observed, likely as a result of steric congestion imposed by the bulky tBu-PNP ligand. However, upon addition of 1 equiv of CO to the iPr-PNP complex 1b, a rapid color change occurred, yielding the unusual neutral pincer Ni(0) carbonyl complex 3b (Scheme 4). Complex 3b exhibits a singlet peak at 68.36 ppm in the 31P{1H} NMR, indicating a metal–ligand cooperative transfer of a proton from the metal back to the unsaturated arm, yielding a symmetric complex. The carbonyl ligand gives rise to a triplet peak at 204.02 ppm in the 13C{1H} NMR spectrum, at a slightly higher field in comparison to that of the recently reported nickel monocarbonyl anionic complex {Na(12-C-4)2}{(acriPNP)Ni(CO)}.66 Slow evaporation of the benzene solution resulted in formation of single crystals suitable for X-ray diffraction (Scheme 4), and these adopt a distorted-tetrahedral geometry. The P–Ni–CO angle is the only characteristic tetrahedral angle (111.3(2)°), while the N–Ni–P and the N–Ni–CO angles are 85.0(1) and 125.8(2)°, respectively. The Ni–CO and C–O bond distances are 1.862(6) and 1.030(6) Å, respectively, and the CO IR band appears at 1888 cm–1 in benzene solution. In comparison, the recently reported monocarbonyl Ni(0) anionic complex [Na(12-C-4)2][(acriPNP)Ni(CO)], which also adopts a distorted-tetrahedral geometry due to the rigid PNP ligand, exhibits Ni–CO and C–O bond lengths of 1.77(1) and 1.18(1) Å, respectively, and an IR CO band appears at 1828 cm–1, indicating a higher degree of π back-donation from the low-valent nickel center in comparison with 3b.66
Scheme 4. Formation of the Ni(0) Complex 3b and Its Single-Crystal X-ray Structure with Thermal Ellipsoids Shown at the 50% Probability Level.
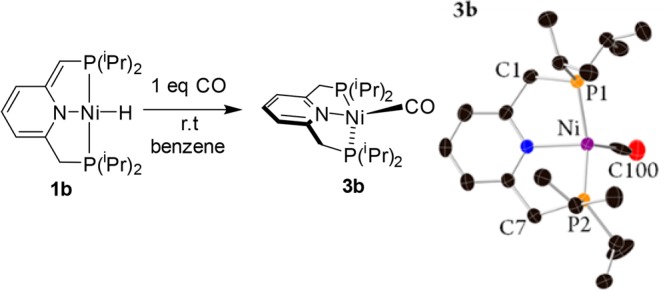
Hydrogen atoms are omitted for clarity. See the Supporting Information for a full structural description.
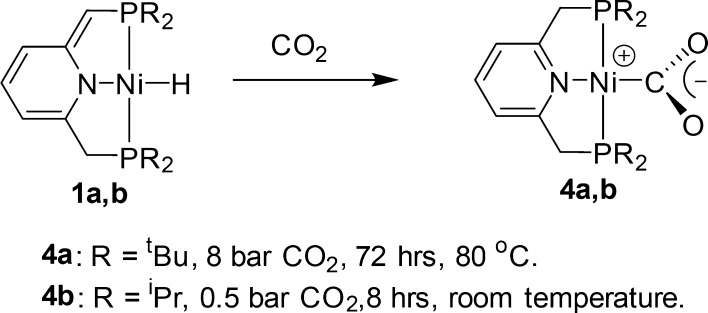
The dearomatized hydride complexes 1a,b react with CO2 to give the aromatized complexes 4a,b with a rare η1 coordination mode of carbon dioxide (Scheme 5). While complex 1b reacts readily with CO2 at ambient temperature with only 0.5 bar of CO2, forming complex 4b in 82% yield (according to 31P NMR) after 8 h, the conversion of 1a to 4a requires the more forcing conditions of 8 bar, 80 °C, and 72 h, yielding 60% of the product.
Scheme 5. Synthesis of Complexes 4a,b.
Whereas the η2-CO2 coordination mode is common, the η1-CO2 coordination mode is rare. It was reported for [Rh(diars)2Cl(CO2)],67 [Ru(bpy)2(CO)(CO2)]·3H2O,68,69 [Ir(dmpe)2Cl(CO2)],70 and [Co(salen)K(η1-CO2)(THF)].71,72 The only Ni-η1-CO2 complex is the recently reported anionic complex [Na(12-C-4)2][(PNP)Ni-η1-CO2] (PNP = N[2-PiPr2-4-Me-C6H3]2),63 which was obtained by a reduction of the carboxylate complex [(PNP)NiCOONa] and not by direct CO2 coordination, unlike complexes 4a,b.
Crystals suitable for X-ray diffraction of 4a,b (Figure 2) were obtained from the crude reaction mixture in a pressure flask under a CO2 atmosphere. Both complexes exhibit a distorted-square-planar geometry with P–Ni–P angles of 171.39(3) and 173.4(1)° and N–Ni–C angles of 179.0(1) and 178.6(2)° for complexes 4a,b, respectively. The CO2 plane is almost perpendicular to the pincer ligand plane. The Ni–CO2 bond lengths in complexes 4a,b are 1.950(3) and 1.912(4) Å, respectively, which are in the range of reported η1-CO2 complexes and longer by 0.11–0.044 Å than those reported for Ni−η2-CO2.57,59 The two C–O bond lengths are similar (1.244(3), 1.254(3) Å in 4a and 1.240(5), 1.250(5) Å in 4b), unlike the C–O bonds in reported Ni-η2-CO2 complexes, in which the difference in length is 0.06 Å.59,60 In addition, the large and similar distances between the oxygen atoms and the metal center (2.737(2), 2.721(1) Å in 4a and 2.749(3), 2.637(3) Å in 4b) also indicate an η1-CO2 coordination mode, as the Ni–O bond lengths in η2-CO2 complexes are shorter by 0.8–0.5 Å.57,59 The bond lengths of the CO2 ligand in complexes 4a,b are similar to the reported bonds of the complex [Na(12-C-4)2][(PNP)Ni-η1-CO2].63 In addition, the Ni–CO2 bond length in complex 4a is longer by 0.04 Å in comparison with 4b. This elongation is probably due to the more sterically demanding tBu-PNP ligand in comparison to the iPr analogue. Aresta et al., in his seminal work on (PR3)2Ni(η2-CO2) complexes (R = (C6H11), Et, Bun), also observed that steric hindrance has a great influence on the Ni–CO2 bond strength.58 We have previously reported that the steric difference between the tBu-PNP and iPr analogues can lead to large differences in reactivity.73 The lower steric hindrance of the iPr-PNP can result in a higher degree of π back-donation from the Ni center to the CO2 ligand,63 which shortens the Ni–CO2 bond length. This is in line with the differences in reaction conditions for the synthesis of complexes 4a,b and the differences in reactivity of complexes 1a,b toward CO. According to NMR studies, whereas complex 1b reacts with substoichiometric amounts of CO2 at room temperature to gradually form complex 4b (Figure 3), complex 1a requires at least 5 bar of CO2 to achieve detectable conversion. In addition, while the formation of 4b under these conditions requires several minutes, detectable formation of 4a requires at least 12 h.
Figure 2.
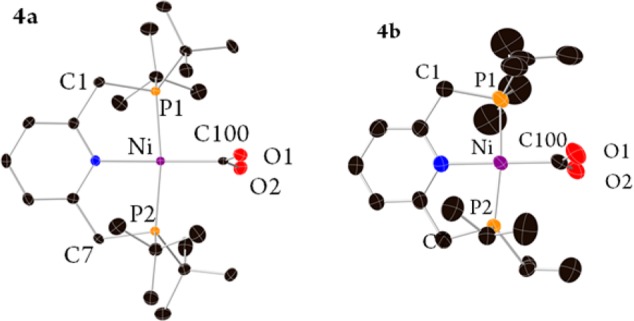
Single-crystal X-ray structures, with thermal ellipsoids shown at the 50% probability level, of complexes 4a (left) and 4b (right). Hydrogen atoms and cocrystallized solvents are omitted for clarity. See the Supporting Information for a full description of the structures.
Figure 3.
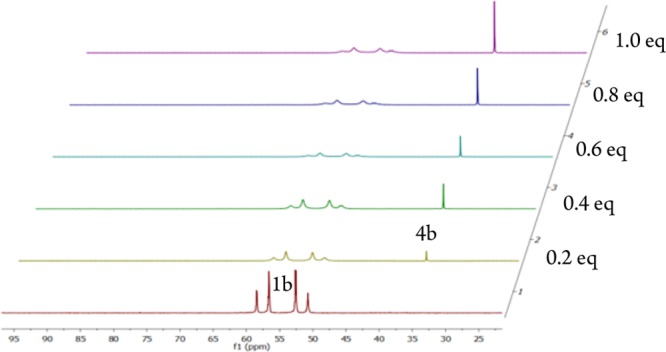
31P{1H} NMR (121.4 MHz) spectra representing gradual injection of about 0.2 equiv of CO2 into a toluene solution of complex 1b in an NMR tube equipped with a septum cap. Similar conditions with complex 1a yielded no detectable conversion.
Complexes 4a,b exhibit symmetric 1H and 13C NMR spectral patterns of the ligand backbone and a singlet signal in the 31P{1H} NMR spectrum at 47.65 (4a) and 35.46 ppm (4b), as expected for aromatic square-planar complexes. The CO2 ligand gives rise to broad peaks at 174.74 and 173.25 ppm, respectively, in the 13C{1H} NMR spectra, which are at a higher field in comparison to the reported chemical shifts of the CO2 ligands in the complexes [Ru(bpy)2(CO)(CO2)]·3H2O68,69 (203.9 ppm) and [Na(12-C-4)2][(PNP)Ni-η1-CO2]63 (197.25 ppm). The reported Ni−η2-CO2 complexes give rise to a signal at 164 ppm.59,60
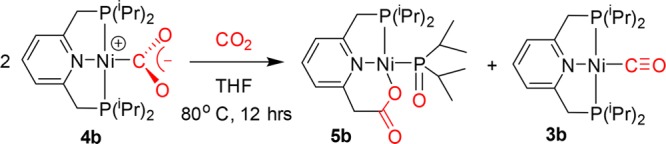
Although complex 4b is stable at room temperature, when a THF solution of 4b is heated to 80 °C under 5 bar of CO2, an unexpected reaction was observed. According to the 31P{1H} NMR spectrum, full conversion to two products took place, giving rise to a singlet signal at 68.3 ppm, assigned as complex 3b, and a pair of doublets at 64.3 and 102.9 ppm (2JPP = 90 Hz), assigned as complex 5b (Scheme 6). Complexes 3b and 5b were obtained from complex 4b also at ambient temperature under 5 bar of CO2, although at lower conversion. Complex 4a demonstrated no such reactivity, despite the use of more forcing conditions of 10 bar of CO2 at 353 K for 7 days.
Scheme 6. CO2 Cleavage by Complex 4b, Involving C=O and C–P Cleavage and C–C Bond Formation.
Complex 3b was extracted from the reaction mixture with pentane, and the two products, 3b and 5b, were isolated and fully characterized. Crystals suitable for X-ray diffraction of [(PiPr2NC2O2)Ni(P(O)iPr2)] (5b, Figure 4) were obtained by layering of pentane over a dichloromethane solution. Complex 5b exhibits a distorted-square-planar geometry with P(1)–Ni–O(1) angle of 167.04(6)° and N–Ni–P(2) angle of 170.51(6)°. The C=O(2) and C–O(1) bond lengths are 1.229(3) and 1.296(3) Å, respectively, both longer than the free CO2 bond length by 0.07–0.14 Å, and the C(7)–C(100) bond length is in the range of a single C–C bond, 1.519(4) Å. The NMR data of 5b fit well with its X-ray structure, and two distinguishable CH2 signals of the benzylic positions, in which only one is coupled to a phosphorus atom, were observed in the 1H and 13C{1H} NMR.
Figure 4.
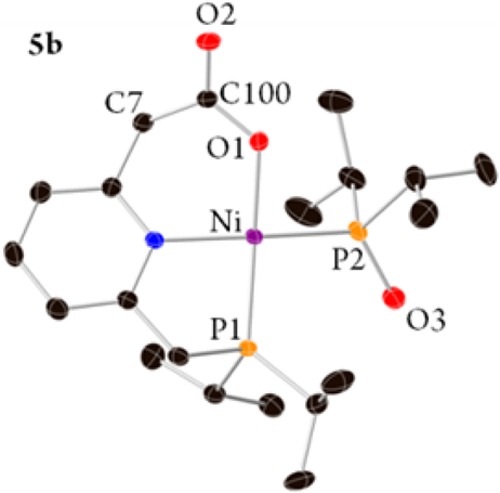
Single-crystal X-ray structures with thermal ellipsoids shown at the 50% probability level of complex 5b. Hydrogen atoms are omitted for clarity. See the Supporting Information for a full description of the structure.
Apparently, two molecules of CO2 are involved, one leading to C–P cleavage followed by C–C bond formation, generating a carboxylato ligand, while the second molecule oxidizes the iPr2P fragment to the iPr2P=O ligand. The resulting CO is then trapped by another molecule of the starting complex to form the carbonyl complex 3b. Similar reactivity was reported recently for the Ir(I) complex [Ir(depe)(dbuP)] (depe = 1,2-bis(diethylphosphino)ethane, dbuP = 1,8-diazabicyclo[5.4.0]undec-7-ene).74 The Ir(I) complex reacts with two CO2 molecules to give the Ir(III) carbonyl phosphoryl complex [Ir(depe)(CO)(Ph2PO)(dbuCO2)], with a carboxylate incorporation into the dbuP ligand. In both complexes, the reported Ir complex and complex 4b, four new bonds were formed, M–C, M–O, C–C, and P–O, and two bonds were cleaved, C=O and P–C. P–C bond cleavage has attracted much attention in the past75 and also more in recent studies.76,77 Complexes bearing phosphoryl ligands (R2P=O)– are not common; examples were reported for Au,78 Ru,79 Ir,80 and Pd.81,82 Ni and Ru bis(phosphinite) pincer complexes were reported to decompose to phosphoryl complexes due to P–O bond cleavage under basic or wet conditions.83,84
In summary, unexpected synthetic and reactivity pathways involving metal–ligand cooperation (MLC) of the (PNP*)NiII–H pincer complexes 1a,b are presented. While complex 1a does not react with CO, the less bulky complex 1b reacts with CO via MLC to give a rare case of a distorted-tetrahedral [(iPrPNP)Ni(CO)] pincer complex. Notably, complexes 1a,b react with CO2 via MLC to form rare examples of η1 binding of CO2 to nickel, complexes 4a,b. Upon heating under a CO2 atmosphere, the CO2 complex 4b undergoes an unexpected CO2 cleavage as well as P–C cleavage, followed by C–C bond formation by carboxylation of the PNP ligand, leading to the formation of the NiIIPO complex 5b and the Ni0CO complex 3b. Further studies are aimed at understanding of the MLC mechanisms of these unusual transformations.
Acknowledgments
This research was supported by the European Research Council (ERC AdG 692775). D.M. holds the Israel Matz Professorial Chair of Organic Chemistry.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.organomet.8b00160.
Experimental procedures and IR and NMR spectra of complexes 1a,b–5a,b (PDF)
Accession Codes
CCDC 1831348–1831353 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
Supplementary Material
References
- Maser L.; Vondung L.; Langer R. The ABC in Pincer Chemistry – From Amine to Borylene and Carbon-Based Pincer-Ligands. Polyhedron 2018, 143, 28–42. 10.1016/j.poly.2017.09.009. [DOI] [Google Scholar]
- Albrecht M.; Lindner M. M. Cleavage of Unreactive Bonds with Pincer Metal Complexes. Dalton Trans. 2011, 40, 8733–8744. 10.1039/c1dt10339c. [DOI] [PubMed] [Google Scholar]
- Albrecht M.; van Koten G. Platinum Group Organometallics Based on “Pincer” Complexes: Sensors, Switches, and Catalysts. Angew. Chem., Int. Ed. 2001, 40, 3750–3781. . [DOI] [PubMed] [Google Scholar]
- van der Boom M. E.; Milstein D. Cyclometalated Phosphine-Based Pincer Complexes: Mechanistic Insight in Catalysis, Coordination, and Bond Activation. Chem. Rev. 2003, 103, 1759–1792. 10.1021/cr960118r. [DOI] [PubMed] [Google Scholar]
- van Koten G. J. Highlights of 45 Years of Research: A Personal Account. J. Organomet. Chem. 2017, 845, 4–18. 10.1016/j.jorganchem.2017.05.001. [DOI] [Google Scholar]
- Morales-Morales D.; Jensen C. M.. The Chemistry of Pincer Compounds, 1st ed.; Elsevier: Oxford, U.K., 2007. [Google Scholar]
- Khusnutdinova J. R.; Milstein D. Metal–Ligand Cooperation. Angew. Chem., Int. Ed. 2015, 54, 12236–12273. 10.1002/anie.201503873. [DOI] [PubMed] [Google Scholar]
- Milstein D. Metal–Ligand Cooperation by Aromatization–Dearomatization as a Tool in Single Bond Activation. Philos. Trans. R. Soc., A 2015, 373, 20140189. 10.1098/rsta.2014.0189. [DOI] [PubMed] [Google Scholar]
- Zell T.; Milstein D. Hydrogenation and Dehydrogenation Iron Pincer Catalysts Capable of Metal–Ligand Cooperation by Aromatization/Dearomatization. Acc. Chem. Res. 2015, 48, 1979–1994. 10.1021/acs.accounts.5b00027. [DOI] [PubMed] [Google Scholar]
- Gunanathan C.; Milstein D. Metal–Ligand Cooperation by Aromatization–Dearomatization: A New Paradigm in Bond Activation and “Green” Catalysis. Acc. Chem. Res. 2011, 44, 588–602. 10.1021/ar2000265. [DOI] [PubMed] [Google Scholar]
- Gunanathan C.; Milstein D. Bond Activation by Metal-Ligand Cooperation: Design of “Green” Catalytic Reactions Based on Aromatization-Dearomatization of Pincer Complexes. Top. Organomet. Chem. 2011, 37, 55–84. 10.1007/3418_2011_6. [DOI] [Google Scholar]
- Milstein D. Discovery of Environmentally Benign Catalytic Reactions of Alcohols Catalyzed by Pyridine-Based Pincer Ru Complexes, Based on Metal–Ligand Cooperation. Top. Catal. 2010, 53, 915–923. 10.1007/s11244-010-9523-7. [DOI] [Google Scholar]
- Montag M.; Zhang J.; Milstein D. Aldehyde Binding through Reversible C–C Coupling with the Pincer Ligand upon Alcohol Dehydrogenation by a PNP–Ruthenium Catalyst. J. Am. Chem. Soc. 2012, 134, 10325–10328. 10.1021/ja303121v. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Leitus G.; Ben-David Y.; Milstein D. Facile Conversion of Alcohols into Esters and Dihydrogen Catalyzed by New Ruthenium Complexes. J. Am. Chem. Soc. 2005, 127, 10840–10841. 10.1021/ja052862b. [DOI] [PubMed] [Google Scholar]
- Gunanathan C.; Ben-David Y.; Milstein D. Direct Synthesis of Amides from Alcohols and Amines with Liberation of H2. Science 2007, 317, 790–792. 10.1126/science.1145295. [DOI] [PubMed] [Google Scholar]
- Fogler E.; Garg J. A.; Hu P.; Leitus G.; Shimon L. J. W.; Milstein D. System with Potential Dual Modes of Metal-Ligand Cooperation: Highly Catalytically Active Pyridine-Based PNNH-Ru Pincer Complexes. Chem. - Eur. J. 2014, 20, 15727–15731. 10.1002/chem.201405295. [DOI] [PubMed] [Google Scholar]
- Hu P.; Diskin-Posner Y.; Ben-David Y.; Milstein D. Reusable Homogeneous Catalytic System for Hydrogen Production from Methanol and Water. ACS Catal. 2014, 4, 2649–2652. 10.1021/cs500937f. [DOI] [Google Scholar]
- Gunanathan C.; Gnanaprakasam B.; Iron M. A.; Shimon L. J. W.; Milstein D. ″Long-Range″ Metal-Ligand Cooperation in H2 Activion. J. Am. Chem. Soc. 2010, 132, 14763–14765. 10.1021/ja107770y. [DOI] [PubMed] [Google Scholar]
- Balaraman E.; Gnanaprakasam B.; Shimon L. J. W.; Milstein D. Direct Hydrogenation of Amides to Alcohols and Amines under Mild Conditions. J. Am. Chem. Soc. 2010, 132, 16756–16758. 10.1021/ja1080019. [DOI] [PubMed] [Google Scholar]
- Feller M.; Diskin-Posner Y.; Shimon L. J. W.; Ben-Ari E.; Milstein D. N–H Activation by Rh(I) via Metal–Ligand Cooperation. Organometallics 2012, 31, 4083–4101. 10.1021/om300248r. [DOI] [Google Scholar]
- Bauer J. O.; Leitus G.; Ben-David Y.; Milstein D. Direct Synthesis of Symmetrical Azines from Alcohols and Hydrazine Catalyzed by a Ruthenium Pincer Complex: Effect of Hydrogen Bonding. ACS Catal. 2016, 6, 8415–8419. 10.1021/acscatal.6b02946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt M.; Nerush A.; Iron M. A.; Leitus G.; Diskin-Posner Y.; Shimon L. J. W.; Ben-David Y.; Milstein D. Activation of Nitriles by Metal Ligand Cooperation. Reversible Formation of Ketimido- and Enamido-Rhenium PNP Pincer Complexes and Relevance to Catalytic Design. J. Am. Chem. Soc. 2013, 135, 17004–17018. 10.1021/ja4071859. [DOI] [PubMed] [Google Scholar]
- Nerush A.; Vogt M.; Gellrich U.; Leitus G.; Ben-David Y.; Milstein D. Template Catalysis by Metal-Ligand Cooperation. C-C Bond Formation via Conjugate Addition of Non-activated Nitriles under Mild, Base-free Conditions Catalyzed by a Manganese Pincer Complex. J. Am. Chem. Soc. 2016, 138, 6985–6997. 10.1021/jacs.5b13208. [DOI] [PubMed] [Google Scholar]
- Anaby A.; Butschke B.; Ben-David Y.; Shimon L. J. W.; Leitus G.; Feller M.; Milstein D. B–H Bond Cleavage via Metal–Ligand Cooperation by Dearomatized Ruthenium Pincer Complexes. Organometallics 2014, 33, 3716–3726. 10.1021/om500311a. [DOI] [Google Scholar]
- Zhang J.; Leitus G.; Ben-David Y.; Milstein D. Efficient Homogeneous Catalytic Hydrogenation of Esters to Alcohols. Angew. Chem., Int. Ed. 2006, 45, 1113–1115. 10.1002/anie.200503771. [DOI] [PubMed] [Google Scholar]
- Schwartsburd L.; Iron M. A.; Konstantinovski L.; Ben-Ari E.; Milstein D. A Dearomatized Anionic PNP Pincer Rhodium Complex: C–H and H–H Bond Activation by Metal–Ligand Cooperation and Inhibition by Dinitrogen. Organometallics 2011, 30, 2721–2729. 10.1021/om200104b. [DOI] [Google Scholar]
- Langer R.; Leitus G.; Ben-David Y.; Milstein D. Efficient Hydrogenation of Ketones Catalyzed by an Iron Pincer Complex. Angew. Chem., Int. Ed. 2011, 50, 2120–2124. 10.1002/anie.201007406. [DOI] [PubMed] [Google Scholar]
- Feller M.; Ben-Ari E.; Diskin-Posner Y.; Carmieli R.; Weiner L.; Milstein D. O2 Activation by Metal–Ligand Cooperation with IrI PNP Pincer Complexes. J. Am. Chem. Soc. 2015, 137, 4634–4637. 10.1021/jacs.5b01585. [DOI] [PubMed] [Google Scholar]
- Ben-Ari E.; Leitus G.; Shimon L. J. W.; Milstein D. Metal–Ligand Cooperation in C–H and H2 Activation by an Electron-Rich PNP Ir(I) System: Facile Ligand Dearomatization–Aromatization as Key Steps. J. Am. Chem. Soc. 2006, 128, 15390–15391. 10.1021/ja066411i. [DOI] [PubMed] [Google Scholar]
- Schwartsburd L.; Iron M. A.; Konstantinovski L.; Diskin-Posner Y.; Leitus G.; Shimon L. J. W.; Milstein D. Synthesis and Reactivity of an Iridium(I) Acetonyl PNP Complex. Experimental and Computational Study of Metal–Ligand Cooperation in H–H and C–H Bond Activation via Reversible Ligand Dearomatization. Organometallics 2010, 29, 3817–3827. 10.1021/om1004435. [DOI] [Google Scholar]
- Liu Q.; Wu L.; Jackstell R.; Beller M. Using Carbon Dioxide as a Building Block in Organic Synthesis. Nat. Commun. 2015, 6, 5933. 10.1038/ncomms6933. [DOI] [PubMed] [Google Scholar]
- Kondratenko E. V.; Mul G.; Baltrusaitis J.; Larrazabal G. O.; Perez-Ramirez J. Status and Perspectives of CO2 Conversion into Fuels and Chemicals by Catalytic, Photocatalytic and Electrocatalytic Processes. Energy Environ. Sci. 2013, 6, 3112–3135. 10.1039/c3ee41272e. [DOI] [Google Scholar]
- Rivada-Wheelaghan O.; Dauth A.; Leitus G.; Diskin-Posner Y.; Milstein D. Synthesis and Reactivity of Iron Complexes with a New Pyrazine-Based Pincer Ligand, and Application in Catalytic Low-Pressure Hydrogenation of Carbon Dioxide. Inorg. Chem. 2015, 54, 4526–4538. 10.1021/acs.inorgchem.5b00366. [DOI] [PubMed] [Google Scholar]
- Vogt M.; Gargir M.; Iron M. A.; Diskin-Posner Y.; Ben-David Y.; Milstein D. A New Mode of Activation of CO2 by Metal–Ligand Cooperation with Reversible C–C and M–O Bond Formation at Ambient Temperature. Chem. - Eur. J. 2012, 18, 9194–9197. 10.1002/chem.201201730. [DOI] [PubMed] [Google Scholar]
- Huff C. A.; Kampf J. W.; Sanford M. S. Role of a Noninnocent Pincer Ligand in the Activation of CO2 at (PNN)Ru(H)(CO). Organometallics 2012, 31, 4643–4645. 10.1021/om300403b. [DOI] [Google Scholar]
- Vogt M.; Nerush A.; Diskin-Posner Y.; Ben-David Y.; Milstein D. Reversible CO2 Binding Triggered by Metal-Ligand Cooperation in a Rhenium(I) PNP Pincer-Type Complex and the Reaction with Dihydrogen. Chem. Sci. 2014, 5, 2043–2051. 10.1039/C4SC00130C. [DOI] [Google Scholar]
- Vogt M.; Rivada-Wheelaghan O.; Iron M. A.; Leitus G.; Diskin-Posner Y.; Shimon L. J. W.; Ben-David Y.; Milstein D. Anionic Nickel(II) Complexes with Doubly Deprotonated PNP Pincer-Type Ligands and Their Reactivity toward CO2. Organometallics 2013, 32, 300–308. 10.1021/om3010838. [DOI] [Google Scholar]
- Feller M.; Gellrich U.; Anaby A.; Diskin-Posner Y.; Milstein D. Reductive Cleavage of CO2 by Metal–Ligand-Cooperation Mediated by an Iridium Pincer Complex. J. Am. Chem. Soc. 2016, 138, 6445–6454. 10.1021/jacs.6b00202. [DOI] [PubMed] [Google Scholar]
- Anaby A.; Feller M.; Ben-David Y.; Leitus G.; Diskin-Posner Y.; Shimon L. J. W.; Milstein D. Bottom-Up Construction of a CO2-Based Cycle for the Photocarbonylation of Benzene, Promoted by a Rhodium(I) Pincer Complex. J. Am. Chem. Soc. 2016, 138, 9941–9950. 10.1021/jacs.6b05128. [DOI] [PubMed] [Google Scholar]
- Paparo A.; Okuda J. Carbon Dioxide Complexes: Bonding Modes and Synthetic Methods. Coord. Chem. Rev. 2017, 334, 136–149. 10.1016/j.ccr.2016.06.005. [DOI] [Google Scholar]
- Guo C.-X.; Yu B.; Ma R.; He L.-N. Metal-promoted Carboxylation of Alkynes/allenes with Carbon Dioxide. Curr. Green Chem. 2015, 2, 14–25. 10.2174/221334610201150209163930. [DOI] [Google Scholar]
- Burgess S. A.; Kendall A. J.; Tyler D. R.; Linehan J. C.; Appel A. M. Hydrogenation of CO2 in Water Using a Bis(diphosphine) Ni–H Complex. ACS Catal. 2017, 7, 3089–3096. 10.1021/acscatal.7b00350. [DOI] [Google Scholar]
- Inoue Y.; Izumida H.; Sasaki Y.; Hashimoto H. Catalytic Fixation of Carbon Dioxide to Formic Acid by Transition-Metal Complexes Under Mild Conditions. Chem. Lett. 1976, 5, 863–864. 10.1246/cl.1976.863. [DOI] [Google Scholar]
- Chakraborty S.; Patel Y. J.; Krause J. A.; Guan H. Catalytic Properties of Nickel Bis(phosphinite) Pincer Complexes in the Reduction of CO2 to Methanol Derivatives. Polyhedron 2012, 32, 30–34. 10.1016/j.poly.2011.04.030. [DOI] [Google Scholar]
- Chakraborty S.; Zhang J.; Patel Y. J.; Krause J. A.; Guan H. Pincer-Ligated Nickel Hydridoborate Complexes: the Dormant Species in Catalytic Reduction of Carbon Dioxide with Boranes. Inorg. Chem. 2013, 52, 37–47. 10.1021/ic300587b. [DOI] [PubMed] [Google Scholar]
- Cao T.; Ma S. Nickel-Catalyzed Alkyl-Zincation and Carboxylation of Diynes. Org. Chem. Front. 2016, 3, 1711–1715. 10.1039/C6QO00484A. [DOI] [Google Scholar]
- Diccianni J. B.; Heitmann T.; Diao T. Nickel-Catalyzed Reductive Cycloisomerization of Enynes with CO2. J. Org. Chem. 2017, 82, 6895–6903. 10.1021/acs.joc.7b01034. [DOI] [PubMed] [Google Scholar]
- Cao T.; Yang Z.; Ma S. Selectivities in Nickel-Catalyzed Hydrocarboxylation of Enynes with Carbon Dioxide. ACS Catal. 2017, 7, 4504–4508. 10.1021/acscatal.7b00556. [DOI] [Google Scholar]
- Cao T.; Ma S. Highly Stereo- and Regioselective Hydrocarboxylation of Diynes with Carbon Dioxide. Org. Lett. 2016, 18, 1510–1513. 10.1021/acs.orglett.6b00028. [DOI] [PubMed] [Google Scholar]
- Makida Y.; Marelli E.; Slawin A. M. Z.; Nolan S. P. Nickel-Catalyzed Carboxylation of Organoboronates. Chem. Commun. 2014, 50, 8010–8013. 10.1039/c4cc03650f. [DOI] [PubMed] [Google Scholar]
- Correa A.; León T.; Martin R. Ni-Catalyzed Carboxylation of C(sp2)– and C(sp3)–O Bonds with CO2. J. Am. Chem. Soc. 2014, 136, 1062–1069. 10.1021/ja410883p. [DOI] [PubMed] [Google Scholar]
- Juliá-Hernández F.; Gaydou M.; Serrano E.; van Gemmeren M.; Martin R. Ni and Fe Catalyzed Carboxylation of Unsaturated Hydrocarbons with CO2. Top. Curr. Chem. 2016, 374, 1–38. 10.1007/s41061-016-0045-z. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Riduan S. N. Catalytic Hydrocarboxylation of Alkenes and Alkynes with CO2. Angew. Chem., Int. Ed. 2011, 50, 6210–6212. 10.1002/anie.201101341. [DOI] [PubMed] [Google Scholar]
- Correa A.; Martín R. Metal-Catalyzed Carboxylation of Organometallic Reagents with Carbon Dioxide. Angew. Chem., Int. Ed. 2009, 48, 6201–6204. 10.1002/anie.200900667. [DOI] [PubMed] [Google Scholar]
- Ochiai H.; Jang M.; Hirano K.; Yorimitsu H.; Oshima K. Nickel-Catalyzed Carboxylation of Organozinc Reagents with CO2. Org. Lett. 2008, 10, 2681–2683. 10.1021/ol800764u. [DOI] [PubMed] [Google Scholar]
- Mori M. Regio- and Stereoselective Synthesis of Tri- and Tetrasubstituted Alkenes by Introduction of CO2 and Alkylzinc Reagents into Alkynes. Eur. J. Org. Chem. 2007, 2007, 4981–4993. 10.1002/ejoc.200700196. [DOI] [Google Scholar]
- Aresta M.; Nobile C. F.; Albano V. G.; Forni E.; Manassero M. New Nickel-Carbon Dioxide Complex: Synthesis, Properties, and Crystallographic Characterization of (Carbon dioxide)-bis(tricyclohexylphosphine)nickel. J. Chem. Soc., Chem. Commun. 1975, 0, 636–637. 10.1039/C39750000636. [DOI] [Google Scholar]
- Aresta M.; Nobile C. F. (Carbon dioxide)bis(trialkylphosphine)nickel complexes. J. Chem. Soc., Dalton Trans. 1977, 7, 708–711. 10.1039/dt9770000708. [DOI] [Google Scholar]
- Anderson J. S.; Iluc V. M.; Hillhouse G. L. Reactions of CO2 and CS2 with 1,2-Bis(di-tert-butylphosphino)ethane Complexes of Nickel(0) and Nickel(I). Inorg. Chem. 2010, 49, 10203–10207. 10.1021/ic101652e. [DOI] [PubMed] [Google Scholar]
- Kim Y.-E.; Kim J.; Lee Y. Formation of a Nickel Carbon Dioxide Adduct and its Transformation Mediated by a Lewis acid. Chem. Commun. 2014, 50, 11458–11461. 10.1039/C4CC04800H. [DOI] [PubMed] [Google Scholar]
- Lee C. H.; Laitar D. S.; Mueller P.; Sadighi J. P. Generation of a Doubly Bridging CO2 Ligand and Deoxygenation of CO2 by an (NHC)Ni(0) Complex. J. Am. Chem. Soc. 2007, 129, 13802–13803. 10.1021/ja075630g. [DOI] [PubMed] [Google Scholar]
- Chiou T.-W.; Tseng Y.-M.; Lu T.-T.; Weng T.-C.; Sokaras D.; Ho W.-C.; Kuo T.-S.; Jang L.-Y.; Lee J.-F.; Liaw W.-F. [NiIII(OMe)]-Mediated Reductive Activation of CO2 Affording a Ni(κ1-OCO) Complex. Chem. Sci. 2016, 7, 3640–3644. 10.1039/C5SC04652A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo C.; Lee Y. Carbon Dioxide Binding at a Ni/Fe Center: Synthesis and Characterization of Ni(η1-CO2-κC) and Ni-μ-CO2-κC:κ2O,O′-Fe. Chem. Sci. 2017, 8, 600–605. 10.1039/C6SC03450K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vlugt J. I.; Lutz M.; Pidko E. A.; Vogt D.; Spek A. L. Cationic and Neutral NiII Complexes Containing a Non-Innocent PNP Ligand: Formation of Alkyl and Thiolate Species. Dalton Trans. 2009, 6, 1016–1023. 10.1039/B814806F. [DOI] [PubMed] [Google Scholar]
- Takeuchi K.; Taguchi H.-o.; Tanigawa I.; Tsujimoto S.; Matsuo T.; Tanaka H.; Yoshizawa K.; Ozawa F. A Square-Planar Complex of Platinum(0). Angew. Chem., Int. Ed. 2016, 55, 15347–15350. 10.1002/anie.201609515. [DOI] [PubMed] [Google Scholar]
- Sahoo D.; Yoo C.; Lee Y. Direct CO2 Addition to a Ni(0)–CO Species Allows the Selective Generation of a Nickel(II) Carboxylate with Expulsion of CO. J. Am. Chem. Soc. 2018, 140, 2179–2185. 10.1021/jacs.7b11074. [DOI] [PubMed] [Google Scholar]
- Calabrese J. C.; Herskovitz T.; Kinney J. B. Carbon Dioxide Coordination Chemistry. 5. The Preparation and Structure of the Rhodium Complex Rh(η1-CO2)(Cl)(diars)2. J. Am. Chem. Soc. 1983, 105, 5914–5915. 10.1021/ja00356a033. [DOI] [Google Scholar]
- Tanaka H.; Nagao H.; Peng S. M.; Tanaka K. Crystal Structure of cis-(carbonyl)(η1-carbon dioxide)bis(2,2′-bipyridyl)ruthenium, an Active Species in Catalytic Carbon Dioxide Reduction Affording Carbon Monoxide and HCOO. Organometallics 1992, 11, 1450–1451. 10.1021/om00040a010. [DOI] [Google Scholar]
- Tanaka H.; Tzeng B. C.; Nagao H.; Peng S. M.; Tanaka K. Comparative Study on Crystal Structures of Ruthenium Bipyridine Carbonyl Complexes [Ru(bpy)2(CO)2](PF6)2, [Ru(bpy)2(CO)(C(O)OCH3)]B(C6H5)4·CH3CN, and [Ru(bpy)2(CO)(η1-CO2)]·3H2O (bpy = 2,2′-bipyridyl). Inorg. Chem. 1993, 32, 1508–1512. 10.1021/ic00060a029. [DOI] [Google Scholar]
- Herskovitz T. Adducts of Carbon Dioxide with Iridium(I) Complexes. J. Am. Chem. Soc. 1977, 99, 2391–2392. 10.1021/ja00449a087. [DOI] [Google Scholar]
- Fachinetti G.; Floriani C.; Zanazzi P. F. Bifunctional Activation of Carbon Dioxide. Synthesis and Structure of a Reversible Carbon Dioxide Carrier. J. Am. Chem. Soc. 1978, 100, 7405–7407. 10.1021/ja00491a045. [DOI] [Google Scholar]
- Gambarotta S.; Arena F.; Floriani C.; Zanazzi P. F. Carbon Dioxide Fixation: Bifunctional Complexes Containing Acidic and Basic Sites Working as Reversible Carriers. J. Am. Chem. Soc. 1982, 104, 5082–5092. 10.1021/ja00383a015. [DOI] [Google Scholar]
- Feller M.; Diskin-Posner Y.; Leitus G.; Shimon L. J. W.; Milstein D. Direct Observation of Reductive Elimination of MeX (X = Cl, Br, I) from RhIII Complexes: Mechanistic Insight and the Importance of Sterics. J. Am. Chem. Soc. 2013, 135, 11040–11047. 10.1021/ja401852c. [DOI] [PubMed] [Google Scholar]
- Langer J.; Hamza A.; Pápai I. RuBisCO-Inspired CO2 Activation and Transformation by an Iridium(I) Complex. Angew. Chem., Int. Ed. 2018, 57, 2455–2458. 10.1002/anie.201712893. [DOI] [PubMed] [Google Scholar]
- Garrou P. E. Transition-Metal-Mediated Phosphorus-Carbon Bond Cleavage and its Relevance to Homogeneous Catalyst Deactivation. Chem. Rev. 1985, 85, 171–185. 10.1021/cr00067a001. [DOI] [Google Scholar]
- Cao J.; Huang X.; Wu L. Nickel-Catalyzed Manipulation of Tertiary Phosphines via Highly Selective C-P Bond Cleavage. Chem. Commun. 2013, 49, 7747–7749. 10.1039/c3cc43640c. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Gan Z.; Su B.; Li J.; Duan Z.; Mathey F. Intramolecular, Pd/Cu-Co-catalyzed P–C Bond Cleavage and Addition onto an Alkyne: A Route to Benzophospholes. Org. Lett. 2015, 17, 5722–5724. 10.1021/acs.orglett.5b02926. [DOI] [PubMed] [Google Scholar]
- Hollatz C.; Schier A.; Schmidbaur H. Neutral Gold(I) Complexes with Mixed Phosphorus Ligands. Chem. Ber. 1997, 130, 1333–1338. 10.1002/cber.19971300925. [DOI] [Google Scholar]
- Sylvain R.; Vendier L.; Bijani C.; Santoro A.; Puntoriero F.; Campagna S.; Sutra P.; Igau A. Evidence of the Unprecedented Conversion of Intermolecular Proton to Water Bridging of two Phosphoryl Ruthenium Complexes. New J. Chem. 2013, 37, 3543–3548. 10.1039/c3nj00522d. [DOI] [Google Scholar]
- Weismann J.; Scharf L. T.; Gessner V. H. Cooperative P–H Bond Activation with Ruthenium and Iridium Carbene Complexes. Organometallics 2016, 35, 2507–2515. 10.1021/acs.organomet.6b00408. [DOI] [Google Scholar]
- Li P.; Li Q.-S.; Xu F.-B.; Song H.-B.; Zhang Z.-Z. Chloro[N-(diphenylphosphino)pyridin-2-amine-κ2N1,P](diphenylphosphoryl)palladium(II). Acta Crystallogr., Sect. E: Struct. Rep. Online 2006, 62, m1825–m1826. 10.1107/S1600536806026055. [DOI] [Google Scholar]
- Kanada J.; Tanaka M. Efficient Addition Reaction of Dibutylphosphane Oxide with Alkynes: New Mechanistic Proposal Involving a Duo of Palladium and Brønsted Acid. Adv. Synth. Catal. 2011, 353, 890–896. 10.1002/adsc.201000758. [DOI] [Google Scholar]
- Zhang J.; Medley C. M.; Krause J. A.; Guan H. Mechanistic Insights into C–S Cross-Coupling Reactions Catalyzed by Nickel Bis(phosphinite) Pincer Complexes. Organometallics 2010, 29, 6393–6401. 10.1021/om100816d. [DOI] [Google Scholar]
- Salem H.; Shimon L. J. W.; Diskin-Posner Y.; Leitus G.; Ben-David Y.; Milstein D. Formation of Stable trans-Dihydride Ruthenium(II) and 16-Electron Ruthenium(0) Complexes Based on Phosphinite PONOP Pincer Ligands. Reactivity toward Water and Electrophiles. Organometallics 2009, 28, 4791–4806. 10.1021/om9004077. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.