Mitochondria have been increasingly recognized as a contributing factor to the pathogenesis of chronic obstructive pulmonary disease (COPD). Patients with COPD manifest chronic airway inflammation and progressive destruction of alveolar structures. Multiple factors are believed to be involved in the development of COPD, including increased levels of reactive oxygen species (ROS), protease–antiprotease imbalance, exaggerated inflammation, cell apoptosis/death, and accelerated cellular senescence. Changes in the mitochondrial respiratory chain complexes and ROS levels also have been implicated in the development of COPD. For example, airway smooth muscle cells cultured from patients with COPD have a reduced expression of complexes I, III, and V; increased mitochondrial ROS; and decreased membrane potential and ATP production (1).
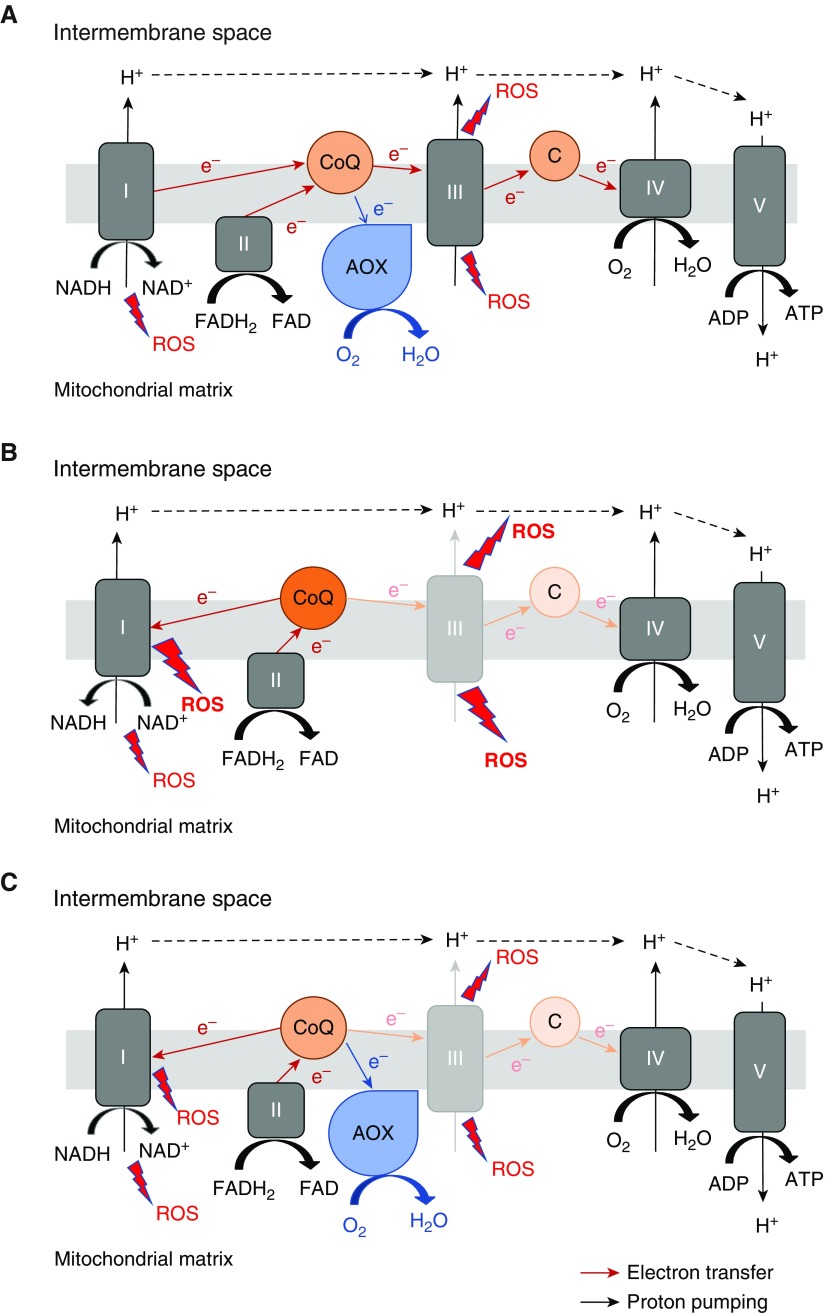
Mitochondria and NADPH oxidases have been implicated as major sites of ROS generation in response to chronic exposure to smoke leading to COPD (2). Mitochondria are major generators of superoxide, predominantly at complexes I and III. Unlike mammals, lower organisms and plants possess an alternative respiratory pathway, in addition to the cytochrome c oxidase-dependent respiratory pathway found in all eukaryotes. Alternative oxidase (AOX) is one of the proteins that can conduct an alternative respiration. It is a single protein that exists in the inner mitochondrial membrane and can transport electrons from ubiquinol to oxygen, allowing mitochondrial respiratory complexes III and IV to be bypassed when they are dysfunctional (Figure 1A). In essence, AOX restores the electron transfer function of complexes III and IV without contributing to proton pumping, and therefore does not generate a proton motive force for ATP synthesis. Because AOX has low affinity for its substrate ubiquinol, as compared with complex III, it does not accept electrons when complex III and the downstream cytochrome pathway are functionally intact (3, 4). AOX transports electrons from ubiquinol to oxygen only when ubiquinol is overreduced, for example, when complex III or IV is dysfunctional, consequently decreasing mitochondrial ROS levels (5). Because of this unique feature, AOX has been suggested as a potential therapeutic modality as well as a useful research tool to study the physiological role of the mitochondrial electron transport chain in isolation from its role in ATP synthesis. AOX has been successfully expressed in human cultured cells (6, 7). Furthermore, AOX can be safely expressed in mice in vivo (8, 9) without disrupting normal physiology. AOX does not seem to participate in electron transfer in the presence of an active complex III function, even though the protein is expressed and enzymatically functional. Thus, AOX expression in vivo, at baseline without stress, has little effect on endogenous electron transport chain activity, including generation of a proton gradient by complex III or IV, or the global metabolome. Indeed, the use of ADP associated with oxygen consumption was not decreased in AOX mice compared with wild-type mice (8). However, under conditions of stress, AOX becomes functionally active. Therefore, AOX-expressing cells produce less ROS when exposed to a respiratory complex inhibitor such as antimycin A (a complex III inhibitor), and AOX-expressing mice are protected from cyanide (a complex IV inhibitor) toxicity (8, 9).
Figure 1.
Schematic diagram of the respiratory chain, illustrating the effect of alternative oxidase (AOX). (A) AOX accepts electrons from reduced ubiquinone (CoQ) and reduces oxygen to water, thus bypassing complexes III and IV. At baseline without stress, AOX expression has little effect on the activities of the endogenous respiratory chain and thus the physiological level of reactive oxygen species (ROS). C = cytochrome c. (B) When complex III is dysfunctional, complex III cannot accept electrons from CoQ efficiently, and therefore the CoQ pool becomes overreduced. Reverse electron transport occurs when electrons from overreduced CoQ are transferred back to complex I. This process generates a significant amount of superoxide. (C) AOX can reoxidize the CoQ pool and prevent electrons from being transferred back to complex I, thus decreasing reverse electron transport–associated ROS production. Also, electrons from the ubiquinone pool are transferred to AOX rather than to complex III, thus decreasing the superoxide production from complex III. FAD = flavin adenine dinucleotide; FADH2 = FAD reduced; NAD+ = nicotinamide adenine dinucleotide; NADH = NAD+ reduced.
In a study presented in this issue of the Journal, Giordano and colleagues (pp. 515–522) used AOX-expressing mice to examine whether AOX expression would decrease mitochondrial ROS production and lung pathology in a smoke-induced model of emphysema (10). With chronic exposure to cigarette smoke (CS), the mice that globally expressed AOX developed less severe emphysema than wild-type mice did, as measured by lung hysteresis and mean chord length. Using immortalized mouse embryonic fibroblasts in vitro, they showed that AOX expression reduced ROS production and cell death induced by CS condensate (CSC). On the other hand, with acute exposure to CS, there was no difference in the number of macrophages and neutrophils in the BAL of wild-type and AOX mice. The authors conclude that expression of AOX attenuates CS-induced lung emphysema, likely by protecting nonimmune alveolar cells from CS-induced cell death through decreased mitochondrial ROS production.
At first glance, the data seem to suggest that complex III is the main culprit site for production of ROS during chronic smoke exposure, causing CS-induced emphysema. With AOX expression, electrons from the ubiquinone pool are transferred to AOX rather than to complex III (Figure 1). This would decrease superoxide generation at complex III, contributing to the overall reduction of mitochondrial ROS generation induced by CS. This suggests that complex III–derived ROS would represent a target for decreasing CS-induced lung destruction. However, by accepting electrons from ubiquinol, AOX quickly generates ubiquinone, which can continue to accept electrons from complex I or II, and in turn prevent complex I from generating superoxide by reverse electron transport (RET), another dominant mechanism for superoxide generation within the mitochondrial respiratory chain (11). Therefore, it is possible that the attenuation of CS-induced emphysema by AOX expression could also be due to decreased RET at complex I (Figures 1B and 1C). The dose-dependent decrease in complex I–driven respiration by CSC, which was partially improved with AOX, may be a reflection of the occurrence of RET during CS exposure, although we do not have direct evidence for this. Hence, the current study does not tell us which is the dominant site of mitochondrial superoxide production during chronic smoke exposure leading to the development of emphysema. However, the study does provide genetic evidence that mitochondria are linked to chronic smoke–induced pathology. Previous studies suggested a strong correlation between mitochondrial function and ROS and the development of COPD, but the causality was difficult to establish due to a lack of research tools.
Multiple cell types are known to interact during chronic smoke–induced injury, including immune, epithelial, mesenchymal, and endothelial cells. A limitation of the study, as pointed out by the authors, is that the mice express AOX globally, and thus it is not clear which cell types are involved in the mitochondrial ROS–dependent pathology. Future studies should use conditional expression of AOX in different cell types to gain mechanistic insight into the cell types that drive chronic smoke–induced pathology.
Overall, the AOX mice they used will be a useful research tool to establish causality between mitochondrial respiratory chain–dependent superoxide production and other lung diseases, including fibrosis, acute lung injury, and pulmonary hypertension. A tempting question is whether nebulized delivery of AOX through gene therapy would ameliorate deleterious effects caused by smoke exposure, or in other diseases characterized by mitochondrial dysfunction.
Supplementary Material
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wiegman CH, Michaeloudes C, Haji G, Narang P, Clarke CJ, Russell KE, et al. COPDMAP. Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2015;136:769–780. doi: 10.1016/j.jaci.2015.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirkham PA, Barnes PJ. Oxidative stress in COPD. Chest. 2013;144:266–273. doi: 10.1378/chest.12-2664. [DOI] [PubMed] [Google Scholar]
- 3.Bahr JT, Bonner WD., Jr Cyanide-insensitive respiration. I. The steady states of skunk cabbage spadix and bean hypocotyl mitochondria. J Biol Chem. 1973;248:3441–3445. [PubMed] [Google Scholar]
- 4.Bahr JT, Bonner WD., Jr Cyanide-insensitive respiration. II. Control of the alternate pathway. J Biol Chem. 1973;248:3446–3450. [PubMed] [Google Scholar]
- 5.Maxwell DP, Wang Y, McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA. 1999;96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martínez-Reyes I, Diebold LP, Kong H, Schieber M, Huang H, Hensley CT, et al. TCA cycle and mitochondrial membrane potential are necessary for diverse biological functions. Mol Cell. 2016;61:199–209. doi: 10.1016/j.molcel.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perales-Clemente E, Bayona-Bafaluy MP, Pérez-Martos A, Barrientos A, Fernández-Silva P, Enriquez JA. Restoration of electron transport without proton pumping in mammalian mitochondria. Proc Natl Acad Sci USA. 2008;105:18735–18739. doi: 10.1073/pnas.0810518105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Khoury R, Dufour E, Rak M, Ramanantsoa N, Grandchamp N, Csaba Z, et al. Alternative oxidase expression in the mouse enables bypassing cytochrome c oxidase blockade and limits mitochondrial ROS overproduction. PLoS Genet. 2013;9:e1003182. doi: 10.1371/journal.pgen.1003182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szibor M, Dhandapani PK, Dufour E, Holmström KM, Zhuang Y, Salwig I, et al. German Mouse Clinic Consortium. Broad AOX expression in a genetically tractable mouse model does not disturb normal physiology. Dis Model Mech. 2017;10:163–171. doi: 10.1242/dmm.027839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giordano L, Farnham A, Dhandapani PK, Salminen L, Bhaskaran J, Voswinckel R, et al. Alternative oxidase attenuates cigarette smoke–induced lung dysfunction and tissue damage. Am J Respir Cell Mol Biol. 2019;60:515–522. doi: 10.1165/rcmb.2018-0261OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scialò F, Sriram A, Fernández-Ayala D, Gubina N, Lõhmus M, Nelson G, et al. Mitochondrial ros produced via reverse electron transport extend animal lifespan. Cell Metab. 2016;23:725–734. doi: 10.1016/j.cmet.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.