Abstract
Background: Depression represents a serious public health concern, imposing a high burden, both in epidemiological and clinical terms. Crocus sativus (Saffron) is a herbal remedy that has anti-cancer, anti-oxidant, anti-inflammatory and anti-platelet properties. However, the exact mechanisms of Saffron in treating depression are not yet clear. This study was conducted to evaluate the effectiveness of Saffron versus placebo and Fluoxetine in the treatment of depressed patients.
Methods: Different bibliographic thesauri, namely the Cochrane Library, Scopus, PubMed/MEDLINE, Centre for Reviews and Dissemination (CRD), EMBASE, and ISI/Web of Science (WoS) were searched up to May 2018. Effect sizes were computed as Standardized Mean Differences (SMD) with their 95% confidence interval (CI). To evaluate the heterogeneity among the studies, I2 test was carried out.
Results: Eight studies were included. The SMD was −0.86 (95% CI: −1.73 to 0.00) concerning the comparison of Saffron with placebo. The SMD was found to be 0.11 (95% CI: −0.20 to 0.43) concerning the comparison of Saffron with Fluoxetine. In both sensitivity analyses, the results did not statistically change, confirming the stability of the findings.
Conclusion: The findings of this study showed that Saffron administration was well comparable with Fluoxetine and placebo.
Keywords: systematic review, meta-analysis, Saffron, Fluoxetine, depression
Introduction
Depression represents a serious public health concern, imposing a high burden, both in epidemiological and clinical terms.1 This common mental disorder causes the affected person to suffer from sleep disorders, feeling tired, losing appetite, feeling grief, having guilty feelings, experiencing weakness in concentration, sadness, loss of interest in doing routine work and usual activities, among others. It can lead to suicide in extreme cases.2,3
According to the World Health Organization (WHO), over 300 million people worldwide are suffering from severe depression and 800,000 deaths occur due to depression-induced suicide annually.4 The relationships between the prevalence of depression and gender, social, economic, and demographic factors have been intensively studied in various studies.5,6
The world’s financial resources in the health sector are limited. Depression affects the working conditions of the individual, resulting in frequent absences, reduced productivity, and absenteeism. At the same time, the disease causes people to consume more health care resources.7,8 It is estimated that by 2020, after cardiovascular disease, depression will be the second most common cause of Disability-Adjusted Life Years (DALYs) in the world.9 Timely diagnosis and treatment of patients with depression can play an important role in reducing disability, increasing survival and quality of life in patients, and contribute to saving the financial resources of the health sector of each country.
Different drugs can be used to treat depression, including Fluoxetine. Studies have shown its clinical effectiveness in the management of depressed patients.10,11 On the other hand, herbal remedies, being safer and with less side-effects, can be considered as a valid alternative for the treatment of depression.12–14
More than 20 herbal remedies have been investigated as potential anti-depressant drugs.15,16 Crocus sativus (Saffron) is one of these herbal remedies that has anti-cancer, anti-oxidant, anti-inflammatory, and anti-platelet properties.17 Saffron can be extracted from the dried elongated stigmas and styles of saffron, a blue-purple flowering plant of the Crocus genus, belonging to the Iridaceae family, which, in some studies, has been used to treat depressed patients.18,19
However, the exact mechanisms of Saffron in treating depression are not yet clear. Several studies have been done to evaluate the effect of Saffron in the treatment of patients with depression. However, sometimes some contrasting findings have been reported. Therefore, using meta-analytic techniques, which enable to pool different studies together, this investigation was conducted to evaluate the effectiveness of Saffron versus placebo and Fluoxetine in the treatment of depressed patients.
Material and methods
Literature search
The results of the current review were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (10). Different bibliographic thesauri, namely the Cochrane Library, Scopus, PubMed/MEDLINE, Centre for Reviews and Dissemination (CRD), EMBASE, and ISI/Web of Science (WOS) were searched by two authors up to May 2018. The Clinical Trial, the Trial Register, as well as international congresses on depression such as the International Depressive Disorder and Anxiety Disorders and Depression were also searched.
Also, since most Saffron-related studies are performed in Iran, Iranian databases like SID, Magiran, and Irandoc were searched. Furthermore, to find relevant studies, reference lists of selected study lists were reviewed. Our search strategy was as follows: (Saffron OR “Crocus sativus”) AND (Depression OR “Major Depression Disorder” OR MDD) AND (Fluoxetine OR Placebo)
No language, location, and time restrictions were applied. After the search was completed, all the articles found were included in the Endnote X7 software and duplicates were deleted. Then, the titles and abstracts of articles were examined based on inclusion and exclusion criteria and finally the main studies were selected. The titles and abstracts of the articles were independently reviewed by two of the authors, and in case of disagreement, discrepancies were resolved through discussion.
Inclusion and exclusion criteria
According to the Patients/Intervention/Comparator/Outcome/Study design (PICOS) criteria, inclusion criteria were the following: P (studies performed in humans and recruiting patients with an official diagnosis of depression, established according to the Diagnostic and Statistical Manual of Mental Disorders – DSM – criteria; any type of depression was considered, without any restriction with regards to the severity – mild or severe depression – or the kind of patient affected – youth or post-partum depression); I (studies in which Saffron was utilized in one arm and placebo or Fluoxetine were used in the other arm); C (Saffron versus placebo or Fluoxetine); O (effect on depression); and, S (studies designed as randomized clinical trial or RCTs).
Studies were excluded if: performed in animals or in non-depressed patients; using saffron in combination with another drug or compound; studies designed as review papers, letters to the editor, case-report and case-series; and, studies results of which were not clear or not sufficiently detailed.
Quality assessment of studies included
Quality assessment of studies included was carried out according to the Cochrane handbook.11
Data extraction
After finding the final studies to be included, a data extraction form was developed based on the information of the articles and the data of the articles were extracted according to the designed form. The title of the article, the name of the author of the first article, the year of the study, the year of the publication of the article, the number of participants, the measured outcomes, the reported complications, the place where the study was conducted, the date of commencement and completion of the study were extracted from each study. The studies were assessed by two authors. Any potential disagreement was resolved through discussion or involving a third author as the referee.
Statistical analysis
Effect sizes were computed as Standardized Mean Differences (SMD) using a random-effects model with their 95% confidence interval (CI).20 The sensitivity analysis was performed to check the stability and reliability of results. To evaluate the heterogeneity of the studies, I2 test was carried out.21 P-values less than 0.05 were considered as significant values. Due to the fact that the number of studies entered was less than 10, there was no possibility to check the publication bias. R environment (version 3.4.0) was used to analyze the data.
Results
Selected studies
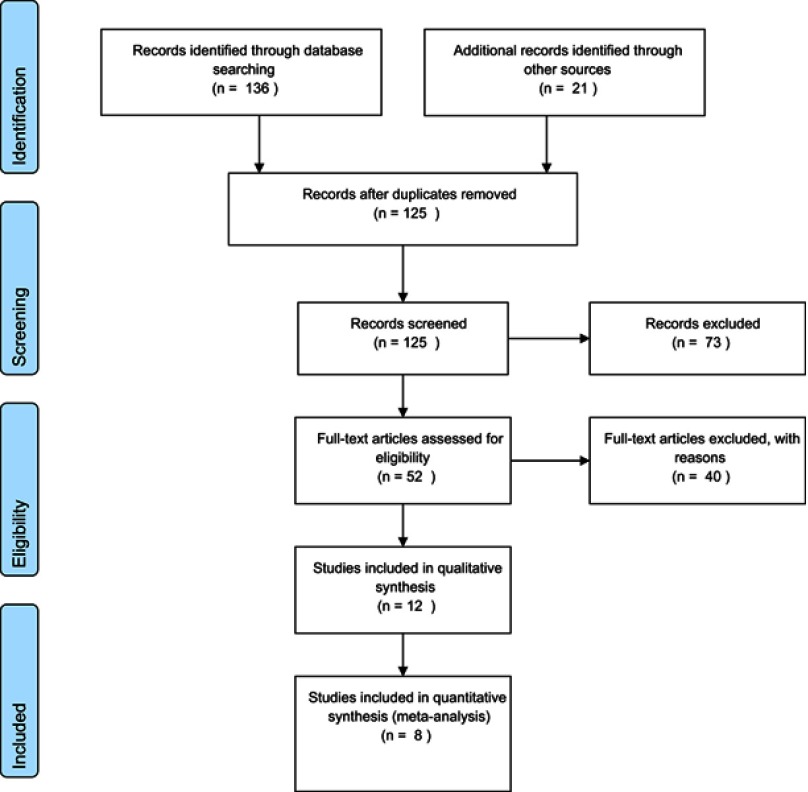
The initial search yielded a pool of 157 studies, which were downloaded; after removing duplicates, 125 remained. At this stage, the title and the abstract of each study were assessed and 73 unrelated studies were discarded. The remaining 52 studies were in-depth reviewed based on the full text. Finally, according to the inclusion/exclusion criteria, 8 studies were selected. The selection process is shown in Figure S1.
Among the included studies, 4 of them compared Saffron and placebo, while four other studies compared Saffron and Fluoxetine 20 or 40 mg.22–29 The main characteristics of these studies are presented in Table 1.
Table 1.
Main characteristics of studies included in the present systematic review and meta-analysis
| Study | Year | Country | Sex | Drugs | Duration | ||
|---|---|---|---|---|---|---|---|
| Female | Male | Experimental | Control | ||||
| Akhondzadeh et al22 | 2005 | Iran | 18 | 22 | Saffron | Placebo | 6 weeks |
| Moshiri et al24 | 2006 | Iran | 17 | 23 | Saffron | Placebo | 6 weeks |
| Akhondzadeh Basti et al26 | 2008 | Iran | 22 | 22 | Saffron | Placebo | 6 weeks |
| Mazidi et al28 | 2016 | Iran | 30 | 30 | Saffron | Placebo | 12 weeks |
| Noorbala et al23 | 2005 | Iran | 20 | 20 | Saffron 30 mg | Fluoxetine 40 mg | 6 weeks |
| Akhondzadeh Basti et al25 | 2007 | Iran | 21 | 19 | Saffron 30 mg | Fluoxetine 20 mg | 8 weeks |
| Shahmansouri et al27 | 2014 | Iran | 25 | 15 | Saffron 30 mg | Fluoxetine 40 mg | 6 weeks |
| Kashani et al29 | 2016 | Iran | 64 | - | Saffron 30 mg | Fluoxetine 40 mg | 6 weeks |
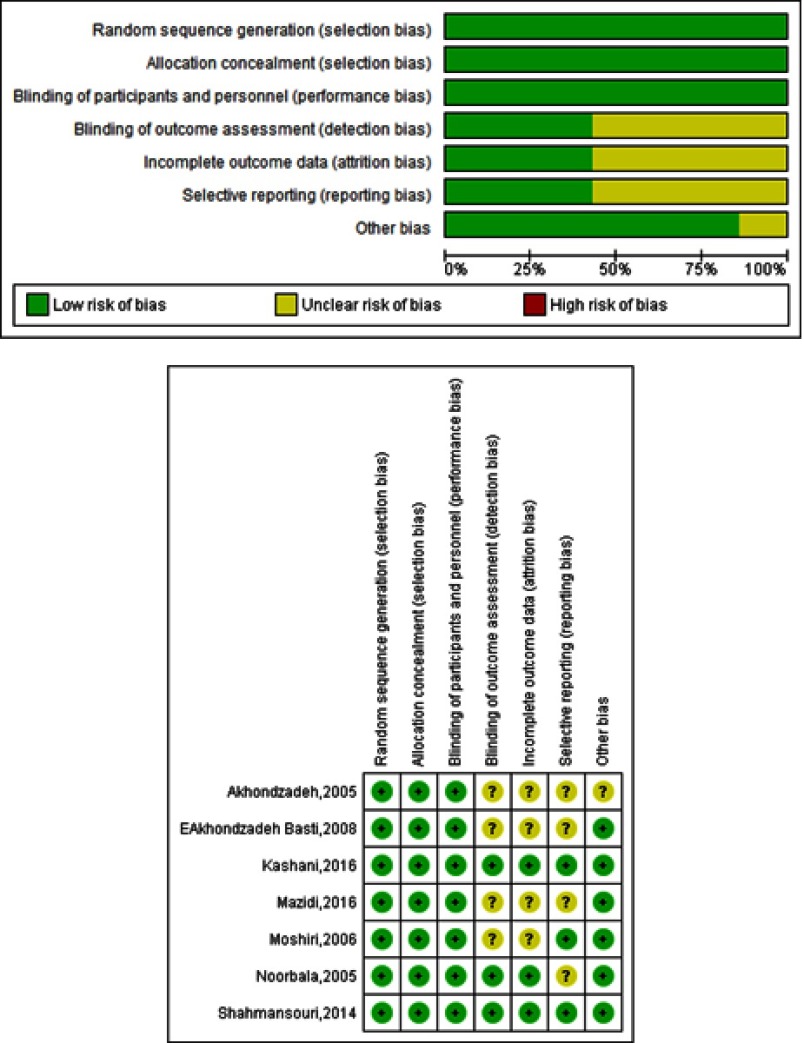
Quality of studies entered
The quality of the studies was evaluated using the Cochrane checklist (Figure S2).
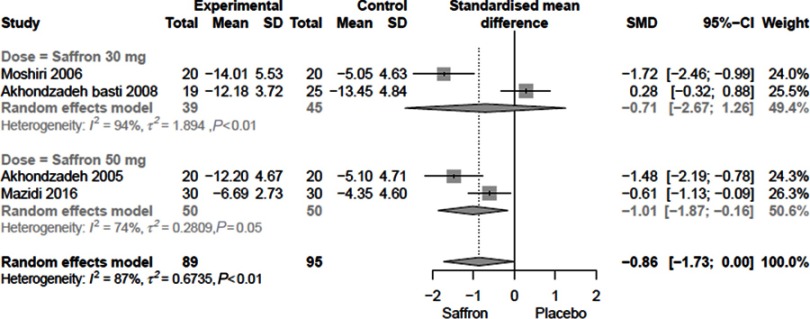
Comparison of Saffron with placebo
Figure 1 shows the effect of Saffron compared to placebo. Heterogeneity among studies was computed to be 87%. The SMD was −0.86 (95% CI: −1.73 to 0.00). Stratifying on the basis of saffron doses, SMDs were computed to be −0.71 (95% CI: −2.67 to 1.26) and −1.01 (95% CI: −1.87 to - 0.16).
Figure 1.
The overall SMD of Saffron compared to placebo.
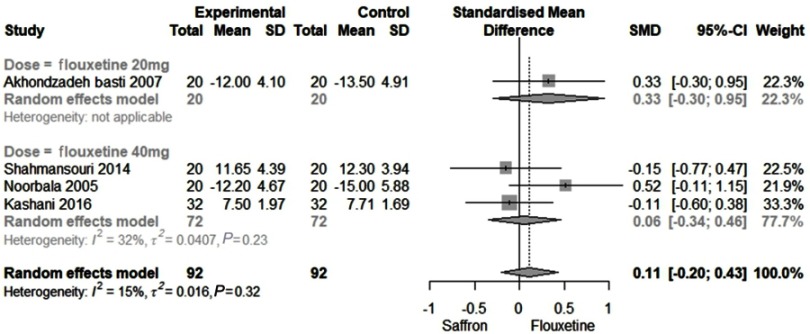
Comparison of Saffron with Fluoxetine
Figure 2 shows the efficacy of Saffron compared to Fluoxetine. Heterogeneity observed among studies was 15% (as such, a fixed model was used). The SMD was found to be 0.11 (95% CI: −0.20 to 0.43). When stratifying according to the doses (20 and 40 mg), SMDs were computed 0.33 (95% CI: −0.30 to 0.95) and 0.06 (95% CI: −0.34 to 0.46), respectively.
Figure 2.
The overall SMD of Saffron compared to Fluoxetine.
Sensitivity analysis
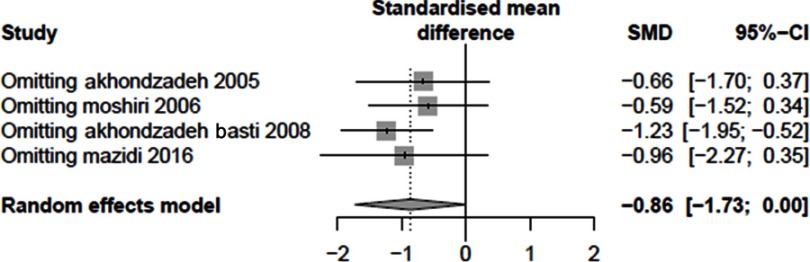
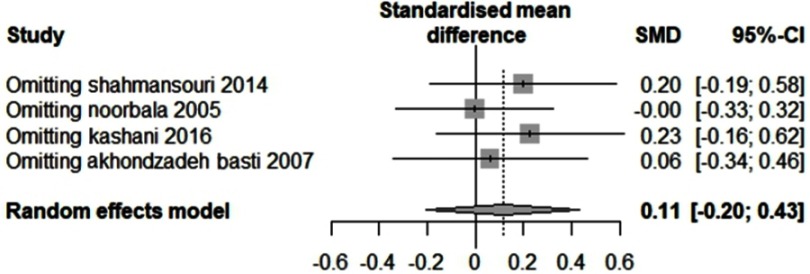
The impact of each study on the overall outcome was evaluated performing sensitivity analysis. To ensure the stability of the results, we conducted a sensitivity analysis (Figure S3 and S4). In both sensitivity analyses, the results did not statistically change, confirming the stability of the findings.
Discussion
Depression is a commonly diagnosed psychiatric disorder that can dramatically impact on quality of life.30 For the treatment of this disease, various drugs can be used, even though they can have different side-effects. Concerning safety, herbal medicines, such as saffron, can represent a valid alternative.22,31,32 Saffron can also be used to relieve menopausal symptoms and to treat other disorders, such as cancer and metabolic syndrome.33
This systematic review and meta-analysis were conducted to evaluate the effectiveness of saffron versus placebo and Fluoxetine 20or 40 mg in treating patients with depression. The findings of this study showed that saffron administration was well comparable with Fluoxetine.
From a biochemical standpoint, in depressed patients, saffron seems to finely tune the level of some neurotransmitters, for example, increasing serotonin levels, inhibiting serotonin reuptake in synapses.34,35 Besides its serotoninergic activity, saffron also modulates hypothalamus-pituitary-adrenal (HPA) axis and exerts neuroprotective effects. Indeed, saffron can increase the concentrations of superoxide dismutase, catalase, and glutathione peroxidase, while lowering malondialdehyde levels and inhibiting the lipid peroxidation pathway. Moreover, saffron positively influences neuronal plasticity and neurogenesis of various brain regions, such as hippocampus, nucleus accumbens, and prefrontal cortex, among others.
Saffron contains more than 150 volatile and nonvolatile, aroma-yielding chemical compounds such as vitamins (like thiamine and riboflavin), amino acids, peptides and proteins, minerals and polysaccharides. Furthermore, saffron contains flavonoids and carotenoids, including zeaxanthin, lycopene, beta-carotenes, crocins (a family of six mono- or di-glycosyl polyene esters, derivatives of 8ʹ-diapocarotene-8,8ʹ- dioic acid, a hydrophilic carotenoid), crocetin (8,8ʹ-diapo-8,8ʹ-carotenoic acid, a carotenoid dicarboxylic acid precursor of crocin), safranal, picrocrocin (4-(β-D-glucopyranosyloxy)-2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde, a molecule derived from the degradation of zeaxanthin and a monoterpene glycoside precursor of safranal) and other substances that have antioxidant properties, thus potentially playing an important role in protecting against free radicals, anti-inflammatory and reactive molecules.28,36 Some studies have shown that the consumption of saffron can result into an improvement of the Hamilton Depression Rating Scale (HDRS) score. Some studies carried out in animal models of depression, such as the study of Hosseinzadeh et al37 have examined the effectiveness of saffron in reducing depressive symptoms.
The findings of this study showed that saffron consumption has similar results with the administration of Fluoxetine, a drug commonly prescribed for the treatment of depression. This seems to suggest that the use of saffron in treating depressed patients can be effective as well, representing a suitable and safer alternative to Fluoxetine. In another study, saffron and Imipramine were used for depressed patients and compared. Results showed that saffron improved the symptoms of the patients in a comparable way to Imipramine.32
To the best of our knowledge, this study was the most comprehensive study on the effectiveness of saffron compared to placebo and Fluoxetine in patients with depression, with respect to previous systematic reviews and meta-analyses,38,39,40,41 specifically focusing on an antidepressant (fluoxetine), for more consistency and accuracy of the findings, instead of pooling together different anti-depressants. Furthermore, we have performed a comprehensive literature search in validated databases, sensitivity analyses, and sub-group analyses. These represent the main strengths of this study.
Limitations
However, there are some limitations in the current systematic review and meta-analysis that should be properly recognized:
The number of studies that have assessed the effectiveness of saffron in treating depressed patients is low. More clinical trials are needed to better evaluate the efficacy of this plant.
Given the highly statistically significant heterogeneity among studies, random-effect models were used and preferred to fixed-effect models. To further minimize the heterogeneity, sub-group analyses stratified according to the dosage of drugs used were carried out.
Due to the small number of included studies, it was not possible to check the existence of publication bias.
All studies have been done in Iran, which accounts for 90% of saffron production, on Iranian patients. Since ethnic differences can impact on drugs efficacy, studies in other countries seem necessary.
The duration of the studies included varied between 6 and 12 weeks. Studies conducted over a longer period of time to assess the efficacy of the drug at longer intervals are, therefore, needed.
Conclusions
The findings of the present systematic review and meta-analysis showed that the use of saffron improved the symptoms of depressed patients. However, on the basis of the abovementioned shortcomings, to ensure the effectiveness of this compound in treating depression, further high-quality studies are needed to provide more solid and valuable evidence.
Acknowledgment
This study was supported by the Razi Herbal Medicines Research Center, Lorestan University of Medical Sciences (No: A-10-1289-1).
Supplementary materials
Figure S1.
The flow-chart of the current systematic review and meta-analysis.
Figure S2.
The risk of bias assessment of studies included.
Figure S3.
The sensitivity analysis of Saffron compared to placebo.
Figure S4.
The sensitivity analysis of Saffron compared to fluoxetine.
Abbreviations list
WHO, World Health Organization; DALY, disability-adjusted life year; PRISMA, preferred reporting items for systematic reviews and meta-analyses; RCT, randomized clinical trial; SMD, standardized mean differences; CI, confidence interval.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547. doi: 10.1371/journal.pmed.1001547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119–138. doi: 10.1146/annurev-publhealth-031912-114409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Depression 2017. Available from: http://www.who.int/mediacentre/factsheets/fs369/en/. Accessed April 10, 2019.
- 5.Marcus SM, Young EA, Kerber KB, et al. Gender differences in depression: findings from the STAR*D study. J Affect Disord. 2005;87(2–3):141–150. doi: 10.1016/j.jad.2004.09.008 [DOI] [PubMed] [Google Scholar]
- 6.Alonso J, Angermeyer MC, Bernert S, et al. Use of mental health services in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;(420):47–54. [DOI] [PubMed] [Google Scholar]
- 7.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851–858. doi: 10.1016/S0140-6736(07)61415-9 [DOI] [PubMed] [Google Scholar]
- 8.Windle M, Windle RC. Recurrent depression, cardiovascular disease, and diabetes among middle-aged and older adult women. J Affect Disord. 2013;150(3):895–902. doi: 10.1016/j.jad.2013.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray CJ, Lopez D. Global Burden of Disease, A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. Boston: Harvard University Press; 1996. [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration‘s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarris J, Panossian A, Schweitzer I, Stough C, Scholey A. Herbal medicine for depression, anxiety and insomnia: a review of psychopharmacology and clinical evidence. Eur Neuropsychopharmacol. 2011;21(12):841–860. doi: 10.1016/j.euroneuro.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 13.Velehorschi C, Bleau P, Vermani M, Furtado M, Klassen LJ. Understanding the role of adjunctive nonpharmacological therapies in management of the multiple pathways to depression. Psychiatry Res. 2014;220(Suppl 1):S34–44. doi: 10.1016/S0165-1781(14)70004-6 [DOI] [PubMed] [Google Scholar]
- 14.Roy T, Lloyd CE. Epidemiology of depression and diabetes: a systematic review. J Affect Disord. 2012;142(Suppl):S8–21. doi: 10.1016/S0165-0327(12)70004-6 [DOI] [PubMed] [Google Scholar]
- 15.Pirotta M, Willis K, Carter M, Forsdike K, Newton D, Gunn J. ‘Less like a drug than a drug‘: the use of St John‘s wort among people who self-identify as having depression and/or anxiety symptoms. Complement Ther Med. 2014;22(5):870–876. doi: 10.1016/j.ctim.2014.07.007 [DOI] [PubMed] [Google Scholar]
- 16.Szafrański T. Herbal remedies in depression–state of the art. Psychiatr Pol. 2014;48(1):59–73. [PubMed] [Google Scholar]
- 17.Kermani T, Mousavi SH, Shemshian M, et al. Saffron supplements modulate serum pro-oxidant-antioxidant balance in patients with metabolic syndrome: a randomized, placebo-controlled clinical trial. Avicenna J Phytomed. 2015;5(5):427–433. [PMC free article] [PubMed] [Google Scholar]
- 18.Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev. 2004;28(6):426–432. doi: 10.1016/j.cdp.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 19.Schmidt M, Betti G, Hensel A. Saffron in phytotherapy: pharmacology and clinical uses. Wien Med Wochenschr. 2007;157(13–14):315–319. doi: 10.1007/s10354-007-0428-4 [DOI] [PubMed] [Google Scholar]
- 20.White IR, Thomas J. Standardized mean differences in individually-randomized and cluster-randomized trials, with applications to meta-analysis. Clin Trials. 2005;2(2):141–151. doi: 10.1191/1740774505cn081oa [DOI] [PubMed] [Google Scholar]
- 21.Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 22.Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, et al. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized and placebo-controlled trial. Phytother Res. 2005;19(2):148–151. doi: 10.1002/ptr.1647 [DOI] [PubMed] [Google Scholar]
- 23.Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, Jamshidi AH. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97(2):281–284. doi: 10.1016/j.jep.2004.11.004 [DOI] [PubMed] [Google Scholar]
- 24.Moshiri E, Basti AA, Noorbala AA, Jamshidi AH, Hesameddin Abbasi S, Akhondzadeh S. Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: a double-blind, randomized and placebo-controlled trial. Phytomedicine. 2006;13(9–10):607–611. doi: 10.1016/j.phymed.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 25.Akhondzadeh Basti A, Moshiri E, Noorbala AA, Jamshidi AH, Abbasi SH, Akhondzadeh S. Comparison of petal of Crocus sativus L. and fluoxetine in the treatment of depressed outpatients: a pilot double-blind randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):439–442. doi: 10.1016/j.pnpbp.2006.11.010 [DOI] [PubMed] [Google Scholar]
- 26.Akhondzadeh Basti A, Ghoreishi SA, Noorbala AA, Akhondzadeh SH, Rezazadeh S. Petal and stigma of Crocus sativus L. in the treatment of depression: a pilot double - blind randomized trial. J Medicinal Plants. 2008;7(4):29–36. [Google Scholar]
- 27.Shahmansouri N, Farokhnia M, Abbasi SH, et al. A randomized, double-blind, clinical trial comparing the efficacy and safety of Crocus sativus L. with fluoxetine for improving mild to moderate depression in post percutaneous coronary intervention patients. J Affect Disord. 2014;155:216–222. doi: 10.1016/j.jad.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 28.Mazidi M, Shemshian M, Mousavi SH, et al. A double-blind, randomized and placebo-controlled trial of Saffron (Crocus sativus L.) in the treatment of anxiety and depression. J Complement Integr Med. 2016;13(2):195–199. doi: 10.1515/jcim-2015-0043 [DOI] [PubMed] [Google Scholar]
- 29.Kashani L, Eslatmanesh S, Saedi N, et al. Comparison of Saffron versus Fluoxetine in treatment of mild to moderate postpar-tum depression: a double-blind, randomized clinical trial. Pharmacopsychiatry. 2017;50(2):64–68. doi: 10.1055/s-0042-115306 [DOI] [PubMed] [Google Scholar]
- 30.Judd L. Mood disorders in the general population represent an important and worldwide public health problem. Int J Clin Psychopharm. 1995;10(suppl 4):5–10. doi: 10.1097/00004850-199512004-00002 [DOI] [PubMed] [Google Scholar]
- 31.Karimi G, Hosseinzadeh H, Khaleghpanah P. Study of antidepressant effect of aqueous and ethanolic extracts of Crocus sativus in mice. Iranian J Basic Med Sci. 2001;4:11–15. [Google Scholar]
- 32.Akhondzadeh S, Fallah-Pour H, Afkham K, Jamshidi AH, Khalighi-Cigaroudi F. Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: a pilot double-blind randomized trial [ISRCTN45683816]. BMC Complement Altern Med. 2004;4:12. doi: 10.1186/1472-6882-4-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rios JL, Recio MC, Giner RM, Manez S. An update review of saffron and its active constituents. Phytotherapy Res. 1996;10(3):189–193. doi: [DOI] [Google Scholar]
- 34.Georgiadou G, Tarantilis PA, Pitsikas N. Effects of the active constituents of Crocus Sativus L., crocins, in an animal model of obsessive-compulsive disorder. Neurosci Lett. 2012;528(1):27–30. doi: 10.1016/j.neulet.2012.08.081 [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Han T, Zhu Y, et al. Antidepressant properties of bioactive fractions from the extract of Crocus sativus L. J Nat Med. 2010;64(1):24–30. doi: 10.1007/s11418-009-0360-6 [DOI] [PubMed] [Google Scholar]
- 36.Samarghandian S, Borji A. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacognosy Res. 2014;6(2):99–107. doi: 10.4103/0974-8490.128963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2:7. doi: 10.1186/1471-2210-2-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hausenblas HA, Saha D, Dubyak PJ, Anton SD. Saffron (Crocus sativus L.) and major depressive disorder: a meta-analysis of randomized clinical trials. J Integr Med. 2013;11(6):377–383. doi: 10.3736/jintegrmed2013056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopresti AL, Drummond PD. Saffron (Crocus sativus) for depression: a systematic review of clinical studies and examination of underlying antidepressant mechanisms of action. Hum Psychopharmacol. 2014;29(6):517–527. doi: 10.1002/hup.2434 [DOI] [PubMed] [Google Scholar]
- 40.Yang X, Chen X, Fu Y, et al. Comparative efficacy and safety of Crocus sativus L. for treating mild to moderate major depressive disorder in adults: a meta-analysis of randomized controlled trials. Neuropsychiatr Dis Treat. 2018;21(14):1297–1305. doi: 10.2147/NDT.S157550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tóth B, Hegyi P, Lantos T, et al. The efficacy of saffron in the treatment of mild to moderate depression: a meta-analysis. Planta Med. 2019;85(1):24–31. doi: 10.1055/a-0660-9565 [DOI] [PubMed] [Google Scholar]