Sickle cell disease (SCD) is the most common inherited blood disorder and affects approximately 100,000 individuals in the United States, primarily those of racial and ethnic minorities.1 Previously associated with high mortality in childhood, greater than 90% of those living with SCD in middle and high-income countries today are expected to survive into adulthood.2 Today, approximately 60% of individuals living with SCD in the United States are adults.1
In 2014, the National Heart Lung and Blood Institute (NHLBI) published “Evidence-Based Management of Sickle Cell Disease: Expert Panel Report (SCD EPR)” to improve the quality of care for individuals with SCD by providing the best science-based recommendations to guide practice.3,4 The NHLBI SCD EPR strongly recommends hydroxyurea therapy as a safe and efficacious treatment for adolescents and adults with sickle cell disease. Despite this recommendation, hydroxyurea therapy remains underutilized – over 75% of patients who could benefit from hydroxyurea fail to receive this drug.5 Providers often have a limited understanding of the optimal use of hydroxyurea,6 while many patients lack general knowledge about hydroxyurea and fear complications or side effects.7 The NHLBI SCD EPR also strongly recommends rapid initiation of opioids for patients visiting the emergency department for a vasoocclusive pain episode. However, a number of studies on patient experiences in the emergency department demonstrate this often does not occur. Patients with SCD are frequently stigmatized, and their pain is often undertreated.8 Providers may perceive patients as being drug seekers or abusers, doubt their pain severity, and hesitate prescribing opioids. These provider perceptions about pain severity, as well as other individual and organizational issues, have been identified as barriers to receiving quality care for patients with SCD.9,10 Patients are often not affiliated with health care providers and health systems with expertise in SCD care or guideline-recommended treatments. Additional barriers to care, including access to transportation, and lack of insurance contribute to the inability to access both routine and specialty care.9 Furthermore, the period of transition from pediatric to adult care poses an additional challenge as adult SCD providers lack in number or expertise, and patients may be unprepared to leave pediatric care during the time when disease complications increase in frequency.11 Ultimately, these gaps in care lead to increased disease burden, higher healthcare costs, and greater mortality of individuals with SCD.
The implementation of evidence-based interventions (EBIs) into usual clinical care is frequently ineffective, with findings that suggest it takes an average of 17 years for 14% of clinical trial research results to reach patients in usual care.12 The implementation gap for EBIs is perhaps greater when related to rare diseases and minority populations.13,14 SCD provides special challenges for clinical care as a rare disease that disproportionately affects underserved minorities in the U.S. where social determinants of health (e.g., socioeconomic status, availability of healthcare resources, and discrimination) contribute to disparities for individuals and in systems of care.
The field of implementation science can help address the quality gap between optimal care (as outlined in guidelines) and the non-standardized care that most patients with SCD currently receive.15 Implementation science aims to study methods to promote the systematic uptake of EBIs into routine practice to improve the quality and effectiveness of health services.16 Implementation studies focus on discovering how to optimally integrate EBIs in real-world contexts, rather than the efficacy or effectiveness of a treatment. While implementation studies may examine the health outcomes associated with the use of an EBI, the emphasis is on evaluating the multi-level influences (e.g., individuals, health care providers, community, social environment, and health system) of the implementation of EBIs.17 Further, with its rigorous clinical trial and quasi-experimental study designs, implementation scientists are able to test the effectiveness of strategies aimed at increasing uptake of EBIs, such as hydroxyurea therapy. This, in combination with the use of frameworks emphasizing stakeholder involvement throughout the implementation process, could break new ground for the sickle cell community by addressing disparities in SCD care and improving health equity for this vulnerable population.18
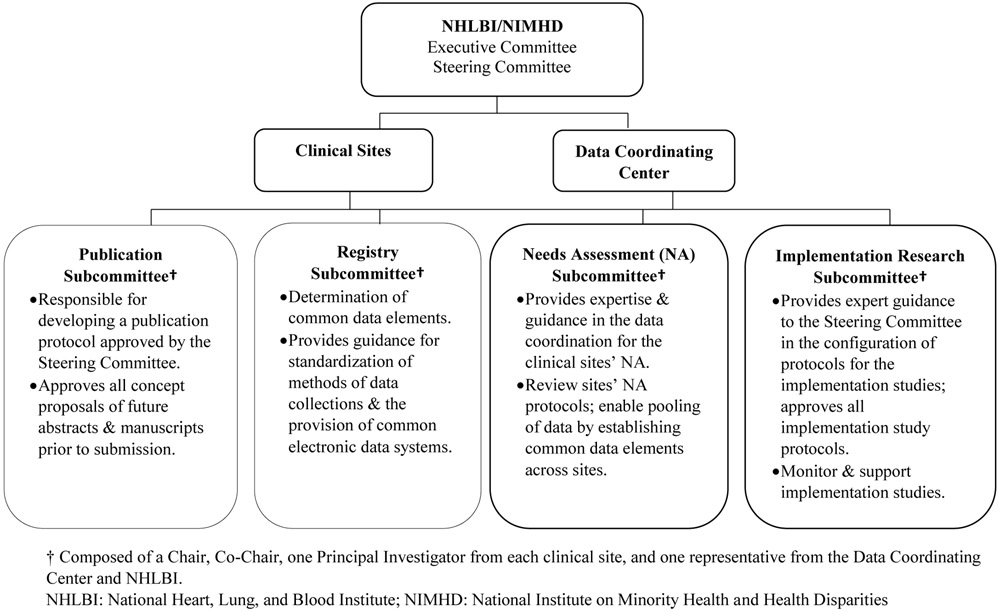
The Sickle Cell Disease Implementation Consortium (SCDIC) History and Structure.
The NHLBI has supported the vast majority of SCD grants in basic, translational, and clinical research that have resulted in the establishment of important clinical milestones over the past 30 years. While previous scientific advances have improved health outcomes of individuals with SCD, the underutilization of evidence-based recommendations and guidelines for adolescent and adult patients has significantly hindered optimal care. As part of its strengthened commitment to implementation research, NHLBI established the Center for Translation Research and Implementation Science (CTRIS) in 2014.19 The primary function of CTRIS is to conduct research to study optimal and sustainable strategies to deliver EBIs and guidelines in diverse multi-level settings, with an emphasis on reducing health inequities among vulnerable populations and in global settings. In 2016, NHLBI funded the Sickle Cell Disease Implementation Consortium (SCDIC) multi-site research study with program leadership from the Division of Blood Resources (DBDR), scientific collaboration from CTRIS, and co-funding from the National Institute of Minority Health and Health Disparities (NIMHD). The RFA-HL-16-010 was written with the goal to support multi-level and multi-component interventions to address the quality gap in the delivery of care for SCD and to develop a longitudinal registry of patients with SCD.
The SCDIC is a cooperative research program composed of eight academic and clinical sites: University of Illinois at Chicago in collaboration with Sinai Health System, Washington University School of Medicine, Augusta University, Icahn School of Medicine at Mount Sinai, St. Jude Children’s Research Hospital, Medical University of South Carolina, Duke University Medical Center, University of California Benioff Children’s Hospital Oakland, and one data coordinating center (DCC), the Research Triangle Institute (RTI). The NHLBI is responsible for providing oversight to the SCDIC and collaborating with the DCC and consortia investigators in the development and execution of the study. An Executive Committee, a Steering Committee, the clinical sites, the DCC, and four subcommittees form the main organizational structure and decision-making body. The Executive and Steering Committees are composed of a Study Chair, NHLBI program officers, DCC Principal Investigator, and clinical site representation. The Steering Committee includes a Principal Investigator from each site. The Executive Committee includes a Principal Investigator from one site that changes on a nine-month rotating basis (Figure 1). The DCC is responsible for major collaborative functions of data management and analytical support of all SCDIC studies, logistics and communications, and support for manuscript and presentation preparation. The longitudinal registry of patients with SCD integrates objective healthcare data with clinical and patient-reported outcomes, including quality of life, access to care, pregnancy outcomes, medication side effects, and sociodemographic variables. The DCC is also responsible for facilitating the registry and guiding all data acquisition.
Figure 1.
Sickle Cell Disease Implementation Consortium (SCDIC) Structure
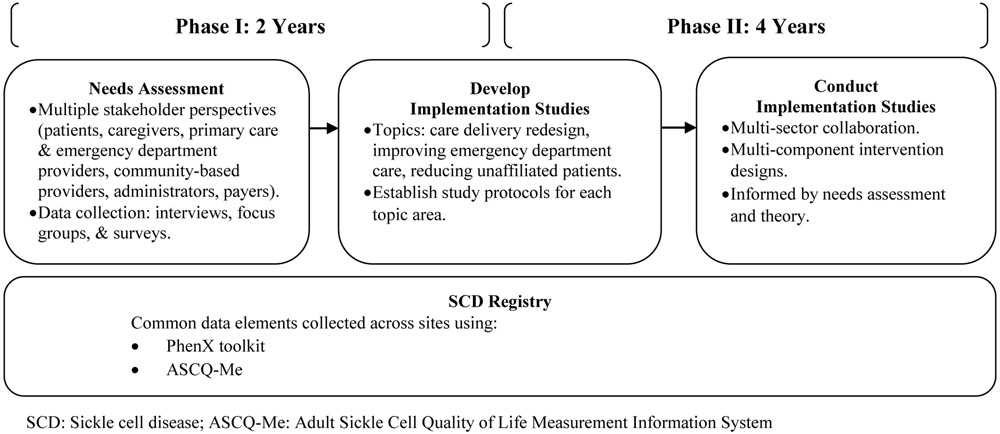
The SCDIC is a six-year study consisting of two phases (Figure 2) with substantial progress and milestones achieved to date. During Phase I (years 1-2), the consortia sites: 1) conducted a community-based needs assessment to investigate stakeholder perspectives on barriers to care for adolescents and adults with SCD; and 2) developed and initiated the registry with over 1,000 patients enrolled to date. Needs assessment data collection regarding barriers to care is complete with over 500 patient and provider participants. During Phase II (years 3-6), registry enrollment will continue, and implementation studies will be conducted addressing multi-level barriers identified in the needs assessment.
Figure 2.
Sickle Cell Disease Implementation Consortium (SCDIC) Project Phases
Based on preliminary findings from the needs assessment data, and principal investigators input regarding topic relevance and feasibility, the Steering Committee selected three themes for SCDIC workgroups: care delivery redesign, improving emergency department care, and reducing the number of patients not currently receiving SCD-specific care (i.e., unaffiliated patients). A defining feature of these workgroups is the pairing of clinical experts in each of these topics with implementation research scientists. Through practical clinical knowledge, evidence-based recommendations, and implementation science methods, the workgroups are developing new approaches to SCD care, particularly for underserved populations.
The operation of a large research consortium encounters many challenges, and the SCDIC is no exception. First, clinical sites and investigators are geographically dispersed across the country. To facilitate communication and collaboration, the SCDIC circulates a monthly bulletin, meets at least monthly, and holds biannual in-person meetings at NHLBI. Also, a website serves as a portal for sharing study information with investigators, including meeting minutes, reports, and protocols (www.scdic.rti.org). Second, improving care delivery for SCD requires multidisciplinary teams of clinical researchers, academic investigators, and community stakeholders, with a broad range of expertise and perspectives. Education about implementation science and SCD using multiple modalities (e.g., presentations, meetings, papers) has been essential to bridging these differences. Third, while preliminary findings from the needs assessment revealed commonalities in barriers to SCD care, variabilities in access and utilization of SCD care across clinical sites were also found. Implementation science designs can accommodate this variability through the adaptation of implementation strategies to local contexts.20 Accounting for differences within the complex environments where SCD care is delivered using models and frameworks to understand why a strategy succeeds or fails is an essential part of the implementation science designs within the SCDIC.17 These lessons may help other large collaborative efforts to accelerate translation of SCD evidence-based guidelines and recommendations into practice, as well as other diseases.
In summary, the complex landscape of care for adolescents and adults with SCD is characterized by barriers and facilitators that exist at multiple levels. The SCDIC has stimulated the sickle cell community with the emerging field of implementation science as a vital step in achieving NHLBT’s goal for the evidenced-based management of SCD for vulnerable populations, and for all. The innovative research infrastructure of the SCDIC will foster the development of new approaches to care and enable important progress towards improving the health and well-being of individuals with SCD informed by the best research evidence.
Funding Acknowledgement
The SCD Implementation Consortium has been supported by US Federal Government cooperative agreements HL133948, HL133964, HL133990, HL133996, HL133994, HL133997, HL134004, HL134007, and HL134042 from the National Heart Lung and Blood Institute and the National Institute on Minority Health and Health Disparities (Bethesda, MD).
Footnotes
Disclosure Statement
JSH receives research funding support from Global Blood Therapeutics and Novartis and is a consultant for Bluebird Bio and National Committee for Quality Assurance; LLH is a consultant for Emmi Corp, Hilton Publishing, Pfizer, Global Blood Therapeutics, and AstraZeneca; JG receives research funding support from Pfizer; AK owns stock in Magenta Therapeutics; TW receives research funding support from Pfizer; NS receives speaker honoraria and research funding support from Novartis. All other authors declare no competing financial interests.
References
- 1.Brousseau DC, Panepinto JA, Nimmer M, Hoffmann RG. The number of people with sickle-cell disease in the United States: national and state estimates. Am J Hematol. 2010;85(1):77–78. [DOI] [PubMed] [Google Scholar]
- 2.American Society of Hematology. State of Sickle Cell Disease: 2016 Report. Accessed June 29, 2018 from http://www.scdcoalition.org/pdfs/ASH%20State%20of%20Sickle%20Cell%20Disease%202016%20Report.pdf.
- 3.National Heart Lung and Blood Institute. Evidence-Based Management of Sickle Cell Disease. Expert Panel Report. 2014; Accessed June 29, 2018 from https://www.nhlbi.nih.gov/sites/default/files/media/docs/sickle-cell-disease-report%200208160.pdf.
- 4.Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033–1048. [DOI] [PubMed] [Google Scholar]
- 5.Stettler N, McKiernan CM, Melin CQ, Adejoro OO, Walczak NB. Proportion of adults with sickle cell anemia and pain crises receiving hydroxyurea. JAMA. 2015;313(16):1671–1672. [DOI] [PubMed] [Google Scholar]
- 6.Lanzkron S, Haywood C Jr., Hassell KL, Rand C. Provider barriers to hydroxyurea use in adults with sickle cell disease: a survey of the Sickle Cell Disease Adult Provider Network. J Natl Med Assoc. 2008;100(8):968–973. [PubMed] [Google Scholar]
- 7.Haywood C Jr., Beach MC, Bediako S, et al. Examining the characteristics and beliefs of hydroxyurea users and nonusers among adults with sickle cell disease. Am J Hematol. 2011;86(1):85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanabe P, Myers R, Zosel A, et al. Emergency department management of acute pain episodes in sickle cell disease. Acad Emerg Med. 2007;14(5):419–425. [DOI] [PubMed] [Google Scholar]
- 9.Adams-Graves P, Bronte-Jordan L. Recent treatment guidelines for managing adult patients with sickle cell disease: challenges in access to care, social issues, and adherence. Expert Rev Hematol. 2016;9(6):541–552. [DOI] [PubMed] [Google Scholar]
- 10.Glassberg JA, Tanabe P, Chow A, et al. Emergency provider analgesic practices and attitudes toward patients with sickle cell disease. Ann Emerg Med. 2013;62(4):293–302 e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porter JS, Wesley KM, Zhao MS, Rupff RJ, Hankins JS. Pediatric to Adult Care Transition: Perspectives of Young Adults With Sickle Cell Disease. J Pediatr Psychol. 2017;42(9):1016–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balas EA, Boren SA. Managing Clinical Knowledge for Health Care Improvement. Yearb Med Inform. 2000(1):65–70. [PubMed] [Google Scholar]
- 13.Sampson UKA, Kaplan RM, Cooper RS, et al. Reducing Health Inequities in the U.S.: Recommendations From the NHLBI's Health Inequities Think Tank Meeting. J Am Coll Cardiol. 2016;68(5):517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potter BK, Khangura SD, Tingley K, Chakraborty P, Little J. Translating rare-disease therapies into improved care for patients and families: what are the right outcomes, designs, and engagement approaches in health-systems research? Genet Med. 2016;18(2):117–123. [DOI] [PubMed] [Google Scholar]
- 15.King AA, Baumann AA. Sickle cell disease and implementation science: A partnership to accelerate advances. Pediatr Blood Cancer. 2017;64(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eccles MP, Mittman BS. Welcome to Implementation Science. Implementation Science. 2006;1(1):1. [Google Scholar]
- 17.Bauer MS, Damschroder L, Hagedorn H, Smith J, Kilbourne AM. An introduction to implementation science for the non-specialist. BMC Psychol. 2015;3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chinman M, Woodward EN, Curran GM, Hausmann LRM. Harnessing Implementation Science to Increase the Impact of Health Equity Research. Med Care. 2017; 55 Suppl 9 Suppl 2:S16–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mensah GA, Engelgau M, Stoney C, et al. News from NIH: a center for translation research and implementation science. Transl Behav Med. 2015;5(2):127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baumann AA, Cabassa LJ, Wiltsey SS. Adaptation in dissemination and implementation science In: Brownson RC, Colditz G, Proctor EK, eds. Dissemination and Implementation Research in Health: Translating Science to Practice Second Edition ed. New York: Oxford University Press; 2017. [Google Scholar]