Abstract
In order to compare connections of premotor cortical areas of New World monkeys with those of Old World macaque monkeys and prosimian galagos, we placed injections of fluorescent tracers and WGA-HRP in dorsal (PMD) and ventral (PMV) premotor areas of owl monkeys. Motor areas and injection sites were defined by patterns of movements electrically evoked from cortex with microelectrodes. Labeled neurons and axon terminals were located in brain sections cut either in the coronal plane or parallel to the surface of flattened cortex, and related to architectonically and electrophysiologically defined cortical areas. Both PMV and PMD had connections with primary motor cortex (M1), the supplementary motor area (SMA), cingulate motor areas, somatosensory areas S2 and PV, and posterior parietal cortex. Only PMV had connections with somatosensory areas 3a, 1, 2, PR and PV. PMD received inputs from more caudal portions of cortex of the lateral sulcus and more medial portions of posterior parietal cortex than PMV. PMD and PMV were only weakly interconnected. New World owl monkeys, Old World macaque monkeys, and galagos share a number of PMV and PMD connections suggesting preservation of a common sensorimotor network from early primates. Comparisons of PMD and PMV connectivity with cortex of the lateral sulcus and posterior parietal cortex of owl monkeys, galagos, and macaques help identify areas that could be homologous.
Keywords: motor cortex, posterior parietal cortex, motor system, primates, primate evolution
INTRODUCTION
The cortical motor system appears to be exceptionally well developed in primates. In addition to primary motor cortex (M1), which is common to all eutherian mammals (Beck et al., 1996), primates have well-differentiated dorsal (PMD) and ventral (PMV) premotor areas, a supplementary motor area (SMA), a frontal eye field (FEF), and two or more cingulate motor fields (Kaas, 2004a). The organization and connections of these areas have been particularly well studied in macaque monkeys (Godschalk et al., 1995; Picard and Strick, 1996; Rizzolatti et al, 1998; for review see Wise et al., 1997; Luppino and Rizzolatti, 2000; Picard and Strick, 2001; Dum and Strick, 2002), but there is good evidence for these areas in New World monkeys (Stepniewska et al., 1993; Preuss et al., 1996, 1997) and in prosimian galagos (Preuss and Goldman –Rakic, 1991a,b,c; Wu et al., 2000; Fang et al., 2005), and humans (e.g. Fink et al., 1997; Geyer et al., 2000). Thus, the cortical motor system has a number of matching subdivisions in prosimian (strepsirhine) primates, platyrhine anthropoids (New World monkeys), and catarhine anthropoids (Old World monkeys, apes and humans). Such a distribution indicates that a constellation of motor areas emerged early in primate evolution, in the period from 65-85 million years ago (Martin, 2004), and has been retained in the major branches.
While the connections of motor and premotor areas have been extensively studied in macaque monkeys, and more recently in galagos, much less is known about such connections in New World monkeys. Major connections of M1 have been described in owl monkeys (Stepniewska et al., 1993) and Cebus monkeys (Dum and Strick, 2005), and the connections of the FEF have been reported for squirrel and owl monkeys (Huerta et al., 1987), but the connections of PMV and PMD are known only for the frontal lobe in Cebus monkeys (Dum and Strick, 2005), and only in part from studies of the connections of M1 in owl monkeys (Stepniewska et al., 1993), and the frontal eye field in owl monkeys and squirrel monkeys (Huerta et al., 1987). In the present study, we directly determined the connections of PMV and PMD of owl monkeys. Injection sites were defined by intracortical microstimulation, and motor areas were delineated electrophysiologically and architectonically in the same monkeys (see Preuss et al., 1996). Our study had two main goals. First, we wanted to compare the cortical connection patterns in owl monkeys with those in galagos and macaques. Similarities across these primates would suggest connectional networks that have been retained since early in primate evolution, while differences would suggest specializations within the different lines of primate evolution. Second, the connections of well-defined cortical areas with cortical regions that are not well understood can help reveal the organization of the latter regions. While the subdivisions of motor and premotor cortex are now reasonably well characterized in owl monkeys (Gould et al., 1986; Stepniewska et al.1993; Preuss et al., 1996), the organization of posterior parietal cortex in these and other New World monkeys is not well known. Since PMD and PMV have dense interconnections with posterior parietal cortex in macaques and galagos, such connections in owl monkeys would indicate the likely locations of areas in posterior parietal cortex that have been defined in other primates.
MATERIAL AND METHODS
Experiments were carried on 6 adult owl monkeys (Aotus trivirgatus). Fluorescent dyes (diamidino yellow, DY, fast blue, FB, fluororuby, FR) and wheat germ agglutinin conjugated to horseradish peroxidase (WGA-HRP) were placed in the forelimb and eye-movement representations of PMD and in the forelimb and face representations of PMV, as determined by microstimulation mapping (see Preuss et al., 1996). In two cases tracers were also placed in the forelimb representation of primary motor cortex (M1). Experimental procedures were approved by the Vanderbilt Animal Care and Use Committee and followed the guidelines of the National Institutes of Health.
Surgical procedures were described in detail by Stepniewska et al. (1993). All procedures were carried out under aseptic conditions. Anesthesia was induced with intramuscular injections of ketamine hydrochloride (30 mg/kg) and xylazine (1mg/kg) and supplemental injections were given as needed to maintain a surgical level of anesthesia. Bone covering part of the frontal lobe was removed and the dura reflected to expose the cortex from the central sulcus (CS) to the dimple of the inferior arcuate sulcus (IAS). Cortex was covered with silicone oil to prevent desiccation and to limit brain pulsation. The locations of electrode penetrations and tracer injection were marked on a photograph of the exposed frontal lobe. After a short microstimulation session to identify areas for placing injections (see below), fluorescent tracers (DY, FB, FR) and WGA-HRP were placed in the forelimb and eye-movement representations of PMD and in the forelimb and face representations of PMV (see Table 1). In two cases, an additional tracer was injected into M1. Tracers were either injected with 1-2 μl Hamilton syringes with glass micropipette attached to the tip of the syringe needle (0.03-0.05 μl of WGA-HRP and 0.1-0.25 μl of fluorescent tracers), or deposited in the cortex in form of a crystal or pellet. Following the injections, the cortex was covered with gelatin film, the skull opening was closed with a dental acrylic cap, and the skin was sutured. After a survival period of 4-9 days to allow tracers to be transported, the skull was opened again and additional locations in the same hemisphere were stimulated with a microelectrode in order to more extensively determine the organization of motor and premotor cortex. At the end of stimulation session, electrolytic lesions were placed to mark the M1 and PM boundaries and the borders between different body representations within these two cortical regions. Lesions to mark cortical locations were made by passing 10 μA of direct current for 10 seconds via the microelectrodes. These lesions and other landmarks subsequently allowed the electrophysiological results to be related to architecture. Results of the physiological and histological investigations are described elsewhere (Preuss et al., 1996). At the end of experiment, each owl monkey was given a lethal dose of barbiturate and perfused through the heart with buffered physiological saline, followed by 2% paraformaldehyde in phosphate buffer, and then by 2% paraformaldehyde in phosphate buffer with 10% sucrose. Perfused brains were removed from the skull and kept in 30% sucrose overnight. In three cases, cortex was separated from the brainstem and flattened manually between glass slides. The cortical tissue was sectioned and processed the next day.
Table 1.
The cases, areas, and tracers injected.
| Case | PMD injection | PMV injection | M1 | Plane of Cut | Survival (in days) |
|---|---|---|---|---|---|
| 90-55L | caudal: DYcrystal | FL: 3%FB (0.25ul) Face. 2%WGA-HRP (0.03ul) |
coronal | 4 | |
| 91-72L | rostral: 10%FR (0.25 ul) | FL: 2%WGA-HRP (0.05 ul) | coronal | 5 | |
| 92-42L | caudal: 10%FR (0.2 ul) | flattened | 7 | ||
| 92-83L | caudal: DY pellet | FL:FR pellet | FL: FB pellet | flattened | 9 |
| 93-4L | FL rep.: DY pellet | coronal | 6 | ||
| 94-26L | caudal: 10%FR (0.1 ul) rostral: 2%DY (0.2ul) |
FL: 2%WGA-HRP (0.03ul) Face: 3%FB (.15ul) |
flattened | 7 |
DY- diamidino yellow ; FB -fast blue; FL- forelimb; FR- fluororuby; WGA-HRP- wheat germ agglutinin
Microstimulation of the left precentral cortical region (premotor and motor cortex) was carried out with low-impedance (0.5-1 MΩ) tungsten microelectrodes inserted perpendicular to the cortical surface to a depth of about 1.7-1.8 mm (corresponding to layer 5 and upper layer 6). This depth was optimal for eliciting low-threshold movement responses from the frontal cortex (Gould et al., 1986, Stepniewska et al. 1993; Preuss et al. 1996). Stimuli consisted of single or multiple trains of cathodal current, with a pulse duration of 0.2 ms, a pulse frequency of 300 Hz, and a train duration of 60 ms delivered by a Grass S88 stimulator. At the start of each experiment, we stimulated the M1 region with currents generally no higher than 90 μA. Then we explored the cortex anterior to the M1 using higher currents, although the large majority of premotor sites were responsive with currents of less than 100 μA. The type of movements and the current thresholds for evoked movements were recorded for each penetration. We considered the movement threshold to be the lowest current that reliably evoked movements detected by each of two observers.
Injected hemispheres were cut at 40 μm either in the coronal plane (3 cases) or were flattened and cut parallel to the cortical surface (3 cases). Series of sections were processed to reveal WGA-HRP (Gibson et al, 1984) or were mounted unstained for detection of fluorescence. For cases cut in the coronal plane, additional series of sections were stained for Nissl substance, cytochrome oxidase (CO) (Wong-Riley, 1979), acetylcholinesterase (Geneser-Jensen and Blackstad, 1971), or myelin (Gallyas, 1979). Flattened tissue was stained for CO, myelinated fibers, and in some cases, for Nissl substance. The locations of cells labeled with fluorochromes were charted with a Leitz microscope connected to an X-Y plotter, with 360nm (for FB, DY) and 530-560nm (for FR) wavelength excitation filters. Sections with WGA-HRP label were studied under dark-field illumination and the locations of HRP injection sites and resulting label were drawn at the same scale as adjacent sections with the fluorescent labeling. Both series of sections were then superimposed by using electrolytic lesions, sulci and blood vessels identified in the tissue sections as guides for alignment. For each case we prepared a composite drawing showing the relationship of injection sites and transported label to the electrophysiological and architectonic maps of motor, premotor, and somatosensory areas. The architectonic definitions of motor and somatosensory areas followed those of Stepniewska et al. (1993) and Preuss et al. (1996). For the brains sectioned in the coronal plane, a flat surface view was constructed by drawing the cortical surface of each section as a straight line segment, and aligning the segments for each section along a perpendicular line representing the crest of the midsagittal fissure. The labeled neurons of each section were projected tangentially onto the appropriate line segment, and the reconstruction was completed by marking architectonic and physiological boundaries. For the brains cut parallel to the surface of flattened cortex, boundaries and labeled neurons were superimposed in representative sections, providing a flattened surface view (see Stepniewska et al., 2005).
Photographs were made using a 35mm Olympus C-35 camera mounted on the Olympus BH-2 microscope. Negatives were scanned at 300 dots per inch with Polaroid Sprint Scan 35 scanner (Polaroid Corporation, Cambridge, MA, USA). The digitized images were adjusted for brightness and contrast, cropped and pasted in the frame, where text was added using Photoshop 7.0 software. Except for contrast adjustment and cropping, the images were not altered in any way.
RESULTS
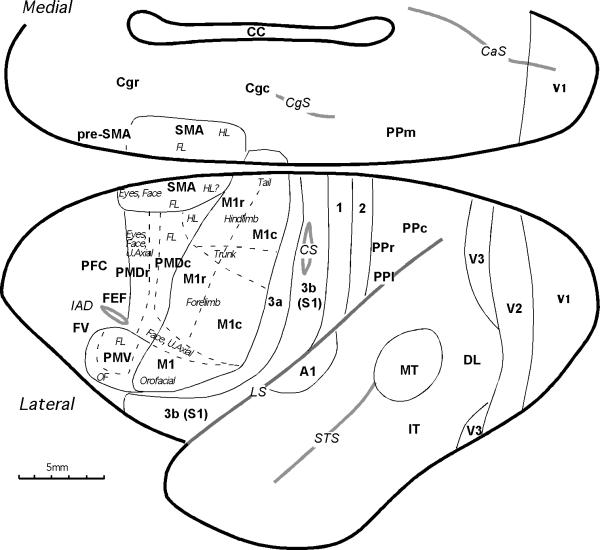
The ipsilateral cortical connections of the ventral (PMV) and dorsal (PMD) premotor areas of cortex were revealed in adult owl monkeys by placing injections of anatomical tracers in these areas and in primary motor cortex (M1). The locations of these areas and some of the fields with connections with premotor cortex are shown in Figure 1. The boundaries of M1, PMD, PMV, and SMA were determined in microelectrode stimulation experiments and correlated with changes in cortical architecture in the owl monkeys (Stepniewska et al., 1993, Preuss at al., 1996). In brief, PMD and PMV were distinguished from M1 by generally higher thresholds for movements evoked by electrical stimulation of cortex and by differences in the somatotopic patterns of evoked movements. Stimulation of PMV elicited forelimb and orofacial movements, while stimulation of PMD elicited movements of the hindlimb, forelimb upper trunk, neck, face and eyes. Microstimulation results from the present cases are illustrated in Preuss et al. (1996). Architectonically, the presence of a thin internal granular layer (layer 4) distinguished PMV from M1, while PMD was distinguished from M1 by a lack of large pyramidal cells in layer 5. Primary sensory areas (3b, A1 and V1) and the middle temporal visual area (MT) were distinguished by their dense CO and myelin staining. Other cortical areas were difficult to reliably identify architectonically and their locations were based on previously established relationships to well-defined cortical areas.
Figure 1.
Subdivisions of cortex in owl monkeys. The locations and somatotopic organizations of motor areas are based on the microstimulation and architectonic mapping results (Gould et al., 1986; Stepniewska et al, 1993; Preuss et al. 1996). They include the primary motor cortex, M1, composed of caudal (M1c) and rostral (M1r) subdivisions; the supplementary (SMA) and pre-supplementary (pre-SMA) motor areas; the dorsal premotor area, PMD, composed of caudal (PMDc) and rostral (PMDr) subdivisions; the ventral premotor area, PMV; the frontal eye field, FEF; and dorsal oculomotor area (OMD). Cingulate motor cortex on the medial wall consists of rostral (Cgr) and caudal (Cgc) areas. The motor region is bordered rostrally by prefrontal cortex (PFC) and caudally and laterally by somatosensory cortex that includes areas 3a, 3b (S1), 1 and 2. Other somatosensory areas (the second area, S2; the parietal ventral area, PV; the parietal rostral area, PR; and the ventral somatosensory area, VS) are hidden within the lateral sulcus. For convenience, we have divided posterior parietal cortex into rostral (PPr), caudal (PPc), medial (PPm) and lateral (PPl) regions. Some visual areas and primary auditory area (A1) are marked for reference. Visual areas include the primary visual cortex (V1), the second visual area (V2), the third visual area (V3), the dorsolateral area (DL), the middle temporal area (MT), and inferior temporal cortex (IT). Cortical areas are demarcated by black solid lines, and somatotopic representations within areas are marked wth thin dashed lines. Sulci are marked with gray solid lines. Additional abbreviations: CaS- calcarine sulcus; CgS- cingulate sulcus; CC- corpus callosum CS- central sulcus; FL- forelimb; HL- hindlimb; IAD- inferior arcuate dimple; LS- lateral sulcus; OF- orofacial; STS-superior temporal sulcus; U.Axial- upper axial.
As each of four of the cases had more than one tracer injected, the connections of two or three different injections could be directly compared within a case. The results indicate that PMV and PMD have sparse cortical connections with each other, and denser connections with M1, SMA, adjoining portions of prefrontal cortex, cingulate motor areas, posterior parietal cortex, and somatosensory areas of the lateral sulcus. PMD was more strongly connected with posterior parietal cortex, while PMV had stronger connections with the somatosensory areas of the lateral sulcus.
Connections of PMV
The connections of PMV, an area that contains mainly forelimb and face representation, were revealed in four cases with a tracer injection centered in the forelimb representation and in one case with an injection in the face representation. As the forelimb representation in PMV is separated from the forelimb representation of M1 by a region of face representation, it was possible to place injections in the physiologically identified forelimb representation of PMV without involvement of the M1 forelimb representation. Results were generally very similar across cases with PMV injections, although there were some minor differences in the locations of labeled neurons. The fluorescent tracers yielded only retrograde labeling of cell bodies, while WGA-HRP labeled both cell bodies and axonal terminals. For ease of comparison, only labeled neurons are illustrated. The cases with WGA-HRP injections did, however, indicate that the connections between PMV and other regions of cortex are reciprocal.
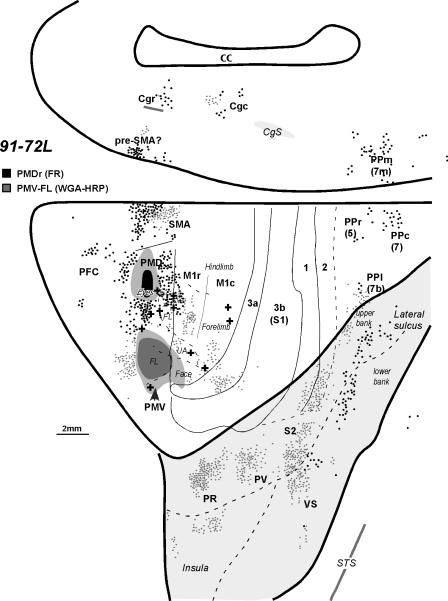
The characteristic connection pattern of PMV can be seen in case 91-72 (Fig. 2). In this owl monkey an injection of WGA-HRP was placed in the forelimb representation in PMV. The injection core included most of the forelimb region, while the injection fringe of dense local transport extended slightly into the face cortex of M1 (see Fig. 9A of Preuss et al., 1996, for the microstimulation results from this case). Results from a FR injection in the eye-movement portion of PMD in this case provides a useful comparison. As the brain was cut in the coronal plane, the distribution of labeled cells from these two injections is shown in a reconstructed flattened view of the cortex with the lateral sulcus opened and cortex of the medial wall adjoining the dorsal view. Labeled neurons were scattered across the forelimb region of the primary motor cortex (M1), including both the rostral (M1r) and caudal (M1c) subdivisions of M1 that are distinguished by differences in architecture, connections, and responsiveness to microstimulation (Stepniewska et al. 1993; Preuss et al., 1996). More neurons were labeled in M1r than M1c. Other labeled neurons were located in the caudal forelimb portion of PMD and the forelimb portion of SMA (see Fig. 1). The labeled neurons in SMA from the forelimb injection in PMV were caudal to those from the injection in the face–eye representation of PMD. This difference in the distribution of labeled neurons is consistent with the microstimulation evidence that the representation of the face and eye is rostral to that of the forelimb in SMA (Gould et al., 1986; Preuss et al., 1996). Other labeled cells were in the forelimb portion of PMD. The cluster of labeled cells just rostral to the PMV injection largely reflects connections with prefrontal cortex. Labeled neurons were also present in the cortex of the medial wall of the cerebral hemisphere, in the location of pre-SMA cortex (Sakai et al., 2000), and in the locations of the caudal and rostral cingulate motor areas, Cgc and Cgr (Fig. 2).
Figure 2.
The distribution of labeled neurons shown on the dorsolateral and medial cortical surface reconstructed from the series of coronal sections of owl monkey 91-72L after injections into the rostral PMD (black dots) and forelimb representation in PMV (gray dots). Uptake cores of injections are marked with dark colors, black for PMD and gray for PMV, and diffusion zones with lighter colors. Solid lines mark architectonic borders determined from sections stained for CO and myelin. The dashed lines indicate estimated borders and the fundus of the lateral sulcus. Electrolytic lesions used to identify borders between physiological zones are marked with crosses (+). For a detailed microstimulation map of this case, see Figure 9A of Preuss et al. (1996). The lateral sulcus is opened to reveal buried cortex, which is highlighted with light gray. UA- upper axial. Other conventions are the same as in Figure 1.
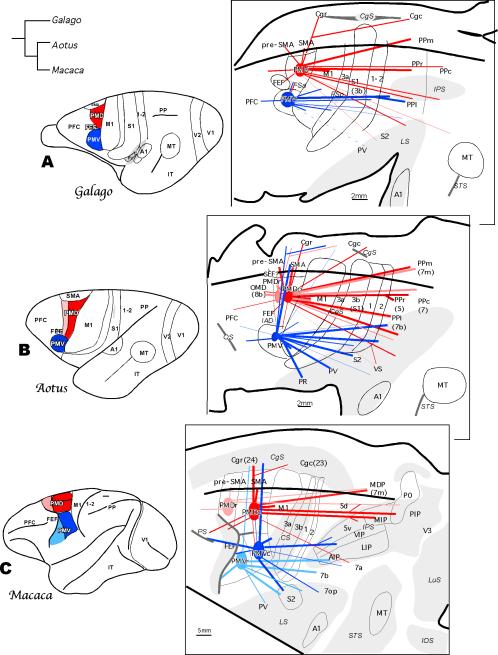
Figure 9.
Summary of the ipsilateral cortical connections to dorsal (red) and ventral (blue) premotor areas in owl monkeys (B) in comparison with such connections in other major branches of primate evolution represented by prosimian galagos (A) and macaque monkeys (C). In the left column premotor areas for each primate are marked on the dorsolateral view of the brain. PMD is divided into rostral (pink) and caudal (red) subdivisions in the owl monkey and the macaque monkey, and PMv is divided into rostral (light blue) and caudal (dark blue) subdivisions in the macaque monkey. The evolutionary relationship of the three taxa is indicated by the tree diagram on the upper left. In the right column the patterns of connections are shown in the flattened cortex with opened sulci. Dense connections between areas are marked with thick lines, and sparse connections are marked with thin lines. Macaque intraparietal areas: AIP, anterior intraparietal area; LIP, lateral intraparietal area; MIP, medial intraparietal area; MDP, medial dorsal parietal area; PIP, posterior intraparietal area; PO, parietal occipital area; VIP, ventral intraparietal area. Macaque sulci: IOS, inferior occipital sulcus; LuS, lunate sulcus; PS, principal sulcus. Other conventions are the same as in Figures 1 and 2.
Other connections of PMV forelimb cortex were with somatosensory areas. The lateral focus of labeled neurons in area 3a was in cortex devoted to the forelimb. A few labeled cells were in the forelimb portion of area 3b (see Jain et al., 1998). A large focus of labeled neurons was located just caudal to area 3b in portions of areas 1 and 2 that represent the forelimb (Merzenich et al., 1978). More of the labeled neurons were in area 2 than area 1. Large numbers of labeled neurons were distributed in several clusters along the upper bank and insula of the cortex within the lateral sulcus in a caudorostral sequence indicating connections with the second somatosensory areas (S2), the parietal ventral area (PV), and the parietal rostral area (PR) (Krubitzer and Kaas, 1990; Qi et al., 2002; Coq et al, 2004). Labeled neurons in the fundus of the lateral sulcus were in the location of the ventral somatosensory area, VS (Cusick et al, 1984; Coq et al, 2004). The more rostrally located cells in the insula were in cortex that is considered to be somatosensory (Jones and Burton, 1976), but the identity of this cortex is uncertain. Finally, two clusters of labeled neurons were in the lateral posterior parietal cortex on the lip of the lateral sulcus. This cortex might correspond to areas 7b and/or AIP, as it is roughly in the position of the anterior intraparietal cortex of macaque monkeys, a region known to project to PMV (e.g., Luppino, et al, 1999).
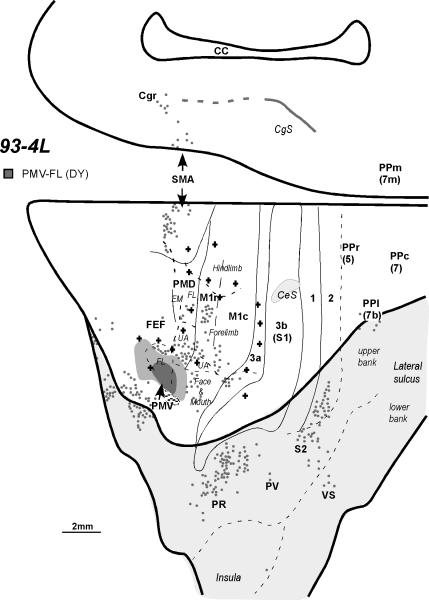
Very similar results were obtained in case 93-4L with an injection of DY in the forelimb portion of PMV (Fig. 3; see Fig. 4A of Preuss et al., 1996 for an extensive microstimulation map of motor and premotor areas in this case). Again, labeled neurons were located in the forelimb portion of M1, especially M1r, with a few cells also in the orofacial portion of M1, possibly due to a slight involvement of the injection core in the orofacial part of PMV. Other labeled neurons were scattered across the forelimb representations of PMD and SMA. This injection also labeled neurons in orbital frontal cortex ventral to PMV. Additional foci of labeled cells were present in the cingulate motor regions. In somatosensory cortex labeled neurons were present in area 3a and in lateral portions of areas 1 and 2 in the lateral sulcus where the forelimb and face are represented. No labeled neurons were present in area 3b. Labeled neurons were widely distributed across the cortex of the upper bank of the lateral sulcus in regions that included somatosensory areas PR, PV, S2 and VS, and a few labeled cells were in lateral locations in the posterior parietal cortex, possibly corresponding to areas 7b and/or AIP, as noted above.
Figure 3.
The distribution of labeled neurons shown on the dorsolateral and medial cortical surfaces reconstructed from the series of coronal sections of monkey 93-4 after an injection into the forelimb representation of PMV. In this case, each dot represents about 5 cells. For a detailed microstimulation map of this case, see Figure 4A of Preuss et al. (1996). EM, eye movements. Other conventions are the same as in Figures 1 and 2.
Figure 4.
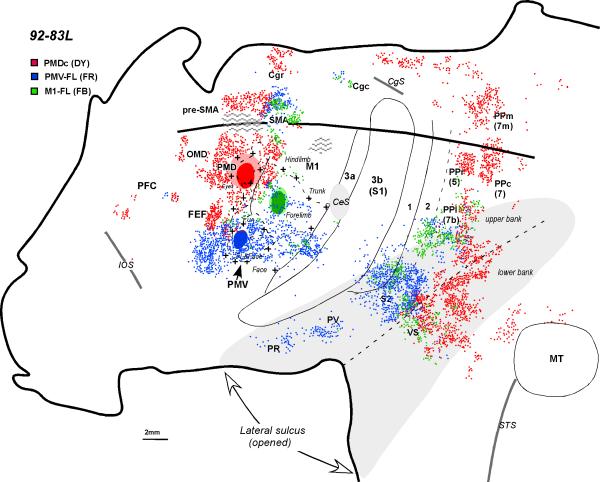
The distribution of labeled neurons shown in the flattened cortex of monkey 92-83L after injections into PMD (red) the PMV forelimb representation (blue), and the M1 forelimb representation (green). For a detailed microstimulation map of this case see Figure 9B of Preuss et al. (1996). The thick line indicates where the dorsal convexity meets the medial wall. Hatching represents tissue damage. Other conventions are the same as in Figures 1 and 2.
In owl monkey 92-83L, an injection of FR was placed in a portion of PMV where shoulder, elbow, and wrist movements were evoked (see Fig. 9B of Preuss et al., 1996). The surface view of the distribution of labeled neurons was reconstructed from brain sections cut parallel to the surface of manually flattened cortex (Fig. 4). Labeled neurons were scattered across the forelimb and adjoining face representations of M1, in the SMA forelimb representation, and in cingulate motor cortex. Other labeled neurons were observed in the cortex lateral and rostral to the injection site. In somatosensory cortex, the forelimb region of area 3a was densely labeled, as were portions of areas 1 and 2 along the lip of the lateral sulcus. A few labeled cells were present in area 3b. The PR, PV, S2, and VS regions contained labeled cells, as did the lateral part of posterior parietal cortex.
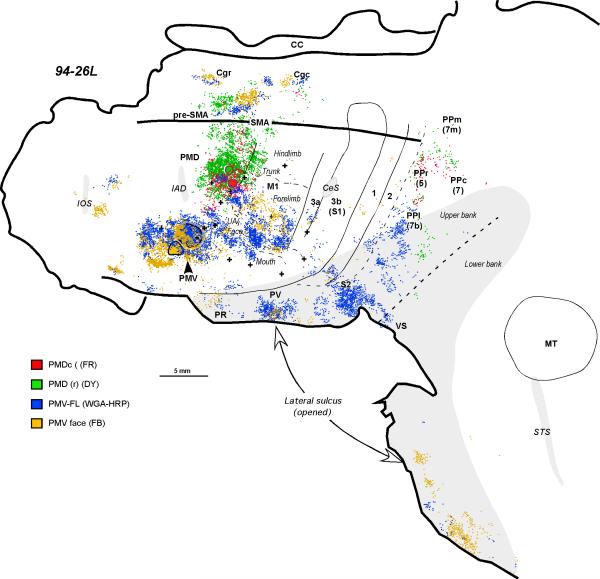
In owl monkey 94-26L (Fig. 5), two injections were placed in PMV. The more dorsal injection of HRP included the digit and wrist representations of PMV while the more ventral injection of FB included the region of PMV devoted to digit and jaw movements (see Fig. 7B of Preuss et al., 1996). The two injections labeled neurons in overlapping regions of the forelimb representations of M1r and M1c, as well as the orofacial region of M1. Neurons labeled by the two injections formed spatially overlapping populations, but there were also regions of little overlap. The more ventral injection did not appear to label proportionately more neurons in face M1 than the more dorsal injection. After both injections in PMV, neurons were labeled in PMD, SMA, and in several locations in cingulate motor cortex. Some neurons were labeled in cortex ventral and rostral to PMV, and in two locations near the rostral pole of the frontal lobe. In somatosensory cortex, neurons were labeled in the forelimb portion of area 3a, a few were in forelimb 3b, and others were labeled in forelimb and possibly face portions of areas 1 and 2. Other neurons were present in the lateral sulcus, including the regions of PR, PV, S2, and VS, and in lateral posterior parietal cortex.
Figure 5.
The distribution of labeled cells in the flattened cortex after injections into caudal (red) and rostral (green) PMD, and the representation of the forelimb (blue) and the face (yellow) in PMV (case 94-26L). For a detailed microstimulation map of this case, see Figure 7B of Preuss et al. (1996). Conventions are the same as in Figures 1 and 2.
Figure 7.
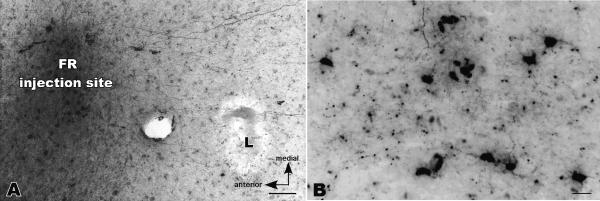
Photomicrographs of an injection site of fluororuby (FR) in the dorsal premotor area (PMD) and labeled neurons in primary motor cortex (M1) of owl monkey 92-42L. Cortex was flattened and cut parallel to the surface in this case. A. The FR injection is to the left of an electrolytic microlesion (L) that was placed at the electrophysiologically defined border of M1 and PMD. A few labeled neurons are apparent in PMD and labeled axons are apparent between PMD and M1. B. Some of the labeled neurons in M1. Only the labeled cell bodies were counted, although labeled axons and cell fragments are also apparent. Scale bar in A = 100μm, and in B = 50μm.
In summary, there was some variability in the distribution of labeled cells produced by the different injections in PMV, but many consistent features. Injections of area PMV, which represents mainly forelimb and face movements, labeled cells in the forelimb and face representations of several somatotopically organized areas, including M1, PMD, SMA, 3a, 3b, 1 and 2. The connections with PV, S2, VS, and the cingulate motor areas may have been somatotopically organized as well, but this is uncertain because the borders and locations of body parts in the representations in these areas were more difficult to estimate. PMV had major connections with M1; these connections were denser with M1r than with M1c. Connections with SMA, PMD and cingulate motor areas were less dense and more variable than those with M1. Dense foci of labeled cells were observed in somatosensory areas of the lateral sulcus (S2, VS, PV, and PR), while fewer labeled cells were in anterior parietal areas 3a, 1 and 2. Few or no labeled neurons were in area 3b. Finally, the lateral portion of posterior parietal cortex, including the cortex along the upper bank of the lateral sulcus, consistently projected to PMV.
Connections of PMD
In an earlier study, Preuss et al. (1996) distinguished rostral and caudal divisions of area PMD, based on differences in architectonics and responses to microstimulation. In particular, limb movements were evoked from caudal PMD (PMDc), while rostral PMD (PMDr) was devoted to face, eye and proximal forelimb movements. In the present study, injections were placed in caudal PMD in three cases, and in rostral PMD in two cases. The distribution of labeled neurons was similar after injections of PMDc or PMDr, but distinctly different from the pattern produced by PMV injections.
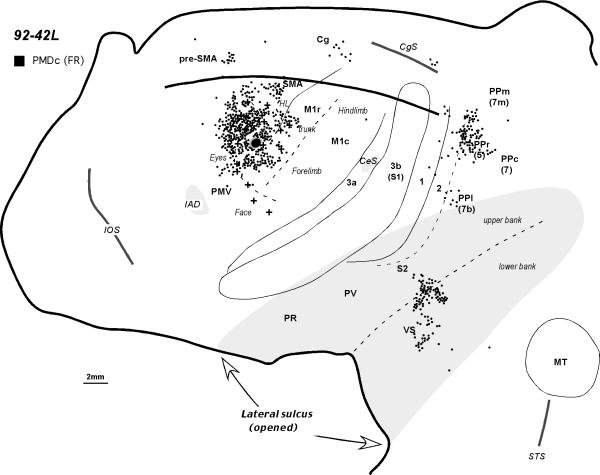
The general pattern of PMDc connections can be seen in owl monkey 92-42L (Fig. 6), where a restricted injection of FR was placed in caudal PMD close to the M1 border (Fig. 7A). At this location, microstimulation produced forelimb movements (see Fig. 7A of Preuss et al., 1996,). The injection labeled many neurons in adjoining parts of PMD, including regions representing movements of the eye, nose, vibrissae, and forelimb. Other connections were with the M1r forelimb (Fig. 7B) and trunk representations. Labeled neurons were also observed in SMA, mostly on the dorsal convexity in a region largely devoted to the forelimb. Only a few labeled neurons were present in PMV, and these were confined to dorsal PMV. Some labeled neurons were present in the cortex lateral to PMD in the region of the frontal eye field (Huerta et al., 1986). A rostral focus of labeled cells on the medial wall of the cerebral hemisphere was in the location of pre-SMA, while a more caudal focus was in the location of the caudal cingulate motor area. A small cluster of labeled neurons ventral to the cingulate sulcus may reflect a cingulate somatosensory area (CSMA), similar to that described in prosimian galagos (see Wu et al., 2003). The injection in PMDc did not label neurons in areas 3a or 3b, and only a few neurons were present in areas 1 and 2. Most or all of PR, PV, and S2 were devoid of labeled neurons. Dense clusters of cells were present in the depths of the lateral sulcus in VS and deep S2. A few cells were labeled in a portion of lateral posterior parietal cortex that also projects to PMV. However, most of the label in the posterior parietal cortex was in a dorsomedial sector that extended from the dorsal convexity onto the adjoining medial wall.
Figure 6.
The distribution of labeled cells in the flattened cortex after an injection into the caudal PMD in monkey 92-42L. For a detailed microstimulation map of this case see Figure 7A in Preuss et al. 1996. Conventions are the same as in Figures 1 and 2.
Results from a larger injection of DY in PMDc can be seen in Figure 4. The injection core filled a portion of PMDc where microstimulation evoked shoulder and elbow movements (see Fig. 9B of Preuss et al., 1996). Labeled neurons were scattered over much of PMD and they extended into the frontal eye field and adjoining cortex just lateral to PMD. A few cells were in the dorsal-most part of PMV devoted to shoulder and elbow movements. Labeled neurons were in the forelimb region of SMA where they overlapped labeled neurons from an injection in forelimb PMV, but the labeled neurons from the PMD injection also extended more rostrally into the regions of the face representations of SMA and pre-SMA. Labeled neurons in M1 were confined to forelimb and hindlimb representations in M1r. A large cluster of labeled neurons was present in cingulate motor cortex. Anterior parietal somatosensory areas 3a, 3b, and 1-2, contained no labeled cells. Among the somatosensory areas of the lateral sulcus, areas PR, PV, and most or all of S2 were unlabeled. However, cortex in the depth and in the lower bank of lateral fissure contained many labeled cells, in a region that includes somatosensory area VS and multisensory areas caudal to primary auditory cortex (Morel and Kaas, 1992; Kaas and Collins, 2004). Labeled neurons were also located more caudally along the fundus and upper bank of the lateral sulcus, spreading onto the lateral convexity of posterior parietal cortex. Large foci of labeled neurons were located more dorsally in the lateral portion of posterior parietal cortex. Other clusters of labeled neurons were in medial posterior parietal cortex and in cingulate cortex below the cingulate sulcus. Finally, some labeled neurons were observed in the cortex rostral to visual area MT, a region that may include visual area MST and the multisensory cortex ventral to MST. Only a few labeled neurons were seen in this region in other cases with PMDc injections (Figs. 6& 8).
Figure 8.
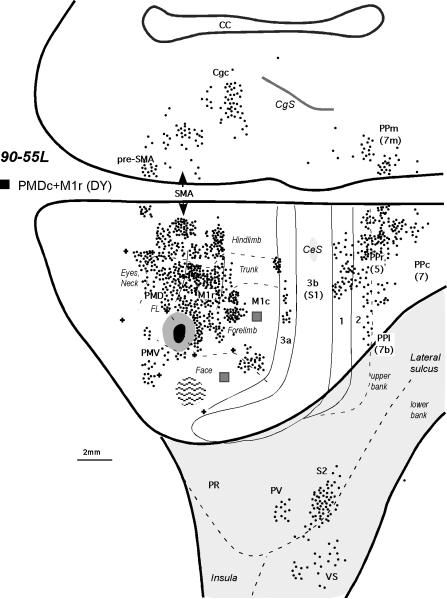
The distribution of labeled cells shown on the dorsolateral and medial cortical surfaces reconstructed from the series of coronal sections of monkey 90-55L after an injection into the caudal PMD (case 90-55). In this case, the injection spread somewhat into the rostral M1. Additional injections, marked with a dark gray squares were in the forelimb and face representations in M1c (for the resulting distributions of labeled neurons, see Figure 12 of Stepniewska et al., 1993). For a detailed microstimulation map of this case, see Figure 11B of Preuss et al. 1996. Conventions are the same as in Figures 1 and 2.
A similar pattern of label was obtained in case 90-55 (Fig.8) with a large injection in the most ventral and caudal portion of PMD, crossing the border into M1r so that the injection site involved the forelimb representations of both areas. This case yielded more labeling of areas PMV, 3a, 1-2, than other PMDc injections, which likely reflects the spread of injection into M1r (see Stepniewska et al. 1993).
In case 94-26L (Fig. 5), two small injections of different tracers were placed in PMD, one just rostral to M1r, centered in the shoulder representation of PMDc, and a second more rostromedially in a region of PMDr representing the upper trunk (Fig. 7B of Preuss et al., 1996). Both injections broadly labeled neurons over PMD, with a tendency for the more medial injection to label neurons more medially in PMD. The more medial injection, which involved PMDr, also labeled neurons more medially in M1r, including the trunk and hindlimb representations. Both the PMDc and PMDr injections labeled the forelimb representation in M1r. Both injections also labeled neurons in SMA, but the rostral injection produced a broader distribution of labeled neurons. There were only a few labeled cells present in the extreme dorsal part of PMV. The PMDr injection produced clusters of labeled neurons in cortex of the medial wall in the regions of pre-SMA, and the rostral and caudal cingulate motor areas. The PMDc injection yielded fewer labeled neurons in these regions of medial cortex. Additional territories with labeled neurons included the posterior parietal cortex on the lateral convexity and cortex deep in the lateral suclus. No labeled neurons were present in any of the somatosensory fields of the lateral sulcus or of anterior parietal cortex.
As a final demonstration of PMD connections, an FR injection was placed in PMDr of case 91-72L (Fig.2). The injection was placed in a portion of PMDr just medial to the FEF, where nose and eye movements predominated (Fig. 9A of Preuss et al, 1996). Labeled neurons were distributed throughout PMD, extending into the adjoining FEF. Other labeled neurons were densely packed in the rostral part of SMA, possibly including the supplementary eye field (SEF). Rostral M1 contained a number of labeled neurons, while PMV was unlabeled. A few foci of labeled neurons were found in frontal granular cortex rostral to PMD. Labeled cells were also noted in posterior parietal cortex on the lateral convexity and along the medial wall of the hemisphere, as well as in the caudal part of the lateral sulcus. As for somatosensory areas, no labeled cells were observed in anterior parietal areas or in areas PR, PV, or S2, but a few were present along the upper limiting sulcus of the insula in or near area VS.
In summary, the pattern of labeling after PMD injections was distinctly different than that after PMV injections. PMD injections labeled several different locations in posterior parietal cortex, as well as the SMA and pre-SMA, cingulate motor areas, and rostral (but not caudal) M1. Injections that involved the eye-movement representation of PMD labeled FEF and perhaps also SEF. Labeling of granular prefrontal cortex was sparse and variable. Labeling of PMV was sparse or absent.
Connections of M1
The present cases with PMV and PMD injections included two owl monkeys that also had injections of M1. These cases allow a comparison in the same case of M1 connections with those of PMV and PMD (see Stepniewska et al., 1993, for a description of M1 connections in other owl monkeys). In owl monkey 92-83 (Fig. 4), the M1 and PMV injections, centered about 4 mm apart, both involved forelimb representations, although some of the cortex involved in the PMV injection represented face movements. The PMV injection labeled neurons in and around the M1 injection core, and the M1 injection labeled neurons around the PMV injection core. In SMA and in the caudal cingulate motor area, the two injections labeled overlapping populations of neurons. In somatosensory cortex of the lateral fissure, the two injections labeled overlapping regions of PV, S2, VS, and lateral 1 and 2. In the lateral part of posterior parietal cortex, along the dorsal rim of the lateral sulcus, neurons projecting to M1 overlapped populations projecting to both PMV and PMD, even though the population projecting to PMD was largely caudal to one projecting to PMV. Similar results were obtained in a second case, 90-55, with M1 (see Stepniewska et al., 1993) and PMD injections. The results indicate that M1 and PMV share several sources of frontal motor and lateral somatosensory connections, while PMV and PMD share a small zone of lateral posterior parietal connectivity. Overlapping populations of neurons in SMA, Cgc, PV, S2, VS, areas 1 and 2, and lateral posterior parietal cortex projected to the hand representations in M1 and PMV.
DISCUSSION
In the present study, we injected tracers in the dorsal (PMD) and ventral (PMV) premotor areas in owl monkeys after these areas were identified by electrical stimulation using microelectrode mapping procedures. The resulting distributions of labeled neurons in ipsilateral neocortex were related to these maps and to architectonic definitions of cortical areas published earlier (Preuss et al., 1996). The results indicate that both PMD and PMV have connections with M1 and other motor areas of frontal cortex. A major difference in the cortical connections of the two areas is that PMV has many connections with somatosensory areas that are early in the hierarchy of cortical processing, while PMD has fewer connections with somatosensory cortex, and these are with higher level and possibly multimodal areas (see Kaas, 2004a,b). Another difference is that the posterior parietal connections of PMV are restricted to a lateral territory along the lip of the lateral sulcus, while PMD has connections with more dorsal and medial regions of posterior parietal cortex. In the following sections, we discuss the present results in owl monkeys in relation to previous studies on cortical organization and connections in owl monkeys and other primates, and then consider the functional implications of the findings. We compare results in New World owl monkeys with those in Old World macaque monkeys and prosimian galagos to identify similar features that were likely present in the earliest primates, as well as differences reflecting the evolutionary specializations of the different primate lineages. Finally, we use similarities in patterns of connectivity to suggest homologies between the posterior parietal fields of New World and Old World monkeys.
Connections of PMV and PMD with other areas of frontal cortex
The cortical connections of PMD and PMV in owl monkeys are summarized in Figure 9B. The patterns of connections revealed by different injections restricted to a single premotor area, either PMD or PMV, were highly similar and Figure 9B reflects this consistency across cases and injections. There were also minor differences in results across injections, which could reflect differences in the efficiency of the tracers employed and variations in the location of injections within an area, as well as individual differences in the connectivity of owl monkeys. In one or two instances, the uptake zone of an injection might have involved another field, such as M1, but the consistencies in results suggest that in most cases other areas were not significantly involved in the injections, nor did the injections involve the white matter.
The results indicate that both PMD and PMV have connections with primary motor cortex, M1. Injections of PMD labeled M1r predominantly or exclusively, while injections of PMV labeled both M1r and M1c, although M1r appeared to be more strongly labeled than M1c in several cases. The interconnections were largely, but not completely, between matching body-part representations. These interconnections were expected because our previous study of connections of M1 in owl monkeys revealed labeled neurons in PMD and PMV that were somatotopically matched and more dense after M1r injections, and injections in M1c sometimes failed to label any neurons in PMD or PMV (Stepniewska et al., 1993). Additional physiological and anatomical evidence that distinctions exist between rostral and caudal M1 has been presented in several previous reports (i.e. Strick and Preston, 1982a,b; Geyer et al., 1996; Zilles et al., 1996; Preuss et al., 1997). The differences in premotor connections may partly reflect differences in the somatotopy of M1r and M1c, as distal (digit) forelimb movements tend to have greater representation in M1c (Stepniewska et al, 1993), but differences in somatotopy would not appear to fully account for the greater interconnections of M1r than M1c with premotor cortex. Instead, it appears that M1r is more directly involved in premotor cortical functions than is M1c, the effects of M1c on premotor function being mediated through its connections with M1r, which are extensive in owl monkeys (Stepniewska et al., 1993) and other primates (e.g. Gatter and Powell, 1978; Huntley and Jones, 1991; Fang et al., 2005).
M1 connections with PMD and PMV have been widely reported in other primates. In particular, PMD have been reported in New World Cebus monkeys (Dum and Strick, 2005) as having the densest input to M1 of all frontal cortical areas, although it is not clear whether these connections were concentrated in rostral M1, as in owl monkeys. These connections involve the digit representations in these areas of Cebus monkeys, which are well developed in highly dextrous, tool-using Cebus monkeys (Moura and Lee, 2004), but not in owl monkeys. Thus, some differences in the somatotopic distribution of connections might be expected. Nevertheless, dense connections of M1 with PMD and PMV were demonstrated in both species of New World monkeys.
In macaque monkeys, the regions of PMD and PMV both connect densely with M1 (e.g. Künzle, 1978; Matsumara and Kubota, 1979; Muakkassa and Strick, 1979; Godschalk et al., 1984; Leichnetz, 1986; Matelli et al., 1986; Barbas and Pandya, 1987; Ghosh et al., 1987; Kurata, 1991; Huntley and Jones, 1991; Tokuno and Tanji, 1993; Ghosh and Gattera, 1995; Johnson and Ferraina, 1996). Digit and orofacial portions of M1 connect with PMV, while proximal forelimb and trunk M1 have more connections with PMD (Tokuno et al., 1997). Both PMD and PMV have been divided into rostral and caudal subareas in macaque monkeys (Barbas and Pandya, 1987; Matelli et al., 1985; Matelli et al., 1998), and these subdivisions reportedly differ in M1 connections (Fig. 9C). In macaques, caudal PMD has stronger M1 connections than rostral PMD (e.g. Barbas and Pandya, 1987). In owl monkeys there is evidence for a rostral (PMDr) and caudal (PMDc) divisions of PMD (Preuss et al., 1996). As in macaques, PMDc of owl monkeys appears to have stronger connections with M1. Finally, in galagos (Wu et al., 2000; Fang et al. 2005) connections of M1 with PMD are largely from the proximal forelimb and trunk representations of M1 while PMV is connected with the forelimb and orofacial representations of M1, which are located ventral to the proximal forelimb representation (Fig. 9A). Moreover, as in New World and Old World monkeys, the connections of M1 with PMD in galagos involve mainly caudal PMD. Thus, there are noteworthy similarities across major primate taxa in the connections of M1 with PMV and PMD.
Owl monkey PMD and PMV are also connected with the supplementary motor area (SMA), which occupies the medial margin of the cerebral hemisphere just rostral to the hindlimb representation in M1 (Preuss et al., 1996). The present results indicate that connections of PMD and PMV with somatotopically matched portion of SMA, may be even denser than those with M1. In New World Cebus monkeys, the projections from PMD and PMV to SMA are also dense (Dum and Strick, 2005). Major connections between SMA and the premotor areas, PMV and PMD, have also been reported in macaques (e.g. Kurata, 1991; Johnson and Ferraina, 1996) and galagos (Fang et al., 2005). The less dense and more variable connections of PMV and PMD with rostral and caudal cingulate motor areas in owl monkeys were also found in Cebus monkeys (Dum and Strick, 2005), galagos (Fang et al., 2005) and macaques (Matelli et al., 1986; Kurata, 1991; Luppino et al., 2003). Other connections of PMD and PMV in the frontal lobe of owl monkey are with adjoining parts of prefrontal cortex. However, these connections were relatively sparse, as in galagos (Fang et al., 2005). In macaques (Barbas and Pandya, 1987; Preuss and Goldman-Rakic, 1989; Luppino et al., 1993; Lu et al, 1994) and Cebus monkeys (Dum and Strick, 2005), however, there are dense connections between premotor and prefrontal cortex, especially between PMV and the cortex surrounding the principal sulcus.
PMD and PMV are interconnected, although these connections are weak compared with the connections of these areas with M1. Generally sparse connections between PMV and PMD have also been reported in macaques (see above), and galagos (Fang et al., 2005). The paucity of connections between PMD and PMV suggest that they are usually involved in distinct and somewhat independent functions. To the extent that PMV and PMD are devoted to different body parts, these premotor areas would be expected to have few interconnections. In contrast to other studied monkeys, Cebus monkeys have distinct digit representations in both PMD ad PMV, and these digit representations are densely interconnected (Dum and Strick, 2005). This apparent specialization of Cebus monkeys suggests an enhanced role for these areas in motor control of digits in Cebus monkeys, known for skillful use of their hands (Westergaard and Fragaszy, 1987).
Connections of PMV and PMD with somatosensory cortex
A major difference between PMD and PMV in owl monkeys is that PMV is strongly connected with the somotosensory areas of the anterior parietal lobe and lateral sulcus, while PMD is not. PMV injections labeled areas 3a, 1 and 2 in the anterior parietal lobe. In some cases, a few labeled cells were also present in areas 3b and 1. The upper bank of the lateral sulcus, in the regions of somatosensory areas S2, the parietal ventral area (PV), and parietal rostral area (PR), was densely labeled after PMV injections. The cells labeled in the anterior parietal somatosensory areas after PMV injections were located in regions consistent with the forelimb and orofacial representations of these areas, and thus appeared to match the injected parts of the representation in PMV. As areas 3a and 2 receive muscle receptor information from the ventroposterior superior nucleus of the thalamus (e.g. Cusick et al., 1985), they would largely relay proprioceptive information to PMV. Inputs from somatosensory areas PR, PV, S2, and VS in the lateral sulcus would provide somatosensory information that is relayed from areas 3a, 3b, 1 and 2 to S2 and PV, and then to PR and VS (Kaas, 2004 a, b).
The results of studies with injections of somatosensory areas provide further evidence for somatosensory input to PMV. In other New World monkeys, area 3b injections labeled few if any neurons in PMV, while injections involving area 3a or areas 1 and 2 did label neurons in the PMV region (Krubitzer and Kaas, 1990; Huffman and Krubitzer, 2001; Coq et al., 2004; Padberg et al., 2005). Likewise, injections in S2 and PV labeled neurons in PMV (Krubitzer and Kaas, 1990; Qi et al., 2002; see Disbrow et al., 2003 for macaques). Thus, PMV receives inputs from an array of somatosensory areas that represent both cutaneous and proprioceptive receptors (see Qi et al., 2002 for review).
Studies based on injections of tracers to PMV in other primates have also revealed connections with somatosensory areas. In galagos PMV injections labeled neurons in areas 3a, 1, 2, S2 and PV (Fang et al., 2005). However, labeling of the cortex in the lateral sulcus was sparse in these animals. In macaques, injections in PMV labeled neurons in the regions of S2 and other somatosensory areas of the upper bank of the lateral sulcus (Godschalk et al., 1984; Matelli et al., 1986; Barbas and Pandya, 1987; Kurata, 1991) and in area 3a (Kurata, 1991). In the study of premotor cortex connections in New World Cebus monkeys (Dum and Strick, 2005), connections with somatosensory cortex were not examined.
In summary, the functions of PMV appear to depend greatly on somatosensory inputs. These inputs could provide important proprioceptive and tactile information for the guidance of grasping and manipulation movements of the hand, some of the proposed functions of PMV (Rizzolatti et al., 1998). The physiological properties of neurons in PMV have been studied in macaques, and many neurons respond to tactile stimuli (Rizzolatti et al., 1981; Gentilucci et al., 1988; Fogassi et al., 1996; Graziano et al., 1997). While deactivation of PMV with muscimol did not notably impair motor performance with the contralateral hand, it did make monkeys less likely to reach with the contralateral hand or reach into contralateral visual space (Schieber, 2000).
In contrast to PMV, PMD has few connections with unimodal somatosensory cortex in owl monkeys. Connections originate in or near the ventral somatosensory area (VS), and in more caudal cortex in the lateral sulcus, a region that may include the retroinsular area and part of area 7b (Robinson and Burton, 1980) and area CS of Cusick et al. (1989). While neurons in these regions are likely to have somatosensory and possibly multisensory functions, they are less directly related to somatosensory inputs than neurons in the areas of the lateral sulcus projecting to PMV. In addition, these somatosensory areas project less strongly to PMD than to PMV. In galagos, similarly, only a few labeled cells were detected in the cortex of the lateral sulcus or in areas 3a and 1-2 after PMD injections (Fang et al., 2005). Macaques also appear to have few or no connections between areas of anterior or lateral parietal cortex and PMD.
Connections of PMV and PMD with posterior parietal cortex
In owl monkeys, both PMV and PMD have substantial connections with posterior parietal cortex. Comparing the parietal connections of owl monkeys to those of macaque monkeys is not straightforward, however, because owl monkeys lack an intraparietal sulcus, which is an important landmark in macaques and many other primates. In owl monkeys, the lateral sulcus extends quite dorsally, and may extend into cortex homologous to the convexity of the inferior parietal lobule of macaques, and possibly even into the homologues of intraparietal sulcal cortex. In owl monkeys, injections of PMV labeled the cortex along the dorsal rim of the lateral sulcus, the region of labeling extending from the buried upper bank of the sulcus across the rim onto the lateral convexity (see especially Figs. 2-4). Injections of area PMD also labeled cells in this region, but much more labeling was observed dorsally and medially on the lateral convexity near the medial margin of the hemisphere and on the medial wall of the hemisphere at the same rostrocaudal level (see especially Fig. 2). In macaques, area PMV is known to be very strongly interconnected with the anterior inferior parietal cortex (area 7b), the ventral intraparietal area (VIP), and the anterior intraparietal area (AIP), while area PMD is connected primarily with area 5 in the superior parietal lobule, and with the cortex of the posterior inferior parietal lobule, caudal cortex of the intraparietal sulcus, and medial parietal lobe (Ghosh and Gattera, 1995; Johnson et al., 1996; Matelli et al., 1998; Luppino et al., 1999; Nakamura et al., 2001; Tanné-Gariepy et al., 2002). The more posterior and medial parietal areas of macaques have gone by a variety of different names; if the nomenclature of Cavada and Goldman-Rakic (1989) is followed, they would include areas 7a, 7ip, and 7m. On this basis, we suggest that (1) the cortex along the dorsal rim of the lateral sulcus in owl monkeys labeled by PMV injections is homologous to area 7b and possibly also areas AIP and VIP of macaques; (2) the cortex on the medial wall labeled after PMD injections corresponds to 7m; (3) the cortex on the dorsal convexity labeled after PMD injections includes area 5; and (4) the cortex in the caudal portion of the lateral sulcus corresponds to areas 7ip and 7a, and possibly areas MIP and lateral PO. (See Padberg et al., 2005 for a recent comparison of posterior parietal areas in New and Old World monkeys, and Cohen and Anderson, 2002, for a recent review of intraparietal areas and their proposed functions).
PMD injections in owl monkeys, especially injections that involved PMDr, labeled an additional region of cortex deep within the lateral sulcus, at the level of, and extending posterior to presumptive area 7b. In macaques, it appears that the ventral intraparietal area (VIP) projects to caudal PMV (PMVc or F4), while the adjoining anterior intraparietal cortex (AIP) projects to rostral PMV (PMVr or F5; Luppino et al, 1999), although others conclude that VIP projects to PMD while the projection to PMV comes from AIP and cortex medial and lateral to AIP (Lewis and Van Essen, 2000; Tanné-Gariepy et al., 2002). These differences in interpretation may reflect difficulties in defining boundaries of areas in the intraparietal cortex. In any case, it appears that cortex in the caudal portion of the lateral sulcus of owl monkeys may correspond to VIP, AIP or both areas of macaques.
In macaques, projections from more caudal parts of posterior parietal cortex, which have more direct visual inputs, project more densely to rostral PMD (PMDr), while more rostromedial parts of posterior parietal cortex, including MIP (the parietal reach region) project more densely to caudal PMD (PMDc) (Johnson et al., 1996; Matelli et al., 1998; Shipp et al, 1998; Battaglia-Mayer et al., 2001; Marconi et al.,2001; Tanné-Gariepy et al., 2002). Current proposals suggest that these posterior parietal regions that project to PMD are part of a network for reaching to objects of interest under visual guidance (Johnson et al., 1996; see also Cohen and Andersen, 2002; Tanné-Gariepy et al., 2002 for review).
As in owl monkeys, PMD injections in galagos labeled neurons in more medial and caudal portions of posterior parietal cortex than PMV injections (Fang et al., 2005). Recent microstimulation experiments in posterior parietal cortex of galagos indicate that reaching movements are evoked from a region of cortex caudal and medial to the region where grasping hand-to-mouth movements were evoked (Stepniewska et al., 2005). Thus, separate parietofrontal circuits for reaching and grasping probably emerged early in primate evolution, and have been maintained in the major branches.
Similarities and difference in the premotor cortex organization of primates
The major connections of dorsal and ventral premotor cortex in New World owl monkeys in comparison with prosimian galagos and Old World macaque monkeys are illustrated in Figure 9. A number of similarities can be noted in the organization of premotor region across these primates. First, in all these primates premotor cortex on the dorsolateral convexity can be divided into dorsal (PMD) and ventral (PMV) regions. In all three primates, PMD represents hindlimb, trunk and forelimb as well as oculomotor movements and is strongly connected to areas in the dorsomedial posterior parietal cortex. In New World and Old World monkeys, and possibly in galagos, PMD can be divided into rostral (PMDr) and caudal (PMDc) regions, each with a different architecture, and distinctive physiological properties and connections. The ventral premotor (PMV) area represents orofacial and forelimb movements, and there is no convincing evidence in any of these primates that PMV represents hindlimb or lower trunk movements to any significant extent. PMV is strongly connected to the areas in the ventral or lateral posterior parietal cortex. In all studied primates except Cebus monkeys, PMD and PMV premotor regions are only weakly interconnected. Thus, there are clear similarities in the organization of premotor region across primates.
There are also differences in premotor cortex organization across primates, and these differences primarily involve the ventral premotor region. In anthropoid primates, especially in macaques, PMV represents proximal and distal forelimb movements, and it has dense connections with somatosensory cortex of the lateral sulcus (S2, PV, PR -all areas with prominent cutaneous receptor inputs), whereas galagos PMV represents only proximal forelimb movements and PMV has few connections with somatosensory areas of the lateral sulcus. Moreover, PMV in Old World macaques and in New World Cebus monkeys has strong connections with the prefrontal association cortex in the ventral bank of the principal sulcus, whereas PMV in owl monkeys or galagos has only few connections with dorsolateral prefrontal cortex. On the bases of regional differences in physiological properties, connections and architecture, PMV has been divided into rostral and caudal subdivisions in macaques, but there is no clear basis for such a distinction in owl monkeys or galagos. These differences in organization of the ventral premotor region in galagos, New World owl monkeys, and macaques suggest that the PMV region changed in evolution to mediate greater control of forelimb movements in reaching, grasping, and retrieving as these behaviors became more important in New World monkeys and especially in Old World monkeys.
AKNOWLEDGEMENTS
The authors thank Judy Ives and Laura Trice for technical assistance.
Supported by: NIH grant NS 16446
REFERENCES
- Barbas H, Pandya DN. Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol. 1987;256:211–228. doi: 10.1002/cne.902560203. [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A, Ferraina S, Genovesio A, Marconi B, Squatrito S, Molinari M, Lacquaniti F, Caminiti R. Eye-hand coordination during reaching. II. An analysis of the relationships between visuomanual signals in parietal cortex and parieto-frontal association projections. Cereb Cortex. 2001;11:528–544. doi: 10.1093/cercor/11.6.528. [DOI] [PubMed] [Google Scholar]
- Beck PD, Pospichal MW, Kaas JH. Topography, architecture, and connections of somatosensory cortex in opossums: evidence for five somatosensory areas. J Comp Neurol. 1996;366:109–133. doi: 10.1002/(SICI)1096-9861(19960226)366:1<109::AID-CNE8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey.I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Cohen YE, Andersen RA. A common reference frame for movement plans in the posterior parietal cortex. Nat Rev Neurosci. 2002;3:553–62. doi: 10.1038/nrn873. [DOI] [PubMed] [Google Scholar]
- Coq JO, Qi H, Collins CE, Kaas JH. Anatomical and functional organization of somatosensory areas of the lateral fissure of the New World titi monkey (Callicebus moloch). J Comp Neurol. 2004;476:363–87. doi: 10.1002/cne.20237. [DOI] [PubMed] [Google Scholar]
- Cusick CG, Steindler DA, Kaas JH. Corticocortical and collateral thalamocortical connections of postcentral somatosensory cortical areas in squirrel monkeys: A double-labeling study with radiolabeled wheatgerm agglutinin and wheatgerm agglutinin conjugated to horseradish peroxidase. Somatosensory Res. 1985;3:1–31. doi: 10.3109/07367228509144574. [DOI] [PubMed] [Google Scholar]
- Cusick CG, Wall JT, Felleman DJ, Kaas JH. Somatotopic organization of the lateral sulcus of owl monkeys: area 3b, S-II, and a ventral somatosensory area. J Comp Neurol. 1989;282:169–90. doi: 10.1002/cne.902820203. [DOI] [PubMed] [Google Scholar]
- Disbrow E, Litinas E, Recanzone GH, Padberg J, Krubitzer L. Cortical connections of the second somatosensory area and the parietal ventral area in macaque monkeys. J Comp Neurol. 2003;462:382–399. doi: 10.1002/cne.10731. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiol Behav. 2002;77:677–682. doi: 10.1016/s0031-9384(02)00929-0. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J Neurosci. 2005;25:1375–1386. doi: 10.1523/JNEUROSCI.3902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P-C, Stepniewska I, Kaas JH. Ipsilateral cortical connections of motor, premotor, frontal eye field and posterior parietal cortex in a prosimian primate Galago garnetti. J Comp Neurol. 2005 doi: 10.1002/cne.20665. (in press) [DOI] [PubMed] [Google Scholar]
- Fink GR, Frackowiak RSJ, Pietrzyk U. Multiple nonprimary motor areas in the human cortex. J. Neurophysiol. 1997;77:2164–2174. doi: 10.1152/jn.1997.77.4.2164. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Fadiga L, Luppino G, Matelli M, Rizzolatti G. Coding of peripersonal space in inferior premotor cortex (area F4) J Neurophysiol. 1996;76:141–157. doi: 10.1152/jn.1996.76.1.141. [DOI] [PubMed] [Google Scholar]
- Gallyas F. Silver staining of myelin by means of physical development. Neurol Res. 1979;1:203–209. doi: 10.1080/01616412.1979.11739553. [DOI] [PubMed] [Google Scholar]
- Gatter KC, Powell TPS. The intrinsic connections of the cortex of area 4 of the monkey. Brain. 1978;101:513–541. doi: 10.1093/brain/101.3.513. [DOI] [PubMed] [Google Scholar]
- Geneser-Jensen FA, Blackstad TW. Distribution of acetyl cholinesterase in the hippocampal region of the guinea pig. I. Entorhinal area, parasubiculum, and presubiculum. Z Zellforsch Mikrosk Anat. 1971;114:460–481. doi: 10.1007/BF00325634. [DOI] [PubMed] [Google Scholar]
- Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R, Rizzolatti G. Functional organization of inferior area 6 in the macaque monkey. I. Somatotopy and the control of proximal movements. Exp Brain Res. 1988;71:475–490. doi: 10.1007/BF00248741. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, Klingberg T, Larsson J, Zilles K, Roland PE. Two different areas within the primary motor cortex of man. Nature. 1996;382:805–807. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- Geyer S, Matelli M, Luppino G, Zilles K. Functional neuroanatomy of the primate isocortical motor system. Anat Embryol. 2000;202:443–474. doi: 10.1007/s004290000127. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Brinkman C, Porter R. A quantitative study of the distribution of neurons projecting to the precentral motor cortex in the monkey (M. fascicularis). J Comp Neurol. 1987;259:424–444. doi: 10.1002/cne.902590309. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Gattera R. A comparison of the ipsilateral cortical projections to the dorsal and ventral subdivisions of the macaque premotor cortex. Somatosens Mot Res. 1995;12:359–378. doi: 10.3109/08990229509093668. [DOI] [PubMed] [Google Scholar]
- Gibson AR, Hansma DI, Houk JC, Robinson FR. A sensitive low artifact TMB procedure for the demonstration of WGA-HRP in the CNS. Brain Res. 1984;298:235–241. doi: 10.1016/0006-8993(84)91423-9. [DOI] [PubMed] [Google Scholar]
- Godschalk M, Lemon RN, Kuypers HG, Ronday HK. Cortical afferents and efferents of monkey postarcuate area: an anatomical and electrophysiological study. Exp Brain Res. 1984;56:410–424. doi: 10.1007/BF00237982. [DOI] [PubMed] [Google Scholar]
- Godschalk M, Mitz AR, van Duin B, van der Burg H. Somatotopy of monkey premotor cortex examined with microstimulation. Neurosci Res. 1995;23:269–279. doi: 10.1016/0168-0102(95)00950-7. [DOI] [PubMed] [Google Scholar]
- Gould HJ, 3rd, Cusick CG, Pons TP, Kaas JH. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. J Comp Neurol. 1986;247:297–325. doi: 10.1002/cne.902470303. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Hu XT, Gross CG. Visuospatial properties of ventral premotor cortex. J Neurophysiol. 1997;77:2268–2292. doi: 10.1152/jn.1997.77.5.2268. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. II. Cortical connections. J Comp Neurol. 1987;265:332–361. doi: 10.1002/cne.902650304. [DOI] [PubMed] [Google Scholar]
- Huffman KJ, Krubitzer L. Area 3a: Topographic organization and cortical connections in marmoset monkeys. Cereb Cortex. 2001;11:849–867. doi: 10.1093/cercor/11.9.849. [DOI] [PubMed] [Google Scholar]
- Huntley GW, Jones EG. Relationship of intrinsic connections to forelimb movement representations in monkey motor cortex: a correlative anatomic and physiological study. J Neurophysiol. 1991;66:390–413. doi: 10.1152/jn.1991.66.2.390. [DOI] [PubMed] [Google Scholar]
- Jain N, Catania KC, Kaas JH. A histologically visible representation of the fingers and palm in primate area 3b and its immutability following long-term deafferentations. Cereb Cortex. 1998;8:227–236. doi: 10.1093/cercor/8.3.227. [DOI] [PubMed] [Google Scholar]
- Johnson PB, Ferraina S. Cortical networks for visual reaching: intrinsic frontal lobe connectivity. Eur J Neurosci. 1996;8:1358–1362. doi: 10.1111/j.1460-9568.1996.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Bianchi L, Caminiti R. Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex. 1996;6:102–119. doi: 10.1093/cercor/6.2.102. [DOI] [PubMed] [Google Scholar]
- Jones EG, Burton H. Areal differences in the laminar distribution of thalamic afferents in cortical fields of the insular, parietal, and temporal regions of primates. J Comp Neurol. 1976;168:197–248. doi: 10.1002/cne.901680203. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Evolution of somatosensory and motor cortex in primates. Anat Rec. 2004a;281A:1148–1156. doi: 10.1002/ar.a.20120. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Somatosensory system. In: Paxinos G, Mai JK, editors. The human nervous system. 2d edition Elsevier Academic Press; London: 2004b. pp. 1054–1092. [Google Scholar]
- Kaas JH, Collins CE. The ressurection of multisensory cortex in primates: Connection patterns that integrates modalities. In: Cavert GA, Spense C, Stein BE, editors. The handbook of multisensory processes. MIT Press; Cambridge, Mass: 2004. pp. 285–293. [Google Scholar]
- Krubitzer LA, Kaas JH. The organization and connections of somatosensory cortex in marmosets. J Neurosci. 1990;10:952–974. doi: 10.1523/JNEUROSCI.10-03-00952.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzle H. An autoradiographic analysis of the efferent connections from premotor and adjacent prefrontal regions (areas 6 and 9) in macaca fascicularis. Brain Behav Evol. 1978;15:185–234. doi: 10.1159/000123779. [DOI] [PubMed] [Google Scholar]
- Kurata K. Corticocortical inputs to the dorsal and ventral aspects of the premotor cortex of macaque monkeys. Neurosci Res. 1991;12:263–280. doi: 10.1016/0168-0102(91)90116-g. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR. Afferent and efferent connections of the dorsolateral precentral gyrus (area 4, hand/arm region) in the macaque monkey, with comparisons to area 8. J Comp Neurol. 1986;254:460–492. doi: 10.1002/cne.902540403. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000;428:112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Lu MT, Preston JB, Strick PL. Interconnections between the prefrontal cortex and the motor areas in the frontal lobe. J Comp Neurol. 1994;341:375–392. doi: 10.1002/cne.903410308. [DOI] [PubMed] [Google Scholar]
- Luppino G, Rizzolatti G. The organization of the frontal motor cortex. News Physiol Sci. 2000;15:219–224. doi: 10.1152/physiologyonline.2000.15.5.219. [DOI] [PubMed] [Google Scholar]
- Luppino G, Murata A, Govoni P, Matelli M. Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4). Exp Brain Res. 1999;128:181–187. doi: 10.1007/s002210050833. [DOI] [PubMed] [Google Scholar]
- Luppino G, Rossi S, Calgavara R, Matelli M. Prefrontal and agranular cingulated projections to the dorsal premotor areas F2 and F7 in the macaque monkey. Eur J Neurosci. 2003;17:559–578. doi: 10.1046/j.1460-9568.2003.02476.x. [DOI] [PubMed] [Google Scholar]
- Marconi B, Genovesio A, Battaglia-Mayer A, Ferraina S, Squatrito S, Molinari M, Lacquaniti F, Caminiti R. Eye-hand coordination during reaching. I. Anatomical relationships between parietal and frontal cortex. Cereb Cortex. 2001;11:513–527. doi: 10.1093/cercor/11.6.513. [DOI] [PubMed] [Google Scholar]
- Martin RD. Palaeontology: Chinese lantern for early primates. Nature. 2004;427:22–23. doi: 10.1038/427022a. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G, Rizzolatti G. Patterns of cytochrome oxidase activity in the frontal agranular cortex of the macaque monkey. Behav Brain Res. 1985;18:125–136. doi: 10.1016/0166-4328(85)90068-3. [DOI] [PubMed] [Google Scholar]
- Matelli M, Camarda R, Glickstein M, Rizzolatti G. Afferent and efferent projections of the inferior area 6 in the macaque monkey. J Comp Neurol. 1986;254:460–492. doi: 10.1002/cne.902510302. [DOI] [PubMed] [Google Scholar]
- Matelli M, Govoni P, Galletti C, Kutz DF, Luppino G. Superior area 6 afferents from the superior parietal lobule in the macaque monkey. J Comp Neurol. 1998;402:327–352. [PubMed] [Google Scholar]
- Matsumura M, Kubota K. Cortical projection to hand-arm motor area from post-arcuate area in macaque monkeys: a histological study of retrograde transport of horseradish peroxidase. Neurosci Lett. 1979;11:241–246. doi: 10.1016/0304-3940(79)90001-6. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Sur M, Lin CS. Double representation of the body surface within cytoarchitectonic areas 3b and 1 in “SI” in the owl monkey (Aotus trivirgatus). J Comp Neurol. 1978;181:41–73. doi: 10.1002/cne.901810104. [DOI] [PubMed] [Google Scholar]
- Morel A, Kaas JH. Subdivisions and connections of auditory cortex in owl monkeys. J Comp Neurol. 1992;318:27–63. doi: 10.1002/cne.903180104. [DOI] [PubMed] [Google Scholar]
- Moura AC, Lee PC. Capuchin stone tool use in Caatinga dry forest. Science. 2004;306:1909. doi: 10.1126/science.1102558. [DOI] [PubMed] [Google Scholar]
- Muakkassa KF, Strick PL. Frontal lobe inputs to primate motor cortex: evidence for four somatotopically organized 'premotor' areas. Brain Res. 1979;177:176–182. doi: 10.1016/0006-8993(79)90928-4. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kuroda T, Wakita M, Kusunoki M, Kato A, Mikami A, Sakata H, Itoh K. From three-dimentional space vision to prehensile hand movements: The lateral intraparietal area links the area V3A and the anterior intraparietal area in macaques. J Neurosci. 2001;21:8174–8187. doi: 10.1523/JNEUROSCI.21-20-08174.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padberg J, Disbrow E, Krubitzer L. The organization and connections of anterior and posterior parietal cortex in titi monkeys: Do New World monkeys have an area 2? Cereb Cortex. 2005 doi: 10.1093/cercor/bhi071. [DOI] [PubMed] [Google Scholar]
- Picard Strick, PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–53. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Picard Strick., PL Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–72. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Stepniewska I, Kaas JH. Movement representation in the dorsal and ventral premotor areas of owl monkeys: a microstimulation study. J Comp Neurol. 1996;371:649–676. doi: 10.1002/(SICI)1096-9861(19960805)371:4<649::AID-CNE12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Connections of ventral granular frontal cortex of macaques with perisylvian premotor and somatosensory areas: anatomical evidence for somatic representation in primate frontal association cortex. J Comp Neurol. 1989;282:293–316. doi: 10.1002/cne.902820210. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate Galago and the anthropoid primate Macaca. J Comp Neurol. 1991a;310:429–474. doi: 10.1002/cne.903100402. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Architectonics of the parietal and temporal association cortex in the strepsirhine primate Galago compared to the anthropoid primate Macaca. J Comp Neurol. 1991b;310:475–506. doi: 10.1002/cne.903100403. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Ipsilateral cortical connections of granular frontal cortex in the strepsirhine primate Galago, with comparative comments on anthropoid primates. J Comp Neurol. 1991c;310:507–549. doi: 10.1002/cne.903100404. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Stepniewska I, Jain N, Kaas JH. Multiple divisions of macaque precentral motor cortex identified with neurofilament antibody SMI-32. Brain Res. 1997;767:148–153. doi: 10.1016/s0006-8993(97)00704-x. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Burton H. Organization of somatosensory receptive fields in cortical areas 7b, retroinsula, postauditory and granular insula of M. fascicularis. J Comp Neurol. 1980;192:69–92. doi: 10.1002/cne.901920105. [DOI] [PubMed] [Google Scholar]
- Qi H-X, Lyon DC, Kaas JH. Cortical and thalamic connections of the parietal ventral somatosensory area in marmoset monkeys (Callithrix jacchus) J Comp Neurol. 2002;443:168–182. doi: 10.1002/cne.10113. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol. 1998;106:283–296. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Scandolara C, Gentilucci M, Camarda R. Response properties and behavioral modulation of mouth neurons of the postarcuate cortex (area 6) in macaque monkeys. Brain Res. 1981;255:421–424. doi: 10.1016/0006-8993(81)90847-7. [DOI] [PubMed] [Google Scholar]
- Sakai ST, Stepniewska I, Qi H-X, Kaas JH. Pallidal and cerebellar afferents to presupplementary motor area thalamocortical neurons in the owl monkey: A multiple labeling study. J. Comp. Neurol. 2000;417:164–180. [PubMed] [Google Scholar]
- Schieber MH. Inactivation of the ventral premotor cortex bisase the laterality of motoric choices. Exp. Brain Res. 2000;130:497–507. doi: 10.1007/s002219900270. [DOI] [PubMed] [Google Scholar]
- Shipp S, Blanton M, Zeki S. A visuo-somatomotor pathway through superior parietal cortex in the macaque monkey: cortical connections of areas V6 and V6A. Eur J Neurosci. 1998;10:3171–3193. doi: 10.1046/j.1460-9568.1998.00327.x. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Preuss TM, Kaas JH. Architectonics, somatotopic organization, and ipsilateral cortical connections of the primary motor area (M1) of owl monkeys. J Comp Neurol. 1993;330:238–271. doi: 10.1002/cne.903300207. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Fang P-C, Kaas JH. Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc Natl Acad Sci USA. 2005;102:4878–4883. doi: 10.1073/pnas.0501048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Preston JB. Two representations of the hand in area 4 of a primate. I. Motor output organization. J Neurophysiol. 1982a;48:139–149. doi: 10.1152/jn.1982.48.1.139. [DOI] [PubMed] [Google Scholar]
- Strick PL, Preston JB. Two representations of the hand in area 4 of a primate. II. Somatosensory input organization. J Neurophysiol. 1982b;48:150–159. doi: 10.1152/jn.1982.48.1.150. [DOI] [PubMed] [Google Scholar]
- Tanné-Gariepy J, Bello A, Fadiga L, Rizzolatti G. Parietal inputs to dorsal versus ventral premotor areas in the macaque monkey: evidence for largely segregated visuomotor pathways. Exp Brain Res. 2002;145:91–103. doi: 10.1007/s00221-002-1078-9. [DOI] [PubMed] [Google Scholar]
- Tokuno H, Tanji J. Input organization of distal and proximal forelimb areas in the monkey primary motor cortex: a retrograde double labeling study. J Comp Neurol. 1993;333:199–209. doi: 10.1002/cne.903330206. [DOI] [PubMed] [Google Scholar]
- Tokuno H, Takada M, Nambu A, Inase M. Reevaluation of ipsilateral corticocortical inputs to the orofacial region of the primary motor cortex in the macaque monkey. J Comp Neurol. 1997;389:34–48. doi: 10.1002/(sici)1096-9861(19971208)389:1<34::aid-cne3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Fragaszy DM. The manufacture and use of tools by capuchin monkeys (Cebus apella). Zoo Biol. 1987;4:317–327. [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Ann Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Wu CW, Bichot NP, Kaas JH. Converging evidence from microstimulation, architecture, and connections for multiple motor areas in the frontal and cingulate cortex of prosimian primates. J Comp Neurol. 2000;423:140–177. doi: 10.1002/1096-9861(20000717)423:1<140::aid-cne12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Wu CW, Kaas JH. Somatosensory cortex of prosimian Galagos: physiological recording, cytoarchitecture, and corticocortical connections of anterior parietal cortex and cortex of the lateral sulcus. J Comp Neurol. 2003;457:263–292. doi: 10.1002/cne.10542. [DOI] [PubMed] [Google Scholar]
- Zilles K, Schlaug G, Matelli M, Luppino G, Schleicher A, Qu M, Dabringhaus A, Seitz R, Roland PE. Mapping of human and macaque sensorimotor areas by integrating architectonic, transmitter receptor, MRI and PET data. J Anat. 1996;187:515–537. [PMC free article] [PubMed] [Google Scholar]