Abstract
Objective:
The study aimed to examine the association based on objective estimates of sleep duration and quality and aortic stiffness while accounting for the potential confounding effect of SDB.
Method:
Participants were part of the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep study. Sleep duration and quality were assessed by 7-day wrist actigraphy, SDB by home polysomnography, and aortic stiffness by magnetic resonance imaging (MRI)-based aortic pulse wave velocity (aPWV), ascending and descending aorta distensibility (AAD and DAD). Aortic stiffness of participants with ‘normal’ sleep duration (6–8 hours) were compared with those of ‘short’ (<6 hours) and ‘long’ sleep duration (>8 hours) adjusting for common cardiovascular risk factors and apnea hypopnea index (AHI).
Results:
The sample consisted of 908 participants (mean age 68.4±9.1 years, 55.3% female). There was a significant linear trend of increased aPWV across short (n=252), normal (n=552), and long sleep durations (n=104) (p for trend=0.008). Multivariable analysis showed that people with short sleep duration had 0.94 m/s lower aPWV (95% CI: −1.54, −0.35), compared with those with normal sleep duration.
Conclusion:
In this ethnically diverse community cohort, habitual short sleep duration as estimated by actigraphy was associated with lower aortic stiffness.
Keywords: Sleep duration, sleep quality, arterial stiffness, pulse wave velocity
Introduction
Arterial stiffness is an important marker of aging and hypertension (Najjar et al., 2008). Because of their proximity to the heart, the aorta and the aorto-illiac pathway make the largest contribution to buffering pulsatile cardiac output (Cavalcante, Lima, Redheuil, & Al-Mallah, 2011). Tonometry-based carotid-femoral pulse wave velocity (cfPWV) has been considered the gold standard for arterial stiffness and has been shown to predict future cardiovascular disease (CVD) and CVD mortality.
Recently, quantification of aortic pulse wave velocity (aPWV) and aortic distensibility (AD) using magnetic resonance imaging (MRI) became available and these measures have been reported to be more strongly related to age than several other noninvasive measures of elastic properties of the aorta, including cfPWV (Redheuil et al., 2010). MRI-based aPWV and AD have also been shown to be independent predictors of CVD outcomes (Ohyama et al., 2017; Redheuil et al., 2014).
Poor sleep is being increasingly recognized as a risk to cardiovascular (CV) health. In efforts to better understand the underlying mechanism by which impaired sleep exerts its adverse impact on CV health, an increasing number of studies have examined various aspects of sleep in relation to subclinical markers of CVD. While most of the studies have focused on sleep disordered breathing (SDB), a limited number of studies have recently addressed other aspects of sleep such as sleep duration and quality (Erden et al., 2010; Nagai, Hoshide, Nishikawa, Shimada, & Kario, 2013; Yamaki, Sato, & Fujii, 2015). In particular, several studies reported that sleep duration and quality are significantly associated with arterial stiffness (Cao, Zhou, Yuan, & Chen, 2016a; Niijima et al., 2016; Tsai et al., 2014a; Yoshioka et al., 2011a). However, in these studies, sleep duration and quality were self-reported. Self-reported sleep duration is weakly associated with objective sleep duration, and self-reported sleep quality (vs. objective estimates) are likely to capture different domains of sleep quality (Landry, Best, & Liu-Ambrose, 2015). No previous studies have used MRI-based aortic stiffness as an arterial stiffness measure in relation to sleep duration and quality. Further, limited by the study designs, previous reports have not accounted for SDB which itself is an independent predictor of increased arterial stiffness (Doonan et al., 2011; Wang et al., 2015). Therefore, we aimed to examine the association between objective estimates of sleep duration and quality from actigraphy, and MRI-based aortic stiffness, taking into account SDB.
Method
Study Sample
The aims and design of the Multi-Ethnic Study of Atherosclerosis (MESA) have been previously published (Bild et al., 2002). In brief, the MESA is a multi-center cohort study of White, African American, Hispanic, and Chinese adults living in six US cities (Winston-Salem, New York, Baltimore, Minneapolis, Chicago, and Los Angeles). At baseline, 6814 men and women 45–84 years of age without known CVD were recruited between 2000 and 2002. All MESA participants other than those reporting regular use of oral devices, nocturnal oxygen, or nightly continuous positive airway pressure were invited to participate in the MESA Sleep study at MESA Exam 5 (2010–2013). We included a subset of participants who underwent sleep assessments mainly wrist actigraphy and polysomnography (PSG) along with a cardiac MRI. Compared with the entire MESA group, the subjects of this study were slightly younger (mean difference =1.8 years, p=<.0001) and there was no difference in gender distribution between two groups (p=.18). All participants provided written informed consent for study participation, which was approved by the institutional review boards (IRBs) of all MESA field centers. This secondary analysis of the MESA data was approved by the University of Virginia IRB.
Assessment of Sleep Parameters
Estimates of sleep duration and quality were derived from wrist actigraphy. Details of actigraphy methodology in MESA have been published previously (Ogilvie et al., 2016). Study participants wore the Actiwatch Spectrum (Philips Respironics, Murrysville, PA) on the dominant wrist for seven consecutive days. Participants with wrist actigraphy data containing at least four weekdays and one weekend day were included. Actigraphy data were analyzed by using Actiware-Sleep version 5.59 software and scored in 30-sec epochs. Estimates of sleep start and end time were determined by decreased and increased activity count, using the Cole-Kripke algorithm (Cole, Kripke, Gruen, Mullaney, & Gillin, 1992). Sleep duration was defined as the duration of main sleep periods between sleep start and end time, averaged across all nights. These times were compared with the event marker, sleep journal bed and wake times, and light level changes.Based on the previously reported U-shaped relationship of self-reported sleep duration with metabolic, cardiovascular, and all-cause mortality (Cao, Zhou, Yuan, & Chen, 2016b; Cappuccio, D’Elia, Strazzullo, & Miller, 2010; Niijima et al., 2016; Qureshi, Giles, Croft, & Bliwise, 1997), sleep duration as estimated by actigraphy was categorized as short (less than 6 hours per night), normal (6 to 8 hours per night), or long (more than 8 hours per night). Estimates of sleep quality included sleep efficiency and wake after sleep onset (WASO). Estimated sleep efficiency (%) was defined as the average proportion of time spent asleep during the in bed time, and was calculated by taking the sum of all sleep time divided by the sum of all in bed time during main sleep intervals across the actigraphy recording. WASO (minutes) was estimated as the average time spent awake between waking up after falling asleep and falling asleep again, and was calculated by dividing the sum of all wake after sleep onset values across the recording by the total number of main sleep periods. The apnea-hypopnea index (AHI), a measure of SDB severity, was assessed by 15-channel home-PSG during the MESA Sleep study as has been described previously (Kwon et al., 2015). Apneas were defined as a greater than or equal to 90% reduction in the thermocouple signal for more than or equal to 10 seconds. Hypopnea was defined as more than or equal to 50% reduction in airflow coupled with 3% oxygen desaturation or an arousal as measured by pulse oximetry.
Aortic Stiffness and Covariate Data
Aortic stiffness was measured by the cardiac MRI using 1.5-T whole-body MRI scanners as described elsewhere (Ohyama et al., 2016). Participants were scanned in a supine position with the following imaging parameters: repetition time, 10 ms; echo time, 1.9 ms; field of view, 34 cm; section thickness, 8 mm; matrix size, 256×224; 2 signal averages; temporal resolution, 20 ms; velocity encoding gradient, 150 cm/s in the superior-to-inferior direction; and receiver bandwidth, ±32 kHz. By using ARTFUN software (INSERM U678), the flow wave transit time between ascending and descending aorta was calculated as the average time difference among all data points on the systolic upslope of the ascending and descending aortic flow curves after peak flow normalization. The distance between ascending and descending aorta was precisely measured at locations where velocities were measured using the oblique sagittal image (perpendicular to the aortic lumen) at the level of the right pulmonary artery during breath hold. aPWV (m/s) was determined by dividing the distance (mm) by the transit time between ascending to descending aorta (ms).
Aortic distensibility was calculated by using the following formula:
aortic distensibility = [(maximum aortic area - minimum aortic area)/ minimum aortic area] / (systolic BP - diastolic BP)
Covariates were based on information obtained from the exam 5 visit and included the following: age, gender, race/ethnicity, body mass index (BMI), smoking status, anti-hypertensive medication use, fasting glucose, HDL-c, LDL-c, triglycerides, lipid lowering medication use, AHI, left ventricular ejection fraction (Ohyama, Ambale-Venkatesh, et al., 2016), and systolic blood pressure (SBP) measured at MRI exam. SBP was based on the average of two SBP measures at the time of MRI session.
Statistical Analysis
Characteristics of study participants across the three estimated sleep duration groups were compared using one-way ANOVA for continuous variables and χ2 test for categorical variables. Multivariable linear regression models were used to model aortic stiffness as a function of actigraphy based estimates of sleep duration (reference group: 6–8 hours), adjusting for all the covariates with and without AHI. Effect modifications by age, gender, race/ethnicity, sleep efficiency, and WASO were tested by including each cross product term in the model. Estimated sleep quality measures [sleep efficiency (%) and WASO (minutes) as a continuous variable] were also tested as predictors using the same model. Sensitivity analysis included exclusion of participants with extreme sleep hours (< 4 hours and/or > 9 hours), using different sleep duration cut-off values (< 5 hours vs. 5–7 vs. > 7 hours) and, modeling sleep duration (minutes) as a continuous measure. All statistical analyses were performed using SAS version 14.1 (SAS Institute, Cary, NC, USA). All reported tests were two-tailed and a threshold of 0.05 was used to define statistical significance.
Results
A total of 908 participants with usable actigraphy data were included. Baseline characteristics are summarized in Table 1. Mean (SD) age was 68.4(9.1) years old and 55.3% of participants were female. The actigraphy estimated mean sleep duration for the study sample was 6 hours 34 minutes. A total of 252 (27.75%) participants were classified as having habitually short sleep duration, 552 (60.79%) with normal sleep duration, and 104 (11.45%) with long sleep duration.
Table 1.
Characteristics of study subjects and group comparisons by sleep duration
| Sleep duration | |||||
|---|---|---|---|---|---|
| Overall (Column %) |
<6 hrs | 6–8 hrs | >8 hrs | P value* | |
| N | 908 (100) | 252 (27.8) | 552 (60.8) | 104 (11.5) | - |
| Age, years | 68.4 ± 9.1 | 68.5±9.5 | 67.63±8.8 | 72.6±8.5 | <0.001 |
| Gender | |||||
| Men | 406 (44.7) | 139(55.2) | 236(42.8) | 31(29.81) | <0.001 |
| Women | 502(55.3) | 113(44.8) | 316(57.2) | 73(70.19) | |
| Race | |||||
| White | 307 (33.8) | 56(22.2) | 206(37.3) | 45(43.3) | <0.001 |
| Chinese | 129 (14.2) | 39(15.5) | 81(14.7) | 9(8.7) | |
| Black | 255 (28.1) | 90(35.7) | 144(26.1) | 21(20.2) | |
| Hispanic | 217 (23.9) | 67(26.6) | 121(21.9) | 29(27.9) | |
| BMI, kg/m2 | 28.0 ± 5.1 | 28.8±5.3 | 27.7±4.9 | 27.9±5.5 | 0.029 |
| Smoking | |||||
| Never smoking | 483 (53.3) | 129(51.2) | 294(53.4) | 60(57.7) | 0.146 |
| Previous smoking | 374 (41.2) | 102(40.5) | 234(42.5) | 38(36.5) | |
| Current smoking | 50 (5.5) | 21(8.3) | 23(4.2) | 6(5.8) | |
| HTN | |||||
| No | 383 (42.2) | 93(36.9) | 257(46.6) | 33(31.7) | 0.003 |
| Yes | 525 (57.8) | 159(63.1) | 295(53.4) | 71(68.3) | |
| HTN med. use | |||||
| No | 420 (46.3) | 101(40.1) | 282(51.1) | 37(35.6) | 0.001 |
| Yes | 488 (53.7) | 151(59.9) | 270(48.9) | 67(64.4) | |
| Lipid lowering med. Use | |||||
| No | 574(63.2) | 165(65.5) | 351(63.6) | 58(55.8) | 0.216 |
| Yes | 334 (36.8) | 87(34.5) | 201(36.4) | 46(44.2) | |
| Total cholesterol (mg/dL) | 185.8±36.1 | 181.4±35.4 | 187.6±36.0 | 187.4±37.7 | 0.073 |
| HDL (mg/dL) | 55.6±15.9 | 53.9±15.1 | 55.6±15.7 | 59.6±17.8 | 0.009 |
| LDL (mg/dL) | 108.5±32.0 | 106.2±30.4 | 109.8±32.3 | 107.2±34.6 | 0.294 |
| Triglycerides (mg/dL) | 109.5±57.2 | 108.6±62.7 | 110.8±54.6 | 104.3±56.8 | 0.544 |
| Fasting glucose (mg/dL) | 100.6±27.1 | 103.5±28.8 | 99.5±27.1 | 98.9±21.8 | 0.125 |
| AHI (/hr) | 23±18.7 | 25.9±20.1 | 21. 8±18.1 | 22.4±17.9 | 0.018 |
| SBP (mmHg) | 127.9±17.6 | 128.9±18.6 | 127.4±16.9 | 128.1±18.4 | 0.509 |
| DBP (mmHg) | 71.9±11.3 | 73.9±11.4 | 71.2±10.8 | 70.8±12.8 | 0.003 |
| LVEF(%) | 62.0±7.3 | 60.9±7.8 | 62.2±7.0 | 63.7±7.2 | 0.004 |
| Ascending AD (%/mmHg) | 1.6 ±1.2 | 1.7±1.2 | 1.6±1.2 | 1.6±0.9 | 0.508 |
| Descending AD (%/mmHg) | 2.1 ± 1.3 | 2.1±1.4 | 2.0±1.3 | 2.1±1.5 | 0.490 |
| aPWV (m/s) | 8.8 ± 4.2 | 8.4 ± 3.6 | 8.7 ± 4.2 | 9.9 ± 4.9 | 0.008 |
Values are mean ± SD or N (Percentage). All percentages are raw percentages except otherwise noted.
BMI, body mass index; HTN, hypertension; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; AHI, apnea hypopnea index; SBP, systolic blood pressure; DBP, diastolic blood pressure; LVEF, left ventricular ejection fraction; AD, aorta distensibility; aPWV, aortic pulse wave velocity
p- values were obtained from one-way analysis of variance for continuous variables and chi-square tests for categorical variables.
Aortic stiffness measures as a function of actigraphy based sleep duration categories are depicted in Figure 1. There were significant differences in aPWV among the three sleep duration categories (p=0.008). Participants with habitually long sleep duration had significantly higher aPWV than participants with habitually normal sleep duration (9.91±4.86 m/s vs. 8.68 ±4.22 m/s, p=0.006). No significant differences were observed in aPWV between participants with habitually short versus normal sleep duration (8.44±3.60 m/s vs. 8.68± 4.22 m/s, p=0.442). Ascending AD (AAD) and descending AD (DAD) were not significantly different among the three categories of sleep duration.
Figure 1. comparison of unadjusted mean of aPWV, AAD, DAD among subjects with different sleep durations by Fisher’s Least Significant Difference (LSD) test.
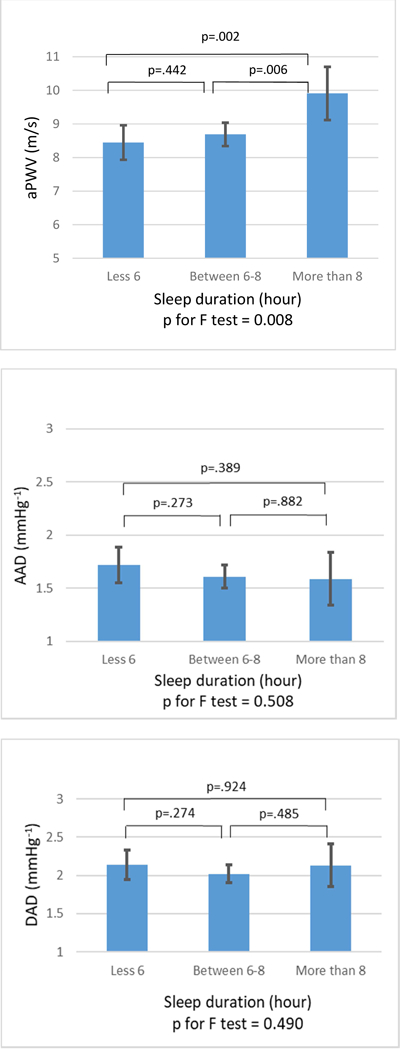
aPWV, aortic pulse wave velocity; AAD, ascending aortic distensibility; DAD, descending aortic distensibility.
In adjusted analyses (Table 2 and Figure 2), participants with short duration had 0.94 m/s (95% CI: [−1.54, −0.35]; p=0.002) lower aPWV compared with participants with normal duration. No significant difference in aPWV was found between participants with normal and long sleep duration (Δ = 0.82 m/s [−0.02, 1.65]; p=0.055). No noticeable association of sleep duration with AAD and DAD was noted (AAD: Δ = 0.13 %/mmHg [−0.07, 0.32]; p=0.197 and DAD: Δ = 0.22 %/mmHg [−0.00, 0.45]; p=0.049, respectively for short vs. normal sleep duration, and AAD: Δ = −0.07 %/mmHg [−0.19, 0.34]; p=0.583 and DAD: Δ = −0.05 %/mmHg [−0.26, 0.35]; p=0.772, respectively for normal vs. long sleep duration). Excluding AHI from the model did not meaningfully change the results. No significant effect modifications were found in sleep duration by age, gender, race, sleep efficiency, and WASO (data not shown).
Table 2.
Adjusted differences* in mean aPWV, AAD, and DAD levels for short and long sleep durations compared to normal sleep duration (N = 908)
| aPWV (m/s) | AAD (%/mmHg) | DAD (%/mmHg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean difference |
95% CI | P | Mean difference |
95% CI | P | Mean difference |
95% CI | P | |
| Sleep duration (h) | |||||||||
| < 6 | −0.94 | −1.54 to -0.35 | 0.002 | 0.13 | −0.07 to 0.32 | 0.197 | 0.22 | 0.00 to 0.45 | 0.049 |
| 6 – 8 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| > 8 | 0.82 | −0.02 to 1.65 | 0.055 | 0.07 | −0.19 to 0.34 | 0.583 | 0.05 | −0.26 to 0.35 | 0.772 |
aPWV, aortic pulse wave velocity; AAD, ascending aortic distensibility; DAD, descending aortic distensibility.
Adjusted for age, gender, race, body mass index, smoking, anti-hypertensive medication use, systolic blood pressure measured at MRI exam, fasting glucose, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, lipid lowering medicine use, left ventricular ejection fraction, and Apnea-Hypopnea index.
Figure 2. Comparison of adjusted mean of aPWV among subjects with different sleep durations by Fisher’s Least Significant Difference (LSD) test.
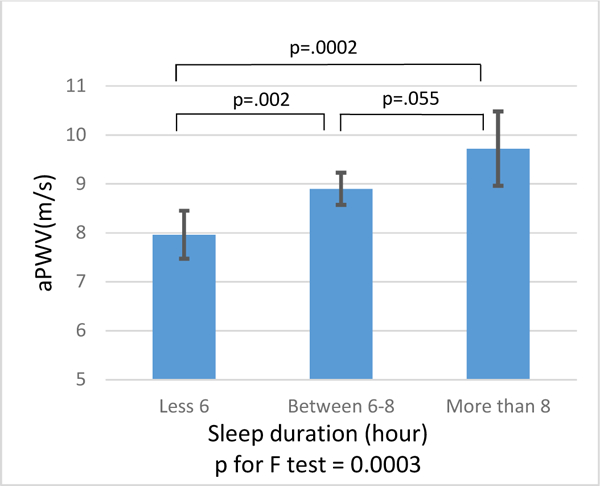
Adjusted for age, gender, race, body mass index, smoking, anti-hypertensive medication use, systolic blood pressure measured at MRI exam, fasting glucose, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, lipid lowering medicine use, left ventricular ejection fraction, and Apnea-Hypopnea index. aPWV, aortic pulse wave velocity; AAD, ascending aortic distensibility; DAD, descending aortic distensibility
Because of the unanticipated findings of an association between habitually short sleep duration and favorable aortic stiffness profiles (i.e., low aPWV), various sensitivity analyses were conducted. Excluding participants with extreme sleep duration (less than 4 hours and/or greater than 9 hours) did not meaningfully change the results (data not shown). When different cut-points were used to categorize the estimated sleep duration from actigraphy (< 5 hr vs. 5–7 hours vs. > 7 hours), neither short nor long sleep duration (vs. normal) were associated with aPWV and DAD (data not shown). When absolute sleepy duration was modeled as a continuous measure, a significant linear relationship was found with aPWV (p<0.001), but not with AAD (p=0.404) or DAD (p=0.632).
Mean sleep efficiency and WASO were 89.99 (3.64) (%) and 39.32 (16.75) (minutes), respectively. Actigraphy estimates of sleep quality (sleep efficiency or WASO) were not associated with any aortic stiffness measures (Table 3).
Table 3.
Multiple linear regression model* of sleep quality on aPWV, AAD, and DAD levels (N = 908)
| aPWV (m/s) | AAD (%/mmHg) | DAD (%/mmHg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | |
| Sleep efficiency (%) | 0.01 | −0.06 to 0.09 | 0.703 | −0.01 | −0.03 to 0.02 | 0.553 | −0.00 | −0.03 to 0.02 | 0.785 |
| WASO (minutes) | 0.01 | −0.00 to 0.03 | 0.135 | −0.00 | −0.01 to 0.00 | 0.968 | 0.00 | −0.01 to 0.01 | 0.942 |
aPWV, aortic pulse wave velocity; AAD, ascending aortic distensibility; DAD, descending aortic distensibility; WASO, wake after sleep onset.
Adjusted for age, gender, race, body mass index, smoking, anti-hypertensive medication use, systolic blood pressure measured at MRI exam, fasting glucose, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, lipid lowering medicine use, left ventricular ejection fraction, and Apnea-Hypopnea index.
Discussion
In this study of an ethnically diverse cohort, actigraphybased habitually short sleep duration was associated with lower aortic stiffness compared with those with normal sleep duration. Specifically, people with shorter average sleep (less than 6 hours) as estimated by one week of actigraphy exhibited 0.94 m/s lower MRI-based aPWV compared with participants with 6 to 8 hours of sleep duration. Given that aPWV had 1.4 m/s increase over 10 years (Ohyama, Teixido- Tura, et al., 2016), the difference of aPWV between short and normal sleep duration is considered clinically significant. This finding was independent of SDB severity and was not influenced by demographic characteristics or other sleep quality measures. No independent associations were found between actigraphybased sleep quality measures and aortic stiffness.
Previous studies linking self-reported sleep duration to arterial stiffness have revealed mixed results. For example, short sleep duration was associated with higher arterial stiffness in middle aged Chinese population with nonalcoholic fatty liver (Cao et al., 2016a), children (Morita et al., 2016), and an elderly population (Zonoozi et al., 2017). On the other hand, several population- based studies have reported associations between long sleep duration and increased arterial stiffness measured by brachial-ankle PWV (baPWV) in middle aged Japanese civil servants (Yoshioka et al., 2011b), Taiwanese males (Tsai et al., 2014b) and elderly in Japan (Niijima et al., 2016). One large study conducted among 18,106 Koreans demonstrated a U-shape association between sleep duration and arterial stiffness supporting both short and long sleep duration are associated with increased arterial stiffness (Kim et al., 2015). Only one study reported no association in ethnic minority groups in the Netherlands (Anujuo et al., 2016).
In contrast with sleep duration, fewer studies have examined sleep quality in relation to arterial stiffness. Self-reported poor sleep quality correlated with higher arterial stiffness as measured by baPWV in Japanese patients with type 2 diabetes mellitus (Osonoi et al., 2015) and middle-aged healthy men and women (Kim et al., 2015). Another study of middle-aged older women also found that self-reported poor sleep (vs. good) was associated with higher arterial stiffness based on cfPWV and femoral-ankle PWV (Choi et al., 2013). Interestingly in that study, while objectively estimated sleep duration was similar between the two groups, subjective sleep duration was longer and actigraphy-based sleep quality such as sleep efficiency and WASO were more abnormal in the poor sleep group compared with the good sleep group. This reinforces the importance of objectively quantifying sleep quality in addition to sleep duration in relation to arterial stiffness. Notably, sleep duration and quality measures from these studies were largely based on self-reported data. In addition, the findings of these studies are partly limited by the lack of SDB measurement. Given the high prevalence of SDB in the community and strong evidence that links SDB to arterial stiffness, results from aforementioned studies need to be carefully interpreted. Our study sought to overcome this limitation by utilizing data from both actigraphy and PSG. Our use of actigraphy data to estimate habitual sleep duration and quality over multiple nights may provide more consistent data on these sleep measures than a single night PSG. Actigraphy based estimation of total sleep time, sleep efficiency and WASO have been shown to correspond reasonably well to PSG in various populations (Blackwell et al., 2008; Jean-Louis, Kripke, Cole, Assmus, & Langer, 2001; Kushida et al., 2001; Pollak, Tryon, Nagaraja, & Dzwonczyk, 2001). The most striking and unexpected finding of our study was the association between short sleep duration and lower aortic stiffness measured by MRI-based aPWV. In contrast, we did not observe any association between long sleep duration and aortic stiffness. Further analyses uncovered a linear association between absolute sleep duration and aortic stiffness measures. A previous study has reported that objective estimates of sleep duration were about one hour shorter than self-reported sleep duration. However, there was no significant difference observed when different sleep duration cut-off values (5 and 7 hours) were used. Excluding participants with extreme sleep duration did not change the study finding, and this association was not influenced by severity of SDB, sleep quality or demographic factors such as age, gender and race/ethnicity. We did not find any meaningful association between sleep quality measures and aortic stiffness.
In previous studies, both short and long sleep duration have generally been considered a risk to cardiovascular health. The underlying mechanisms of the association between abnormal sleep duration and arterial stiffness remain unclear but we speculate it would involve other mediating pathways such as neurohormonal, autonomic or endothelial dysfunction. Previous studies have suggested short sleep duration is associated with elevated BP (Gangwisch et al., 2006), altered cortisol levels (Vgontzas et al., 1999), endothelial dysfunction (Calvin et al., 2014), and increased levels of endothelin-1 (Weil et al., 2010), which may contribute to increased arterial stiffness. Vgontzas et al. (2004) demonstrated that even modest sleep restriction (from 8 hours to 6 hours) were associated with increased secretion of inflammatory cytokines (Vgontzas et al., 2004). On the other hand, increased arterial stiffness related to long sleep duration has been attributed to poor sleep quality such as sleep fragmentation and low sleep efficiency which may cause increased sympathetic activity (Zhang et al., 2011) and endothelial dysfunction (Cooper et al., 2014). Increased inflammatory markers were also observed as risk factors of increased arterial stiffness in those with long sleep duration (Irwin, Olmstead, & Carroll, 2016). These studies raise the possibility that the association of sleep duration with subclinical markers such as arterial stiffness may be confounded by other aspects of sleep such as SDB and sleep quality.
Our finding of lower aortic stiffness in people with shorter sleep duration is unexpected and physiological mechanisms that underlie this association is not clear. While we tried to exclude as many alternative explanations as possible through our sensitivity analyses, this association may have in part resulted from residual confounding due to unmeasured factors. On the other hand, it raises an important question about the possible bidirectional and dynamic relationship between sleep and cardiovascular health. Just as longer sleep duration can be a marker of poor cardiovascular health (Stamatakis & Punjabi, 2007), modestly short sleep duration may be a marker of favorable cardiovascular health. Existing evidence suggests that shorter sleep duration is reflective of favorable neurocognitive function. For example, a study on healthy young adults reported that shorter sleep duration was associated with better executive functioning and lower mean diffusivity (which reflects greater tissue density) of the brain in 1201 healthy young adults (Takeuchi et al., 2018). Future studies using objectively estimated sleep duration (vs. self-eport) and vascular health may provide more insights into this question.
It is unknown whether the inconsistent findings of our study from those of the previous studies are attributable to the difference between subjective vs. objective measure of sleep duration. Subjective reports of sleep may reflect self-perceived adequacy of sleep duration and can be inherently different from objectively assessed sleep. Given the discrepancy between two methods reported in previous studies (Guedes et al., 2016; Palesh et al., 2017), further studies are required to investigate the differences between subjective and objective measures of sleep parameters and their effects on aortic stiffness within the same subjects.
The difference between our results and those of previous studies may be also attributed to the method we used to measure aortic stiffness. To the best of our knowledge, this study is the first to use MRI-based aPWV and AD in relation to sleep duration and quality. The simultaneous comparison of aPWV and aortic distensibility in 111 subjects without acute or chronic disease in MESA demonstrated aPWV was a more sensitive marker of vascular aging in older individuals (≥50 years of age), while AAD was a better predictor of vascular aging in younger individuals (<50 years of age) (Redheuil et al., 2010). Since our study cohort included older participants, aPWV may be more pertinent arterial stiffness marker. The current study findings, which reflect participants primarily above age 50, showed that short sleep duration was associated with low aPWV (i.e., lower aortic stiffness), but not with AAD. For that reason, aPWV may represent the most sensitive marker for subclinical large artery stiffening among this population.
The strength of the present study includes the cohort size and ethnic diversity, the use of robust objective estimates of sleep, and controlling for SDB, as well as state-of-the-art cardiac MRI- based aortic stiffness measurement. Several limitations should be also noted. While SDB was taken into account in the analyses, other potentially important PSG-metrics beyond AHI, such as measures of intermittent hypoxemia severity, were not considered. However, AHI is the most commonly used metric for SDB and is a reasonably good indicator of overall SDB severity. The lack of representation of younger-aged populations may limit the generalizability of the study.
In addition, our cross-sectional nature of the study suffers from inherent limitations including inability to infer direction and residual confounding due to unmeasured inadequate adjustment.
In conclusion, we found that actigraphy based short sleep duration was associated with lower aortic stiffness independent of SDB. Since sleep duration is an important modifiable risk factor for CVD, further elucidation of the causal relationship between sleep duration and arterial stiffness likely has important clinical implications.
Highlights.
Objectively estimated sleep duration had a positive linear relationship with aortic stiffness measured by MRI-based pulse wave velocity in the community-dwelling elderly population.
People with short sleep duration (≤ 6 hours) had 10.6% lower MRI-based pulse wave velocity compared to those with normal sleep duration (6–8 hours).
No statistically significant associations were found between objectively measured sleep quality measures and aortic stiffness.
Acknowledgement
We thank all the investigators, the staff, and the research participants of the MESA for their valuable contributions. A full list of investigators and institutions are found at http://www.mesa-nhlbi.org.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ declarations of interest: None
Contributor Information
Jeongok G. Logan, University of Virginia School of Nursing, McLeod Building, Room 4011, 225 Jeanette Lancaster Way, Charlottesville, VA 22908, jl3zj@virginia.edu 434-924-0082.
Hyojung Kang, Systems and Information Engineering University of Virginia, Olsson 102A 151 Engineers’ Way Charlottesville 22901, hkang@virginia.edu 1-434-297-6313.
Jennifer Mason Lobo, Department of Public Health Sciences, Division of Biomedical Informatics, University of Virginia Health System West Complex, Room 3003, Charlottesville, Virginia 22908, jem4yb@virginia. edu 1-434-924-2813.
Min-Woong Sohn, Department of Public Health Sciences, University of Virginia, Old Med School, Room 3874, Charlottesville, Virginia 22908, ms5vs@Virginia. edu 1-434-924-8753.
Gen-Min Lin, Hualien Armed Forces General Hospital No. 100, Jinfeng str Hualien 970, Taiwan Northwestern University, Chicago, IL 60611, USA gen-min. lin@northwestern. edu.
Joao Lima, School of Medicine Johns Hopkins University 733 N Broadway, Baltimore, MD 21205 jlima@jhmi.edu 410-614-1284.
Naresh Punjabi, Johns Hopkins Medicine Johns Hopkins Bloomberg School of Public Health, 615 N. Wolfe Street, Baltimore, MD 21205 npunjabi@jhmi.edu.
Susan Redline, Harvard Medical School 164 Longwood Avenue Boston, MA 02115 sredline1@rics.bwh.harvard.edu 1-617-983-7420.
Younghoon Kwon, University of Virginia UVA Heart and Vascular Center Fontaine Fontaine Research Park 500 Ray C. Hunt Drive Charlottesville, VA 22908 YK2J@hscmail.mcc.virginia.edu 1-434-243-1000.
References
- Anujuo K, Stronks K, Snijder MB, Jean-Louis G, van den Born BJ, Peters RJ, & Agyemang C (2016). Relationship between sleep duration and arterial stiffness in a multiethnic population: The HELIUS study. Chronobiology International, 33(5), 543–552. doi: 10.3109/07420528.2016.1158721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz M, Ali SS, Das S, Younus A, Malik R, Latif MA, . . . Nasir K (2016). Association of subjective and objective sleep duration as well as sleep quality with non-invasive markers of sub-clinical cardiovascular disease (CVD): A systematic review. Journal of Atherosclerosis and Thrombosis, doi: 10.5551/jat.36194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell V, McCabe EL, Larson MG, Rong J, Merz AA, Osypiuk E, . . . Cheng S (2017). Relations between aortic stiffness and left ventricular mechanical function in the community. Journal of the American Heart Association, 6(1), 10.1161/JAHA.116.004903. doi:e004903 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, . . . Tracy RP (2002). Multi-ethnic study of atherosclerosis: Objectives and design. American Journal of Epidemiology, 156(9), 871–881. [DOI] [PubMed] [Google Scholar]
- Calvin AD, Covassin N, Kremers WK, Adachi T, Macedo P, Albuquerque FN, . . . Somers VK (2014). Experimental sleep restriction causes endothelial dysfunction in healthy humans. Journal of the American Heart Association, 3(6), e001143. doi: 10.1161/JAHA.114.001143[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Zhou J, Yuan H, & Chen Z (2016a). Association between sleep condition and arterial stiffness in chinese adult with nonalcoholic fatty liver disease. Journal of Thrombosis and Thrombolysis, 42(1), 127–134. doi:10.1007/s11239-016-1356-1 [doi] [DOI] [PubMed] [Google Scholar]
- Cao X, Zhou J, Yuan H, & Chen Z (2016b). Association between sleep condition and arterial stiffness in chinese adult with nonalcoholic fatty liver disease. Journal of Thrombosis and Thrombolysis, 42(1), 127–134. doi: 10.1007/s11239-016-1356-1 [doi] [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, D’Elia L, Strazzullo P, & Miller MA (2010). Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep, 33(5), 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcante JL, Lima JA, Redheuil A, & Al-Mallah MH (2011). Aortic stiffness: Current understanding and future directions. Journal of the American College of Cardiology, 57(14), 1511–1522. doi: 10.1016/j.jacc.2010.12.017 [doi] [DOI] [PubMed] [Google Scholar]
- Cole RJ, Kripke DF, Gruen W, Mullaney DJ, & Gillin JC (1992). Automatic sleep/wake identification from wrist activity. Sleep, 15(5), 461–469. [DOI] [PubMed] [Google Scholar]
- Cooper DC, Ziegler MG, Milic MS, Ancoli-Israel S, Mills PJ, Loredo JS, . . . Dimsdale JE (2014). Endothelial function and sleep: Associations of flow-mediated dilation with perceived sleep quality and rapid eye movement (REM) sleep. Journal of Sleep Research, 23(1), 84–93. doi: 10.1111/jsr. 12083 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doonan RJ, Scheffler P, Lalli M, Kimoff RJ, Petridou ET, Daskalopoulos ME, & Daskalopoulou SS (2011). Increased arterial stiffness in obstructive sleep apnea: A systematic review. Hypertension Research : Official Journal of the Japanese Society of Hypertension, 34(1), 23–32. doi: 10.1038/hr.2010.200 [doi] [DOI] [PubMed] [Google Scholar]
- Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, . . . Malaspina D (2006). Short sleep duration as a risk factor for hypertension: Analyses of the first national health and nutrition examination survey. Hypertension (Dallas, Tex.: 1979), 47(5), 833–839. doi:01.HYP.0000217362.34748.e0 [pii] [DOI] [PubMed] [Google Scholar]
- Guedes LG, Abreu Gde A, Rodrigues DF, Teixeira LR, Luiz RR, & Bloch KV (2016). Comparison between self-reported sleep duration and actigraphy among adolescents: Gender differences. Revista Brasileira De Epidemiologia = Brazilian Journal of Epidemiology, 19(2), 339–347. doi: 10.1590/1980-5497201600020011 [doi] [DOI] [PubMed] [Google Scholar]
- Hayashi K, Sugawara J, Komine H, Maeda S, & Yokoi T (2005). Effects of aerobic exercise training on the stiffness of central and peripheral arteries in middle-aged sedentary men. The Japanese Journal of Physiology, 55(4), 235–239. doi:jjphysiol/S2116 [pii] [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, & Carroll JE (2016). Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biological Psychiatry, 80(1), 40–52. doi: 10.1016/j.biopsych.2015.05.014 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal M, Urbina EM, Wadwa RP, Talton JW, D’Agostino RB Jr., Hamman RF, . . . Dabelea D (2013). Reduced heart rate variability is associated with increased arterial stiffness in youth with type 1 diabetes: The SEARCH CVD study. 36(8), 2351–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CW, Chang Y, Zhao D, Cainzos-Achirica M, Ryu S, Jung HS, . . . Sung E (2015). Sleep duration, sleep quality, and markers of subclinical arterial disease in healthy men and women. Arteriosclerosis, Thrombosis, and Vascular Biology, 35(10), 2238–2245. doi: 10.1161/ATVBAHA.115.306110 [doi] [DOI] [PubMed] [Google Scholar]
- Kwon Y, Gharib SA, Biggs ML, Jacobs DR Jr, Alonso A, Duprez D, . . . Heckbert SR (2015). Association of sleep characteristics with atrial fibrillation: The multi-ethnic study of atherosclerosis. Thorax, 70(9), 873–879. doi: 10.1136/thoraxjnl-2014-206655 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhu M, Bai B, Chi C, Yu S, Teliewubai J, . . . Xu Y (2017). Comparison of carotid-femoral and brachial-ankle pulse-wave velocity in association with target organ damage in the community-dwelling elderly chinese: The northern shanghai study. Journal of the American Heart Association, 6(2), 10.1161/JAHA.116.004168. doi:e004168 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, . . . Witteman JC (2006). Arterial stiffness and risk of coronary heart disease and stroke: The rotterdam study. Circulation, 113(5), 657–663. doi:113/5/657 [pii] [DOI] [PubMed] [Google Scholar]
- Morita N, Kambayashi I, Okuda T, Oda S, Takada S, Nakajima T, . . . Okita K (2016). Inverse relationship between sleep duration and cardio-ankle vascular index in children. Journal of Atherosclerosis and Thrombosis, doi: 10.5551/jat.36517 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, . . . Lakatta EG (2008). Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the baltimore longitudinal study of aging. Journal of the American College of Cardiology, 51(14), 1377–1383. doi: 10.1016/j.jacc.2007.10.065 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao M, Nomura K, Karita K, Nishikitani M, & Yano E (2004). Relationship between brachial-ankle pulse wave velocity and heart rate variability in young japanese men. 27(12), 925–931. Retrieved from http://www http://ncbi.nlm.nih.gov/pubmed/15894832; http://www.ncbi.nlm.nih.gov/pubmed/15894832 [DOI] [PubMed] [Google Scholar]
- Niijima S, Nagai M, Hoshide S, Takahashi M, Shimpo M, Kario K, & Japan Morning Surge-Home Blood Pressure Study Investigators Group. (2016a). Long sleep duration: A nonconventional indicator of arterial stiffness in japanese at high risk of cardiovascular disease: The J-HOP study. Journal of the American Society of Hypertension : JASH, 10(5), 429–437. doi: 10.1016/j.jash.2016.02.010 [doi] [DOI] [PubMed] [Google Scholar]
- Niijima S, Nagai M, Hoshide S, Takahashi M, Shimpo M, Kario K, & Japan Morning Surge-Home Blood Pressure Study Investigators Group. (2016b). Long sleep duration: A nonconventional indicator of arterial stiffness in japanese at high risk of cardiovascular disease: The J-HOP study. Journal of the American Society of Hypertension : JASH, 10(5), 429–437. doi: 10.1016/j.jash.2016.02.010 [doi] [DOI] [PubMed] [Google Scholar]
- Ogilvie RP, Redline S, Bertoni AG, Chen X, Ouyang P, Szklo M, & Lutsey PL (2016). Actigraphy measured sleep indices and adiposity: The multi-ethnic study of atherosclerosis (MESA). Sleep, 39(9), 1701–1708. doi: 10.5665/sleep.6096 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama Y, Ambale-Venkatesh B, Noda C, Chugh AR, Teixido-Tura G, Kim JY, . . . Lima JA (2016). Association of aortic stiffness with left ventricular remodeling and reduced left ventricular function measured by magnetic resonance imaging: The multi-ethnic study of atherosclerosis. Circulation.Cardiovascular Imaging, 9(7), 10.1161/ CIRCIMAGING. 115.004426. doi: 10.1161/CIRCIMAGING.115.004426 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama Y, Ambale-Venkatesh B, Noda C, Kim JY, Tanami Y, Teixido-Tura G, . . . Lima JAC (2017). Aortic arch pulse wave velocity assessed by magnetic resonance imaging as a predictor of incident cardiovascular events: The MESA (multi-ethnic study of atherosclerosis). Hypertension (Dallas, Tex..: 1979), 70(3), 524–530. doi: 10.1161/HYPERTENSIONAHA.116.08749 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama Y, Teixido-Tura G, Ambale-Venkatesh B, Noda C, Chugh AR, Liu C-Y, ... Lima JAC (2016). Ten-year longitudinal change in aortic stiffness assessed by cardiac MRI in the second half of the human lifespan: the multi-ethnic study of atherosclerosis. European Heart Journal Cardiovascular Imaging, 17(9), 1044–1053. http s://doi.org/10.1093/ehjci/jev332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palesh O, Haitz K, Levi F, Bjarnason GA, Deguzman C, Alizeh I, . . . Innominato PF (2017). Relationship between subjective and actigraphy-measured sleep in 237 patients with metastatic colorectal cancer. Quality of Life Research : An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, doi: 10.1007/s11136-017-1617-2 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi AI, Giles WH, Croft JB, & Bliwise DL (1997). Habitual sleep patterns and risk for stroke and coronary heart disease: A 10-year follow-up from NHANES I. Neurology, 48(4), 904–911. [DOI] [PubMed] [Google Scholar]
- Redheuil A, Wu CO, Kachenoura N, Ohyama Y, Yan RT, Bertoni AG, . . . Lima JA (2014). Proximal aortic distensibility is an independent predictor of all-cause mortality and incident CV events: The MESA study. Journal of the American College of Cardiology, 64(24), 2619–2629. doi: 10.1016/j.jacc.2014.09.060 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redheuil A, Yu WC, Wu CO, Mousseaux E, de Cesare A, Yan R, . . . Lima JA (2010). Reduced ascending aortic strain and distensibility: Earliest manifestations of vascular aging in humans. Hypertension (Dallas, Tex.: 1979), 55(2), 319–326. doi: 10.1161/HYPERTENSIONAHA.109.141275 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safar ME, & O’Rourke MF (2009). The brachial-ankle pulse wave velocity. 27(10), 1960–1961. Retrieved from http: // www.ncbi.nlm.nih.gov/ pubmed/19893429; http://www.ncbi.nlm.nih.gov/pubmed/19893429 [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Nouchi R, Yokoyama R, Kotozaki Y, Nakagawa S, ... Kawashima R (2018). Shorter sleep duration and better sleep quality are associated with greater tissue density in the brain. Scientific Reports, 8(1), 5833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, DeSouza CA, & Seals DR (1998). Absence of age-related increase in central arterial stiffness in physically active women. Arteriosclerosis, Thrombosis, and Vascular Biology 18(1), 127–132. [DOI] [PubMed] [Google Scholar]
- Trinder J, Kleiman J, Carrington M, Smith S, Breen S, Tan N, & Kim Y (2001). Autonomic activity during human sleep as a function of time and sleep stage. Journal of Sleep Research, 10(4), 253–264. doi:263 [pii] [DOI] [PubMed] [Google Scholar]
- Tsai TC, Wu JS, Yang YC, Huang YH, Lu FH, & Chang CJ (2014a). Long sleep duration associated with a higher risk of increased arterial stiffness in males. Sleep, 37(8), 1315–1320. doi: 10.5665/sleep.3920 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai TC, Wu JS, Yang YC, Huang YH, Lu FH, & Chang CJ (2014b). Long sleep duration associated with a higher risk of increased arterial stiffness in males. Sleep, 37(8), 1315–1320. doi: 10.5665/sleep.3920 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ittersum FJ, Schram MT, van der Heijden-Spek JJ, Van Bortel LM, Elte JW, Biemond P, . . . Stehouwer CD (2004). Autonomic nervous function, arterial stiffness and blood pressure in patients with type I diabetes mellitus and normal urinary albumin excretion. 18(11), 761–768. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15175635; http://www.ncbi.nlm.nih.gov/pubmed/15175635 [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Mastorakos G, Bixler EO, Kales A, Gold PW, & Chrousos GP (1999). Sleep deprivation effects on the activity of the hypothalamic-pituitary-adrenal and growth axes: Potential clinical implications. Clinical Endocrinology, 51(2), 205–215. doi:cen763 [pii] [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, & Chrousos GP (2004). Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. The Journal of Clinical Endocrinology and Metabolism, 89(5), 2119–2126. doi: 10.1210/jc.2003-031562 [doi] [DOI] [PubMed] [Google Scholar]
- Wang J, Yu W, Gao M, Zhang F, Gu C, Yu Y, & Wei Y (2015). Impact of obstructive sleep apnea syndrome on endothelial function, arterial stiffening, and serum inflammatory markers: An updated meta-analysis and metaregression of 18 studies. Journal of the American Heart Association, 4(11), 10.1161/JAHA.115.002454.10.1161/JAHA.115.002454.10.1161/JAHA.115.002454.10.1161/JAHA.115.002454. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil BR, Mestek ML, Westby CM, Van Guilder GP, Greiner JJ, Stauffer BL, & DeSouza CA (2010). Short sleep duration is associated with enhanced endothelin-1 vasoconstrictor tone. Canadian Journal of Physiology and Pharmacology, 88(8), 777–781. doi: 10.1139/Y10-046 [doi] [DOI] [PubMed] [Google Scholar]
- Yoshioka E, Saijo Y, Kita T, Okada E, Satoh H, Kawaharada M, & Kishi R (2011a). Relation between self-reported sleep duration and arterial stiffness: A cross-sectional study of middle-aged japanese civil servants. Sleep, 34(12), 1681–1686. doi: 10.5665/sleep.1434 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka E, Saijo Y, Kita T, Okada E, Satoh H, Kawaharada M, & Kishi R (2011b). Relation between self-reported sleep duration and arterial stiffness: A cross-sectional study of middle-aged japanese civil servants. Sleep, 34(12), 1681–1686. doi: 10.5665/sleep.1434 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ma RC, Kong AP, So WY, Li AM, Lam SP, . . . Wing YK (2011). Relationship of sleep quantity and quality with 24-hour urinary catecholamines and salivary awakening cortisol in healthy middle-aged adults. Sleep, 34(2), 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonoozi S, Ramsay SE, Papacosta O, Lennon L, Ellins EA, Halcox JPJ, . . . Goya Wannamethee S (2017). Self-reported sleep duration and napping, cardiac risk factors and markers of subclinical vascular disease: Cross-sectional study in older men. BMJ Open, 7(6), e016396–2017-016396. doi: 10.1136/bmjopen-2017-016396 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]


