Abstract
Fragile X syndrome (FXS) is a neurodevelopmental disorder caused by a single genetic mutation in the Fmr1 gene. Mutations in the Fmr1 gene are the largest monogenic cause of Autism spectrum disorder (ASD), and thus both disorders share many of the same cognitive and behavioral impairments. There is increasing evidence suggesting dysregulated immune responses play a role in the pathophysiology of ASD, however, the association between FXS and altered immunity requires further investigation. This study examined whether Fmr1 knockout (KO) and wild type (WT) mice on a FVB/NJ background strain had altered cytokine expression at baseline levels in the hippocampus. Results showed Fmr1 KO mice to have decreased pro-inflammatory cytokine hippocampal mRNA expression, specifically IL-6 and TNFα, compared to WT mice. However, no differences were detected in the expression levels of IL-1β, MCP-1, IFNγ, or IL-10. Despite the high comorbidity between FXS and ASD, these results suggest that the Fmr1 KO mouse does not mimic the increased pro-inflammatory cytokine expression commonly found in ASD mouse models and patients. Further investigation of the immune profile of the Fmr1 KO mouse is critical to understand whether this deficiency of cytokines in the hippocampus is indicative of a broader immunologic deficit associated with FXS.
Keywords: Fragile X syndrome, FXS, autism, cytokines, IL-6, TNFα, inflammation
Introduction
Fragile X syndrome (FXS) is the most common inherited cause of intellectual disability and is characterized by a broad spectrum of cognitive and behavioral impairments. The near-absence of fragile X mental retardation protein (FMRP) results in a diagnosis of the syndrome and varying degrees of intellectual disability [1]. A notable 21 to 50% of FXS patients meet criteria for Autism spectrum disorder (ASD) [2]. In addition, approximately 2 to 6% of individuals with ASD have a FMR1 gene mutation, making it the largest genetic contributor to ASD [3]. These disorders also share many of the same core characteristics such as altered social interactions, repetitive or stereotypical behaviors, and impaired language and communication [2].
The etiology and underlying neuropathology of both FXS and ASD are largely unknown. In addition, no clear or consistent biological markers have been discovered to be characteristic of either disorder. There is, however, substantial evidence for altered immune responses in patients with ASD, such as increased levels of pro-inflammatory cytokines in the brain tissue, plasma, and peripheral blood mononuclear cells (PBMCs) [4–6]. Despite the high comorbidity rate between the disorders, there are a relatively limited number of studies investigating whether these alterations could also contribute to FXS pathogenesis. Patients with FXS have been found to have increases in gastrointestinal (GI) difficulties including gastroesophageal reflux, similar to findings in those with ASD [7]. One study examining a subgroup of boys with FXS found them to have increased frequency of infections in early childhood, such as otitis media, suggesting they are at high risk for recurrent infectious ear diseases [8]. Further support of the involvement of the immune system in FXS is supported by preliminary studies of minocycline as a treatment for FXS. Minocycline is a commonly prescribed antibiotic with anti-inflammatory effects that has been shown to reverse dendritic spine and synaptic abnormalities in the Fmr1 knockout (KO) mouse, as well as reduce anxiety and increase exploratory behavior in the mice [9]. Furthermore, preliminary clinical studies have found minocycline to improve language, social communication, attention, and mood-related behaviors in those with FXS [10].
Dysregulated expression of small secreted or membrane-bound proteins, known as cytokines, has been noted in many neurological disorders, as they are essential for aspects of normal neurodevelopment such as cellular migration within the central nervous system (CNS) and synaptic network formation [11]. Imbalances in cytokine levels can have profound effects on neural activity and it is possible that these effects could ultimately mediate behavioral aspects of FXS. However, there has yet to be thorough investigation of cytokine expression in this animal model of the syndrome, preventing future manipulations that could involve targeting aspects of the immune system to treat FXS. In the present study, we examined baseline hippocampal gene expression of key cytokines that are often found to be altered in ASD, to determine whether a similar cytokine profile is found in the Fmr1 KO mouse. While immune disturbances exist in FXS patients, there is minimal evidence for whether the Fmr1 KO mouse encapsulates these deficits and can stand as a viable animal model to investigate the involvement of the immune system in FXS.
Methods
Animals
Adult male Fmr1 knockout (KO) (n = 6–8) and wild type (WT) (n = 7–8) mice on a FVB/NJ background were utilized in this study. All mice were bred and group housed at Baylor University at an ambient temperature of 22°C and a 12-hour light/dark diurnal cycle. Mice were given ad libitum access to food and water. Procedures were conducted in compliance with the Baylor University Institutional Animal Care and Use Committee and the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
Cytokine analysis
Subject mice were euthanized by rapid decapitation and the hippocampus was dissected from the brain, rinsed in 1X PBS, and placed on dry ice. Hippocampal tissue was stored in −80°C until processed. Total RNA was isolated from hippocampal tissue samples using the RNeasy Mini Kit (RNeasy, Qiagen, Hilden, Germany) according to established protocols. The RNA was subsequently measured for concentration and purity using a NanoDrop ND-1000 Spectrophotometer (Thermo Scientific, NanoDrop Products, Wilmington, DE). Extracted RNA was reverse transcribed into single-stranded complementary DNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA). Expression of mRNA was determined by quantitative real-time polymerase chain reaction (qRT-PCR) using TaqMan probe and primer chemistry on a QuantStudio 5 Real-Time PCR System (Applied Biosystems, Carlsbad, CA). Reactions were performed in triplicate for each sample in a 384-well plate with an endogenous control gene (β-actin) used for normalization. The relative expression levels of each target gene (IL-1β, IL-6, TNFα, MCP-1, IL-10, IFNγ) were calculated by normalizing quantified mRNA transcripts to β-actin using the 2-ΔΔCt method of quantitation.
Statistical analysis
Data were analyzed using GraphPad Prism 7 software (La Jolla, CA) or SPSS 21.0 (IBM, USA). Relative gene expression was determined using the 2-ΔΔCt method of quantitation and t-tests were utilized to test significance between groups. In cases in which the homogeneity of variance assumption was violated, nonparametric Mann-Whitney U tests were conducted. For all analyses a value of p < 0.05 was considered significant.
Results
Adult Fmr1 knockout (KO) mice showed decreased baseline gene expression of select cytokines in the hippocampus compared to WT mice. Pro-inflammatory cytokines IL-6 and TNFα were significantly decreased in Fmr1 KO mice (IL-6: U = 5, p = 0.003, TNFα: U = 3, p = 0.001). No differences were detected in expression of other pro-inflammatory cytokines, including IL-1β (t[14] = 1.13, p = 0.277) or IFNγ (t[12] = 0.13, p = 0.903). Relative expression levels of chemokine MCP-1 (t[13] = 0.31, p = 0.761) or anti-inflammatory cytokine IL-10 (t[12] = 0.11, p = 0.911) were also not different between Fmr1 KO and WT mice (Fig. 1).
Figure 1. Hippocampal cytokine mRNA expression in adult male Fmr1 knockout (KO) and wild type (WT) mice.
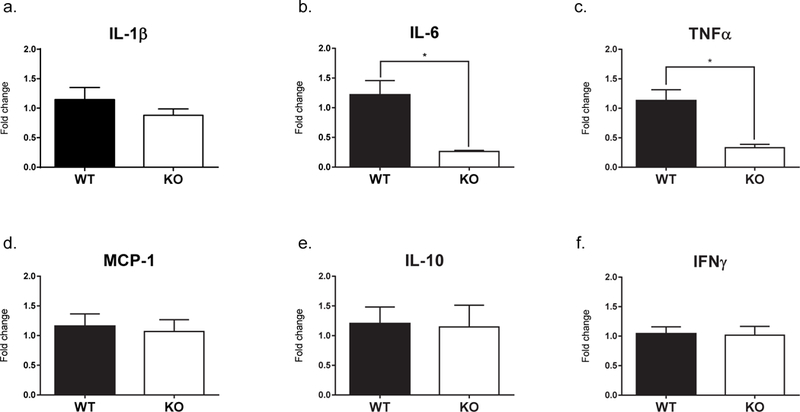
The Fmr1 KO mice (n=6–8) display significantly decreased expression of pro-inflammatory cytokines IL-6 (b) and TNFα (c) compared to WT mice (n=7–8). There was no effect of genotype on the expression of IL-1β (a), MCP-1 (d), IL-10 (e), or IFNγ (f). The expression levels were normalized to β-actin. The bars represent the mean and the error bars represent the standard error of the mean. * p < 0.01.
Discussion
We measured hippocampal cytokine levels in adult Fmr1 knockout (KO) and wild type (WT) mice and found that KO mice had significantly decreased baseline expression of pro-inflammatory cytokines IL-6 and TNFα. However, no changes were detected in the gene expression of any other cytokines, including IL-1β, IFNγ, MCP-1, or IL-10. There is evidence of altered cytokine and chemokine levels in those with ASD, however, few studies have investigated whether differences in these molecules could also be implicated in the pathophysiology of FXS. The first study to examine whether cytokines could play a role in FXS also found distinct cytokine profiles in the plasma of male FXS patients with and without autism compared to controls [12]. They found patients with FXS to have significantly decreased levels of RANTES and IP-10, along with elevated IL-1α compared to controls. In addition, patients with FXS and ASD had increased IL-12 (p40) and decreased eotaxin and MCP-1α levels. Contrastingly, one study found TNFα and IFNγ serum protein levels in Fmr1 KO and WT mice on a C57BL/6J background strain to not differ between genotypes [13]. It is important to note that our study was conducted on a FVB/NJ background strain. Previous studies have found considerable strain differences in the behavior of the Fmr1 KO mouse, suggesting there may be underlying gene-strain interactions that could also manifest in immune-related findings in these mice [14]. These results corroborate the growing body of evidence that suggests dysregulated cytokine expression and signaling could be contributing to deficits seen in FXS.
Pro-inflammatory cytokines are involved in the amplification of many inflammatory reactions and contribute to CNS signaling cascades which downstream can ultimately effect cognition and behavior [15]. Our results contradict numerous studies examining these molecules in humans and rodent models of ASD, which have found cytokines to be primarily elevated in brain tissue, plasma, and the GI tract compared to controls [16, 17]. Rather, our findings appear to mimic a similar cytokine profile of FXS premutation carriers, those with only 55 to 200 repeats on the FMR1 gene instead of the full mutation. While they often have normal intelligence levels, premutation carriers are at risk for developing many neurological symptoms and associated disorders, such as fragile X-associated tremor/ataxia syndrome (FXTAS) and immune-mediated disorders, such as hypothyroidism and fibromyalgia [18]. Increased rates of autoimmune and autoinflammatory disorders have also been noted in premutation carriers [19]. One study by Careaga et al. (2014), found numerous pro-inflammatory cytokines to be decreased in female premutation monocytes and peripheral blood leukocytes at baseline, such as GM-CSF, IL-12 (p40), IFNγ, and MCP-1. Similar findings of decreased cytokine expression was found in CGG knock-in male and female mice splenocytes (IL-6, IL-13, and IL-17) [20].
The mechanism by which decreased IL-6 and TNFα may contribute to dysregulated immune function and FXS disease pathophysiology is unknown. In the CNS, IL-6 can be secreted by a variety of cells (microglia, astrocytes, neurons, etc.) and contributes to numerous complex cellular processes [21, 22]. While IL-6 is typically considered to be a pro-inflammatory cytokine with elevated levels contributing to neurodegeneration, gliosis, and microglial activation; physiological levels of the cytokine have been shown to have anti-inflammatory properties and to be critical for normal CNS development [23]. Interleukin-6 is a member of the neuropoietic family of cytokines and has been shown to promote neural growth and differentiation, as well as protect against excitotoxicity in cerebellar neurons [23, 24]. Furthermore, alterations in IL-6 levels have been associated with behavioral outcomes, such as mice deficient in the cytokine demonstrating increased aggression, and mice overexpressing the cytokine appearing to be more social [25]. A reduction in baseline IL-6 and TNFα expression levels could be associated with reduced neuroprotective abilities, ultimately contributing to a deficit in cross-communication between the innate immune and nervous systems.
It is unknown whether dysregulated immune function, and specifically cellular processes mediated by cytokines, contributes to the pathophysiology of FXS. Our study is the first to report altered hippocampal cytokine expression in male Fmr1 KO mice on a FVB/NJ background strain. There has been considerable research investigating aspects of immunity in premutation carriers due to the high prevalence of immune-related conditions in these patients. It is possible that the complex mechanism by which Fmr1 and the protein, FMRP, alter immune processes in premutation carriers may also be evident in humans with the full mutation. Beyond investigation of cytokine expression at baseline levels, future studies may examine whether innate immune stimulation may lead to a similar deficiency in cytokine levels in the hippocampus. Based on our findings, it is apparent there is a deficit in select pro-inflammatory cytokine expression, however, whether this deficiency effects activation of the immune system and downstream signaling cascades needs to be explored. Additional research examining cytokine levels in the Fmr1 KO mouse is critical in order to establish whether this mouse model accurately mimics the immune dysfunction that has been observed in patients. This will allow for subsequent manipulation and therapeutic interventions aimed at alleviating potential immune deficits that could be linked to behavioral outcomes and pathophysiology of the disorder.
Acknowledgments
We would like to acknowledge the Baylor University Molecular Biosciences Core for the use of equipment for this study. SLH and SON collected the data, SLH, JHT, & JNL analyzed the data. SLH and JNL wrote the manuscript.
Source of Funding: This work was supported by the National Institutes of Health (NIH) [Grant Number: NS088776]
Footnotes
Statement of Conflicts of Interest: None Declared
References
- [1].Weiler IJ, Greenough WT, Synaptic synthesis of the fragile X protein: possible involvement in synapse maturation and elimination, Am. J. Med. Genet. 83 (1999) 248–252. [DOI] [PubMed] [Google Scholar]
- [2].Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, et al. , Autism profiles of males with fragile X syndrome, Am. J. Ment. Retard. 113 (2008) 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hagerman RJ, Lessons from fragile X regarding neurobiology, autism, and neurodegeneration, J. Dev. Behav. Pediatr. 27 (2006) 63–74. [DOI] [PubMed] [Google Scholar]
- [4].Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J, Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome, Brain. Behav. Immun. 25 (2011) 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jyonouchi H, Sun S, Itokazu N, Innate immunity associated with inflammatory responses and cytokine production against common dietary proteins in patients with autism spectrum disorder, Neuropsychobiology 46 (2002). [DOI] [PubMed] [Google Scholar]
- [6].Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, et al. , Elevated immune response in the brain of autistic patients, J. Neuroimmunol. 207 (2009) 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kidd SA, Lachiewicz A, Barbouth D, Blitz RK, Delahunty C, McBrien D, et al. , Fragile X syndrome: a review of associated medical problems, Pediatrics 134 (2014) 995–1005. [DOI] [PubMed] [Google Scholar]
- [8].Hagerman RJ, Altshul-Stark D, McBogg P, Recurrent otitis media in the fragile X syndrome, Am. J. Dis. Child. 141 (1987) 184–187. [DOI] [PubMed] [Google Scholar]
- [9].Bilousova TV, Dansie L, Ngo M, Aye J, Charles JR, Ethell DW, et al. , Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model, J. Med. Genet. 46 (2009) 94–102. [DOI] [PubMed] [Google Scholar]
- [10].Leigh MJ, Nguyen DV, Mu Y, Winarni TI, Schneider A, Chechi T, et al. , A randomized double-blind, placebo-controlled trial of minocycline in children and adolescents with fragile X syndrome, J. Dev. Behav. Pediatr. 34 (2013) 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Deverman BE, Patterson PH, Cytokines and CNS development, Neuron 64 (2009) 61–78. [DOI] [PubMed] [Google Scholar]
- [12].Ashwood P, Nguyen DV, Hessl D, Hagerman RJ, Tassone F, Plasma cytokine profiles in fragile X subjects: is there a role for cytokines in the pathogenesis?, Brain. Behav. Immun. 24 (2010) 898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yuskaitis CJ, Beurel E, Jope RS, Evidence of reactive astrocytes but not peripheral immune system activation in a mouse model of fragile X syndrome, Biochim. Biophys. Acta 1802 (2010) 1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST, Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function, Neuroscience 94 (1999) 185–192. [DOI] [PubMed] [Google Scholar]
- [15].Goines PE, Ashwood P, Cytokine dysregulation in autism spectrum disorders (ASD): possible role of the environment, Neurotoxicol. Teratol. 36 (2013) 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA, Neuroglial activation and neuroinflammation in the brain of patients with autism, Ann. Neurol. 57 (2005) 67–81. [DOI] [PubMed] [Google Scholar]
- [17].Croonenberghs J, Bosmans E, Deboutte D, Kenis G, Maes M, Activation of the inflammatory response system in autism, Neuropsychobiology 45 (2002) 1–6. [DOI] [PubMed] [Google Scholar]
- [18].Coffey SM, Cook K, Tartaglia N, Tassone F, Nguyen DV, Pan R, et al. , Expanded clinical phenotype of women with the FMR1 premutation, Am. J. Med. Genet. A 146a (2008) 1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Winarni TI, Chonchaiya W, Sumekar TA, Ashwood P, Morales GM, Tassone F, et al. , Immune-mediated disorders among women carrier of fragile X premutation alleles, Am. J. Med. Genet. A 0 (2012) 2473–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Careaga M, Rose D, Tassone F, Berman RF, Hagerman R, Ashwood P, Immune dysregulation as a cause of autoinflammation in fragile X premutation carriers: link between FMRI CGG repeat number and decreased cytokine responses, PLoS One 9 (2014) e94475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Erta M, Quintana A, Hidalgo J, Interleukin-6, a major cytokine in the central nervous system, Int. J. Biol. Sci. 8 (2012) 1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Benveniste EN, Cytokine actions in the central nervous system, Cytokine Growth Factor Rev. 9 (1998) 259–275. [DOI] [PubMed] [Google Scholar]
- [23].Juttler E, Tarabin V, Schwaninger M, Interleukin-6 (IL-6): a possible neuromodulator induced by neuronal activity, Neuroscientist 8 (2002) 268–275. [DOI] [PubMed] [Google Scholar]
- [24].Peng Y-P, Qiu Y-H, Lu J-H, Wang J-J, Interleukin-6 protects cultured cerebellar granule neurons against glutamate-induced neurotoxicity, Neurosci. Lett. 374 (2005) 192–196. [DOI] [PubMed] [Google Scholar]
- [25].Alleva E, Cirulli F, Bianchi M, Bondiolotti GP, Chiarotti F, De Acetis L, et al. , Behavioural characterization of interleukin-6 overexpressing or deficient mice during agonistic encounters, Eur. J. Neurosci. 10 (1998) 3664–3672. [DOI] [PubMed] [Google Scholar]


