Abstract
This study evaluated the effect of the alpha-2A-adrenoceptor agonist guanfacine on prefrontally-mediated cognitive functions, as well as quality of life and global function in healthy older participants. One hundred twenty-three participants 75-years and older were randomly assigned to guanfacine 0.5 mg, 0.1 mg, or placebo daily for 12 weeks. The primary outcome measure was the change in z-score for 6 prefrontal executive function tasks over 12 weeks (PEF6). Neither dose of guanfacine improved PEF6 z-score relative to placebo. The rate of mean change [95% confidence interval] in PEF6 z-score over 12 weeks was 0.270 [0.159, 0.380] for placebo, compared with 0.121 [0.011, 0.232] for guanfacine 0.1 mg (p = 0.06, compared to placebo), and 0.213 [0.101, 0.324] for 0.5 mg (p = 0.47). Neither dose of guanfacine improved quality of life or global function relative to placebo. Among common adverse events, only dry mouth was significantly more frequent on guanfacine compared to placebo. Guanfacine failed to ameliorate prefrontal cognitive function in older individuals, who were cognitively normal for age.
Keywords: Cognitive aging, Executive function, Prefrontal cortex, Brain aging, Guanfacine
1. Introduction
A core feature of cognitive aging is the decline in executive functions and working memory mediated by the prefrontal cortex (PFC) (reviewed in (West, 1996)). These functions include the ability to keep in mind an event that has just occurred, or bring to mind an event from long-term stores, and temporarily use this information to guide behavior, thought and affect (Fuster, 1985,Goldman-Rakic, 1995).
In large-scale studies older individuals have been observed to perform worse than younger individuals on PFC tasks, while scoring comparably on many nonPFC tasks (Whelihan and Lesher, 1985). Some abilities, such as vocabulary show remarkable stability into old age. These findings have been corroborated by a multitude of studies of single tasks thought to be sensitive to frontal lobe damage. For example, older adults—compared to younger adults—generally perform worse on tasks of spatial working memory (Owen, et al., 1990,Robbins, et al., 1998), show greater interference on incongruent trials of the Stroop task (Van der Elst, et al., 2006), have greater difficulty on sequential planning tasks (Owen, et al., 1990,Robbins, et al., 1998), and commit significantly more errors on tests of extradimensional vs. intradimensional set shifting (Robbins, et al., 1998). These findings have increasingly led theorists to conclude that the cognitive processes supported by the PFC decline earlier and more profoundly than other cognitive abilities. PFC cognitive deficits begin in middle age and become increasingly evident with advancing age—most apparent after age 75 (Robbins, et al., 1998,Scuteri, et al., 2005), the target age of the present study. The functional significance of impairment in executive functions is evident both cross-sectionally and longitudinally. Measures of executive function in older individuals predict performance in instrumental activities of daily living (IADLs) (Cahn-Weiner, et al., 2000) and correlate with driving safety (Daigneault, et al., 2002). The rates of longitudinal change in measures of executive function in aged cohorts are closely related to decline in IADLs (Royall, et al., 2004) and predict subsequent conversion to Alzheimer’s disease (Rapp and Reischies, 2005).
Although PFC cognitive dysfunction is a characteristic and disabling feature of normal aging (Royall, et al., 2004,West, 1996), no treatment has been developed to date for the amelioration of these symptoms. It has been appreciated for over three decades of animal research that the working memory and executive functions of the PFC can be improved by alpha-2-adrenoceptor agonists such as guanfacine (Arnsten, et al., 1988,Arnsten and Goldman-Rakic, 1985,Franowicz and Arnsten, 1998,Rama, et al., 1996,Ramos, et al., 2006,Tanila, et al., 1996,Wang, et al., 2007). Guanfacine acts at post-synaptic alpha-2A receptors on PFC spines, where it strengthens PFC connections by inhibiting cAMP opening of potassium channels (Wang, et al., 2007). A similar enhancing profile has been observed with acute treatment in humans, whereby guanfacine has been shown to improve PFC cognitive functions including spatial working memory and sequential planning (Jäkälä, et al., 1999a), paired associates learning (Jäkälä, et al., 1999b), sustained attention and response inhibition (Scahill, et al., 2001), and Stroop interference (Taylor and Russo, 2001). Guanfacine is well-tolerated and has already been shown to improve cognitive function in one clinical disorder—attention deficit hyperactivity disorder (ADHD) (Biederman, et al., 2008,Sallee, et al., 2009,Scahill, et al., 2001,Taylor and Russo, 2001). It has also been shown to improve prefrontal cognitive functions in patients with schizotypal disorder (McClure, et al., 2007) and early abstinent cocaine-dependent individuals (Fox, et al., 2015). However, guanfacine has thus far not been tested for its ability to restore working memory and executive function in older adults, despite the evidence from nonhuman primate research that they are the most likely to experience its benefit (Arnsten, et al., 1988,Franowicz and Arnsten, 1998). Guanfacine studies in aged human samples have generally focused on the dose range of 0.1–0.5 mg daily (Crook, et al., 1992,McEntee, et al., 1991), with one study suggesting a weak trend for global benefit on doses of 0.4 and 0.5 mg daily in age-associated memory impairment (AAMI) (McEntee, et al., 1991). We therefore tested guanfacine doses of 0.1 and 0.5 mg daily, administered at bedtime as in previous studies of older participants to minimize sedative effects (Crook, et al., 1992,McEntee, et al., 1991).
The primary aim of the present pilot study was to determine whether low doses of the alpha-2A-adrenoceptor agonist guanfacine could improve PFC-mediated working memory and executive control functions, in healthy older participants. Secondarily, it aimed to determine whether guanfacine could favorably influence quality of life and global function and could be safe and well tolerated.
2. Methods
2.1. Participants
Eligible participants were at least 75 years of age, fluent in English, and in stable general medical health. All participants provided written informed consent in a protocol approved by the Yale University School of Medicine Human Investigation Committee. They then received a screening evaluation that included medical history, physical/neurological examinations, clinical laboratories (hematology, chemistry panel 20, urinalysis, thyroid function studies, serum B12, RPR, electrocardiogram, and brain MRI or CT (if not done within 36 months).
Participants were excluded if they scored <24 on the Mini-Mental State Examination (MMSE) (Folstein, et al., 1975), met DSM IV criteria for dementia (American Psychiatric Association, 1994) or Petersen criteria for amnestic Mild Cognitive Impairment (Petersen, 2004), as evidenced by abnormal memory function documented by scoring 1.5 SD below the education adjusted cutoff on the Logical Memory II subscale (Delayed Paragraph Recall) from the Wechsler Memory Scale – Revised (the maximum score is 25) (Wechsler, 1987). Participants scoring below this cutoff completed a Clinical Dementia Rating Scale (CDR) (Morris, 1993) and were excluded for a global score ≥0.5. CANTAB Motor Screening was administered to ensure ability to touch a screen in response to visual cues for more complex CANTAB tasks. Participants were also excluded who had significant neurologic disease, unstable medical conditions, a history of alcohol or substance abuse within the past 5 years, or active major psychiatric disorders, including major depression (or a score of ≥5 on the Geriatric Depression Scale, range 0–15) (Sheikh and Yesavage, 1986). Participants were excluded who were taking antipsychotics, anti-Parkinson’s drugs, anticonvulsants for seizure disorder, narcotic analgesics, systemic corticosteroids, centrally active beta-blockers, alpha-2 agonists, cholinesterase inhibitors, or memantine.
2.2. Randomization and Interventions
Participants were assigned to receive placebo or guanfacine treatment (0.1 mg or 0.5 mg) daily at bedtime in a double-blind randomized fashion. Four participants (two in the 0.1 mg group and two in the 0.5 mg group) had difficulty complying with bedtime dosing and were permitted to take study medication in the morning with their other medications. Treatment groups were balanced with respect to sex and age (75–79; 80–84; 85–89; 90–94 years). Randomization was performed by the Investigational Drug Service (IDS) in the Yale-New Haven Medical Center using a computerized random-number generator, and treatment was assigned by a computer. Dosing of study medication began after the Baseline visit. Participants then returned to clinic at Weeks 1, 6, 12, and 13 (± 3 days) for safety evaluations and dispensing of ongoing study medication. At Weeks 6 and 12 the outcome Neuropsychological Test Battery was repeated.
2.3. Outcome Measures
The primary outcome measure was a composite z-score from a 6-item neuropsychological battery targeting prefrontal executive function (PEF6). The neuropsychological test battery consisted of 4 items from the Cambridge Neuropsychological Test Automated Battery (CANTAB) (Robbins, et al., 1994): Spatial Working Memory (total between-search errors, for the 6- and 8-box levels) (Owen, et al., 1990,Robbins, et al., 1998), Stockings of Cambridge (mean number of excess moves made for four and five move problems) (Owen, et al., 1995), Intradimensional/Extradimensional Shift (ID/ED Shift, total extra-dimensional shift errors) (Robbins, et al., 1998) Paired Associates Learning (total trials to criterion on the 6- and 8-box problems) (Blackwell, et al., 2004), as well as the Stroop Interference Task (Color Word score) (Golden, 1976) and the Trail Making Test B (time in seconds) (Reitan, 1958). The tests that comprised this battery were selected from those thought to reflect prefrontally-mediated executive or working memory function (Owen, et al., 1990,Owen, et al., 1991,Owen, et al., 1995,Robbins, et al., 1998,Van der Elst, et al., 2006) and demonstrating sensitivity to aging effects (at least by age 75) (Robbins, et al., 1998,Van der Elst, et al., 2006) and—in most cases— guanfacine treatment effects (Jäkälä, et al., 1999a,Jäkälä, et al., 1999b,Taylor and Russo, 2001) in previous human studies. Secondary efficacy outcomes included quality of life as measured by the Mental Component Score of the 36-Item Short-Form Health Survey (SF-36-MCS; QualityMetric, Lincoln, RI) (Ware and Sherbourne, 1992) and global function as assessed by the Alzheimer’s Disease Cooperative Study—Clinical Global Impression of Change (ADCS-CGIC) (Schneider, et al., 1997).
Safety outcomes included adverse events, which also encompassed significant clinical laboratory and electrocardiographic findings. Systolic and diastolic blood pressure were measured with participant supine (average of 3 consecutive readings with participant lying down for ≥5 minutes) and standing (average of 3 consecutive readings obtained immediately upon standing). Orthostatic blood pressure readings (difference of supine and standing readings) were obtained for both systolic and diastolic blood pressures. Sedation was monitored using the Epworth Sleepiness Scale (range 0–24) (Johns, 1991). Instrumental Activities of Daily Living (IADLs) were assessed by the participant’s self-ratings of difficulty from the Minimum Data Set–Home Care scale (range 0–38) (Teresi, et al., 1997). Depression symptoms were monitored using the Geriatric Depression Scale (range 0–15) (Sheikh and Yesavage, 1986). Blood was obtained at Week-12 endpoint for the measurement of plasma guanfacine concentration by GMA in collaboration with the Mass Spectrometry facility of the Department of Pharmacology, Yale University School of Medicine. Guanfacine standards were measured with high linearity (r2=0.9993) and the concentration (3.57 ng/mL) of a quality assessment (“QC”) sample was determined with intra-assay and inter-assay coefficients of variation (CVs) of 5.2% (n=5) and 3.4% (n=5), respectively. This assay became available when the study was in progress and was therefore obtained in a subset of participants (n=101).
2.4. Statistical analyses
The primary aim of the statistical analysis was to determine if participants treated with guanfacine (0.1 mg or 0.5 mg daily) showed improved performance in working memory and executive functions relative to placebo at study endpoint. A composite measure of executive function was generated by converting raw scores for each of the six prefrontal executive function tests (PEF6) to z-scores (using the sample baseline mean and SD for each test) and then averaging to obtain a PEF6 z-score. The primary outcome measure was the change in the PEF6 z-score between Baseline and Week 12 endpoint. A multivariable linear regression model using maximum likelihood estimation was fit with medication treatment group as the main predictor and baseline score, age, sex, and education as pre-specified covariates. To graphically display adjusted longitudinal results (including the intermediate Week 6 time point), the rate of change in PEF6 z-scores was compared between treatment groups using longitudinal multivariable regression.
Due to the limited availability of preliminary data for the PEF6 z-score, power calculations were based on a standardized effect size measured in units of SD. To attain 80% power to detect a 0.66 SD increase in the change in PEF6 z-score (two tailed α=0.05) in either treatment group compared to placebo, the study required 37 completers or 41 randomized participants per treatment group (assuming an overall attrition rate of ~10%).
The original pre-planned primary analysis followed the intention-to-treat (ITT) approach, in which all randomized individuals were included in the final analysis. Missing scores were to be imputed using the multiple imputation method of Rubin (Rubin, 1976). However, with the approval of the DSMB, the primary analysis was changed to an analysis of protocol completers because: 1) they represented 119 of 123 (97%) of participants, and 2) the Little MCAR screening test failed to reject the null hypothesis of MCAR missingness (Little, 1988). Multiple imputation was performed, and results were compared to the completer’s analysis to assess the impact of missing data. Several post hoc analyses were performed to provide additional insight into the causal efficacy of treatment. These included an analysis of compliers (those who ingested ≥80% of the prescribed study medication based on return pill count).
Secondary efficacy aims determined if participants treated with guanfacine showed improved quality of life or global status at study endpoint. As a measure of quality of life, the SF-36-MCS was analyzed as described above for the PEF6 z-score. The effect of treatment assignment on global function (ADCS-CGIC at Week 12 endpoint) was analyzed using ordinal logistic regression, and specifically the proportional odds model. The seven-point scale was collapsed to 3 ordered groups: worsened, no change, and improved for the analysis.
Secondary safety aims sought to determine if participants treated with guanfacine showed statistically significant differences in safety measures. The effect of treatment assignment on the following individual safety measures was analyzed in a manner similar to the PEF6 z-score above: Blood Pressure: Systolic Blood Pressure, Diastolic Blood Pressure, Orthostatic Blood Pressure Drop; Sedation Ratings: Epworth Sleepiness Scale; Geriatric Depression Scale; IADLs: Minimum Data Set–Home Care scale. In addition, frequencies of adverse events in the three treatment groups were compared using Fisher’s exact test.
SAS® 9.4 (SAS/STAT 14.1) statistical software was used for all analyses. p-Values <0.05 in two-sided tests were interpreted as being statistically significant.
3. Results
3.1. Participant characteristics
Participants were recruited between May 22, 2009 and May 11, 2012; the last participant was randomized on May 21, 2012 and completed the study on August 13, 2012. As shown in Figure 1, a total of 154 participants were screened for the study: 19 were found ineligible, 12 withdrew consent prior to randomization, and 123 were randomized. All 123 randomized participants (54 women and 69 men) were included in the safety analysis set and comprised the modified intention-to-treat analysis set for efficacy, and 119 (96.7%) completed the study (Figure 1). These 119 participants provided outcome data and were included in the study’s analytical sample. One participant discontinued the study while receiving guanfacine 0.1 mg, two while receiving 0.5 mg, and one while receiving placebo—all for adverse events. In addition, one participant completed all study procedures off study medication (guanfacine 0.1 mg) following an adverse event. Participant baseline characteristics were generally well balanced across treatment groups—with the exception of baseline Epworth Sleepiness Scale scores, which differed by treatment groups (Table 1).
Figure 1.
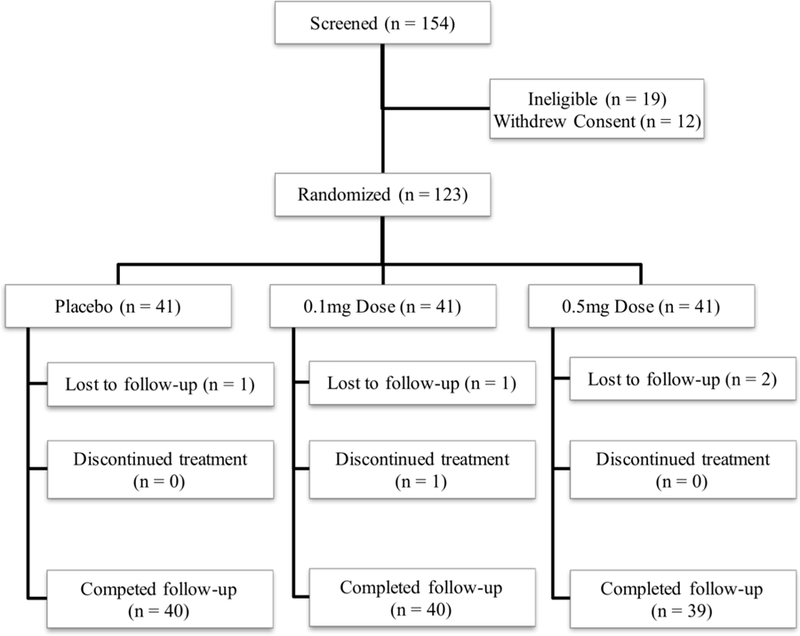
CONSORT diagram reflecting flow of study participants through the study.
Table 1.
Participant Characteristics
| Characteristic | Placebo n = 41 | 0.1mg n = 41 | 0.5mg n = 41 | p-Value* | |||
|---|---|---|---|---|---|---|---|
| mean | SD | mean | SD | mean | SD | ||
| Age (y) | 80.5 | 4.0 | 80.0 | 4.0 | 80.1 | 4.0 | 0.824 |
| Sex (% male) | 58.5% | 53.7% | 56.1% | 0.906 | |||
| Education (y) | 17.0 | 2.5 | 16.2 | 2.5 | 16.2 | 2.7 | 0.265 |
| Weight (lbs) | 165.2 | 31.7 | 177.3 | 34.1 | 164.4 | 37.7 | 0.168 |
| Geriatric Depression Scale | 1.1 | 1.3 | 1.1 | 1.4 | 1.3 | 1.5 | 0.834 |
| Epworth Sleepiness Scale | 6.8 | 3.3 | 6.0 | 3.3 | 5.0 | 2.7 | 0.032 |
| Cognitive Status | |||||||
| MMSE | 29.5 | 0.7 | 29.3 | 1.2 | 29.6 | 0.6 | 0.418 |
| Logical Memory 1 | 13.2 | 3.9 | 13.0 | 4.1 | 11.9 | 4.0 | 0.247 |
| Logical Memory 2 | 11.9 | 4.3 | 11.5 | 3.8 | 10.5 | 4.0 | 0.279 |
| WAIS - Vocabulary | 52.9 | 7.8 | 48.4 | 11.0 | 52.0 | 11.7 | 0.116 |
| WAIS - Block Design | 30.3 | 11.7 | 28.1 | 8.8 | 29.5 | 7.6 | 0.581 |
| Executive Function Measures | |||||||
| Spatial Working Memory | 43.9 | 16.0 | 46.5 | 15.5 | 42.2 | 16.9 | 0.477 |
| Stockings of Cambridge | 4.3 | 2.3 | 4.2 | 2.5 | 4.0 | 2.1 | 0.875 |
| ID/ED Shift | 11.9 | 10.3 | 11.8 | 10.2 | 11.9 | 10.3 | 0.997 |
| Paired Associates Learning | 11.3 | 3.9 | 12.6 | 4.4 | 12.7 | 4.7 | 0.245 |
| Stroop Color Word | 30.2 | 8.2 | 29.9 | 9.0 | 30.5 | 9.5 | 0.952 |
| Trail Making Test B | 94.4 | 35.6 | 106.4 | 48.8 | 88.8 | 32.0 | 0.122 |
| Quality of Life | |||||||
| SF-36-PCS | 48.3 | 6.9 | 46.4 | 8.8 | 48.1 | 8.3 | 0.525 |
| SF-36-MCS | 56.0 | 5.7 | 56.1 | 6.9 | 55.5 | 6.6 | 0.920 |
Notes: MMSE = Mini-Mental State Examination; ID/ED Shift = Intradimensional/Extradimensional Shift; SF-36-MCS and SF-36-PCS = Mental Component Score and Physical Component Score of the 36-Item Short-Form Health Survey
p-Values are for ANOVA for continuous variables and chi-square for categorical variables and are uncorrected for multiplicity.
3.2. Effect of Guanfacine Treatment on Prefrontal Executive Function (PEF6 z-score)
Guanfacine treatment had no significant effect on the primary outcome measure of prefrontal executive function, as shown in Figure 2 and Table 2. The rate of mean change [95% confidence interval] in PEF6 z-score over 12 weeks was 0.270 [0.159, 0.380] for the placebo group, compared with 0.121 [0.011, 0.232] for the guanfacine 0.1 mg group (p = 0.06), and 0.213 [0.101, 0.324] for the guanfacine 0.5 mg group (p = 0.47; Figure 2). A multivariable linear regression model was employed, whose main predictor was medication treatment group, and whose covariates included baseline PEF6 z-score, age, sex, and education. One hundred nineteen of the 123 trial participants had outcome data recorded at 12 weeks of follow-up; multiple imputation of missing values yielded very similar results, with the p-value for the 0.1 mg dose estimate being 0.10 and for the 0.5 mg dose estimate being 0.56. These analyses did not show a benefit of either dose of guanfacine relative to placebo. One hundred fifteen of the 123 participants met the 80% requirement for study medication compliance. A “compliers” analysis also revealed no benefit of either dose of guanfacine relative to placebo.
Figure 2. Effect of guanfacine treatment on prefrontal executive function (PEF6 z-score) in cognitively normal older participants.
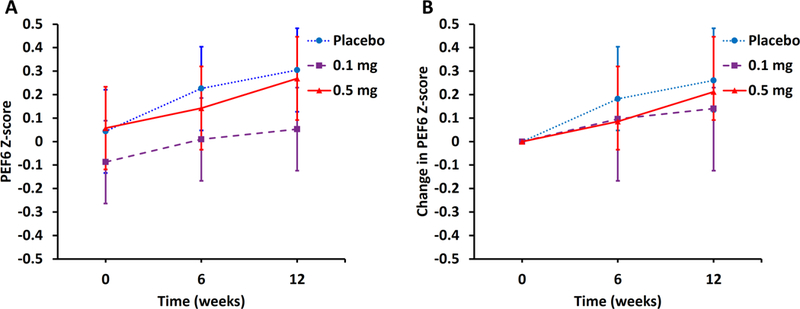
Least squares means and 95% confidence limits are estimated from linear mixed effects models accounting for serial correlation among repeated PEF6 z-score measurements at three time-points (baseline, 6 weeks, and 12 weeks). Both the observed means (A) and the difference (from baseline) means (B) are shown after multivariable adjustments for age, education, and sex. Abbreviations: PEF6 z-score, mean of z-scores for 6 executive function tasks (CANTAB: Spatial Working Memory, Stockings of Cambridge, Intradimensional/Extradimensional Shift, Paired Associates Learning; Stroop Color Word Score, Trail Making Test B).
Table 2.
Effect of Guanfacine Treatment on Primary and Secondary Outcomes
| Outcome Measure | Placebo (n=40) | 0.1 mg (n=40) | 0.5 mg (n=39) |
|---|---|---|---|
| Prefrontal Executive Function (PEF6 z-score) | |||
| Unadjusted Baseline | 0.039 | −0.084 | 0.102 |
| Unadjusted Week 12 | 0.305 | 0.053 | 0.303 |
| Unadjusted Change | 0.266 | 0.137 | 0.201 |
| Adjusted Change | 0.270 | 0.121 | 0.213 |
| 95% confidence interval | 0.159, 0.380 | 0.011, 0.232 | 0.101, 0.324 |
| p-Value* | –––– | 0.06 | 0.47 |
| Quality of Life (SF-36-MCS) | |||
| Unadjusted Baseline | 55.878 | 56.617 | 55.462 |
| Unadjusted Week 12 | 55.985 | 55.677 | 54.638 |
| Unadjusted Change | 0.107 | −0.940 | −0.824 |
| Adjusted Change | −0.083 | −0.618 | −0.960 |
| 95% confidence interval | −1.725, 1.559 | −2.253, 1.017 | −2.614, 0.694 |
| p-Value* | –––– | 0.65 | 0.46 |
| Global Function (ADCS-CGIC) | |||
| Worsened (%) | 3 (7.5) | 4 (10.0) | 2 (5.1) |
| No Change (%) | 25 (62.5) | 20 (50.0) | 22 (56.4) |
| Improved (%) | 12 (30.0) | 16 (40.0) | 15 (38.5) |
| Odds Ratio | –––– | 1.43 | 1.46 |
| p-Value** | –––– | 0.67 | 0.60 |
Notes: PEF6 z-score = the mean of z-scores for 6 executive function tasks (CANTAB: Spatial Working Memory, Stockings of Cambridge, Intradimensiona/Extradimensional Shift, Paired Associates Learning; Stroop Color Word Score, Trail Making Test B); SF-36-MCS = Mental Component Score of the 36-Item Short-Form Health Survey (higher scores represent better mental health); ADCS-CGIC scores are for Week 12 compared to baseline. The seven-point scale was collapsed to 3 groups: improved (1–3), no-change (4), and worsening (5–7).
p-Values for regression coefficients representing the differences in least squares means between each dosing group and the placebo group. A multivariable linear regression model was employed, whose main predictor was medication treatment group, and whose covariates included baseline score, age, sex, and education.
p-Values for regression coefficients representing log odds ratios for each dosing group as compared to the placebo group. A multivariable proportional odds model was employed, whose main predictor is medication treatment group, and whose covariates include age, sex, and education.
Post-hoc analyses were undertaken to evaluate a possible selective treatment effect for participants who were younger or had lower baseline executive function (PEF6 z-score) but were not significant. Additional post-hoc analyses were performed for each of the 6 components of the PEF6 z-score and were not significant. Finally, Week-12 plasma concentrations were obtained in 101 participants and were considered valid if study drug had not been missed in the days prior to the Week-12 visit and had not been taken on the day of the visit. The elapsed time from last dose to plasma level ranged from 11.0 to 19.9h (15.0 ± 2.1, elimination half-life of guanfacine 17h (Kiechel, 1980)). Plasma levels for the three dose groups were essentially non-overlapping: all undetectable for the placebo group, undetectable to 0.79 ng/mL for the 0.1 mg group, 1.13 to 3.84 ng/mL for the 0.5 mg group (Supplementary Figure 1). Plasma concentrations were not associated with change in PEF6 z-score (Spearman’s ρ = –0.022, p = 0.83 N = 96, Supplementary Figure 1).
3.3. Effects of Guanfacine Treatment on Quality of Life and Global Function
The effects of guanfacine treatment on the secondary efficacy measures of quality of life and global function are shown in Table 2. On the SF-36-MCS the rate of mean change [95% confidence interval] over 12 weeks for the placebo group (–0.083 [–1.725, 1.559]) did not differ from that for the guanfacine 0.1 mg group (–0.618 [2.253, 1.017], p = 0.65) or the guanfacine 0.5 mg group (–0.960 [–2.614, 0.694], p = 0.46). A multivariable linear regression model was employed, whose main predictor was medication treatment group, and whose covariates included baseline SF-36-MCS score, age, sex, and education. One hundred nineteen of the 123 trial participants had outcome data recorded at 12 weeks of follow-up.
On the ADCS-CGIC, participants in the placebo group were not rated differently over 12 weeks from either the guanfacine 0.1 mg group (OR 1.43, p = 0.67, compared to placebo) or the guanfacine 0.5 mg group: (OR 1.46, p = 0.60, compared to placebo). A multivariable proportional odds model was employed, whose main predictor was medication treatment group, and whose covariates included age, sex, and education. One hundred nineteen of the 123 trial participants had outcome data recorded at 12 weeks of follow-up.
3.4. Effect of Guanfacine Treatment on Safety Measures
Adverse events occurring in at least 5% of any treatment group are shown in Table 3. The only event that was statistically more common with guanfacine treatment was dry mouth, which occurred in 0 participants receiving placebo, 3 participants receiving guanfacine 0.1 mg, and 7 participants receiving guanfacine 0.5 mg (p = 0.02, Fisher’s Exact Test). Gastroenteritis was statistically more common in the placebo group (p = 0.03). A total of 6 serious adverse events occurred during the trial, including 1 prior to randomization (pneumonia), 2 in the placebo group (myocardial infarction, subarachnoid hemorrhage), 2 in the guanfacine 0.1 mg group (stroke, pneumonia), and 1 in the guanfacine 0.5 mg group (hyponatremia due to adrenal insufficiency).
Table 3.
Adverse Events Occurring in at least 5% of any Treatment Group
| Adverse Event Categorya count (%) | Placebo (n=41) | 0.1 mg (n=41) | 0.5 mg (n=41) | Total (N=123) |
|---|---|---|---|---|
| Constipation | 1 (2.4) | 3 (7.3) | 0 (0.0) | 4 |
| Dry Mouth | 0 (0.0) | 3 (7.3) | 7 (17.1) | 10 |
| Fall | 1 (2.4) | 2 (4.9) | 3 (7.3) | 6 |
| Fatigue | 3 (7.3) | 3 (7.3) | 7 (17.1) | 13 |
| Gastroenteritis | 4 (9.8) | 0 (0.0) | 0 (0.0) | 4 |
| Joint Pain | 1 (2.4) | 1 (2.4) | 4 (9.8) | 6 |
| Lightheadedness | 4 (9.8) | 0 (0.0) | 4 (9.8) | 8 |
| Low Back Pain | 3 (7.3) | 0 (0.0) | 0 (0.0) | 3 |
| Sedation | 1 (2.4) | 3 (7.3) | 1 (2.4) | 5 |
| Upper Respiratory Infection | 9 (22.0) | 4 (9.8) | 3 (7.3) | 16 |
| Urinary Tract Infection | 3 (7.3) | 1 (2.4) | 1 (2.4) | 5 |
Fisher’s Exact Test: Dry Mouth (p = 0.02) & Gastroenteritis (p = 0.03). No Fisher’s Exact Test has an associated p < 0.05 after a False Discovery Rate correction for multiplicity.
The effect of guanfacine treatment on blood pressure was analyzed both short-term (change from baseline to Week 1) and long-term (change from baseline to Week 12). At Week 1 there was a significant effect of guanfacine 0.5 mg treatment compared to placebo on change in standing systolic (–8.1 mmHg greater change on 0.5 mg compared to placebo, p = 0.01, multivariable linear regression model, covariates: baseline measurement, age, sex, and education) and diastolic (–5.8 mmHg, p = 0.002), supine systolic (–8.9 mmHg, p = 0.002) and diastolic (–4.1 mmHg, p = 0.02), but not on orthostatic systolic BP. At the 0.1 mg dose, the only significant effect was on change in supine diastolic BP (–3.4 mmHg, p = 0.049). At Week 12 the only effect that remained significant was for the 0.5 mg dose in supine diastolic BP (–4.6 mmHg greater change on 0.5 mg compared to placebo, p = 0.01).
There was no overall effect of guanfacine treatment on sedation as evaluated using the Epworth Sleepiness Scale measured at 1, 3, 6, 9 and 12 weeks of follow-up for either the guanfacine 0.1 mg group (p = 0.97 compared to placebo, generalized linear regression model with repeated measurements; covariates: baseline outcome score, age, sex, and education) or the guanfacine 0.5 mg group: (p = 0.29). Short-term sedative effects at 1 week were analyzed specifically and were also non-significant for either the guanfacine 0.1 mg group (p = 0.71) or the guanfacine 0.5 mg group: (p = 0.98). There was no effect of guanfacine treatment on depression symptoms as evaluated using the Geriatric Depression Scale (0.1 mg: p = 0.33; 0.5 mg: p = 0.59 compared to placebo) or on ADLs as evaluated using the Minimum Data Set–Home Care scale (0.1 mg: p = 0.16; 0.5 mg: p = 0.48 compared to placebo). Both scales were measured at 6 and 12 weeks of follow-up (generalized linear regression model, with repeated measurements, covariates include baseline outcome score, age, sex, and education).
4. Discussion
Guanfacine treatment for 12 weeks had no effect on working memory and executive functions in cognitively normal older individuals. We observed no benefit of either dose of guanfacine (0.1 mg daily or 0.5 mg daily) relative to placebo on the primary outcome—a composite measure of prefrontal executive function—or on secondary measures of quality of life or global function. The trend for worsening in prefrontal executive function at 12 weeks for the 0.1 mg but not the 0.5 mg dose (Figure 2, Table 2) is puzzling but probably represents a statistical aberration, as previous human (McEntee, et al., 1991) and primate (Arnsten, et al., 1988) studies have not suggested the potential for cognitive impairment in this low-dose range (~0.001 mg/kg). Of note was the relatively benign side effect profile of low doses of guanfacine in this frail older population. Among common adverse events, only dry mouth was significantly more frequent on guanfacine compared to placebo. Although guanfacine 0.5 mg daily (but not 0.1 mg daily) significantly lowered blood pressure from Baseline to Week 1, tolerance appeared to develop to acute effects, as they were largely absent at Week 12. The clinical importance of these physiological effects appears small, as adverse events commonly associated with hypotension (lightheadedness, falls) were infrequent.
We chose not to select exclusively for prefrontal executive impairment relative to norms for older (i.e. for a non-amnestic, single-domain—executive—MCI) or younger individuals, instead studying a more representative older sample. We therefore conceptualized executive dysfunction as an inherent part of aging. However, a post-hoc analysis of those participants with the greatest impairment (bottom half by median split) did not differ, suggesting that this decision probably did not account for the absence of a treatment effect.
4.1. Comparison to Animal Studies
The absence of a treatment effect in older humans despite the observed benefits of guanfacine and other alpha-2A-adrenoceptor agonists in aged animals is difficult to explain. However, possible explanations include dose differences, the age-range of participants, and the choice of outcome measures.
One possibility is that we tested guanfacine at incorrect doses (0.1 mg and 0.5 mg daily). Guanfacine has been evaluated for its cognitive and behavioral effects in several clinical studies and is approved in an extended-release formulation for the treatment of pediatric attention-deficit/hyperactivity disorder (ADHD) (Biederman, et al., 2008,Sallee, et al., 2009,Scahill, et al., 2001,Taylor and Russo, 2001). Previous human cognitive trials have examined a wide range of doses from 0.1 mg daily to 4.0 mg daily. Several studies in young humans have suggested benefit in the range of 1.0–2.5 mg daily (Jäkälä, et al., 1999a,Jäkälä, et al., 1999b,McClure, et al., 2007,Scahill, et al., 2001,Taylor and Russo, 2001). In a study in which clinicians titrated blindly (balancing efficacy against side effects), the mean final dose was 1.1 mg daily in participants aged 41.2±11.4 (21–57) (Taylor and Russo, 2001). However, studies in aged non-human primates have demonstrated that the dose-response curve is significantly shifted compared to that of young animals (Arnsten, et al., 1988,Franowicz and Arnsten, 1998). The optimal guanfacine dose for improving delayed response performance in aged nonhuman primates without sedative or hypotensive effects is ~0.001 mg/kg (single intramuscular dose) (Arnsten, et al., 1988). This corresponds to a human dose in the order of 0.1 mg daily (although the conversion from an acute intramuscular dose to a chronic oral dose is unknown). Guanfacine studies in older samples have generally focused on the dose range of 0.1–0.5 mg daily (Crook, et al., 1992,McEntee, et al., 1991), with one study suggesting a weak trend for global benefit on doses of 0.4 and 0.5 mg daily in age-associated memory impairment (AAMI) (McEntee, et al., 1991). We therefore tested guanfacine doses of 0.1 and 0.5 mg daily, administered at bedtime as in previous studies of older participants to minimize sedative effects (Crook, et al., 1992,McEntee, et al., 1991). Although our two active doses had a five-fold difference, it is unlikely that an optimal intermediate dose was missed, as post-hoc analysis of plasma levels revealed no favorable trends at intermediate levels (Supplementary Figure 1). The higher doses used to treat ADHD (Biederman, et al., 2008,Sallee, et al., 2009,Scahill, et al., 2001,Taylor and Russo, 2001) may have had beneficial effects on prefrontal executive function, although these likely would have been limited by safety and tolerability.
A second possibility is that the participants in the present study were too old to replicate the effect observed in animal studies. Guanfacine has been shown to improve the working memory performance of aged monkeys in several studies (Arnsten, et al., 1988,Rama, et al., 1996,Ramos, et al., 2006,Wang, et al., 2007) and is more potent and more efficacious in aged than young adult monkeys (Arnsten, et al., 1988,Franowicz and Arnsten, 1998). A similar profile is found in rodents, where alpha-2A-adrenoceptor agonists have especially powerful effects in aged rats (Tanila, et al., 1996). However, these studies have been conducted in animals whose average age (~22 years in rhesus monkeys, 18 years in stump-tailed macaques, and 22 months in rats) probably corresponds to humans in their 50s and 60s and may not apply to the 75+ bracket. While guanfacine’s enhanced effects in aged animals have been shown to derive from inhibition of dysregulated cAMP-K+ signaling in dendritic spines (Carlyle, et al., 2014,Wang, et al., 2011), the loss of spines (Morrison and Baxter, 2012) and alpha-2A receptors (Moore, et al., 2005) that occurs with age may eventually exceed a threshold such that guanfacine would lose its therapeutic target and cease to be effective. The 75+ age range was chosen for the present study to ensure robust age-related decline in PEF outcome measures (7, 10). Within this range there was no evidence of differential aging effects, as a post-hoc median-split analysis of drug effects in the younger 50% of participants (~aged 75–80) did not reveal favorable treatment effects in relatively younger participants. Nonetheless, further study in older adults <75 years may be worthwhile. Measurable decline in prefrontal executive function is evident by middle-age. However, specific impairment criteria may be required to identify individuals with relative dysfunction in executive function.
A third possibility is that the outcome measures employed in the present study differed from those utilized in animal studies. The vast majority of non-human primate studies have utilized delayed response tasks, for which the closest human task in the present study is spatial working memory. A post hoc analysis of the spatial working memory task alone revealed no effect of guanfacine treatment. Most of the tasks contained in the PEF6 outcome measure have no direct animal counterpart. However, the battery was selected largely from tests that have shown guanfacine treatment effects in previous human studies (Jäkälä, et al., 1999a,Jäkälä, et al., 1999b,Taylor and Russo, 2001).
4.2. Conclusion
Guanfacine failed to ameliorate prefrontal cognitive function in older individuals, who were cognitively normal for age. Further studies may be worthwhile in older adults <75 years— especially those with relative deficits in executive function.
Supplementary Material
Acknowledgements
The authors wish to thank Peter Charpentier and Shu Chen for informatics support and Ronald Thomas and Heather Allore for biostatistical consultation. ClinicalTrials.gov Identifier: NCT00935493. This work was supported by the National Institute on Aging [R01-AG030457 (CHvD), P50-AG047270, and P30-AG021342].
Footnotes
Disclosure Statement
Dr. Arnsten and Yale University receive royalties from Shire Pharmaceuticals for the US sales of extended release guanfacine (Intuniv®) for the treatment of ADHD and related disorders. They do not receive royalties for non-US sales, generic extended release guanfacine, or immediate release guanfacine. Dr. Arnsten is the spouse of Dr. van Dyck.
References
- American Psychiatric Association; 1994. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. American Psychiatric Association, Washington, DC. [Google Scholar]
- Arnsten AFT, Cai JX, Goldman-Rakic PS 1988. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects. J Neurosci 8, 4287–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Goldman-Rakic PS 1985. Alpha-2 adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science 230, 1273–6. [DOI] [PubMed] [Google Scholar]
- Biederman J, Melmed RD, Patel A, McBurnett K, Konow J, Lyne A, Scherer N 2008. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics 121(1), e73–84. [DOI] [PubMed] [Google Scholar]
- Blackwell AD, Sahakian BJ, Vesey R, Semple JM, Robbins TW, Hodges JR 2004. Detecting dementia: novel neuropsychological markers of preclinical Alzheimer’s disease. Dement Geriatr Cogn Disord 17(1–2), 42–8. [DOI] [PubMed] [Google Scholar]
- Cahn-Weiner DA, Malloy PF, Boyle PA, Marran M, Salloway S 2000. Prediction of functional status from neuropsychological tests in community-dwelling elderly individuals. Clin Neuropsychol 14(2), 187–95. [DOI] [PubMed] [Google Scholar]
- Carlyle BC, Nairn AC, Wang M, Yang Y, Jin LE, Simen AA, Ramos BP, Bordner KA, Craft GE, Davies P, Pletikos M, Sestan N, Arnsten AF, Paspalas CD 2014. cAMP-PKA phosphorylation of tau confers risk for degeneration in aging association cortex. Proceedings of the National Academy of Sciences of the United States of America 111(13), 5036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook T, Wilner E, Rothwell A, Winterling D, McEntee W 1992. Noradrenergic intervention in Alzheimer’s Disease. Psychopharmacol Bull 28, 67–70. [PubMed] [Google Scholar]
- Daigneault G, Joly P, Frigon JY 2002. Executive functions in the evaluation of accident risk of older drivers. J Clin Exp Neuropsychol 24(2), 221–38. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR 1975. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3), 189–98. [DOI] [PubMed] [Google Scholar]
- Fox H, Sofuoglu M, Sinha R 2015. Guanfacine enhances inhibitory control and attentional shifting in early abstinent cocaine-dependent individuals. Journal of psychopharmacology (Oxford, England) 29(3), 312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franowicz JCS, Arnsten AFT 1998. The alpha-2A noradrenergic agonist, guanfacine, improves delayed response performance in young adult rhesus monkeys. Psychopharmacology 136, 8–14. [DOI] [PubMed] [Google Scholar]
- Fuster JM 1985. The prefrontal cortex, mediator of cross-temporal contingencies. Human Neurobiol 4, 169–79. [PubMed] [Google Scholar]
- Golden CJ 1976. Identification of brain disorders by the Stroop Color and Word Test. J Clin Psychol 32, 654–8. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS 1995. Cellular basis of working memory. Neuron 14, 477–85. [DOI] [PubMed] [Google Scholar]
- Jäkälä P, Riekkinen M, Sirviö J, Koivisto E, Kejonen K, Vanhanen M, Riekkinen PJ 1999a. Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology 20, 460–70. [DOI] [PubMed] [Google Scholar]
- Jäkälä P, Sirviö J, Riekkinen M, Koivisto E, Kejonen K, Vanhanen M, Riekkinen PJ 1999b. Guanfacine and clonidine, alpha-2 agonists, improve paired associates learning, but not delayed matching to sample, in humans. Neuropsychopharmacology 20, 119–30. [DOI] [PubMed] [Google Scholar]
- Johns MW 1991. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14(6), 540–5. [DOI] [PubMed] [Google Scholar]
- Kiechel JR 1980. Pharmacokinetics and metabolism of guanfacine in man: a review. Br J Clin Pharmacol 10 (Suppl 1), 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJA 1988. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc 83(404), 1198–202. [Google Scholar]
- McClure MM, Barch DM, Romero MJ, Minzenberg MJ, Triebwasser J, Harvey PD, Siever LJ 2007. The effects of guanfacine on context processing abnormalities in schizotypal personality disorder. Biol Psychiatry 61(10), 1157–60. [DOI] [PubMed] [Google Scholar]
- McEntee WJ, Crook TH, Jenkyn LR, Petrie W, Larrabee GJ, Coffey DJ 1991. Treatment of age-associated memory impairment with guanfacine. Psychopharmacol Bull 27(1), 41–6. [PubMed] [Google Scholar]
- Moore TL, Schettler SP, Killiany RJ, Herndon JG, Luebke JI, Moss MB, Rosene DL 2005. Cognitive impairment in aged rhesus monkeys associated with monoamine receptors in the prefrontal cortex. Behavioural brain research 160(2), 208–21. [DOI] [PubMed] [Google Scholar]
- Morris JC 1993. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43(11), 2412–4. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG 2012. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nature reviews Neuroscience 13(4), 240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW 1990. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia 28(10), 1021–34. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW 1991. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia 29(10), 993–1006. [DOI] [PubMed] [Google Scholar]
- Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW 1995. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia 33(1), 1–24. [DOI] [PubMed] [Google Scholar]
- Petersen RC 2004. Mild cognitive impairment as a diagnostic entity. J Intern Med 256(3), 183–94. [DOI] [PubMed] [Google Scholar]
- Rama P, Linnankoski I, Tanila H, Pertovaara A, Carlson S 1996. Medetomidine, atipamezole, and guanfacine in delayed response performance of aged monkeys. Pharmacol Biochem Behav 54, 1–7. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Stark D, Verduzco L, van Dyck CH, Arnsten AF 2006. Guanfacine improves prefrontal cortical regulation of behavior through inhibition of cAMP/PKA signaling. Learn Mem 13(6), 770–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp MA, Reischies FM 2005. Attention and executive control predict Alzheimer disease in late life: results from the Berlin Aging Study (BASE). Am J Geriatr Psychiatry 13(2), 134–41. [DOI] [PubMed] [Google Scholar]
- Reitan RM 1958. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills 8, 271–6. [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, Lawrence AD, McInnes L, Rabbitt PM 1998. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. J Int Neuropsychol Soc 4(5), 474–90. [DOI] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P 1994. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia 5(5), 266–81. [DOI] [PubMed] [Google Scholar]
- Royall DR, Palmer R, Chiodo LK, Polk MJ 2004. Declining executive control in normal aging predicts change in functional status: the Freedom House Study. J Am Geriatr Soc 52(3), 346–52. [DOI] [PubMed] [Google Scholar]
- Rubin DB 1976. Inference and missing data. Biometrika 63, 581–92. [Google Scholar]
- Sallee FR, McGough J, Wigal T, Donahue J, Lyne A, Biederman J 2009. Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: a placebo-controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry 48(2), 155–65. [DOI] [PubMed] [Google Scholar]
- Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, Arnsten AFT, Cohen DJ, Leckman JF 2001. Guanfacine in the treatment of children with tic disorders and ADHD: A placebo-controlled study. Amer J Psychiatry 158, 1067–74. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, Schmitt FA, Grundman M, Thomas RG, Ferris SH 1997. Validity and reliability of the Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 11 Suppl 2, S22–32. [DOI] [PubMed] [Google Scholar]
- Scuteri A, Palmieri L, Lo Noce C, Giampaoli S 2005. Age-related changes in cognitive domains. A population-based study. Aging Clin Exp Res 17(5), 367–73. [DOI] [PubMed] [Google Scholar]
- Sheikh J, Yesavage J 1986. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. in: Brind TL (Ed.). Clinical gerontology: A guide to assessment and intervention Haworth Press, New York, pp 165–73. [Google Scholar]
- Tanila H, Rama P, Carlson S 1996. The effects of prefrontal intracortical microinjections of an alpha-2 agonist, alpha-2 antagonist and lidocaine on the delayed alternation performance of aged rats. Brain Res Bull 40, 117–9. [DOI] [PubMed] [Google Scholar]
- Taylor FB, Russo J 2001. Comparing guanfacine and dextroamphetamine for the treatment of adult Attention Deficit-Hyperactivity Disorder. J Clin Psychopharm 21, 223–8. [DOI] [PubMed] [Google Scholar]
- Teresi J, Lawton M, Holmes D, Ory M 1997. Measurement in elderly chronic care populations. in: Morris J, Morris S (Eds.). Assessment Measures for Use With Frail Elders Springer Publishing Co, New York, NY. [Google Scholar]
- Van der Elst W, Van Boxtel MP, Van Breukelen GJ, Jolles J 2006. The Stroop color-word test: influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment 13(1), 62–79. [DOI] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AF 2011. Neuronal basis of age-related working memory decline. Nature 476(7359), 210–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, Mazer JA, McCormick DA, Arnsten AF 2007. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell 129(2), 397–410. [DOI] [PubMed] [Google Scholar]
- Ware JE, Sherbourne CD 1992. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30(6), 473–83. [PubMed] [Google Scholar]
- Wechsler D 1987. WMS-R Wechsler Memory Scale - Revised Manual The Psychological Corporation, Harcourt Brace Jovanovich, Inc., New York. [Google Scholar]
- West RL 1996. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull 120, 272–92. [DOI] [PubMed] [Google Scholar]
- Whelihan WM, Lesher EL 1985. Neuropsychological changes in frontal functions with aging. Developmental Neuropsychology 1, 371–80. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


