Abstract
Background
Estrogen levels regulate changes in osteoarthritis (OA) by inhibiting degradation of the extracellular matrix. Recent in vitro studies have also shown the role of microRNA-140-5p (miR-140-5p). This study aimed to investigate the role of estrogen deficiency, selective modulation of expression of the estrogen receptor (ER), and expression of miR-140-5p in cartilage and subchondral bone remodeling in an ovariectomized rat model of postmenopausal OA.
Material/Methods
Female Sprague-Dawley rats included two model groups, ovariectomized (OVX) rats and rats with destabilization of the medial meniscus (DMM) rats. Two months after surgery, estrogen levels were measured by the enzyme-linked immunosorbent assay (ELISA). Three-dimensional (3D) micro-computed tomography (micro-CT) was used to image the knee joints. Rats were treated with subcutaneous injection of estrogen (E2) or the selective estrogen receptor modulator (SERM), raloxifene (RAL), for one month. Quantitative real-time polymerase chain reaction (qRT-PCR) was used to detect miR-140-5p in serum, and histology of the knee joint cartilage and bone was performed.
Results
In the ovariectomized rat model of OA, estrogen therapy reduced the degree of cartilaginous degeneration, while treatment with raloxifene showed no significant effect. Expression levels of miR-140-5p in the OA model group were significantly lower than the control group. Micro-CT showed that in the model group, anterior cruciate ligament dislocation and subchondral bone density were significantly reduced.
Conclusions
In an ovariectomized rat model of postmenopausal OA, estrogen deficiency resulted in resorption of subchondral bone and degeneration of articular cartilage.
MeSH Keywords: Estrogens, MicroRNAs, Osteoarthritis, Raloxifene
Background
Osteoarthritis (OA) is characterized by a progressive loss of cartilage and bone that occurs more commonly in the elderly, particularly in postmenopausal women [1,2]. In the population aged more than 60 years, 18% of women and 9.6% of men suffer from OA [3]. Previous studies have shown that the pathogenesis and progression of OA involve degradation of the joint and also subchondral bone [4,5]. Because cartilage lacks blood vessels, lymphatics, and nerves, cartilage is primarily supplied by vessels in the subchondral bone [6]. Multiple factors have been found to influence the development of OA, including genetics, age, gender, sex hormones, dietary intake, bone mineral density, muscle weakness, obesity, and joint laxity. Currently, increasing attention has been paid to the role of sex hormones in OA and epidemiological studies have shown that a low level of estrogen may result in OA [7,8].
Estrogen therapy carries more health risks than benefits and studies have shown that women using estrogens after the menopause have an increased risk of developing breast cancer and ovarian cancer [9–11]. Therefore, synthetic nonsteroidal agents with a different chemical structure have been developed, selective estrogen receptor modulators (SERMs). SERMs act as estrogen agonists, especially in joint tissues and have been shown to have fewer and less severe adverse effects compared with estrogen when used to prevent postmenopausal OA.
Few previous studies have been performed to investigate the in vivo effect of estrogen and SERMS on subchondral bone and cartilage matrix remodeling. Previous studies from our research group have indicated that estrogen inhibits the catabolism of cartilage extracellular matrix by estrogen receptors (ERs) and miR-140-5p [12]. Therefore, this study aimed to investigate the role of estrogen deficiency, selective modulation of expression of the estrogen receptor (ER), and expression of miR-140-5p in cartilage and subchondral bone remodeling in an ovariectomized rat model of postmenopausal OA.
Material and Methods
Animals
Six-month-old female Sprague-Dawley rats were purchased from the Experimental Animal Center of Guangzhou Medical University. The experimental protocol was conducted in accordance with the guidelines for the care of laboratory animals and was approved by the Institutional Animal Care and Use Committee of Shenzhen Second Peoples’ Hospital.
All the rats were maintained for one week before the experiments began at 23±1°C with a 12-hourly light/dark cycle and were given free access to food and water. No more than three rats were kept in each cage to ensure they had enough activity and to avoid biting. The experiment included two steps. The first step was to establish the OA animal model. The ovariectomized (OVX) rat model and the destabilization of the medial meniscus (DMM) rat model were used to study OA. Two months after the models were successfully established, rats were injected with estrogen (0.1mg/kg/day) (Sigma-Aldrich, St. Louis MO, USA) or raloxifene (3mg/kg/day) (MedChem Express, Monmouth Junction, NJ, USA) for one month.
Establishment of the rat model of OA
Rats were randomly divided into two groups. The rats in the model group were subjected to ovariectomy and medial meniscectomy. The rats in the control group were treated with a mock operation. Anesthesia was performed by intraperitoneal injection of aqueous chloral hydrate (40 mg/kg). Ovariectomy was performed according to the method previously described. Firstly, an electric razor was used to remove hair from the knee area and the surgical site was disinfected with povidone-iodine solution and 75% ethanol. Ovariectomy was performed via bilateral incisions in the skin and small bilateral sections through the muscle layer at the angle between the last rib and vertebral column. The skin was incised together with the dorsal muscles and the peritoneal cavity was accessed. The ovary was identified, surrounded by a variable amount of fat. After vascular ligation, the connection between the fallopian tube and the uterine horn was cut and the ovary removed. The surgical approach for DMM was a 3 mm longitudinal incision over the distal patella to the proximal tibial plateau. The joint capsule immediately medial to the patellar tendon was incised. The joint capsule was opened with microsurgery scissors. Blunt dissection of the fat pad over the intercondylar area was used to expose the intercondylar region. The medial meniscus was surgically removed. The surgical incisions were closed in two layers using absorbable sutures. After surgery, the rats were divided into a control group, the OA model group, the estrogen treatment group (OVX/DMM+E2), and the raloxifene treatment (RAL) group.
Micro-computed tomography (micro-CT) joint imaging
The isolated tibia was imaged using a Skyscan 1176 Micro-CT (Bruker, Billerica, MA, USA). Scans were performed using a PANalytica Microfocus X-ray tube (Malvern Panalytica, Malvern, UK) and 18 μm voxel size, 60 KV, 385 μA, and 0.5° rotation step (180° angular range). The 3D images were reconstructed using SkyScan volumetric NRecon reconstruction software version 1.6 (Bruker, Billerica, MA, USA). After 3D reconstruction, the region of interest (ROI) of subchondral bone in the tibial epiphysis was selected. The ROI included all trabecular bone enclosed by the growth plate and included 1 mm to 4 mm of the distal tibia. The bone mineral density (BMD) was measured within the ROI.
Estrogen or raloxifene injection
Two months after the models were successfully established, rats were injected intraperitoneally with estrogen (0.1mg/kg/day) or raloxifene (3mg/kg/day) for one month.
Histology
After one month of intraperitoneal injections, all rats were euthanized and all the knees were harvested. The rat knee specimen was fixed with 10% neutral buffered formalin for 48 hours and decalcified with EDTA for one month. All specimens were processed and sectioned at 4 μm thickness and stained with hematoxylin and eosin (H&E) and observed by light microscopy.
Enzyme-linked immunosorbent assay (ELISA)
To determine the changes in estrogen levels after surgery, the estrogen levels in the rat serum were determined using an estradiol (rat) ELISA kit (Biovision Inc., Milpitas, CA, USA). The protein concentration was quantified by BCA before ELISA, and the same amount of protein from different culture conditions was loaded. Absorbance was measured at a wavelength of 450 and 550 nm. The 450 nm values were subtracted from the 550nm values for correction of the optical errors.
Quantitative real-time polymerase chain reaction (qRT-PCR)
RNA was extracted from serum samples using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. cDNA was synthesized from total RNA using an Omniscript RT kit (Qiagen, Hilden, Germany). The qRT-PCR was performed in a ViiA 7 Real-time PCR System (Applied Biosystems, Foster City, CA, USA) using qRT-PCR SYBR Green master mix (CloudSeq Pte Ltd). Gene expression was quantified by the comparative 2−ΔΔCt method using miR-16-5p as a normalization control. Melting curves were used to check for PCR specificity. The primer sequences used for stem-loop RT-PCR of miR-16-5p and miR-140-5p are listed in Table 1.
Table 1.
The primer sequences used for real-time polymerase chain reaction (RT-PCR) of miR-16-5p and miR-140-5p.
| Genes | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| miR-16-5p | GGGTAGCAGCACGTAAATA | CAGTGCGTGTCGTGGAGT |
| miR-140-5p | GGGCAGTGGTTTTACCCTA | CAGTGCGTGTCGTGGAGT |
Statistical analysis
Data were analyzed using SPSS version18.0 (IBM Corp, Armonk, NY, USA). All experiments were performed in triplicate. Statistical analysis was performed using two-way analysis of variance (ANOVA) with Bonferroni’s post hoc analysis when more than two groups were compared, or one-way ANOVA when more than two groups were compared. All data were presented as the mean ±SE. P<0.05 was considered statistically significant.
Results
Establishment of the rat model of osteoarthritis (OA)
Serum estrogen level decreased after ovariectomy when compared with serum estrogen levels in the normal rats. Three months after surgery, the serum estrogen levels in the OA model group (OVX/DMM) decreased significantly. The levels of serum estrogen in the OVX/DMM+E2 group were significantly higher compared with the control group and the OVX/DMM group (P<0.01) (Figure 1). Ovariectomy was found to reduce the plasma levels of estrogen and estrogen injection increased the serum estrogen levels.
Figure 1.

Enzyme-linked immunosorbent assay (ELISA) for the detection of changes in serum estrogen levels in the rats. One month after injection, the serum levels of estrogen are compared between the ovariectomized/destabilization of the medial meniscus (OVX/DMM) rat group and the normal rat group (** P<0.01).
Radiographic signs of OA after surgery in the rat model
Two months after ovariectomy, radiographic signs of OA were present in the OVX/DMM group (Figure 2). Degenerative changes in the joint cartilage, osteophyte formation, and joint space narrowing were observed in the OA model group.
Figure 2.
Three-dimensional (3D) micro-computed tomography (micro-CT) images of the rat knee joints. (A) The normal rat knee joint. (B–D) The rat knee joints after ovariectomy. Osteophyte formation and joint space narrowing are seen in the joints.
Three-dimensional (3D) reconstruction of the damaged subchondral bone after surgery
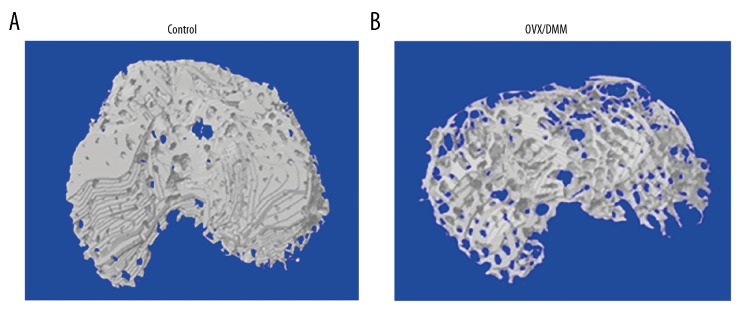
There were significant differences in the subchondral bone between the control group (Figure 3A) and the OVX/DMM group (Figure 3B). In the OVX/DMM group, a large amount of bone loss was observed in the subchondral bone, and the normal bone structure was lost.
Figure 3.
The representative three-dimensional (3D) micro-computed tomography (micro-CT) images of the control rat group and the ovariectomized/destabilization of the medial meniscus (OVX/DMM) rat group. (A) Images of the knee joints from the control group induced by the sham operation. The structural integrity of subchondral bone is not changed. (B) Images of the knee joints from the ovariectomized/destabilization of the medial meniscus (OVX/DMM) rat group. Osteoporosis of the subchondral bone and destroyed areas of the cartilage are shown.
Therapeutic effects of estrogen and raloxifene
Estrogen replacement therapy restored the integrity of subchondral bone at the early stage of OA. Significant structural changes and bone loss in the subchondral bone were observed in the OVX/DMM group two weeks after injection with estrogen (Figure 4A). In the estrogen treatment (E2) group, subchondral bone showed no obvious pathological changes at the junction with the cartilage (Figure 4B). Changes in bone loss and bone structure changes were observed in the raloxifene treatment (RAL) group (Figure 4C). All rats had significant osteoporosis after four weeks (Figure 4D–4F).
Figure 4.
Three-dimensional (3D) micro-computed tomography (micro-CT) images of the subchondral bone in the ovariectomized/destabilization of the medial meniscus (OVX/DMM) rat group with and without estrogen (E2) and raloxifene (RAL) treatment and the control group. (A) Severe bone erosion, cyst formation, and bone sclerosis are shown in the OVX/DMM group. (B) Near-normal subchondral bone in the OVX/DMM+E2 group. (C) The subchondral bone shows osteoporosis and the bone structure is destroyed in the OVX/DMM+RAL group. (D–F) The degree of osteoporosis in the subchondral bone was increased.
Bone mass density (BMD) of subchondral bone decreased after ovariectomy
Four weeks after ovariectomy surgery, the BMD of the subchondral bone was significantly greater in the control group compared with the OVX/DMM group, the OVX/DMM+E2 group, and the OVX/DMM+RAL group (Figure 5).
Figure 5.

The bone mineral density (BMD) of the subchondral bone at 0, 2, and 4 weeks after surgery. Compared with the control group, the ovariectomized/destabilization of the medial meniscus (OVX/DMM) rat group, the OVX/DMM+estrogen (E2) group, and the raloxifene (RAL) treated group all show a loss of bone mass. Maintenance of bone mass showed no significant difference between the OA group and the OVX/DMM+RAL group, but the OVX/DMM+E2 group show significant improvement in bone mass (* P<0.05, ** P<0.01).
Estrogen replacement therapy upregulated the expression levels of miR-140-5p
The level of miR-140-5p expression was significantly down-regulated in the OVX/DMM group compared with the control group (P<0.01). miR-140-5p expression in the OVX/DMM+E2 group was significantly lower than that in the control group (P<0.01). However, compared with the OVX/DMM group, there was a significant increase in miR-140-5p expression in the OVX/DMM+E2 group (Figure 6).
Figure 6.

The expression of microRNA-140-5p (miR-140-5p) determined by quantitative real-time polymerase chain reaction (qRT-PCR). Compared with the control group, the expression level of miR-140-5p is significantly reduced in the OVX/DMM and the OVX/DMM+E2 group. The expression level of miR-140-5p in the OVX/DMM+E2 group is significantly greater than in the OVX/DMM group (** P<0.01, *** P<0.001).
Estrogen replacement therapy maintained the structure of the joints
Four weeks after ovariectomy surgery, histology showed that the articular cartilage of the control group was normal and the cartilage matrix was uniform in appearance (Figure 7A). In the OVX/DMM group, the articular cartilage was destroyed (Figure 7B). However, the joint structure was maintained and was uniform in appearance in the estrogen replacement therapy group. The E2 group showed normal histology of proteoglycans and a smooth cartilage surface with no osteophyte development (Figure 7C). After raloxifene treatment, the joint matrix structure was maintained, but unevenly stained and the cartilage surface was rough (Figure 7D).
Figure 7.
Photomicrographs of the histology of the rat knee joints in the groups treated with estrogen (E2) and raloxifene (RAL). (A) The histology of the knee joint in the normal control rat group. Hematoxylin and eosin (H&E) ×100. (B) The histology of the knee joint in the ovariectomized/destabilization of the medial meniscus (OVX/DMM) rat group. H&E ×100. (C) The histology of the knee joint in the estrogen (E2)-treated rat group. H&E ×100. (D) The histology of the knee joint in the raloxifene (RAL)-treated rat group, H&E ×100.
Discussion
There are still mechanisms involved in the pathogenesis of osteoarthritis (OA) that remain to be investigated. A complex interaction exists between cartilage and subchondral bone, and the exact mechanisms remain unknown. Epidemiological studies have shown a correlation between estrogen levels and matrix remodeling in OA [13]. The aims of the present study were to investigate the role of estrogen in the development of OA by establishing an ovariectomized rat model of postmenopausal OA and the role of the selective estrogen receptor modulator (SERM), raloxifene, in the treatment of OA.
The beneficial therapeutic effects of estrogen on the articular cartilage have been previously recognized, and include the inhibition of the degradation of the cartilage matrix [14–16]. In the present study, the pathological changes of OA were shown to occur after a reduction in estrogen levels. Joint histology showed that subchondral bone developed osteoporosis when estrogen levels were reduced, and after estrogen supplementation, osteoporosis was alleviated. This finding was confirmed by evaluation of bone mineral density (BMD). From the three-dimensional (3D) reconstruction of micro-computed tomography (micro-CT) imaging, the basic structure of subchondral bone was retained in the estrogen replacement therapy group. These findings showed that estrogen had a protective effect both on cartilage and subchondral bone.
The SERM, raloxifene, is effective for the prevention and treatment of osteoporosis and because it is without the side effects of estrogen treatment, raloxifene is widely used to prevent osteoporosis in postmenopausal women. Raloxifene acts on bone and inhibits bone loss and bone fracture risk. In different tissues, raloxifene can bind the two isoforms of ERs, ERα, and ERβ. Ligand-binding structural differences of ERs determine whether raloxifene act as estrogen agonist or antagonist. In clinical studies, raloxifene has been used for prevention of OA in postmenopausal women. In this study, after injection of raloxifene in the OA rat model, the lesions in the cartilage were not reduced, and osteopenia of the subchondral bone was even more apparent. Based on findings from other studies, three months of treatment of raloxifene can help to prevent osteoporosis in postmenopausal women [17]. In the present study, which used an ovariectomized rat model of postmenopausal OA, the rats were treated with raloxifene for only one month, which might have been too short a time to develop a treatment effect.
The findings of the present study also showed that estrogen deficiency resulted in subchondral bone loss, and estrogen replacement prevented bone loss, as measured by the bone density index, resulting from estrogen deficiency. Therefore, it may be hypothesized that the estrogen level was closely related to the dynamic balance of bone remodeling. Our previous study showed that estrogen acts on the ER and miR-140-5p to inhibit the catabolic activity of proteases in the extracellular matrix of chondrocytes. Reduced levels of estrogen in vivo resulted in OA and a significant reduction in the levels of miR-140-5p expression. With estrogen replacement treatment, miR-140-5p expression was upregulated and cartilage erosions were significantly reduced. Therefore, it may be hypothesized that the signaling pathways of ER/miR-140-5p are involved in the complex effects of estrogen on the cartilage in the in vivo ovariectomized rat model of postmenopausal OA.
Subchondral bone played an important role in the maintenance of the normal structure of cartilage and its physiological function [18], but the effect of estrogen for subchondral bone had not been previously well studied. In the present study, an ovariectomized rat model of postmenopausal OA was established, which showed that reduced estrogen levels resulted in subchondral bone loss and accelerated the degeneration of articular cartilage. The protective effect of estrogen on cartilage was confirmed, while raloxifene had no effect on cartilage. These findings support that there should be further studies to develop new selective estrogen receptor modulators to provide improved treatment strategies for postmenopausal OA.
Conclusions
This study aimed to investigate the role of estrogen deficiency, selective modulation of expression of the estrogen receptor (ER), and expression of miR-140-5p in cartilage and subchondral bone remodeling in an ovariectomized rat model of postmenopausal osteoarthritis (OA). Estrogen depletion resulted in increased damage to articular cartilage and abnormal subchondral bone remodeling, leading to the changes of OA. Estrogen deficiency resulted in resorption of subchondral bone and degeneration of articular cartilage, but there was a nonsignificant protective effect of raloxifene on the development of osteophytes.
Footnotes
Source of support: The National Natural Science Foundation of China (No. 81572198; No. 81772394), the Key Program of Natural Science Foundation of Guangdong Province (No.2018B0303110003), the Shenzhen Peacock Project (KQTD20170331100838136), the Shenzhen Science and Technology Projects (No. JCYJ20170817172023838, No. JCYJ20170306092215436, No. JCYJ20170412150609690, No. JCYJ20170413161649437, No. JCYJ20170413161800287, No. SGLH20161209105517753, No. JCYJ20160301111338144), the Fund for High Level Medical Discipline Construction of Shenzhen University (No. 2016031638). Animal license held by the Shenzhen Second Peoples’ Hospital (No. 075583366388)
References
- 1.Li S, Ou Y, Zhang H, et al. Vitamin D status and its relationship with body composition, bone mineral density and fracture risk in urban central South Chinese postmenopausal women. Ann Nutr Metab. 2014;64:13–19. doi: 10.1159/000358340. [DOI] [PubMed] [Google Scholar]
- 2.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) Chronic Rheumatic Conditions. Available at [URL]: http://www.who.int/chp/topics/rheumatic/en/
- 4.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: An update with relevance for clinical practice. Lancet. 2011;377:2115–26. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 5.Cooper C, Adachi JD, Bardin T, et al. How to define responders in osteoarthritis. Curr Med Res Opin. 2013;29:719–29. doi: 10.1185/03007995.2013.792793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen NP, Foster RJ, Mow VC. Composition and dynamics of articular cartilage: Structure, function, and maintaining healthy state. J Orthop Sport Phys. 1998;28:203–15. doi: 10.2519/jospt.1998.28.4.203. [DOI] [PubMed] [Google Scholar]
- 7.Richette P, Corvol M, Bardin T. Estrogens, cartilage, and osteoarthritis. Joint Bone Spine. 2003;70:257–62. doi: 10.1016/s1297-319x(03)00067-8. [DOI] [PubMed] [Google Scholar]
- 8.Bay-Jensen AC, Slagboom E, Chen-An P, et al. Role of hormones in cartilage and joint metabolism: Understanding an unhealthy metabolic phenotype in osteoarthritis. Menopause. 2013;20:578–86. doi: 10.1097/GME.0b013e3182745993. [DOI] [PubMed] [Google Scholar]
- 9.Sniekers YH, Weinans H, Bierma-Zeinstra SM, et al. Animal models for osteoarthritis: The effect of ovariectomy and estrogen treatment – a systematic approach. Osteoarthr Cartilage. 2008;16:533–41. doi: 10.1016/j.joca.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Ma HL, Blanchet TJ, Peluso D, et al. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthr Cartilage. 2007;15:695–700. doi: 10.1016/j.joca.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Lugo L, Villalvilla A, Largo R, et al. Selective estrogen receptor modulators (SERMs): New alternatives for osteoarthritis? Maturitas. 2014;77:380–84. doi: 10.1016/j.maturitas.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Liang Y, Duan L, Xiong J, et al. E2 regulates MMP-13 via targeting miR-140 in IL-1β-induced extracellular matrix degradation in human chondrocytes. Arthritis Res Ther. 2016;18:105. doi: 10.1186/s13075-016-0997-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van der Meij L, Almela M, Buunk AP, et al. Men with elevated testosterone levels show more affiliative behaviours during interactions with women. Proc Biol Sci. 2011;279:202–8. doi: 10.1098/rspb.2011.0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 15.Rasanen T, Messner K. Articular cartilage compressive stiffness following oophorectomy or treatment with 17beta-estradiol in young postpubertal rabbits. Acta Obstet Gynecol Scand. 1999;78:357–62. [PubMed] [Google Scholar]
- 16.Turner AS, Athanasiou KA, Zhu CF, et al. Biochemical effects of estrogen on articular cartilage in ovariectomized sheep. Osteoarthr Cartilage. 1997;5:63–69. doi: 10.1016/s1063-4584(97)80032-5. [DOI] [PubMed] [Google Scholar]
- 17.Bitto A, Burnett BP, Polito F, et al. Effects of genistein aglycone in osteoporotic, ovariectomized rats: A comparison with alendronate, raloxifene and oestradiol. Br J Pharmacol. 2008;155:896–905. doi: 10.1038/bjp.2008.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bettica P, Cline G, Hart DJ, et al. Evidence for increased bone resorption in patients with progressive knee osteoarthritis: Longitudinal results from the Chingford study. Arthritis Rheumatol. 2002;46:3178–84. doi: 10.1002/art.10630. [DOI] [PubMed] [Google Scholar]