Abstract
Context:
It is important to evaluate the role of stromal myofibroblasts (MFs) in carcinogenesis and also as a predictive marker for lymph node (LN) metastasis at the invasive front of oral squamous cell carcinoma (OSCC).
Aims:
To demonstrate the expression of α-smooth muscle actin (α-SMA) by MFs in the tissues of oral leukoplakia (OL) with dysplasia and OSCC. To record and compare the distribution of MFs in OSCC with LN metastasis and without LN metastasis.
Settings and Design:
Fifty paraffin-embedded tissue blocks with 10 cases of normal oral mucosa, 10 cases of OL with dysplasia and 30 diagnosed cases of OSCCs were studied.
Subjects and Methods:
The samples were subjected to heat-induced antigen retrieval method followed by staining using primary mouse monoclonal antibodies against α-SMA and visualized using super sensitive polymer–HRP detection system.
Statistical Analysis:
Descriptive statistical analysis and ANOVA test were used for statistical analysis.
Results:
There was no α-SMA expression in normal oral mucosa or in OL with dysplasia. All tissues of OSCC were positive for α-SMA expression. The difference in the expression between OL with dysplasia and OSCC was statistically significant (P < 0.05). The mean α-SMA count of OSCC with LN metastasis is significantly greater than in the OSCC without LN metastasis (P = 0.001). In OSCC without LN metastasis, focal and spindle patterns were predominant and in OSCC with LN metastasis network pattern was more.
Conclusion:
α-SMA expression by MFs in OSCCs indicates its role in tumor growth and invasion. The mean α-SMA count was found to correlate with tumor invasiveness and locoregional LN metastasis.
Keywords: Myofibroblasts, oral leukoplakia, oral squamous cell carcinoma, α-smooth muscle actin
INTRODUCTION
The structural organization and function of normal tissue is determined by interactions between cells and extracellular matrix (ECM). The tumor cells should therefore interact with the ECM at several stages of the metastatic cascade.[1] Cross talk between epithelial and stromal cells is known to be essential for differentiation and development of normal organs and tissue as well as for growth and progression of tumors.[2]
Oral leukoplakia (OL) is the most common potentially malignant disorder of the oral mucosa and has been recognized and established as a definitively precancerous lesion with a rate of malignant transformation ranging from 0.13% to 2.2% per year on global level.[3,4]
Oral squamous cell carcinoma (OSCC) is the most common malignant neoplasm of the oral cavity. It represents about 90%–95% of all malignant neoplasms of the oral cavity.[5]
Metastasis is a complex process that depends on many interactions among tumor cells and the microenvironment. The dynamic process of ECM remodeling, called stromagenesis, is orchestrated by stromal myofibroblasts (MFs) and creates a permissive environment for tumor growth, invasion and metastasis.[6]
MFs are fibroblasts with smooth muscle-like features characterized by the presence of a contractile apparatus.[7] They exhibit a phenotype between fibroblasts and smooth muscle cells and are characterized by the expression of a specific smooth muscle isoform of alpha smooth muscle actin (α-SMA). The presence of MFs has been detected in the stroma of breast, kidney, liver, bladder, colon and prostate cancers.[8]
The microenvironment or stroma of neoplastic tissues plays an active role in tumor progression. Fibroblasts are considered as one of the most important mesenchymal cells involved in tumor progression. Transdifferentiation of fibroblasts to MFs is a crucial event in tumorigenesis, which is mediated by growth factors and cytokines expressed by tumor cells. The MFs, in turn, secrete numerous growth factors and inflammatory mediators that stimulate epithelial cell proliferation. Thus, MFs have been implicated in the vicinity of invasive OSCC.[9]
The transdifferentiated MFs significantly upregulate the secretion of hepatocyte growth factor or scatter factor (HGF/SF), which, in turn, promotes SCC invasion through basement membrane proteins.[10]
The present study is undertaken to evaluate the significance of presence of MFs at the invasive front of OSCCs. It is also to assess the comparative distribution of myofibroblasts in OSCCs with and without Lymph Node (LN) involvement in terms of quantity and pattern, thus trying to establish its role as a predictive marker for LN metastasis.
SUBJECTS AND METHODS
This descriptive study was carried out on tissue sections obtained from diagnosed cases of OL with dysplasia and various grades of OSCC retrieved from the archives of our department.
Specimens of 10 cases of OL with dysplasia, 30 cases of OSCC (15 with lymph node [LN] metastasis and 15 without LN metastasis) and 10 cases of normal oral mucosa (Gingiva) were obtained by random sampling [Table 1]. Sample size was decided after discussion with the statistician, so that the study was adequately powered. Cases of OL were graded as mild, moderate and severe as per the classification by Pindborg et al. Squamous cell carcinoma of the lip, benign neoplasms arising from oral mucosa and metastatic tumors of oral cavity and jawbones other than OSCC were excluded from the study. The study was carried out from March 2011 to August 2012 in our institution. As per the rules of the institution, ethical clearance was not required for this study as it involves retrospective analysis of tissue samples.
Table 1.
The final study sample
| Sample type | Number of cases |
|---|---|
| Normal oral mucosa (gingiva) | 10 |
| OL with dysplasia | 10 |
| OSCC without LN metastasis | 15 |
| OSCC with LN metastasis | 15 |
| Total | 50 |
OSCC: Oral squamous cell carcinoma, LN: Lymph node, OL: Oral leukoplakia
Monoclonal (1A4) mouse antihuman α-SMA primary antibody (BIOGENEX USA) was used for the study. α-SMA is a marker for stromal MFs.
The presence of brown-colored precipitate at the site of target antigens was indicative of positive immunoreactivity. α-SMA-positive cells within blood vessel walls served as internal positive control for the specificity of the stain. Tissue sections of normal colon were taken as external positive control. Tissues of normal oral mucosa were treated as external negative control. The evaluation of the study cases was done subsequently in a similar way.
Stromal spindle cells positive for cytoplasmic immunostaining for α-SMA were considered as MFs. MFs were assessed by selecting five random fields in the connective tissue below the epithelium for both normal oral mucosa and oral dysplasias. Samples in which no stromal MFs detected were classified as negative. A comparative study of the expression pattern of α-SMA was made between OSCC showing nodal metastasis and without nodal metastasis. The results were presented as the mean number of α-SMA-positive cells per field. To eliminate observer bias, the count was performed by three different observers in five varied random fields with good interobserver agreement. Distribution pattern of SMA-positive cells was also detected.
Statistical methods
Descriptive statistical analysis has been carried out in the present study; the results of α-SMA-positive cell counts per field are presented using t-test. The patterns of MF distribution are presented as percentage of each pattern in each of the groups using Chi-square test. Statistical significance was at P < 0.05. The statistical software, namely statistical package for the social sciences SPSS for Windows, (version 13.0, Chicago, SPSS Inc., IBM 2004), was used for the analysis of the data and required computations.
RESULTS
In the present study, a total of 50 previously histologically diagnosed, paraffin-embedded tissue blocks including 10 cases of normal oral mucosa (Gingival mucosa), 10 cases of OL with dysplasia, 15 cases of OSCC without LN metastasis and 15 cases of OSCC with LN metastasis were selected and studied for the expression of α-SMA.
There was no α-SMA expression in any of the tissues of normal oral mucosa (gingiva), except α-SMA-positive cells within blood vessel walls (internal positive control) [Figure 1]
There was no α-SMA expression in any of the tissues of OL with dysplasia (20% mild, 40% moderate and 40% severe dysplasia) [Figure 2]
All the OSCC tissue samples were positive for α-SMA. Thus, the difference between OL group and OSCC group was statistically significant (P < 0.05)
OSCC with LN metastasis had a significantly higher α-SMA count than the OSCC group without LN metastasis (P = 0.001) [Table 2]
In samples of OSCC group without LN metastasis, Network pattern [Figure 3] was 20%, Spindle pattern [Figure 4] and Focal pattern [Figure 5] were 40% each and in samples of OSCC with LN metastasis 47% was network type and 33% and 20% of spindle and focal patterns, respectively. No significant association is observed between the pattern of MFs and the groups mentioned (P = 0.260) [Table 3].
Figure 1.
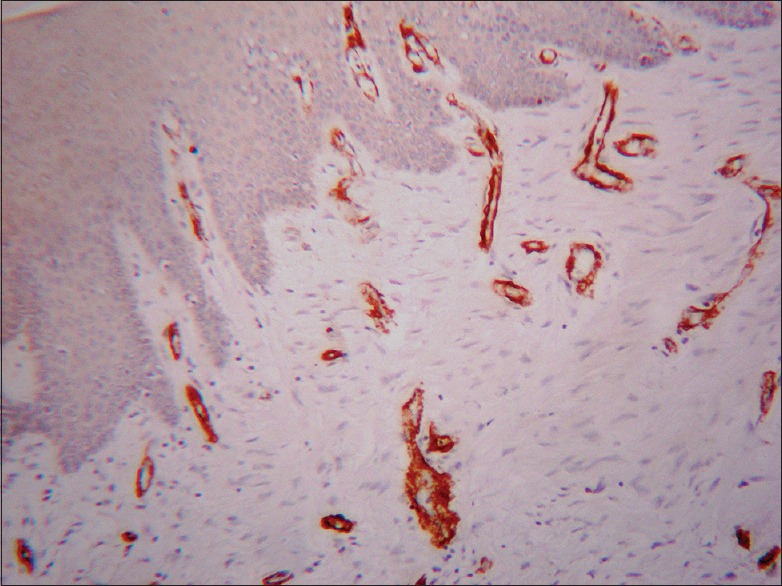
Normal oral mucosa (gingiva) showing strong positive staining of blood vessel walls (α-smooth muscle actin immunostain, ×10)
Figure 2.
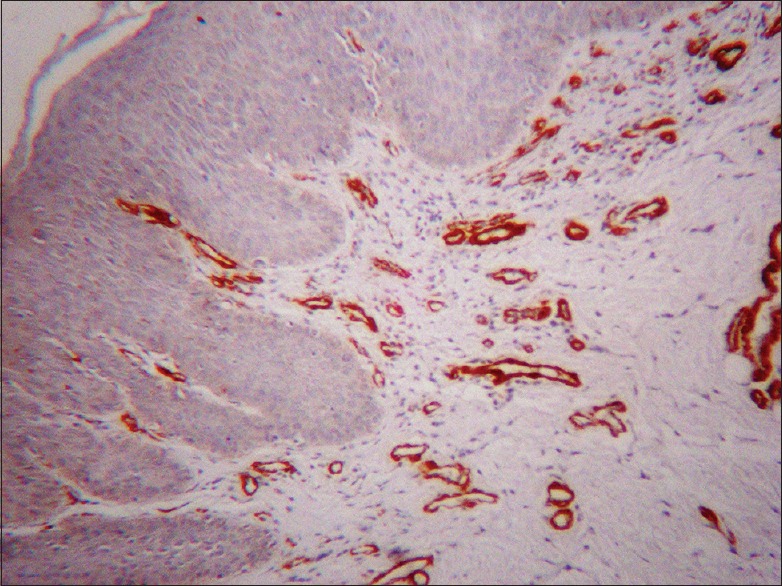
Oral leukoplakia with dysplasia showing strong positive staining of blood vessel walls (α-smooth muscle actin immunostain, ×10)
Table 2.
Mean α-smooth muscle actin count in the two groups
| Group | Mean | SD | SEM | 95% CI for mean | P-Value | |
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| OSCC without LN metastasis | 6.53 | 4.41 | 1.14 | 4.09 | 8.98 | 0.001* |
| OSCC with LN metastasis | 14.01 | 6.47 | 1.67 | 10.43 | 17.60 | |
*P value ≤ 0.001 is Highly Significant. OSCC: Oral squamous cell carcinoma, LN: Lymph node, SD: Standard deviation, SEM: Standard error of mean, CI: Confidence interval
Figure 3.
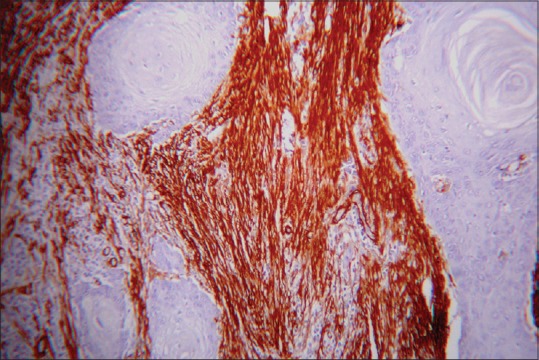
Myofibroblasts showing network pattern (α-smooth muscle actin immunostain, ×10)
Figure 4.
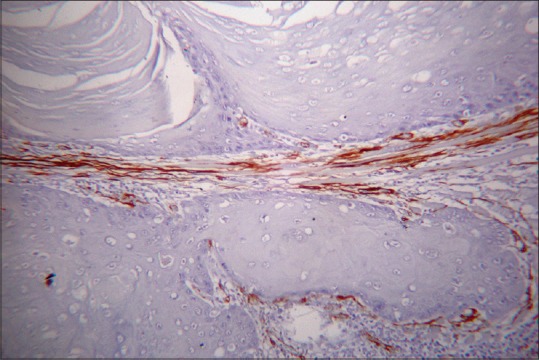
Myofibroblasts showing spindle pattern (α-smooth muscle actin immunostain, ×10)
Figure 5.
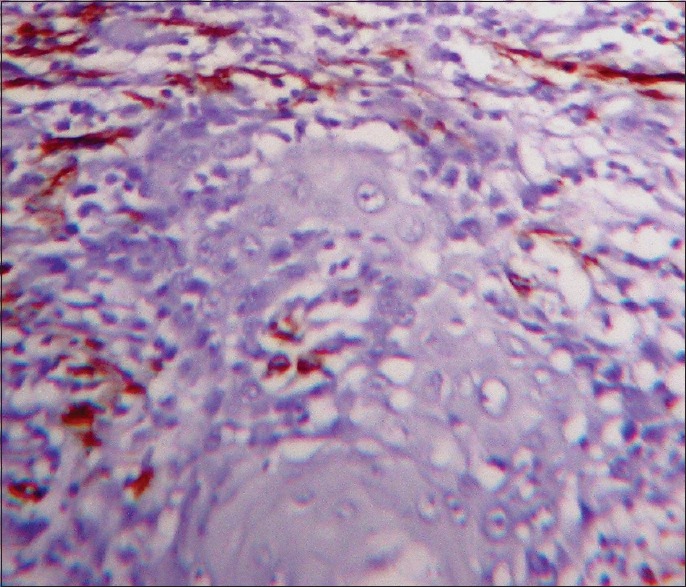
Myofibroblasts showing focal pattern (α-smooth muscle actin immunostain, ×10)
Table 3.
Pattern distribution of myofibroblasts between oral squamous cell carcinoma groups
| Pattern of MFs | OSCC without LN metastasis, n (%) | OSCC with LN metastasis, n (%) | Total | χ2 | P-Value |
|---|---|---|---|---|---|
| Focal | 6 (40) | 3 (20) | 9 | 2.691 | 0.260 |
| Network | 3 (20) | 7 (47) | 10 | ||
| Spindle | 6 (40) | 5 (33) | 11 | ||
| Total | 15 (100) | 15 (100) | 30 |
MFs: Myofibroblasts, OSCC: Oral squamous cell carcinoma, LN: Lymph node
DISCUSSION
The present study was undertaken to investigate α-SMA expression in oral epithelial dysplasia and OSCC. OSCC samples were further subclassified based on the presence or absence of LN involvement. Quantitative and pattern-wise analysis of α-SMA by MFs in these two subgroups, especially at the invasive front, were done.
In the present study, all the ten samples of OL were negative for α-SMA expression, thus showing lack of MFs, while all the thirty samples of OSCC were positive for its expression, thus establishing its role in tumor stroma.
Zidar et al.[11] found lack of MFs in normal, epithelial hyperplastic lesions and dysplastic laryngeal epithelium regardless of their severity while MFs were found exclusively in invasive SCC.
Lewis et al.[10] found that α-SMA-positive stromal cells in OSCC are commonly found at the invasive margin directly abutting tumor cells and are absent in areas distant from tumor.
Kellermann et al.[12] reported that no MFs were found in the stroma of normal mucosa and epithelial dysplasia; however, they were detected at the invasive front of the SCCs.
Kellermann et al.[8] demonstrated a complete negative expression of α-SMA in tumor-free stroma while its numbers were elevated in stroma adjacent to tumor islands, thus suggesting that a close contact between epithelial and stromal cells is required for the induction of MF differentiation.
Etemad-Moghadam et al.[9] showed that the presence of MFs was significantly higher in OSCCs compared to both dysplasia and normal mucosa samples, and they were detected more in the tumor invasive front.
Vered et al.[13] found that stromal MFs were almost exclusively associated with established carcinomas and not with premalignant lesions.
De-Assis et al.[4] found that stromal MFs were not detected in OL irrespective of the grade but were heterogeneously detected in OSCC, and its presence was higher in tumors with a more diffuse histological pattern of invasion.
In our study, MF's count was significantly more in OSCC with LN metastasis when compared with OSCC without LN involvement group with P < 0.05.
Kellermann et al.[12] showed that abundance of MFs at the invasive front leads to more aggressive behavior of the SCCs, including an elevated proliferative potential. Kellermann et al.[8] demonstrated that abundant presence of MFs in tumor stroma significantly correlated with N stage, disease stage and regional recurrence of OSCC. They further showed that its presence leads to a more aggressive behavior and was significantly associated with an increase in LN metastasis.
Kawashiri et al.[14] in their study on OSCC found that MF appearance also increased with increasing tumor invasiveness and locoregional LN metastasis.
Our study is in agreement with that of Vered et al.[15] and Seifi et al.[16] with the following observations: focal (40%) and spindle (40%) patterns predominated in OSCC without LN involvement and network (47%) pattern predominated in OSCC with LN involvement. However, no significant association is observed between the patterns of MF distribution and the groups (P > 0.05).
The present study was an attempt to reinforce the hypothesis that MFs are essentially a part of reactive tumor stroma and to establish the quantitative and qualitative relationship of MFs at the invasive front with the biological behavior of OSCC.
The results of our study showed an increase in the number of α-SMA-positive MFs and change in their distribution pattern during the process of tissue invasion and metastasis. MFs establish a permissive environment supportive of tumor growth, through the production of chemokines, growth factors and matrix-degrading enzymes. In addition, they act in concert with immune cells to support blood vessel formation, breaks down basement membrane barriers and attract tumor cells to distant sites. They significantly upregulate the secretion of HGF/SF, which promotes invasion of squamous cell carcinoma.[17] N-Cadherin, expressed by MFs, promotes matrix invasion, perineural invasion, muscular invasion and transendothelial migration.[18]
The network pattern of MF arrangement is responsible for higher invasiveness and metastasis due to higher number of MFs in these arrangements in comparison to spindle and focal patterns.[16]
CONCLUSION
The findings from this study are suggestive of the role of MFs in creating a permissive environment for tumor invasion and metastasis in OSCC. Presence of MFs is thus a prognostic marker. Its evaluation in terms of frequency and distribution pattern in the tumor stroma will enable us to identify more precise diagnostic modalities and define new therapeutic targets.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Stricker TP, Kumar V. In: Robbins and Cotran Pathologic Basis of Disease. 8th ed. Kumar V, Abbas AK, Fausto N, editors. Philadelphia: Saunders Elsiever; 2009. pp. 298–9. [Google Scholar]
- 2.Hu M, Polyak K. Microenvironmental regulation of cancer development. Curr Opin Genet Dev. 2008;18:27–34. doi: 10.1016/j.gde.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sciubba JJ. Oral leukoplakia. Crit Rev Oral Biol Med. 1995;6:147–60. doi: 10.1177/10454411950060020401. [DOI] [PubMed] [Google Scholar]
- 4.de-Assis EM, Pimenta LG, Costa-e-Silva E, Souza PE, Horta MC. Stromal myofibroblasts in oral leukoplakia and oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal. 2012;17:e733–8. doi: 10.4317/medoral.17834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seyedmajidi M, Faizabadi M. Squamous cell carcinoma of the tongue in a 13-year-old boy. Arch Iran Med. 2008;11:341–3. [PubMed] [Google Scholar]
- 6.Ziober BL, Silverman SS, Jr, Kramer RH. Adhesive mechanisms regulating invasion and metastasis in oral cancer. Crit Rev Oral Biol Med. 2001;12:499–510. doi: 10.1177/10454411010120060401. [DOI] [PubMed] [Google Scholar]
- 7.Vered M, Shohat I, Buchner A, Dayan D. Myofibroblasts in stroma of odontogenic cysts and tumors can contribute to variations in the biological behavior of lesions. Oral Oncol. 2005;41:1028–33. doi: 10.1016/j.oraloncology.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Kellermann MG, Sobral LM, da Silva SD, Zecchin KG, Graner E, Lopes MA, et al. Mutual paracrine effects of oral squamous cell carcinoma cells and normal oral fibroblasts: Induction of fibroblast to myofibroblast transdifferentiation and modulation of tumor cell proliferation. Oral Oncol. 2008;44:509–17. doi: 10.1016/j.oraloncology.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Etemad-Moghadam S, Khalili M, Tirgary F, Alaeddini M. Evaluation of myofibroblasts in oral epithelial dysplasia and squamous cell carcinoma. J Oral Pathol Med. 2009;38:639–43. doi: 10.1111/j.1600-0714.2009.00768.x. [DOI] [PubMed] [Google Scholar]
- 10.Lewis MP, Lygoe KA, Nystrom ML, Anderson WP, Speight PM, Marshall JF, et al. Tumour-derived TGF-beta1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br J Cancer. 2004;90:822–32. doi: 10.1038/sj.bjc.6601611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zidar N, Kojc N, Gale N. Diagnostic and prognostic significance of stromal myofibroblasts in laryngeal carcinogenesis. Zdrav Vestn. 2002;71(Suppl 3):49–51. [Google Scholar]
- 12.Kellermann MG, Sobral LM, da Silva SD, Zecchin KG, Graner E, Lopes MA. Myofibroblasts in the stroma of oral squamous cell carcinoma are associated with poor prognosis. Histopathology. 2007;51:849–53. doi: 10.1111/j.1365-2559.2007.02873.x. [DOI] [PubMed] [Google Scholar]
- 13.Vered M, Allon I, Buchner A, Dayan D. Stromal myofibroblasts accompany modification in the epithelial phenotype of tongue dysplastic and malignant lesions. Cancer Microenviron. 2009;2:49–57. doi: 10.1007/s12307-009-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawashiri S, Tanaka A, Noguchi N, Hase T, Nakaya H, Ohara T. Significance of stromal desmoplasia and myofibroblast appearance at the invasive front in squamous cell carcinoma of the oral cavity. Head Neck. 2009;31:1346–53. doi: 10.1002/hed.21097. [DOI] [PubMed] [Google Scholar]
- 15.Vered M, Dayan D, Dobriyan A, Yahalom R, Talmi YP, Bedrin L. Myofibroblasts and phenotypical changes in the expression of epithelial markers in lymph node metastases from patients with tongue cancer. Oral Oncol. 2009;3(Suppl 3):S201–36. [Google Scholar]
- 16.Seifi S, Shafaei S, Shafigh E, Sahabi SM, Ghasemi H. Myofibroblast stromal presence and distribution in squamous epithelial carcinomas, oral dysplasia and hyperkeratosis. Asian Pac J Cancer Prev. 2010;11:359–64. [PubMed] [Google Scholar]
- 17.Thode C, Jørgensen TG, Dabelsteen E, Mackenzie I, Dabelsteen S. Significance of myofibroblasts in oral squamous cell carcinoma. J Oral Pathol Med. 2011;40:201–7. doi: 10.1111/j.1600-0714.2010.00999.x. [DOI] [PubMed] [Google Scholar]
- 18.De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429–47. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]


