Abstract
Context:
The exact factors that determine the biological behavior of odontogenic lesions have not been thoroughly established yet. The influence of the matrix metalloproteinases (MMPs) on the clinical behavior of these lesions was recently brought to light.
Aims:
We did a pioneer study to investigate the association of MMP9 (rs3918242 [−1562 C/T] and rs17576) and MMP2 (rs243865 [−1306 C/T] and rs865094) gene polymorphisms and aggressiveness of ameloblastomas, keratocystic odontogenic tumors (KCOT) and dentigerous cysts (DC).
Settings and Design:
A case–control study conducted in the Department of Oral Pathology and Microbiology, Government Dental College, Trivandrum and Human Molecular Genetics Laboratory, Rajiv Gandhi Institute of Biotechnology and Poojappura, Trivandrum, Kerala.
Subjects and Methods:
DNA from the blood samples of histopathologically proven ameloblastoma (n = 15), KCOT (n = 11) and DC (n = 13) patients were extracted using standard protocols. Primers were designed based on the functionality and relevance for polymerase chain reaction (PCR). PCR products were analyzed by PCR-restriction fragment length polymorphism and sequencing.
Statistical Analysis Used:
Chi-square analysis was done to assess the association of gene polymorphisms among the cases and controls.
Results:
Ameloblastomas showed a higher frequency of mutant allele (T = 0.43; P = 0.05) of MMP9 rs3918242 (−1562C/T) compared to the control population. All the cases showed a statistically significant difference in the distribution of genotype (P = 0.046) and allele (P = 0.03; odds ratio [OR] = 2.06 [1.08–3.95]) frequency of MMP2 rs2438659 (−1306C/T). KCOT samples also showed a significant association in distribution of both genotype (P = 0.01) and allele (P = 0.01 with an OR at 3.42 [1.31–8.92]) frequency, on comparison with control population.
Conclusions:
MMP2 rs243865 polymorphism has a plausible role in increasing the aggressiveness of ameloblastomas and KCOT compared to that of the control population. Furthermore, MMP9 rs3918242 polymorphism may contribute to the aggressive behavior of ameloblastomas.
Keywords: Ameloblastoma, matrix metalloproteinase, odontogenic, promoter, single-nucleotide polymorphism
INTRODUCTION
Odontogenic tumors constitute <1% of all pathologies in an oral and maxillofacial setup.[1] Ameloblastoma is reportedly the most frequent tumor in the Asian and African population.[2] Odontogenic cysts are relatively common in an oral and maxillofacial setup, constituting 7%–12% of all oral and maxillofacial biopsies.[3] The initiation and progression of odontogenic pathology are thought to be determined by complicated interactions among various molecular entities, which also have a definite role in physiological tooth formation.[4] Altered expression of genes such as sonic hedgehog, bone morphogenetic protein, fibroblasts growth factor, wingless and patched, otherwise involved in physiologic tooth development, has been proposed involved in the pathogenesis of ameloblastoma and keratocystic odontogenic tumor (KCOT).[5] The growth mechanism of odontogenic cysts, as well as the invasion and destructive potential of these odontogenic tumors, might be influenced by the secretion of biologically active molecules such as matrix metalloproteinases (MMPs), proteins that can be produced by both epithelial and mesenchymal cells.[6,7,8] Promoter level polymorphisms in MMP2 and MMP9 genes have been reported to have an influence on the development and progression of potentially malignant lesions of head and neck.[9,10] There are differences in the prevalence of MMP polymorphisms across different populations.[11] MMP2 and MMP9 gene polymorphisms in odontogenic lesions have not been studied in our population till date. It was, therefore, envisaged to do a pioneer study intended to find the frequency of polymorphism in our population in the normal and cases and also to assess the association, if any, between gene polymorphism and aggressiveness of ameloblastomas and KCOT and dentigerous cysts (DC).
SUBJECTS AND METHODS
Subjects and controls
A case–control study was conducted in the Government Dental College, Trivandrum in collaboration with Rajiv Gandhi Centre for Biotechnology, Trivandrum with a total of 145 participants, including 15 ameloblastoma, 11 KCOT and 13 DC patients and 106 controls. The diagnosis of odontogenic lesions was confirmed clinically as well as histopathologically by the WHO (2005) criteria. Patients belonged to the State of Kerala by domicile and birth. No patient with compromised systemic health or coexisting cystic lesions or neoplasms of the jaw was included in the study. The study protocol was approved by the Institutional Ethics Committee, and informed consent was obtained from the participants.
DNA isolation and genotyping
A volume of 5 ml peripheral blood sample was collected by venipuncture from both patients and controls. Genomic DNA was isolated by a modified salting-out method.[12]
Polymerase chain reaction (PCR) was done with diluted DNA samples and the specific primers designed to amplify the area of interest of the rs3918242 (−1562 C>T) and rs17576 of MMP9 and rs243865 (−1306 C>T) and rs865094 of MMP2 genes. Except for MMP9 rs17576, the amplified products were further subjected to sequencing PCR using BigDye® Terminator v3.1. The PCR products of MMP9 rs17576 were digested with restriction enzyme Sma 1. The details of the primers used are shown in Table 1.
Table 1.
Primers of MMP 9 and MMP 2 genes used
| Primer | Sequence 5’- 3’ | Tm | Method |
|---|---|---|---|
| mmp9rs3918242F | 5’-ATgCCTggCACATAgTAggC-3’ | 56 | Sequencing |
| mmp9rs3918242R | 5’-TCgggCAgggTCTATATTCA-3’ | ||
| mmp9rs17576F | 5’-ACCATCCATgggTCAAAgAA-3’ | 56 | RFLP (Sma1) AA=296 bp AG=90 + 206 + 296 bp GG=90 + 206 bp |
| mmp9rs17576R | 5’-gggCTgAACCTggTAgACAg-3’ | ||
| mmp2rs865094F | 5’-CCTTgACCCATgCATTCTCT-3’ | 56 | Sequencing |
| mmp2rs865094R | 5’-CCATCCCAATgACCTCATCTA-3’ | ||
| mmp2rs243865F | 5’-ATTCTTTCAgCCCCTgACCT-3’ | 56 | Sequencing |
| mmp2rs243865R | 5’-CCTgTgACAACCgTCTCTgA-3’ |
Statistical analysis
The data were analyzed using the computer software, Graph Pad Prism version 5 (Graph Pad software, San Diego, California, USA). The data were expressed in terms of frequency and percentage. Frequencies of the individual alleles as well as the homozygous and heterozygous genotypes of the MMP2 and MMP9 polymorphisms were determined in both patient groups and controls. Those samples which could not be genotyped, due to technical errors, were not included in the statistical analysis.
To elucidate the associations and comparisons between different parameters, Chi-square (χ2) test was used as nonparametric test. For all statistical evaluations, a two-tailed probability of value, P < 0.05 was considered as statistically significant. Hardy–Weinberg equilibrium analysis was also carried out in the control population.
RESULTS
The demographic details signifying the gender, age, site and type of odontogenic lesion in the patient group are shown in Table 2.
Table 2.
Demographic data of the patient group
| Characteristics | Cases (%) |
|---|---|
| Type of lesion | |
| Ameloblastoma | 15 (38.46) |
| KCOT | 11 (28.20) |
| DC | 13 (33.34) |
| Gender | |
| Males | 28 (71.79) |
| Females | 11 (28.21) |
| Site of lesion | |
| Maxilla | 8 (20.52) |
| Mandible | 29 (74.35) |
| Both | 2* (5.13) |
| Age (years) | |
| <20 | 16 (41.02) |
| 21-40 | 12 (30.77) |
| >40 | 11 (28.21) |
*Extensive lesion. KCOT: Keratocystic odontogenic tumors, DC: Dentigerous cysts
All the patients and controls were genotyped for rs3918242 (−1562 C >T) and rs17576 of MMP9 and rs243865 (−1306 C>T) and rs865094 of MMP2 gene polymorphism. The control population was found to be in the Hardy–Weinberg equilibrium for genotype frequencies of the MMP2 (rs243865 [−1306 C>T] and rs865094) and MMP9 (rs3918242 [−1562 C>T] and rs17576) polymorphism.
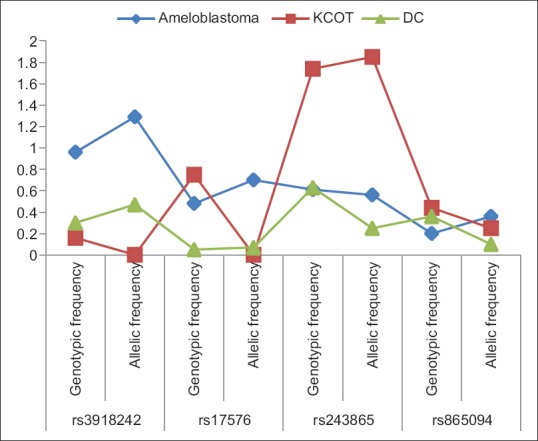
While comparing the genotype and allele frequencies of the MMP 2 and MMP 9 polymorphism in patients and control population, we observed that MMP2 rs243865 (−1306 C>T) polymorphism was significantly associated with odontogenic lesions at both allelic and genotype levels [Table 3]. There was a higher frequency of the genotype TT (0.08) and CT (0.36) and allele T (0.25) when compared to the control group with an odds ratio (OR) of 2.06 (1.08–3.95) in patients. However, none of the other single-nucleotide polymorphisms (SNPs) was found to have any association with the cases in total.
Table 3.
Comparison of genotype and allele frequencies of MMP 9 and MMP 2 gene variants between total patients and controls
| MMP 9 | CC | CT | TT | P | C | T | OR (95% CI) | P | |
|---|---|---|---|---|---|---|---|---|---|
| rs3918242 | Cases | 19 | 18 | 2 | 0.75 | 56 | 22 | 0.87 (0.48-1.56) | 0.65 |
| 0.49 | 0.46 | 0.05 | 0.72 | 0.28 | |||||
| Controls | 56 | 40 | 6 | 152 | 52 | ||||
| 0.55 | 0.39 | 0.06 | 0.75 | 0.25 | |||||
| MMP 9 | AA | AG | GG | P | A | G | OR (95% CI) | P | |
| rs17576 | Cases | 3 | 24 | 12 | 0.43 | 30 | 48 | 0.81 (0.48-1.39) | 0.50 |
| 0.08 | 0.61 | 0.31 | 0.38 | 0.62 | |||||
| Controls | 17 | 58 | 31 | 92 | 120 | ||||
| 0.16 | 0.55 | 0.29 | 0.43 | 0.57 | |||||
| MMP 2 | CC | CT | TT | P | C | T | OR (95% CI) | P | |
| rs243865 | Cases | 22 | 14 | 3 | 0.046 | 58 | 20 | 2.07 (1.08-3.95) | 0.034 |
| 0.56 | 0.36 | 0.08 | 0.74 | 0.26 | |||||
| Controls | 71 | 26 | 1 | 168 | 28 | ||||
| 0.72 | 0.27 | 0.01 | 0.86 | 0.14 | |||||
| MMP 2 | AA | AG | GG | P | A | G | OR (95% CI) | P | |
| rs865094 | Cases | 28 | 9 | 2 | 0.46 | 65 | 13 | 1 (0.49-2.01) | 1 |
| 0.72 | 0.23 | 0.05 | 0.83 | 0.17 | |||||
| Controls | 72 | 31 | 2 | 175 | 35 | ||||
| 0.69 | 0.29 | 0.02 | 0.83 | 0.17 | |||||
OR: Odds ratio, CI: Confidence interval
Each odontogenic lesion was compared individually with the control group. In the case of ameloblastoma, the allele frequencies of MMP9 rs3918242 were 0.57 for C and 0.43 for T. On comparison with controls, the value of P was observed to be significant at 0.05 (OR-2.23 [1.01–4.91]).
In the case of KCOT, the genotype frequencies of MMP2 rs243865 (−1306 C>T) gene polymorphism, CC, CT and TT were 0.36, 0.55 and 0.09, respectively. The Chi-square analysis gave a significant P = 0.01. The allele frequency of C was 0.64 and T was 0.36. A statistically significant P = 0.01 with an OR at 3.429 (1.31–8.92) was observed. No statistically significant associations were seen on comparison of DC cases and controls. The values of P obtained, on comparison of each odontogenic lesion with control group are shown in Figure 1.
Figure 1.
Comparison of P values among the odontogenic lesions, on Chi-square analysis with control group, individually
As the Chi-square analysis gave a statistically significant value of P on comparison of cases with controls, a functional prediction of the SNP MMP2 rs243865 was done using an functional SNP database.
The function of the MMP2 gene was predicted to be changed because of the promoter level polymorphism at rs243865–1306 C/T by prediction tools (transcription factor search) and consite, and the function was predicted to be unchanged by the Golden Path. A functional significance score of 0.208 was observed.
DISCUSSION
Ameloblastoma is a benign but clinically persistent and locally aggressive neoplasm of odontogenic origin seen in gnathic bones. KCOT has always remained a controversial pathology. The rapacious and disruptive clinical course of KCOT vindicates its addition to odontogenic tumors, though histologically and surgically it is closer to a cystic pathology. DC is the clinically most common odontogenic cyst of developmental origin, which rarely shows an aggressive course.
Henriques et al.[13] quoted numerous factors to have a role in the aggressive behavior of ameloblastomas, such as an increased proliferation potential, alterations in the expression of tumor suppressor genes, and the aberrant expression of the cell cycle regulating proteins, adhesion molecules and MMPs and their inhibitors. Silveira et al.[6] suggested MMPs have an inevitable role in the expansion of KCOT and DC. Thus the past decade saw numerous studies devoted to explore the role of MMPs in the progression and expansion of various odontogenic lesions.
To the best of our knowledge, till date, no studies have been done to investigate the role of MMP2 and MMP9 gene polymorphisms in odontogenic lesions. This study explored the role of variants of MMP2 and MMP9 gene in ameloblastoma, KCOT, DC and controls. An SNP is a DNA sequence variation occurring commonly within a population and can act as biological markers to locate genes that are associated with disease pathogenesis.[14] Two SNPs each for each gene were selected on the basis of functional and tagging status. These SNPs have already been studied in numerous other pathologies in various populations.
MMP9 gene is located on chromosome 20, and MMP2 gene is located on chromosome 16. rs3918242 (−1562 C>T) is a SNP located in the promoter region of MMP9 gene and rs17576 in the exon region. rs243865 (−1306 C>T) is located in the promoter region of MMP2 and rs865094 in the intron region. The exact functions of the noncoding regulatory region SNPs are not clear yet. Kim et al.[15] suggested that such SNPs may be related to genes by influencing the binding affinity of transcription factors. Such alterations in the binding or production of transcription factors might have a change in the function of the gene involved.
Many studies on the association of MMP9 polymorphism with cancer susceptibility have been published. In chronic oral inflammatory conditions,[16] also −1562 C>T polymorphism in MMP9 has been established. In odontogenic lesions, only protein expression studies of MMP-9 have been reported so far. Pinheiro et al.[17] through immunohistochemical studies have revealed increased proliferative activity of ameloblastic cells in the presence of MMP-9 enzyme. Henriques et al.[13] have demonstrated increased staining in the epithelium (P = 0.058) and mesenchyme (P = 0.005) of KCOTs and ameloblastomas compared to DCs and radicular cysts (RCs).
In this study, no variation was found in the frequency distribution of MMP9 rs3918242 genotype (P = 0.75) or allele (P = 0.65) among total cases and controls. However, ameloblastoma cases showed a significant association (P = 0.050; OR = 2.23 [1.01–4.91]) in the distribution of allelic frequency on comparison with controls. The frequency of T (0.43) allele and genotypes CT (0.60) and TT (0.13) was found to be higher in ameloblastomas when compared to the control (0.25) data. The mutant TT homozygous genotype was not found in KCOTs or DCs.
Folgueras et al.[18] have reported that promoter site plays a regulatory role in the expression of MMP genes. Among cancer studies,[19] the 1562 (rs3918242) C to T substitution has been shown to up-regulate the promoter activity, and the presence of the 1562T allele has also been reported to associated with the decreased capacity of a putative transcription repressor protein with a subsequent increase in gene expression. This might lead to increased protein production, which can affect the clinical nature of the disease. Thus, a change in the promoter site of the MMP9 gene might be a reason for aggressive clinical course of ameloblastomas.
MMP9 rs17576 is an SNP in the exon region of the gene. An allelic change from A to G at MMP9 rs17576 substitutes glutamine with arginine. Sun et al.[20] studied MMP9 rs17576 in Han Chinese participants and found it to be significantly associated (P ≤ 0.001) with an increased risk of lung cancer.
In this study, no significant association was observed in the allelic (P = 0.50) and genotype (P = 0.42) distribution between cases and controls. On further evaluation, among individual odontogenic lesions also, no statistically significant association was found. As the immunohistochemical studies[13,17] suggest, altered MMP enzyme activity certainly affects the biological behavior of odontogenic lesions. As no other documented studies on the role of this particular SNP on odontogenic lesions are available, further studies with increased sample size are warranted to establish our observation.
MMP2 rs243865 (−1306 C>T) is an SNP in the promoter region of MMP2 gene. Hence, it might have a role in the regulation of the function of MMP2 gene by affecting its transcriptional activity. Zhou et al.[21] suggested that MMP-2–1306 C/T polymorphism may contribute to breast cancer susceptibility (P = 0.001). Beeghly-Fadiel et al.[22] reported that allele homozygotes for rs243865 (−1306 C/T) tended to have an increased risk of breast cancer (OR, 1.4; 95% CI, 0.9–2.4). Wang et al.[23] reported that inhibition of MMP-2 activity suppresses the local invasiveness of ameloblastoma cells. In the study conducted by Khalifa et al.,[24] the highest MMP-2 immunoexpression was shown by ameloblastomas followed by KCOT and lowest in RCs.
In this study, we found a significant difference in the distribution of genotypes (P = 0.04) and alleles (P = 0.03; OR = 2.06 [1.08–3.95]) in cases compared to that of controls. The cases showed a higher frequency of genotype TT (0.08) and CT (0.36) and allele T (0.25) when compared to the control group. On further comparison, KCOTs also showed an increased frequency of genotype TT (0.09) and CT (0.55) and allele T (0.36) compared to the control population. A statistically significant P = 0.01 with an OR at 3.41 (1.31–8.92) was observed in the allelic distribution. Functional prediction tool suggested this variant to be a polymorphism which affected the function of the MMP2 gene.
Numerous studies have been done on KCOTs to substantiate its neoplastic nature and differences from other odontogenic cystic lesions. The increased presence of MMP-2 and MMP-9 enzymes in keratocystic extracts ha already been established.[25] Ameloblastoma is a neoplasm with established genetic pathogenesis. Increased MMP activity in ameloblastomas as a cause for its aggressiveness has been substantiated by numerous immunohistochemical studies. One of the causes for the higher aggressive nature of ameloblastomas and KCOTs, compared to DCs may be attributed to the polymorphism in the promoter region of the MMP2 gene.
MMP2 rs865094 is an SNP in the intron region of MMP 2 gene. We found no significant difference in the distribution of allele (P = 1.00) and genotype (P = 0.46) frequencies between the cases and controls. Individual odontogenic lesions also did not show any statistical significance. Similarly, Beeghly-Fadiel et al.[22] found no association between rs865094 and breast cancer risk. Although the effect of SNPs in the noncoding region is unclear, they are presumed to have a regulatory effect on gene function. Hence, a polymorphism at MMP2 rs865094 does not seem to have any effect in the aggressiveness of ameloblastomas, KCOT or DC.
CONCLUSIONS
We did a pioneer study to investigate the role of MMP2 and MMP9 gene polymorphisms in the biological behavior of ameloblastoma, KCOT and DC. We found that ameloblastomas showed a higher frequency of a mutant allele (T) of MMP9 rs 3918242–1562 compared to the control population. All the cases showed a statistically significant difference in the distribution of genotype and allele frequency of MMP2 rs 243865–1306. Both of the above are promoter level polymorphisms of the respective genes. These observations give evidence to our initial hypothesis that polymorphisms in MMP9 and MMP2 genes are associated with clinically aggressive odontogenic lesions. However, no significant association was found between polymorphisms at MMP9 rs 17576 and MMP2 rs 865094 and aggressiveness of odontogenic lesions in the samples studied. However, it is not feasible to draw a profound conclusion based on the presence of such a limited number of samples and SNPs. Further studies with larger sample size and more SNPs for genotyping are warranted to establish an impeccable role of MMP2 and MMP9 gene alterations in the biological behavior of odontogenic lesions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Neville BW, Damm DD, Allen CM, Bouquot JE. 2nd ed. Philadelphia, PA: W.B. Saunders Co; 2002. Odontogenic cysts and Tumors. Oral and Maxillofacial Pathology; pp. 590–610. [Google Scholar]
- 2.Sriram G, Shetty RP. Odontogenic tumors: A study of 250 cases in an Indian teaching hospital. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e14–21. doi: 10.1016/j.tripleo.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Selvamani M, Donoghue M, Basandi PS. Analysis of 153 cases of odontogenic cysts in a South Indian sample population: A retrospective study over a decade. Braz Oral Res. 2012;26:330–4. doi: 10.1590/s1806-83242012005000007. [DOI] [PubMed] [Google Scholar]
- 4.Jahagirdar PB, Kale AD, Hallikerimath S. Stromal characterization and comparison of odontogenic cysts and odontogenic tumors using picrosirius red stain and polarizing microscopy: A retrospective and histochemical study. Indian J Cancer. 2015;52:408–12. doi: 10.4103/0019-509X.176742. [DOI] [PubMed] [Google Scholar]
- 5.Stolf DP, Karim AC, Banerjee AG. Genetic aspects of ameloblastoma: A brief review. Biotechnol Mol Biol Rev. 2007;2:116–22. [Google Scholar]
- 6.Silveira EJ, Piva MR, Galvão HC, Souza LB, Freitas RA. Role of matrix metalloproteinases in the etiopathogeny of odontogenic cysts. J Bras Pathol Med Lab. 2007;43:203–9. [Google Scholar]
- 7.Souza Freitas V, Ferreira de Araújo CR, Alves PM, de Souza LB, Galvão HC, de Almeida Freitas R, et al. Immunohistochemical expression of matrilysins (MMP-7 and MMP-26) in ameloblastomas and adenomatoid odontogenic tumors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:417–24. doi: 10.1016/j.tripleo.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 8.Biljana E, Boris V, Cena D, Veleska-Stefkovska D. Matrix metalloproteinases (with accent to collagenases) J Cell Anim Biol. 2011;5:113–20. [Google Scholar]
- 9.Alaeddini M, Salah S, Dehghan F, Eshghyar N, Etemad-Moghadam S. Comparison of angiogenesis in keratocystic odontogenic tumours, dentigerous cysts and ameloblastomas. Oral Dis. 2009;15:422–7. doi: 10.1111/j.1601-0825.2009.01566.x. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhary AK, Singh M, Bharti AC, Asotra K, Sundaram S, Mehrotra R, et al. Genetic polymorphisms of matrix metalloproteinases and their inhibitors in potentially malignant and malignant lesions of the head and neck. J Biomed Sci. 2010;17:10. doi: 10.1186/1423-0127-17-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava P, Kapoor R, Mittal RD. Influence of matrix metalloproteinase gene polymorphisms in healthy North Indians compared to variations in other ethnic groups worldwide. Asian Pac J Cancer Prev. 2009;10:1127–30. [PubMed] [Google Scholar]
- 12.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henriques ÁC, Vasconcelos MG, Galvão HC, de Souza LB, de Almeida Freitas R. Comparative analysis of the immunohistochemical expression of collagen IV, MMP-9, and TIMP-2 in odontogenic cysts and tumors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:468–75. doi: 10.1016/j.tripleo.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 14.Barreiro LB, Laval G, Quach H, Patin E, Quintana-Murci L. Natural selection has driven population differentiation in modern humans. Nat Genet. 2008;40:340–5. doi: 10.1038/ng.78. [DOI] [PubMed] [Google Scholar]
- 15.Kim BC, Kim WY, Park D, Chung WH, Shin KS, Bhak J, et al. SNP@Promoter: A database of human SNPs (single nucleotide polymorphisms) within the putative promoter regions. BMC Bioinformatics. 2008;9(Suppl 1):S2. doi: 10.1186/1471-2105-9-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keles GC, Gunes S, Sumer AP, Sumer M, Kara N, Bagci H, et al. Association of matrix metalloproteinase-9 promoter gene polymorphism with chronic periodontitis. J Periodontol. 2006;77:1510–4. doi: 10.1902/jop.2006.050378. [DOI] [PubMed] [Google Scholar]
- 17.Pinheiro JJ, Freitas VM, Moretti AI, Jorge AG, Jaeger RG. Local invasiveness of ameloblastoma. Role played by matrix metalloproteinases and proliferative activity. Histopathology. 2004;45:65–72. doi: 10.1111/j.1365-2559.2004.01902.x. [DOI] [PubMed] [Google Scholar]
- 18.Folgueras AR, Pendás AM, Sánchez LM, López-Otín C. Matrix metalloproteinases in cancer: From new functions to improved inhibition strategies. Int J Dev Biol. 2004;48:411–24. doi: 10.1387/ijdb.041811af. [DOI] [PubMed] [Google Scholar]
- 19.Zhang LF, Mi YY, Cao Q, Wang W, Qin C, Wei JF, et al. Update analysis of studies on the MMP-9 -1562 C&T polymorphism and cancer risk. Mol Biol Rep. 2012;39:3435–41. doi: 10.1007/s11033-011-1115-5. [DOI] [PubMed] [Google Scholar]
- 20.Sun JZ, Yang XX, Hu NY, Li X, Li FX, Li M, et al. Genetic variants in MMP9 and TCF2 contribute to susceptibility to lung cancer. Chin J Cancer Res. 2011;23:183–7. doi: 10.1007/s11670-011-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou P, Du LF, Lv GQ, Yu XM, Gu YL, Li JP, et al. Current evidence on the relationship between four polymorphisms in the matrix metalloproteinases (MMP) gene and breast cancer risk: A meta-analysis. Breast Cancer Res Treat. 2011;127:813–8. doi: 10.1007/s10549-010-1294-0. [DOI] [PubMed] [Google Scholar]
- 22.Beeghly-Fadiel A, Lu W, Long JR, Shu XO, Zheng Y, Cai Q, et al. Matrix metalloproteinase-2 polymorphisms and breast cancer susceptibility. Cancer Epidemiol Biomarkers Prev. 2009;18:1770–6. doi: 10.1158/1055-9965.EPI-09-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang A, Zhang B, Huang H, Zhang L, Zeng D, Tao Q, et al. Suppression of local invasion of ameloblastoma by inhibition of matrix metalloproteinase-2 in vitro . BMC Cancer. 2008;8:182. doi: 10.1186/1471-2407-8-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khalifa GA, Shokier HM, Abo-Hager EA. Evaluation of neoplastic nature of keratocystic odontogenic tumor versus ameloblastoma. J Egypt Natl Canc Inst. 2010;22:61–72. [PubMed] [Google Scholar]
- 25.Kumamoto H, Yamauchi K, Yoshida M, Ooya K. Immunohistochemical detection of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in ameloblastomas. J Oral Pathol Med. 2003;32:114–20. doi: 10.1034/j.1600-0714.2003.00086.x. [DOI] [PubMed] [Google Scholar]


