Abstract
Background:
The extent of involvement of cervical lymph nodes is known to be the most important prognosticator in oral squamous cell carcinoma (SCC) that significantly affects the survival rate of patients. The clinical, radiological and pathological factors that can predict cervical lymph node metastasis are yet to be ascertained clearly, which poses a challenge for the surgeon to determine the extent of neck dissection.
Aim:
This study aims to identify the clinical and histopathologic predictors of lymph node metastasis among patients with oral SCC and to devise a scoring system based on those predictors to aid in better clinical decision-making regarding the extent of neck dissection.
Setting:
Malabar Cancer Centre, a specialized tertiary cancer care center in Kerala, India.
Methods:
A retrospective review of 160 patient records and biopsy slides collected and preserved between June 2014 and May 2016.
Conclusion:
The clinicopathologic parameters such as site of cancer (P = 0.03), histologic differentiation (P = 0.03), shape of rete pegs (P = 0.002), pattern of invasion (P = 0.0001) and depth of invasion >3 mm (P = 0.016) were significantly associated with the risk of lymph node metastasis. The risk score devised based on these predictors serves as an efficient tool in aiding clinical decision-making regarding the extent of neck dissection.
Keywords: Cervical lymph node, Malabar Cancer Centre, oral cancer, risk score
INTRODUCTION
Cancers related to the oral cavity are a major public health issue in India, with tobacco and betel chewing being the significant risk factors. Approximately, 77,000 new cases and 52,000 deaths are reported annually.[1] Squamous cell carcinomas (SCCs) encompass at least 90% of all oral malignancies.[2] With the World Health Organization expecting a worldwide increase in oral SCC incidence in the next decade,[2] oral cancer, predominantly SCC, is the major malignancy in India and South East Asia, accounting for up to 50% of all cancers.[3] The overall 5-year survival rate of oral cancers, including all the stages, has shown little improvement over the past several decades, hovering around 50%. The extent of the cervical lymph node involvement is known to be the significant prognosticator in oral SCC which affects the survival rate of patients.[4,5] The clinical, radiological or pathological factors that can predict the cervical lymph node metastasis are yet to be clearly ascertained. This poses a clinical challenge in determining the extent of neck dissection as it is associated with functional side effects such as neck pain, fibrosis and shoulder dysfunction.[6,7] Hence, it is imperative to decide the extent and type of neck dissection to limit the occurrence of functional disability.
Apart from grading carcinomas, many studies have attempted to determine which histologic parameter correlates most strongly with the aggressive biologic behavior. However, certain studies have found that tumor grading based on the histologic and cytologic features of differentiation has only limited value in predicting lymph node metastasis due to intra- and inter-observer disagreements.[8] Spiro et al. found that oral SCC patients with a high-grade pattern of invasion were more likely to present with concomitant nodal metastasis, develop distant metastasis and result in death.[9] The mode of tumor invasion within the stroma as well as the thickness, depth of invasion and budding of the tumor has been described as the key histopathologic feature which can predict lymph node metastasis.[10,11,12,13] Therefore, we aim to identify the specific clinical and histopathologic predictors of lymph node metastasis and devise a scoring system based on the key significant predictors to aid in better clinical decision-making regarding the extent of neck dissection.
METHODS
Study design
This is a retrospective review of 160 patient records and preserved biopsy slides.
General setting
Kerala is a South Indian state with the lowest population (3.44%), highest Human Development Index of 0.79 in 2011, highest literacy rate (93.9%) in the 2011 census and highest life expectancy (77 years).[14] According to the recent Global Burden of Disease estimates, Kerala has the highest crude cancer incidence. Among all other cancers, oral cancer is one of the top five causes of deaths and disabilities in the state.[15]
Study setting
Malabar Cancer Centre (MCC) is a specialized cancer treatment center in the southern part of the country. Every year, it treats around 5000 patients for cancer, with nearly 300 patients suffering from oral cancer.
Study population
All patients with oral SCC involving lips, buccal mucosa, tongue, hard palate, retromolar trigone or floor of mouth who had undergone a wide excision with neck node dissection (selective, modified and radical) at MCC between June 2014 and May 2016 were recruited into the study.
Study period
The study was carried out between June 2016 and October 2016.
Sources of data and data collection
Patients who visited the head-and-neck outpatient department of MCC with suspected oral cancer were subjected to a diagnostic procedure namely incision or wedge biopsy. Once a histopathologic diagnosis of SCC is done, the patients are subjected to wide excision of primary tumor with dissection of the draining cervical lymph nodes. After the surgery, the dissected lymph node specimen is sent to the pathology department. Specimen status regarding the number and size is ascertained and is recorded in the histopathology report (HPR).
The sociodemographic and clinical characteristics of the patients who underwent neck dissection for oral SCC were collected from the Medical Records Division, MCC. The lymph node specimens were dissected, and the histopathological factors were noted. Specific histopathological parameters of incision biopsy specimen were ascertained by reexamining the hematoxylin- and eosin-stained glass slides preserved in the archives.
The three parameters namely pattern of invasion (pushing/minimally invasive/frankly invasive), shape of rete pegs (bulbous/uniform to irregular) and the number of keratin pearls were evaluated at 40× (4 × 10) (scanner). Mitosis (both increased and abnormal) and depth of invasion were evaluated at 400× (40 × 10) (high power).
Outcome variable
Lymph node status was the outcome variable. Routinely, after head-and-neck surgery, a lymph node dissection specimen is sent to the pathology department where its number and size were determined and recorded in the HPR. Thereafter, it was accordingly categorized into low risk, moderate risk and high risk based on the number and size of the lymph nodes (AJCC TNM stage 7th Edition).
Operational definitions
The rete pegs are classified into slender (elongated, length more than width), bulbous and uniform (rounded, length equal to or less than width) and irregular (of varying shapes and sizes) based on their shape. The pattern of invasion is classified into pushing (leading edge of the tumor is regular), minimally invasive (mild-to-moderate disarray of the leading edge of tumor) and frankly invasive (marked disarray and widely splayed cell groups/single cells at the leading edge).
Data analysis
Data were entered using EpiData entry version 3.1 (EpiData Association, Odense, Denmark) and analyzed using EpiData analysis v2.2.2.182. Double data entry and validation was done to avoid data entry errors. Descriptive statistics such as percentages were used to summarize the demographic and clinicopathologic characteristics. Univariate analysis was done to study the association between various patient characteristics (clinical and histopathologic parameters) and the risk of lymph node metastasis. A logistic regression model was developed to predict the risk of cervical node metastasis. Risk factors that were significantly related to nodal metastasis (P < 0.1) were chosen as candidate variables which were used to develop the logistic regression model. The regression coefficients were used to assign relative scores to predictors. The individual scores were then added to obtain the overall score. Predicted risk (%) was then calculated for each overall score.
Ethical approval
The ethical approval for this study was obtained from the institute's Ethics Committee, MCC, Kerala, and the Union Ethics Advisory Group, Paris, France.
RESULTS
A total of 160 cases of oral squamous cell cancer were included in the study. Majority of the patients were males (106, 66%), belonging to the elderly age group of 60 years and above (102, 64%). A total of 52 (32%) patients were clinically diagnosed as Stage IV followed by 48 (30%) cases in Stage II. The most common site of tumor was buccal mucosa (80, 50%) followed by tongue (63, 39%). Histological analysis showed predominant well-differentiated tumors in 85% (136) of the cases. The most common shape of rete pegs was irregular (75, 47%) followed by bulbous and uniform (70, 44%). The invasion pattern varied from being pushing in 62 (39%) patients to minimally invasive in 56 (35%) and frankly invasive in 41 (26%) cases. Nodal involvement was not seen in 101 (63%) patients [Table 1].
Table 1.
Sociodemographic, clinical and pathological characteristics of patients with oral squamous cell carcinoma diagnosed from June 2014 to May 2016 at Malabar Cancer Centre, Kerala, India
| Characteristics | n (%) |
|---|---|
| Total | 160 (100) |
| Gender | |
| Male | 106 (66) |
| Female | 54 (34) |
| Age group (years) | |
| 30-44 | 12 (7) |
| 45-59 | 46 (29) |
| 60 and above | 102 (64) |
| Clinical staging | |
| I | 22 (14) |
| II | 48 (30) |
| III | 38 (24) |
| IV | 52 (32) |
| Site of tumor | |
| Tongue | 63 (39) |
| Buccal mucosa | 80 (50) |
| Others | 17 (24) |
| Histologic differentiation | |
| Well differentiated | 136 (85) |
| Moderately differentiated | 17 (11) |
| Poorly differentiated | 5 (3) |
| Not recorded | 2 (1) |
| Shape of rete pegs | |
| Slender and fused | 14 (9) |
| Bulbous and uniform | 70 (44) |
| Irregular | 75 (47) |
| Not recorded | 1 (1) |
| Pattern of invasion | |
| Pushing | 62 (39) |
| Minimally invasive | 56 (35) |
| Frankly invasive | 41 (26) |
| Not recorded | 1 (0) |
| Depth of invasion (mm) | |
| 0-3 | 123 (77) |
| >3 | 37 (23) |
| Mitotic count | |
| 0-1 mitosis | 71 (45) |
| 2-3 mitoses | 74 (46) |
| >3 mitoses | 13 (8) |
| Missing | 2 (1) |
| Keratin pearls | |
| 0-1 | 36 (22) |
| 2-3 | 42 (26) |
| >3 | 81 (51) |
| Missing | 1 (1) |
| Lymph node status | |
| N0 | 101 (63) |
| N1 | 21 (13) |
| N2a | 3 (2) |
| N2b | 28 (18) |
| N2c | 7 (4) |
| N3 | 0 |
The clinical and pathologic parameters such as site of cancer (P = 0.03), histologic differentiation (P = 0.03), shape of rete pegs (P = 0.002), pattern of invasion (P = 0.0001) and depth of invasion >3 mm (P = 0.016) were found to be significantly associated with the risk of lymph node metastasis [Table 2].
Table 2.
Demographic, clinical and pathological factors associated with the risk of cervical lymph node metastasis among patients with oral squamous cell carcinoma at Malabar Cancer center, Kerala, India, 2014-2016
| Characteristics | Low risk, n (%) | High risk, n (%) | Total, n | P | Regression coefficients |
|---|---|---|---|---|---|
| Age group | |||||
| 30-44 | 7 (58) | 5 (42) | 12 | 0.67 | - |
| 45-59 | 36 (78) | 10 (22) | 46 | - | |
| 60 and above | 79 (77) | 23 (23) | 102 | - | |
| Clinical staging | |||||
| I | 18 (82) | 4 (18) | 22 | 0.38 | - |
| II | 41 (85) | 7 (15) | 48 | - | |
| III | 29 (76) | 9 (24) | 38 | - | |
| IV | 34 (67) | 17 (33) | 51 | - | |
| Tumor site | |||||
| Tongue | 44 (70) | 19 (30) | 63 | 0.03 | 0.12 |
| Buccal mucosa | 66 (83) | 14 (17) | 80 | 0.03 | |
| Others | 10 (59) | 7 (41) | 17 | 0.19 | |
| Histologic differentiation | |||||
| Well | 109 (80) | 27 (20) | 136 | 0.03 | 0.09 |
| Moderate | 9 (53) | 8 (47) | 17 | 0.32 | |
| Poor | 2 (40) | 3 (60) | 5 | 0.47 | |
| Shape of rete pegs | 0.002 | ||||
| Slender and fused | 12 (86) | 2 (14) | 14 | 0.10 | |
| Bulbous and uniform | 58 (83) | 12 (17) | 70 | 0.22 | |
| Irregular | 52 (69) | 23 (31) | 75 | 0.29 | |
| Pattern of invasion | |||||
| Pushing | 54 (87) | 8 (13) | 62 | 0.0001 | 0.08 |
| Minimally invasive | 44 (79) | 12 (21) | 56 | 0.17 | |
| Frankly invasive | 24 (58) | 17 (42) | 41 | 0.49 | |
| Depth of invasion (mm) | |||||
| 0-3 | 99 (80) | 24 (20) | 123 | 0.016 | 0.09 |
| >3 | 23 (62) | 14 (38) | 37 | 0.27 | |
| No of keratin pearls | |||||
| 0-1 | 26 (72) | 10 (28) | 36 | 0.29 | - |
| 2-3 | 35 (83) | 7 (17) | 42 | - | |
| >3 | 60 (74) | 21 (26) | 81 | - | |
| Mitotic count | |||||
| 0-1 | 55 (77) | 16 (23) | 71 | 0.35 | - |
| 2-3 | 54 (73) | 20 (27) | 74 | - | |
| >3 | 11 (85) | 2 (15) | 13 | - |
Predictors of nodal metastasis were scored relative to the regression coefficients in the logistic regression model, which are listed in Table 3. The minimum total risk score is 4 for a patient without any risk factors, whereas the maximum possible score is 18. The predicted probabilities of nodal metastasis for each risk score ranged from 5% for a patient with a score of 4 to 91% for a patient with the highest possible score of 18 [Table 4].
Table 3.
Risk scores for cervical node metastasis among patients with oral squamous cell carcinoma, Malabar Cancer Centre, Kerala, India, during 2014-2016
| Risk factor | Score |
|---|---|
| Shape of rete pegs | |
| Slender and fused | 1 |
| Bulbous and uniform | 2 |
| Irregular | 3 |
| Pattern of invasion | |
| Pushing | 1 |
| Minimally invasive | 2 |
| Frankly invasive | 5 |
| Depth of invasion (mm) | |
| 0-3 | 1 |
| >3 | 3 |
| Histologic differentiation | |
| Well | 1 |
| Moderate | 3 |
| Poor | 5 |
| Site of cancer | |
| Buccal mucosa | 0 |
| Tongue | 1 |
| Others | 2 |
Table 4.
Predicted risk of cervical node metastasis among the patients with oral squamous cell carcinoma, Malabar Cancer Centre, Kerala, India, during 2014-2016
| Total risk score | Predicted risk (%) |
|---|---|
| 4 | 5 |
| 5 | 9 |
| 6 | 13 |
| 7 | 18 |
| 8 | 21 |
| 9 | 27 |
| 10 | 31 |
| 11 | 39 |
| 12 | 48 |
| 13 | 54 |
| 14 | 67 |
| 15 | 79 |
| 16 | 83 |
| 17 | 86 |
| 18 | 91 |
Figures 1-4 depict the depth and pattern of invasion and tumor differentiation.
Figure 1.
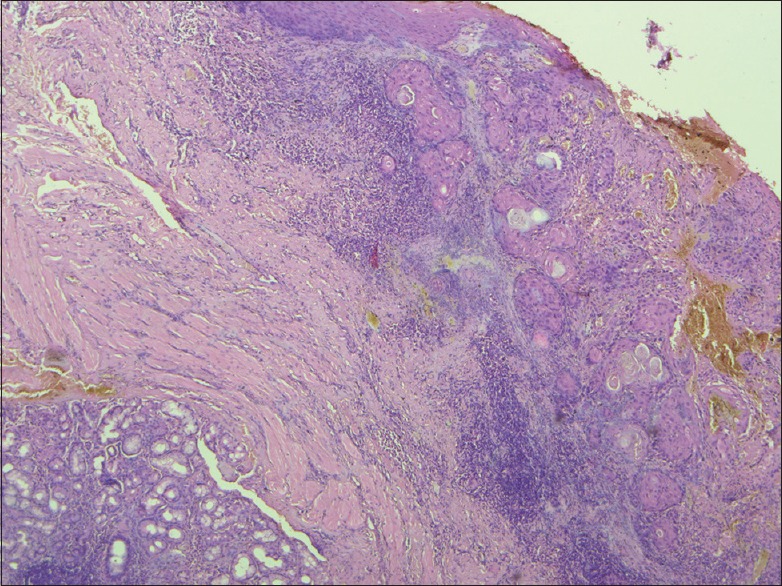
Depth of invasion of 3 mm, measured by micrometer (H and E, ×400)
Figure 4.
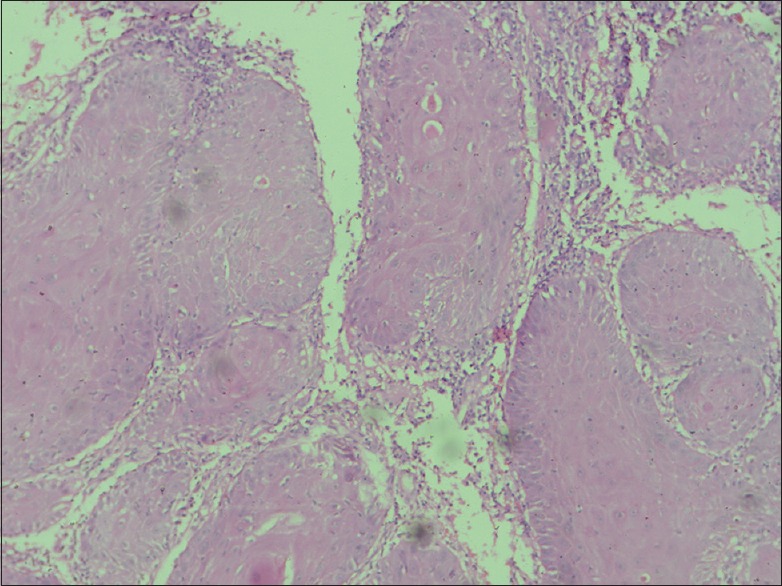
Round and bulbous rete pegs (H and E, ×40)
Figure 2.
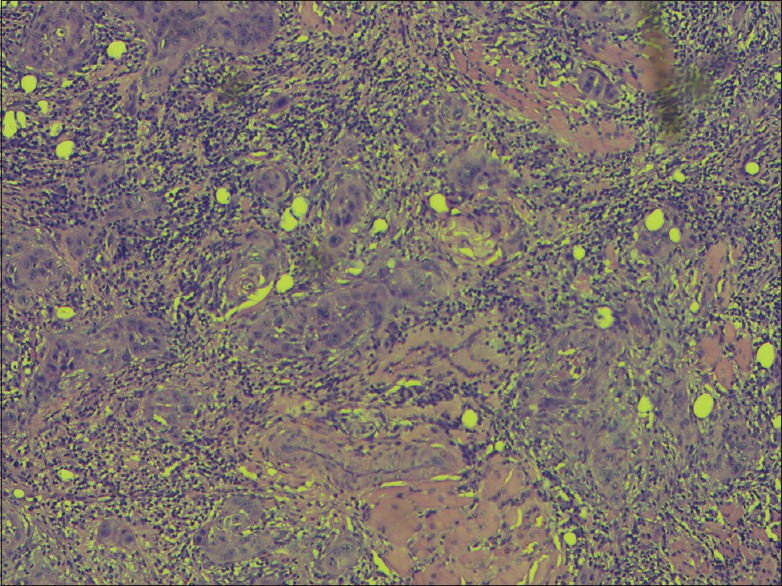
Frankly invasive pattern (H and E, ×400)
Figure 3.
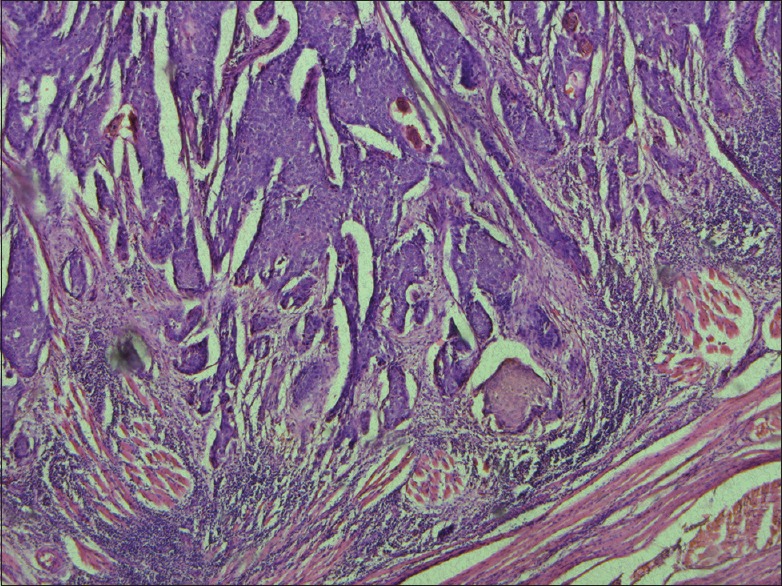
Minimally invasive pattern (H and E, ×40)
DISCUSSION
In this study, we have attempted to predict the biologic behavior of oral SCC seen in the different subsites of the oral cavity based on major histologic characteristics. A significant statistical association was found between the risk of cervical lymph node metastasis and the four histological parameters of differentiation, namely shape of rete pegs, pattern of invasion and depth of invasion as well as the site of cancer.[16] For patients with pN0, majority were well differentiated with depth of invasion <3 mm; almost half of the total number of patients had uniform rete pegs with pushing margins. A scoring system was also devised to aid in clinical decision-making.
Depth of invasion (≥3 mm) was significantly associated with the risk of cervical lymph node metastasis, similar to the findings of Seki et al.[10] Several studies showed that a tumor depth of >4 mm showed a high risk for nodal metastasis, whereas in those by Kane et al. and Fukano et al., it was >5 mm.[13,17,18,19,20] In contrast, Goerkem et al. found no significant association between tumor depth and risk of nodal metastasis.[21]
Similar to the findings in previous studies, moderate-to-poor histologic differentiation was also associated with lymph node metastasis.[21,22,23] Of the pN0 cases (T1–T4), the overwhelming majority were histologically well differentiated [Figure 5]. In the same way, pattern of invasion also had a prognostic implication on nodal metastasis, thus supporting the findings from literature.[17,21,24,25]
Figure 5.
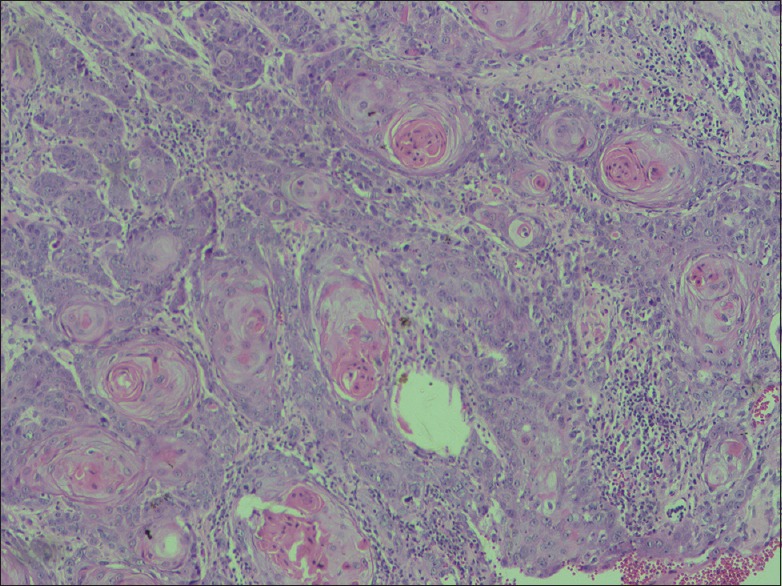
Well-differentiated oral squamous cell carcinoma (H and E, ×40)
The risk score developed in this study is designed to be a convenient tool to predict the risk of cervical node metastasis based on the clinicopathologic parameters. The point score assigned to each risk factor was derived using a well-fit logistic regression model, which included risk factors consistent with other literature. The scores were assigned relative to the regression coefficients in the model consistent with the methodology outlined by Moons et al.[26] This score might aid the surgeon in deciding the extent of neck dissection, thereby limiting the functional side effects of a radical neck dissection.
It is worth mentioning that although a high-risk score can fairly predict the risk of nodal metastasis, it is not designed to precisely predict a single patient's treatment outcome. In addition, an individual patient may have other clinical factors not included in the model that are associated with the outcome. Therefore, the risk score should be used as a tool to help physicians and patients make informed decisions, but not to predict a patient's disease outcome. We look forward to test the generalizability of the risk score developed in this study by testing it on other patient populations.
The limitation of the study was that other clinical factors could not be assessed for their role in predicting the risk of nodal metastasis.
CONCLUSION
The clinical and histopathologic factors studied herein permit greater selectivity and more informed decision-making while addressing elective neck treatment for oral cancers. Although the risk score needs to be tested in other patient populations, it is a convenient tool that aids in better clinical decision-making regarding the extent of neck dissection.
Financial support and sponsorship
This research paper is an outcome of SORT-IT Oncology program funded by the Academy for Public Health, Kozhikode, Kerala, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This research was supported through an operational research course that was jointly developed and run by the Academy for Public Health, Kozhikode, Kerala, India; MCC, Thalassery, Kerala, India; and Centre for Operational Research, International Union Against Tuberculosis and Lung Disease, France. The authors thank the staff of MCC for their support in the process of data collection. This course is under the umbrella of the World Health Organization – TDR Structured Operational Research and Training IniTiative (SORT-IT) programme for capacity building in low- and middle-income countries.
REFERENCES
- 1.Laprise C, Madathil SA, Allison P, Abraham P, Raghavendran A, Shahul HP, et al. No role for human papillomavirus infection in oral cancers in a region in Southern India. Int J Cancer. 2016;138:912–7. doi: 10.1002/ijc.29827. [DOI] [PubMed] [Google Scholar]
- 2.Barnes L, Eveson JW, editors. Lyon: Peterreichart, David Sidransky, International Agency for Research on Cancer (IARC); 2005. World Health Organization Classification of Tumours: Pathology & Genetics Head and Neck Tumours. [Google Scholar]
- 3.Johnson NW. Cambridge UK: Cambridge University Press; 1991. A Global View of the Epidemiology of Oral Cancer; pp. 3–326. [Google Scholar]
- 4.Bogenrieder T, Herlyn M. Axis of evil: Molecular mechanisms of cancer metastasis. Oncogene. 2003;22:6524–36. doi: 10.1038/sj.onc.1206757. [DOI] [PubMed] [Google Scholar]
- 5.Kapoor C, Vaidya S, Wadhwan V, Malik S. Lymph node metastasis: A bearing on prognosis in squamous cell carcinoma. Indian J Cancer. 2015;52:417–24. doi: 10.4103/0019-509X.176750. [DOI] [PubMed] [Google Scholar]
- 6.Barber B, McNeely M, Chan KM, Beaudry R, Olson J, Harris J, et al. Intraoperative brief electrical stimulation (BES) for prevention of shoulder dysfunction after oncologic neck dissection: Study protocol for a randomized controlled trial. Trials. 2015;16:240. doi: 10.1186/s13063-015-0745-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baggi F, Santoro L, Grosso E, Zanetti C, Bonacossa E, Sandrin F, et al. Motor and functional recovery after neck dissection: Comparison of two early physical rehabilitation programmes. Acta Otorhinolaryngol Ital. 2014;34:230–40. [PMC free article] [PubMed] [Google Scholar]
- 8.Acharya S, Sivakumar AT, Shetty S. Cervical lymph node metastasis in oral squamous cell carcinoma: A correlative study between histopathological malignancy grading and lymph node metastasis. Indian J Dent Res. 2013;24:599–604. doi: 10.4103/0970-9290.123385. [DOI] [PubMed] [Google Scholar]
- 9.Spiro RH, Guillamondegui O, Jr, Paulino AF, Huvos AG. Pattern of invasion and margin assessment in patients with oral tongue cancer. Head Neck. 1999;21:408–13. doi: 10.1002/(sici)1097-0347(199908)21:5<408::aid-hed5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Seki M, Sano T, Yokoo S, Oyama T. Histologic assessment of tumor budding in preoperative biopsies to predict nodal metastasis in squamous cell carcinoma of the tongue and floor of the mouth. Head Neck. 2016;38(Suppl 1):E1582–90. doi: 10.1002/hed.24282. [DOI] [PubMed] [Google Scholar]
- 11.O-Charoenrat P, Pillai G, Patel S, Fisher C, Archer D, Eccles S, et al. Tumour thickness predicts cervical nodal metastases and survival in early oral tongue cancer. Oral Oncol. 2003;39:386–90. doi: 10.1016/s1368-8375(02)00142-2. [DOI] [PubMed] [Google Scholar]
- 12.Spiro RH, Huvos AG, Wong GY, Spiro JD, Gnecco CA, Strong EW, et al. Predictive value of tumor thickness in squamous carcinoma confined to the tongue and floor of the mouth. Am J Surg. 1986;152:345–50. doi: 10.1016/0002-9610(86)90302-8. [DOI] [PubMed] [Google Scholar]
- 13.Fukano H, Matsuura H, Hasegawa Y, Nakamura S. Depth of invasion as a predictive factor for cervical lymph node metastasis in tongue carcinoma. Head Neck. 1997;19:205–10. doi: 10.1002/(sici)1097-0347(199705)19:3<205::aid-hed7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Wikipedia Contributors. Kerala. Wikipedia, The Free Encyclopedia. [Last accessed on 2016 Jun 02]. Available from: https://www.en.wikipedia.org/w/index.php?title=Special: CiteThisPage and page=Kerala&id=723279141 .
- 15.India State-Level Disease Burden Initiative Cancer Collaborators. The burden of cancers and their variations across the states of India: The global burden of disease study 1990-2016. Lancet Oncol. 2018;19:1289–306. doi: 10.1016/S1470-2045(18)30447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rousseau A, Badoual C. Head and neck: Squamous cell carcinoma: An overview. Atlas Genet Cytogenet Oncol Haematol. 2012;16:145–55. [Google Scholar]
- 17.Kurokawa H, Yamashita Y, Takeda S, Zhang M, Fukuyama H, Takahashi T, et al. Risk factors for late cervical lymph node metastases in patients with stage I or II carcinoma of the tongue. Head Neck. 2002;24:731–6. doi: 10.1002/hed.10130. [DOI] [PubMed] [Google Scholar]
- 18.Fakih AR, Rao RS, Borges AM, Patel AR. Elective versus therapeutic neck dissection in early carcinoma of the oral tongue. Am J Surg. 1989;158:309–13. doi: 10.1016/0002-9610(89)90122-0. [DOI] [PubMed] [Google Scholar]
- 19.Lim SC, Zhang S, Ishii G, Endoh Y, Kodama K, Miyamoto S, et al. Predictive markers for late cervical metastasis in stage I and II invasive squamous cell carcinoma of the oral tongue. Clin Cancer Res. 2004;10:166–72. doi: 10.1158/1078-0432.ccr-0533-3. [DOI] [PubMed] [Google Scholar]
- 20.Kane SV, Gupta M, Kakade AC, D’Cruz A. Depth of invasion is the most significant histological predictor of subclinical cervical lymph node metastasis in early squamous carcinomas of the oral cavity. Eur J Surg Oncol. 2006;32:795–803. doi: 10.1016/j.ejso.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Goerkem M, Braun J, Stoeckli SJ. Evaluation of clinical and histomorphological parameters as potential predictors of occult metastases in sentinel lymph nodes of early squamous cell carcinoma of the oral cavity. Ann Surg Oncol. 2010;17:527–35. doi: 10.1245/s10434-009-0755-3. [DOI] [PubMed] [Google Scholar]
- 22.Troeltzsch M, Knösel T, Woodlock T, Troeltzsch M, Pianka A, Probst FA, et al. Are there clinical or pathological parameters of maxillary oral squamous cell carcinoma with an influence on the occurrence of neck node metastasis? An appraisal of 92 patients. J Oral Maxillofac Surg. 2016;74:79–86. doi: 10.1016/j.joms.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Kurokawa H, Yamashita Y, Takeda S, Zhang M, Fukuyama H, Takahashi T. Risk factors for late cervical lymph node metastases in patients with stage I or II carcinoma of the tongue. Head Neck. 2002;24:731–6. doi: 10.1002/hed.10130. [DOI] [PubMed] [Google Scholar]
- 24.Nagata T, Schmelzeisen R, Mattern D, Schwarzer G, Ohishi M. Application of fuzzy inference to European patients to predict cervical lymph node metastasis in carcinoma of the tongue. Int J Oral Maxillofac Surg. 2005;34:138–42. doi: 10.1016/j.ijom.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Akhter M, Hossain S, Rahman QB, Molla MR. A study on histological grading of oral squamous cell carcinoma and its co-relationship with regional metastasis. J Oral Maxillofac Pathol. 2011;15:168–76. doi: 10.4103/0973-029X.84485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moons KG, Harrell FE, Steyerberg EW. Should scoring rules be based on odds ratios or regression coefficients? J Clin Epidemiol. 2002;55:1054–5. doi: 10.1016/s0895-4356(02)00453-5. [DOI] [PubMed] [Google Scholar]


