Abstract
Background:
Mucins are high-molecular-weight glycoproteins and are implicated in diverse biological functions. MUC4, a member of transmembrane mucin family, is expressed in airway epithelial cells and body fluids such as the saliva, tear film, ear fluid and breast milk. In addition to its normal expression, an aberrant expression of MUC4 has been reported in a variety of carcinomas. Till date, very few studies have discussed about MUC4 expression in normal and cancerous oral mucosa.
Aim:
The aim of the study is to evaluate the expression of MUC4 in varying grades of oral squamous cell carcinoma (OSCC) and also to analyze its role played in oral carcinogenesis.
Materials and Methods:
Formalin-fixed paraffin-embedded tissues of five cases of normal tissue, ten cases of well-differentiated OSCC, ten cases of moderately differentiated OSCC and ten cases of poorly differentiated OSCC were retrieved from the archives of the department, and MUC4 antigen was immunohistochemically localized.
Statistical Analysis:
The result was subjected to statistical analysis using Pearson's Chi-square test and one-way analysis of variance.
Results:
About 70% of OSCC were stained positive with MUC4 antigen. Maximum intensity of staining was noted in well-differentiated OSCC. A steady decrease in MUC4 staining was noted with the increase in histological grading of OSCC.
Conclusion:
The findings of the study suggest that MUC4 plays a vital role in the pathogenesis of OSCC and can be regarded as a novel marker for OSCC.
Keywords: MUC4, oral squamous cell carcinoma, transmembrane
INTRODUCTION
Oral cancer accounts for 2%–4% of all cancer cases worldwide. It includes a group of neoplasms affecting any region of the oral cavity. However, this term tends to be used interchangeably with oral squamous cell carcinoma (OSCC), which accounts for 90% of all oral neoplasms.[1]
Mucins are high-molecular-weight glycoproteins that play a major role in growth, differentiation and signaling of a cell. Based on the structural properties, they are classified into gel-forming, membrane-bound/transmembrane and soluble mucins.[2] MUC4 is a membrane-bound mucin mapped on chromosome 3q29. The largest and distinct feature of it is its extracellular tandem repeat domain, comprising a stretch of 16 amino acids repeated in tandem up to 500 times.[3]
An aberrant expression of MUC4 is seen in various human cancers including breast, pancreas, lung and salivary gland and squamous cell carcinoma of the oral cavity, esophagus and cervix. The cancer cells use mucin for cell proliferation, survival, invasion and protection against innate immunity.[4] The present study aimed to evaluate the expression of MUC4 in varying grades of OSCC and also to analyze its role played in oral carcinogenesis.
MATERIALS AND METHODS
The study was conducted in the Department of Oral and Maxillofacial Pathology and Microbiology, I.T.S Dental College, Hospital and Research Centre, Greater Noida, Delhi-NCR, using archival retrieved formalin-fixed paraffin-embedded biopsy specimens of histopathologically diagnosed ten cases each of varying grades of OSCC which were submitted for histopathology. Detailed history of all these patients related to habits and duration was also retrieved from the department. Five cases of normal tissues from volunteers with no oral lesions and related oral habits were obtained and processed in the similar way as the pathological specimens.
Immunohistochemistry method
All tissue sections (4 μm) were cut and taken on poly-L-lysine-coated slides. Sections were air-dried at room temperature. The sections were deparaffinized by heating on the slide-warming table at 60°C for 15–20 min and then passed through two changes of xylene for 5 min each. Sections were rehydrated by taking them through three changes of 100% alcohol for 10 min each, followed by 90% and 70% alcohol for 10 min each. Sections were then brought down to phosphate-buffered saline (PBS) wash for 5 min. The deparaffinized tissues were placed in tanks containing citrate buffer (retrieval solution) at pH 6.2–6.4 and brought to a boil in an EZ antigen retrieval machine in two cycles: Cycle I – 85°C for 5 min and Cycle II – 98°C for 10 min. They were cooled for 30-45min and washed in PBS (3 × 3 changes). Sections were treated with peroxide block for 10 min and then washed in PBS (3 × 3 changes). Sections were treated with power block for 10 min. Sections were treated with primary antibody (MUC4) for 50–60 min and again washed with PBS (3 × 3 changes). The sections were then treated with super-enhancer for 25 min. The sections were again washed with PBS (3 × 3 changes) and then treated with SS-labeled poly-horseradish peroxidase substrate (secondary antibody) for 30 min and again washed with PBS (3 × 3 changes). Sections were covered with freshly prepared substrate chromogen solution for 10 min. The slides were then washed with PBS and counterstained in Harris hematoxylin for 30 s to 1 min. The sections were dehydrated, dipped in xylene and were later mounted using (Di-N-butyle phthalate in xylene) DPX, a nonaqueous permanent mounting medium. Adenocarcinoma of the colon was used as positive control [Figures 1-6].
Figure 1.
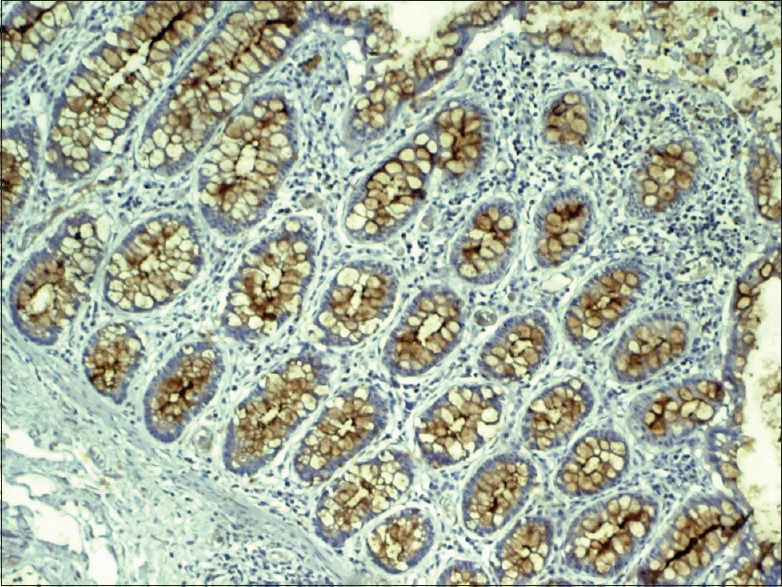
Photomicrograph showing membrane and cytoplasmic staining of MUC4 in adenocarcinoma of the colon
Figure 6.
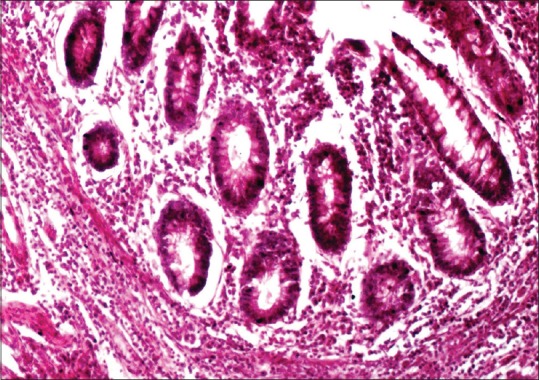
The corresponding (H and E section, ×10)
Interpretation of staining
The membrane and cytoplasm were stained brown against a blue background in the MUC4 antibody-positive cells. The staining pattern in colon carcinoma was used as the standard to interpret the study sections [Figure 1]. The staining was graded as mild when <25% of the cells stained positive, moderate when 25%–50% of the cells stained positive and intense when >50% of the cells stained positive. The staining pattern was statistically analyzed using SPSS software version 21 (IBM, New Delhi). Statistical analysis was performed using Pearson's Chi-square test, and an intergroup analysis was performed using one-way analysis of variance (ANOVA).
OBSERVATIONS AND RESULTS
The study comprised ten cases each of varying grades of OSCC. Eight of ten cases of well-differentiated OSCC (WDOSCC) stained positive for MUC4 expression [Figures 2a and 7a]. Five of eight cases exhibited intense membrane and cytoplasmic staining, whereas three of eight cases exhibited mild membrane and cytoplasmic staining. Two of ten cases showed the absence of MUC4 expression [Figures 2b and 7b].
Figure 2.
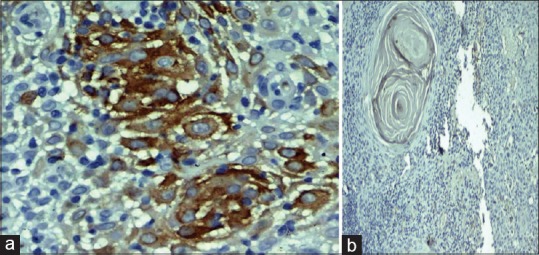
(a) Photomicrograph showing intense membrane and cytoplasmic staining of MUC4 in the keratin pearl of well-differentiated squamous cell carcinoma (IHC, ×40). (b) Absence of MUC4 expression in the keratin pearl of well-differentiated squamous cell carcinoma (IHC, ×40)
Figure 7.
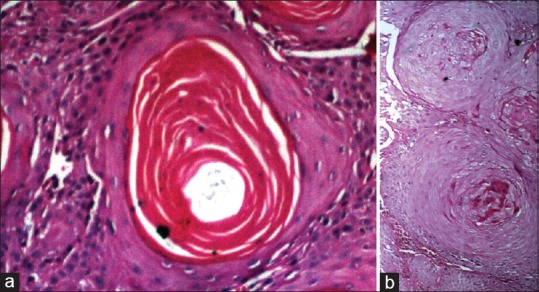
(a and b) The corresponding (H and E section, ×40)
In moderately differentiated OSCC (MDOSCC), seven of ten cases stained positive for MUC4 expression [Figures 3a and 8a]. Four of seven cases exhibited moderate membrane and cytoplasmic staining, two of seven cases exhibited mild membrane and cytoplasmic staining and one of the cases exhibited intense membrane and cytoplasmic staining. Three of ten cases showed the absence of MUC4 expression [Figures 3b and 8b].
Figure 3.
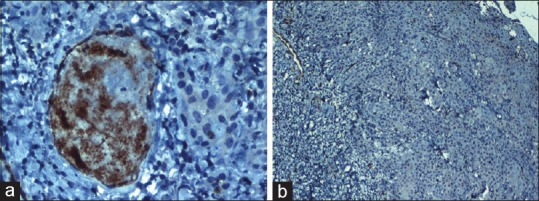
(a) Photomicrograph showing moderate membrane and cytoplasmic staining of MUC4 in moderately differentiated squamous cell carcinoma (IHC, ×40). (b) Absence of MUC4 expression in moderately differentiated squamous cell carcinoma (IHC, ×40)
Figure 8.
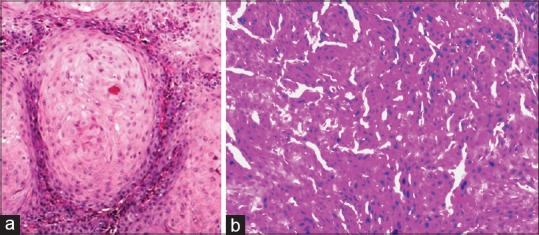
(a and b) The corresponding (H and E section, ×40)
In poorly differentiated OSCC (PDOSCC), five of ten cases stained positive for MUC4 expression [Figures 4a and 9a]. Three of five cases exhibited mild membrane and cytoplasmic staining and two of five cases exhibited moderate membrane and cytoplasmic staining. Five of ten cases showed the absence of MUC4 expression [Figures 4b and 9b].
Figure 4.
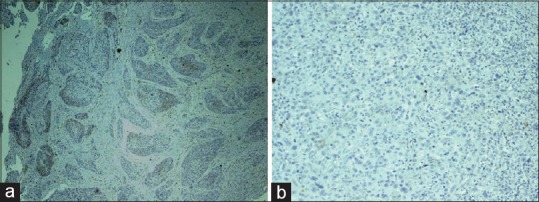
(a) Photomicrograph showing poor membrane and cytoplasmic staining of MUC4 in poorly differentiated squamous cell carcinoma (IHC, ×10). (b) Absence of MUC4 expression in poorly differentiated squamous cell carcinoma (IHC, ×10)
Figure 9.
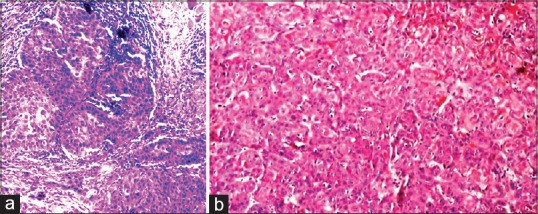
(a) The corresponding (H and E section, ×40). (b) The corresponding (H and E section, ×10)
The staining pattern among varying grades of OSCC was statistically significant (χ2 = 13.345, DF = 6 and P = 0.015).
One-way ANOVA test was applied for the intra- and intergroup assessment of the expression of MUC4 in varying grades of OSCC where it was found to be statistically significant (P = 0.041) [Figure 5].
Figure 5.
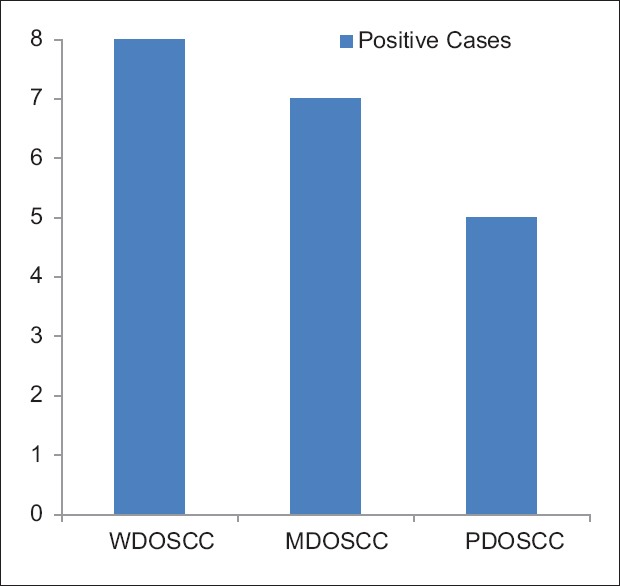
Bar diagram showing decreased expression of MUC4 with increased severity of oral squamous cell carcinoma
DISCUSSION
Neoplasms of diverse cellular origin arise in the oral cavity, and among these, OSCC arising from the mucosa of the oral cavity constitutes to over 90%.[5] Mucins are large glycosylated proteins and engage themselves in morphogenetic signal transduction pathways at the epithelial surface. They play a vital role in the carcinogenesis, which has been proved by its aberrant expression in various human cancers.[6] The membrane and cytoplasm staining of MUC4 in the squamous cells corresponds to its transmembrane and cytoplasmic subunits, respectively. The transmembrane segment anchors it to the cell surface, whereas the cytoplasmic tail participates in signal transduction pathways.[4]
The role of MUC4 differs significantly under normal and pathological conditions. In normal conditions, there is a controlled interaction between neighboring cells and the cell and the extracellular matrix. Whereas during cancer development such interactions are interrupted, while the novel interactions occur as a result of alterations in the cell surface proteins, extracellular matrix composition and loss of cell polarity. All these molecular and cytoarchitectural changes create a favorable environment for tumor progression.[2]
The multiple roles of MUC4 include its ability to modulate the function of other adhesion-associated signaling molecules through steric hindrance.[7] It has been shown that rMUC4 physically interacts with the receptor tyrosine kinase ErbB2, induces limited phosphorylation leading to an upregulation of the cyclin-dependent kinase inhibitor p27kip and did not activate protein kinase B/Akt pathways. In contrast, in the presence of neuregulin, activation of both neuregulin receptor (ErbB3) and ErbB2 was seen, which was further potentiated by rMuc4 and led to the downregulation of the p27kip and an enhanced activation of extracellular-regulated kinase (ERK) and Akt pathways. These changes in signaling pathways facilitated the cell cycle progression.[7,8]
A couple of studies have reported the expression of MUC4 in cancer such as Chu et al. who demonstrated an overexpression of ErbB2 in OSCC. An increased expression of ErbB2 along with its ligand MUC4 thus feeds the squamous cells with continuous growth signals that are transmitted to the nucleus through its cytoplasmic tail imparting limitless replicating potential to the tumor cells.[9] Bafna et al. demonstrated an increased cellular proliferation and decreased apoptosis in MUC4 overexpressing mouse fibroblast cells. According to the Authors, (MUC4-ErbB2- Grb2/sos-Ras-Raf1-MEK-ERK1/2) pathway caused oncogenic transformation in mouse fibroblasts cells and an increased expression of Cox 3 and ND1 genes caused a depression in apoptosis.[10]
In the current study, MUC4 positivity in the OSCC samples was highly restricted to the keratin pearls and well-differentiated areas of the tumors [Figure 2]. A decrease in positivity of MUC4 was noted in MDOSCC and PDOSCC [Figure 5] compared with WDOSCC cases, which was in accordance with a study done by Donald et al., in which he demonstrated a significant decrease in MUC4 expression with an increase in the histological grade of the OSCC, thus confirming the association of MUC4 with the well-differentiated cells of OSCC.[11]
Thus, a decrease in the expression of MUC4 in MDOSCC and PDOSCC might be attributed to the inability of the less-differentiated squamous cells to express MUC4 compared with that of the well-differentiated cells of OSCC. This finding of our study is consistent with a study conducted by Guillem et al. in esophageal squamous cell carcinoma using immunohistochemistry and northern blot analysis.[12]
CONCLUSION
The findings of this study have provided us with supportive evidence to consider MUC4 as a novel marker for tumor cell differentiation and thus determine the prognosis of OSCC.
Further studies are required that would help in establishing MUC4 as a diagnostic and prognostic marker.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Markopoulos AK. Current aspects on oral squamous cell carcinoma. Open Dent J. 2012;6:126–30. doi: 10.2174/1874210601206010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaturvedi P, Singh AP, Batra SK. Structure, evolution, and biology of the MUC4 mucin. FASEB J. 2008;22:966–81. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamada T, Wakamatsu T, Miyahara M, Nagata S, Nomura M, Kamikawa Y, et al. MUC4: A novel prognostic factor of oral squamous cell carcinoma. Int J Cancer. 2012;130:1768–76. doi: 10.1002/ijc.26187. [DOI] [PubMed] [Google Scholar]
- 4.Narashiman S, Narasimhan M, Venkatraman G. Expression of mucin 4 in leukoplakia and oral squamous cell carcinoma: An immunohistochemical study. J Oral Maxillofac Pathol. 2014;18:25–31. doi: 10.4103/0973-029X.131887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalu UE, Christopher B, editors. Oral cancer. Intech open. (1st ed) 2012:48. [Google Scholar]
- 6.Hollingsworth MA, Swanson BJ. Mucins in cancer: Protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 7.Carraway KL, Theodoropoulos G, Kozloski GA, Carothers Carraway CA. Muc4/MUC4 functions and regulation in cancer. Future Oncol. 2009;5:1631–40. doi: 10.2217/fon.09.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramsauer VP, Pino V, Farooq A, Carothers Carraway CA, Salas PJ, Carraway KL, et al. Muc4-ErbB2 complex formation and signaling in polarized CACO-2 epithelial cells indicate that Muc4 acts as an unorthodox ligand for ErbB2. Mol Biol Cell. 2006;17:2931–41. doi: 10.1091/mbc.E05-09-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu F, Feng Q, Qian Y, Zhang C, Fang Z, Shen G, et al. ERBB2 gene amplification in oral squamous cell malignancies: A correlation with tumor progression and gene expression. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:90–5. doi: 10.1016/j.tripleo.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 10.Bafna S, Singh AP, Moniaux N, Eudy JD, Meza JL, Batra SK, et al. MUC4, a multifunctional transmembrane glycoprotein, induces oncogenic transformation of NIH3T3 mouse fibroblast cells. Cancer Res. 2008;68:9231–8. doi: 10.1158/0008-5472.CAN-08-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weed DT, Gomez-Fernandez C, Yasin M, Hamilton-Nelson K, Rodriguez M, Zhang J, et al. MUC4 and ErbB2 expression in squamous cell carcinoma of the upper aerodigestive tract: Correlation with clinical outcomes. Laryngoscope. 2004;114:1–32. doi: 10.1097/00005537-200408001-00001. [DOI] [PubMed] [Google Scholar]
- 12.Guillem P, Billeret V, Buisine MP, Flejou JF, Lecomte-Houcke M, Degand P, et al. Mucin gene expression and cell differentiation in human normal, premalignant and malignant esophagus. Int J Cancer. 2000;88:856–61. doi: 10.1002/1097-0215(20001215)88:6<856::aid-ijc3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]


