Abstract
Background:
Recently, studies have concentrated on complementary medicine in treating a large number of diseases, including cancer. Unfortunately, many of these treatment methods do not provide a permanent solution, and even if they do, many do not have a scientific corroboration. A shift toward alternative medicine and natural methods is being preferred to reduce if not counter the toxic effects of the drugs that are used to treat such diseases. Studies have shown that amygdalin is a natural plant product to have an anticarcinogenic effect on many types of cancers. However, the effect of amygdalin on oral cancers has not been studied, and hence, the present study aimed to evaluate the anticarcinogenic effect of amygdalin extracted from apricots and almonds on human oral cancer cell lines.
Materials and Methods:
Oral cancer cell lines (KB cell line) were used in the present study. Ethanolic extracts of apricots and almonds were prepared using Soxhlet extraction method. The antiproliferative and cytotoxic activity on KB cell line was evaluated using 3-(4,5-dimethylthiazol-2-YL)-2,5-diphenyltetrazolium bromide assay.
Results:
Both the extracts exhibited cytotoxic and antiproliferative activity on KB cell lines. While almonds exhibited maximum efficacy at 50 μg/mL, apricots extract required 100 μg/mL concentrations.
Conclusion:
Ethanolic extracts of amygdalin from almonds and apricots were effective as an antiproliferative agent which caused apoptosis in oral cancer cell line.
Keywords: Almonds, amygdalin, apricots, oral cancer, squamous cell carcinoma
INTRODUCTION
Squamous cell carcinoma (SCC) is the most common type of oral cancer with increased mortality and morbidity rates.[1,2] Globally, it is the 6th most frequent cancer[3] with highest incidence and prevalence in the Indian subcontinent.[1] Various risk factors such as tobacco, betel quid, areca nut and human papillomavirus have been implicated in the etiology of oral cancer.[4] Despite advances in conventional treatments such as surgery, radiotherapy and chemotherapy, problems related to these therapies such as side effects, opportunistic infections and the development of drug resistance remained unsolved.[5,6] Therefore, the need of the hour is to develop novel treatment modalities using plant derivatives which act as effective therapeutic agents and have minimal side effects.
Amygdalin is one such natural anticancerous plant product of glycosides and found in the seeds of sweet almond, apricots, blackberries, peaches, apples and other plants.[7] It is composed of two molecules of glucose, one molecule of benzaldehyde and one molecule of hydrocyanide. Studies have shown that the benzaldehyde in amygdalin is able to induce an analgesic effect, and the hydrocyanide in amygdalin is able to induce an anticancerous effect.[8]
Many studies have shown that amygdalin can inhibit the proliferation of prostate cancer,[8] cervical cancer,[9] liver cancer,[10] bladder cancer,[11] non-small cell lung cancer,[12] colon cancer[13] and SCC in buccal pouch of hamsters[14] by promoting apoptosis.
The anticancer effect and mechanism of amygdalin in oral cancer has never been reported. The present research was designed to study the apoptotic effect of amygdalin on human oral cancer cells in vitro to provide a rationale for its use in the treatment of oral cancer.
Objectives
To evaluate the antiproliferative activity of amygdalin obtained from extracts of apricot and almonds on oral SCC cell line (KB mouth cell line)
To compare the effectiveness between apricots and almonds.
MATERIALS AND METHODS
Preparation of extracts
Fresh almonds and apricots were washed with distilled water and air-dried and finely powdered. The extracts were prepared according to Othman et al.[15] Fifty grams of the prepared powder was mixed to thrice the volume of ethanol (i.e., 150 mL). Soxhlation was carried out for 6 h. The ethanolic extract was evaporated using a Rotary Evaporator (BUCHI, German Company) at 60°C. The residue was collected and stored at −20°C until further use. Five concentrations (10, 50, 100, 150 and 200 μg/mL) of extracts were prepared. A similar procedure was carried out for apricots.
Cell line and cell culture
Oral SCC cell line (KB mouth cell line) produced from the National Centre for Cell Sciences, Pune, India, was used for the study. Human oral cancer cell line (KB mouth cell line) was seeded into a 96-well microtiter plate containing Minimum Essential Medium Eagle supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin. The cells were maintained in a CO2 incubator of 5% humidity at 37°C for 48–72 h.
Activity of extracts on oral cancer cell line
Oral SCC cell lines (KB mouth cell line) were seeded into 96-well plates at a density of 5 × 104 cells/well. After 24 h, the cells were treated with prepared apricot and almond extracts at five concentrations (10, 50, 100, 150 and 200 μg/mL) diluted with dimethyl sulfoxide (DMSO) and incubated for 24–48 h. The proliferation activity of cell population under different concentrations was determined based on the mitochondrial dehydrogenase activity in the living cells.
Cell viability assay
Cell viability assay was done to determine the sublethal concentrations of inhibitory concentration (IC50) of extracts and proliferative activity of the cells in the presence of extracts. After completion of incubation period, working solution 10 μl of 3-(4,5-dimethylthiazol-2-YL)-2,5-diphenyltetrazolium bromide was added to each culture well to detect the cell viability. The color was allowed to develop for an additional 4 h of incubation. Equal volume of DMSO was added to stop this reaction to solubilize the crystals. Absorbance was recorded at 570 nm using ultraviolet-visible spectrophotometer.[16]
RESULTS
Both almond and apricot extracts showed antitumor activity on KB cell line. The viability decreased in a dose-dependent manner. The cell viability decreased as the concentration of the extracts increased.
Almond exhibited maximum efficacy at 50 μg/mL by killing 78% of cells, whereas apricot required a concentration of 100 μg/mL to attain 82% of activity [Figures 1-3]. The IC50 values were also calculated, and almond and apricot extracts showed a maximum IC50 at 32 and 61 μg/mL, respectively, showing that the sublethal cytotoxic effect (IC50) was twice as better in almond extract than apricot extract, but the apricot extract showed maximum cytotoxic efficacy than almond extract.
Figure 1.
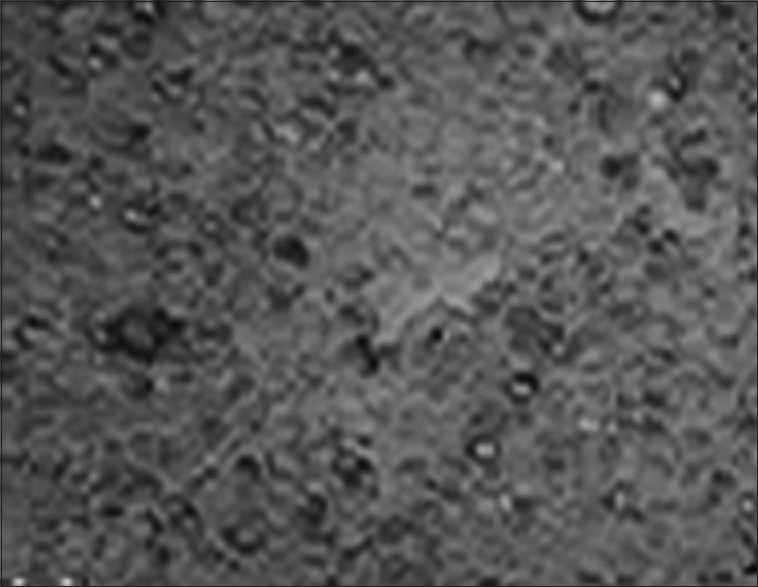
Oral cancer cell line (KB cell line) – control
Figure 3.
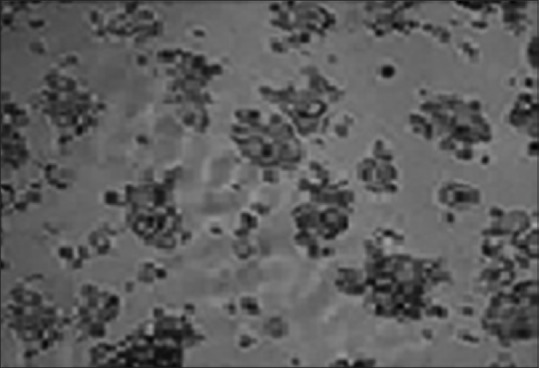
Apricot extract showing inhibition of cell growth at 100 μg/mL
Figure 2.
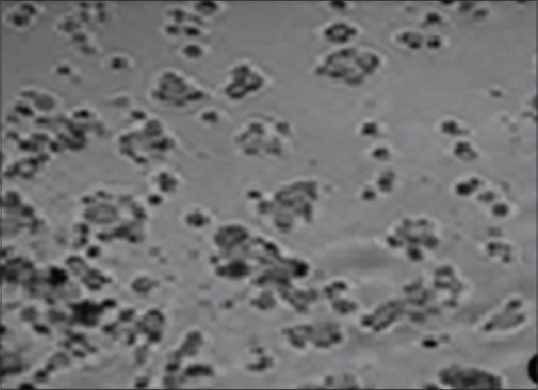
Almond extract showing inhibition of cell growth at 50 μg/mL
DISCUSSION
In the 1970s, amygdalin was one of the most common nonconventional anticancer drugs in Western countries.[12] However, the antitumor efficacy and mechanisms of action of amygdalin have remained controversial since then. Some recent studies provide positive evidence to resolve the controversy. Studies have shown that the hydrocyanic acid present in amygdalin could serve as an anticancerous agent by decomposing carcinogenic substances in the body, killing cancer cells, blocking the nutrient source of tumor cells and inhibiting cancer cell growth.[17]
Recently, the antitumor mechanism of amygdalin was tested in various cancers. Kwon et al. confirmed that amygdalin can induce apoptosis in human promyelocytic leukemia-60 cells.[18] Park et al. have shown that amygdalin inhibited the proliferation of human colon cancer SNU-C4 cell, by inhibiting the expression of cell cycle-related genes.[13] Chang et al. identified that amygdalin can induce apoptotic cell death in human DU145 and LNCaP prostate cancer cells by caspase-3 activation through downregulation of Bcl-2, an antiapoptotic protein, and upregulation of bax, a pro-apoptotic protein.[8] Chen et al. also observed that amygdalin could induce apoptosis in human cervical cancer cell line HeLa cells by caspase-3 activity.[9] Another study by Qian et al. showed that amygdalin inhibited the in vitro proliferation, invasion and migration of non-small cell lung cancer cells by regulating integrin and E-cadherin expression and the downstream key signaling pathways such as Akt-mTOR.[12] An in vitro study by Makarević et al. explained the effect of amygdalin in blocking bladder cancer through diminishing cyclin A and cyclin-dependent kinase 2, the cell cycle regulating proteins in cancer cells.[11]
Ours was a preliminary in vitro study wherein we evaluated the effectiveness of amygdalin as a cytotoxic agent for the first time against human oral cancer cells. There was an overwhelmingly positive response of amygdalin-rich almond and apricot extract on oral cancer cells. Almond exhibited maximum efficacy at 50 μg/mL by killing 78% of cells, whereas apricot exhibited at 100 μg/mL with 82% of activity. Sublethal cytotoxic effect (IC50) was twice better in almond extract than apricot extract, but apricot extract showed maximum cytotoxic efficacy than almond extract. The cytotoxic effect was mainly because of the apoptosis caused by the release of secondary metabolite hydrocyanide.
Studies have shown that the presence of β-glucosidase in cancer cells is responsible for the breakdown of amygdalin to release hydrocyanide and to exert cytotoxicity on cancer cells[19,20,21] as reflected in our study. Some studies suggest that the presence of rhodanese in normal cells has the ability to detoxify cyanide. The combined action of the two enzymes may be responsible for inducing cyanide-related toxicity in cancer cells treated with amygdalin while normal cells remain undamaged.[13,22]
A study by Nour et al. revealed that amygdalin causes apoptosis of SCC cells, induced in the buccal pouch of hamster by downregulating the expression of P53 and reducing the rate of mitotic index (Ki67).[14] Because most of the human oral cancers are squamous cell in origin, this could be implied to our study also. However, more studies are required to find the exact cytotoxic effect of amygdalin on human oral cancer cells.
CONCLUSION
Based on the results of our study and literature review, amygdalin extracted from the seeds of almonds and apricots showed cytotoxic effect on human oral cancer cell lines. However, controlled clinical trials are required to further substantiate the anticancer property of amygdalin on oral cancer.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We acknowledge the assistance of our laboratory technician Mr. Padmanabha Reddy.
REFERENCES
- 1.Attar E, Dey S, Hablas A, Seifeldin IA, Ramadan M, Rozek LS, et al. Head and neck cancer in a developing country: A population-based perspective across 8 years. Oral Oncol. 2010;46:591–6. doi: 10.1016/j.oraloncology.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagan J, Sarrion G, Jimenez Y. Oral cancer: Clinical features. Oral Oncol. 2010;46:414–7. doi: 10.1016/j.oraloncology.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Shah JP, Gil Z. Current concepts in management of oral cancer – Surgery. Oral Oncol. 2009;45:394–401. doi: 10.1016/j.oraloncology.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petti S. Lifestyle risk factors for oral cancer. Oral Oncol. 2009;45:340–50. doi: 10.1016/j.oraloncology.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The functional assessment of cancer therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–9. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 6.Islam M, Datta J, Lang JC, Teknos TN. Down regulation of RhoC by microRNA-138 results in de-activation of FAK, Src and Erk1/2 signaling pathway in head and neck squamous cell carcinoma. Oral Oncol. 2014;50:448–56. doi: 10.1016/j.oraloncology.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirmiranpour H, Khaghani S, Zandieh A, Khalilzadeh OO, Gerayesh-Nejad S, Morteza A. Amygdalin inhibits angiogenesis in the cultured endothelial cells of diabetic rats. Indian J Pathol Microbiol. 2012;55:211–4. doi: 10.4103/0377-4929.97874. [DOI] [PubMed] [Google Scholar]
- 8.Chang HK, Shin MS, Yang HY, Lee JW, Kim YS, Lee MH, et al. Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expressions in human DU145 and LNCaP prostate cancer cells. Biol Pharm Bull. 2006;29:1597–602. doi: 10.1248/bpb.29.1597. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Ma J, Wang F, Hu J, Cui A, Wei C, et al. Amygdalin induces apoptosis in human cervical cancer cell line HeLa cells. Immunopharmacol Immunotoxicol. 2013;35:43–51. doi: 10.3109/08923973.2012.738688. [DOI] [PubMed] [Google Scholar]
- 10.Yang C, Li X, Rong J. Amygdalin isolated from Semen Persicae (Tao Ren) extracts induces the expression of follistatin in HepG2 and C2C12 cell lines. Chin Med. 2014;9:23. doi: 10.1186/1749-8546-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makarević J, Rutz J, Juengel E, Kaulfuss S, Reiter M, Tsaur I, et al. Amygdalin blocks bladder cancer cell growth in vitro by diminishing cyclin A and Cdk2. PLoS One. 2014;9:e105590. doi: 10.1371/journal.pone.0105590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian L, Xie B, Wang Y, Qian J. Amygdalin-mediated inhibition of non-small cell lung cancer cell invasion in vitro . Int J Clin Exp Pathol. 2015;8:5363–70. [PMC free article] [PubMed] [Google Scholar]
- 13.Park HJ, Yoon SH, Han LS, Zheng LT, Jung KH, Uhm YK, et al. Amygdalin inhibits genes related to cell cycle in SNU-C4 human colon cancer cells. World J Gastroenterol. 2005;11:5156–61. doi: 10.3748/wjg.v11.i33.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nour A, Azar B, Rabata A, Manadili A. The effect of amygdalin in the treatment of squamous cell carcinoma induced in the buccal pouch of golden Syrian hamster. IOSR J Dent Med Sci. 2016;15:75–9. [Google Scholar]
- 15.Othman M, Loh HS, Wiart C, Khoo TJ, Lim KH, Ting KN. Optimal methods for evaluating antimicrobial activities from plant extracts. J Microbiol Methods. 2011;84:161–6. doi: 10.1016/j.mimet.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Alan D, Brayan G. England: John Wiley and Sons Ltd; 2000. Cell quantification – An overview – MTT assay. Cell and Tissue Culture for Medical Research; p. 49. [Google Scholar]
- 17.Song Z, Xu X. Advanced research on anti-tumor effects of amygdalin. J Cancer Res Ther. 2014;10(Suppl 1):3–7. doi: 10.4103/0973-1482.139743. [DOI] [PubMed] [Google Scholar]
- 18.Kwon HY, Hong SP, Hahn DH, Kim JH. Apoptosis induction of Persicae Semen extract in human promyelocytic leukemia (HL-60) cells. Arch Pharm Res. 2003;26:157–61. doi: 10.1007/BF02976663. [DOI] [PubMed] [Google Scholar]
- 19.Dorr RT, Paxinos J. The current status of laetrile. Ann Intern Med. 1978;89:389–97. doi: 10.7326/0003-4819-89-3-389. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg DM. The case against laetrile: The fraudulent cancer remedy. Cancer. 1980;45:799–807. doi: 10.1002/1097-0142(19800215)45:4<799::aid-cncr2820450432>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Herbert V. The nutritionally and metabolically destructive “nutritional and metabolic antineoplastic diet” of laetrile proponents. Am J Clin Nutr. 1979;32:96–8. doi: 10.1093/ajcn/32.1.96. [DOI] [PubMed] [Google Scholar]
- 22.Newmark J, Brady RO, Grimley PM, Gal AE, Waller SG, Thistlethwaite JR. Amygdalin (Laetrile) and prunasin beta-glucosidases: Distribution in germ-free rat and in human tumor tissue. Proc Natl Acad Sci U S A. 1981;78:6513–6. doi: 10.1073/pnas.78.10.6513. [DOI] [PMC free article] [PubMed] [Google Scholar]


