Abstract
Heme oxygenase-1 (HO-1) catalyzes the degradation of heme and forms antioxidant bile pigments as well as the signaling molecule carbon monoxide. HO-1 is inducible in response to a variety of chemical and physical stress conditions to function as a cytoprotective molecule. Therefore, it is important to maintain the basal level of HO-1 expression even when substrate availability is limited. We hypothesized that the HO-1 protein itself could regulate its own expression in a positive feedback manner, and that this positive feedback was important in the HO-1 gene induction in response to oxidative stress. In cultured NIH 3T3 cells, transfection of HO-1 cDNA or intracellular delivery of pure HO-1 protein resulted in activation of a 15kb HO-1 promoter upstream of luciferase as visualized by bioluminescent technology and increased HO-1 mRNA and protein levels. These effects were independent of HO activity because an enzymatically inactive mutant form of HO-1 similarly activated the HO-1 promoter and incubation with HO inhibitor metalloporphyrin SnPP did not affect the promoter activation. In addition, HO-1 specific siRNA significantly reduced hemin and cadmium chloride mediated HO-1 induction. Furthermore, deletion analyses demonstrated that the E1 and E2 distal enhancers of the HO-1 promoter are required for this HO-1 auto-regulation. These experiments document feed-forward auto-regulation of HO-1 in oxidative stress and suggest that HO-1 protein has a role in the induction process. We speculate that this mechanism may be useful to maintain HO-1 expression when substrate is limited and may also serve to up-regulate other genes to promote cytoprotection and to modulate cell proliferation.
Keywords: Heme Oxygenase-1, transcription regulation, protein delivery, promoter, siRNA, cell roliferation
INTRODUCTION
Heme oxygenase (HO) is the rate-limiting enzyme in heme degradation to biliverdin. This reaction generates equimolar concentrations of ferrous iron, biliverdin and carbon monoxide (CO). The inducible form, HO-1, is regulated by various stimuli such as heavy metals, inflammation, UV radiation and oxidative stress (1, 2, 3, Ricchetti, 2004 #490, 4). Many have suggested that HO-1 mediates its cytoprotective effects via the degradation of heme and the formation of the reaction byproducts, however, when substrate availability is limited, the cytoprotective functions of HO are difficult to explain and the induction of HO-1 should not occur yet it does. Enzymatically inactive HO-1 mutant protein is cytoprotective against hydrogen peroxide-induced oxidative stress (5). Furthermore, transfection of a cDNA encoding for an inactive HO-1 mutant protein increases catalase gene expression, suggesting a regulatory role for the HO-1 protein itself (5). Recent evidence documents nuclear localization of a truncated form of the HO-1 protein after cleavage of the membrane bound C-terminus (6). Nuclear extracts enriched with this form of HO-1 protein were devoid of normal HO activity. This further suggests that there is a role for the HO-1 protein itself in nuclear functions. Because HO-1 is readily inducible even when limited substrate is present, we hypothesized that HO-1 protein could serve to regulate its own expression. Recent studies showed that non-heme induced HO-1 does not alter cellular iron metabolism, further suggesting non-enzymatic roles of HO-1 in cytoprotection (7). Autoregulation has been demonstrated in various examples (8, 9) where this leads to amplification or maintenance of gene function. Kravets et al have also reported that biliverdin reductase (BVR), which catalyzes reduction of the HO activity product, biliverdin, to bilirubin, can induce the expression of HO-1 protein, suggesting that various components of the bilirubin system could regulate the expression of genes in the pathway (10).
Previous studies in rodents have identified key regulatory regions within the genomic sequence upstream of the HO-1 gene. Distal enhancer (DE) 1 and DE2 located at −4kb and −10 kb upstream of the transcription start site respectively are critical for HO-1 induction by most stimuli, including heme, heavy metals, and hydrogen peroxide (11, 12). Both DE1 and DE2 regions contain multiple stress-responsive elements (StRE) that represent binding sites of regulatory proteins such as AP-1, Jun, CREB, Maf and the Cap’n’collar/basic-leucine zipper (CNC-bZIP) transcription factors. Among the CNC-bZIP proteins, the NF-E2 related factor-2 (Nrf2) functions as a transcriptional activator of HO-1 promoter whereas Bach-1 serves as negative regulator of HO-1 transcription (13–15). Several binding partners have been reported to interact with Nrf2 and Bach-1 in response to different cellular stimulations. It is conceivable that transcription regulatory protein complexes composed of multiple factors are crucial to the regulation of HO-1 expression. MAPK signaling pathways have been shown to be activated during HO-1 inductions including after ischemia-reperfusion lung injury (16). It is important to test whether the MAPK pathways, including p38 and ERK signaling, are involved in the HO-1 self regulation.
In this study, we evaluated whether transfection with HO-1 cDNA or intracytoplasmic delivery of HO-1 protein could modulate the activation of a 15kb mouse HO-1 promoter upstream of luciferase. By comparing the effects of a wild type (active) and inactive mutant HO-1 protein, we determined whether HO enzymatic activity was required to mediate these effects. We also assessed the role of HO-1 protein on enhancing HO-1 gene transcription resulting from common HO-1 inducers such as hemin and cadmium chloride. Furthermore, we have identified the genomic regions in the HO-1 promoter that respond to HO-1 protein and the essential domains within the HO-1 protein required for the self-activation. Lastly, we examined the activation of MAPK signaling pathway in the process of this HO-1 self-regulation.
EXPERIMENTAL PROCEDURES
Cell Culture
NIH 3T3 cells were obtained from American Type Culture Collection (Manassas, VA). NIH3T3 cells stably transfected with a 15kb HO-1 promoter-luciferase construct (3T3-HO-1/luc cells) were grown in Dulbecco's minimal essential medium (DMEM; Life Technologies, Rockville, MD) supplemented with 10% FBS and 1% antibiotic-antimycotic.
HO-1 cDNA constructs
Full-length rat HO-1 cDNA was amplified by PCR with primers 5’- AGGATCCAGATGGAGCGCCCACAG and 3’- GACTCGAGCCAGATCCTCTTCTGAGATG. The PCR product was cloned into the BamHI/XhoI site of pcDNA3.1/His (Invitrogen) to generate HisHO1myc construct. The HisHO1myc fragment was then subcloned into p3XFLAGCMV (Sigma) to generate HisHO1FLAG. A rat HO-1 cDNA (HisHO1mutFLAG) expressing an enzymatically inactive HO-1 with a substitution of Histidine 25 to Alanine was derived from the HisHO1FLAG by PCR using a site-directed mutagenesis kit from Stratagene (QuickChange II, catalog # 200523-4).
Transient transfection with HO-1 cDNA
Transient transfection was performed in NIH3T3 cells using Lipofectamine 2000 (Invitrogen). Briefly, one day before transfection, 105 cells were seeded in 24-well plates with antibiotics free growth medium. DNA (0.8 µg) and 2 µl of lipofectamine 2000 reagent were diluted into 50 µl of DMEM separately. After 5 minutes incubation, the DNA and lipofectamine solution were mixed and incubated for additional 20 minutes at room temperature. The DNA/Lipofectamine complex was then added to the cells. Cells were grown for 48 hours before being subjected to assays.
Intracytoplasmic delivery of HO-1 Protein
A GST HO-1 fusion construct was expressed in the E.coli strain BL21 (Invitrogen.). Bacteria were grown to an OD of 0.6–0.8. Thereafter, the fusion protein was induced with 100 µM IPTG at 30°C. After 5 hrs, bacteria were harvested and sonicated for 5 minutes. The fusion protein was purified using a GST-purification module (Amersham Biosciences, Piscataway, NJ) according to the manufacturer’s instruction. Ten μg of GST HO-1 protein was delivered in the 3T3-HO-1/luc cells using the Pro-Ject™ System (Pierce, Rockford, IL, USA) as per the manufacturer. Briefly, the Pro-Ject™ reagent was solubized in 250µl methanol. The methanol was evaporated overnight under a sterile hood and the dried Pro-Ject was stored at −20°C until use. Purified HO-1 protein was diluted in PBS and added to the dried Pro-Ject reagent. After 5 minutes incubation, the mixture was added to the cell culture medium with 5% serum. After 3 h, cells were washed with PBS, and maintained in serum-free medium for the first 4 h of the incubation. Thereafter, 5% FBS was reincorporated. In other experiments, purified mutant HO-1 protein (His25Ala) devoid of catalytic activity (gift of Paul Ortiz de Montellano, UCSF, San Francisco, CA), was delivered to the cells as described above.
siRNA mediated gene knockdown of HO-1
Control siRNA and HO-1 specific siRNA oligos were purchased from Dharmacon, Inc. The sequences of the HO-1 siRNA were: 5'-AAGCCACACAGCACUAUGUAAdTdT-3' and 5'- UUACAUAGUGCUGUGUGGCUUdTdT-3'. siGLO Cyclophilin B siRNA (Cat # D-001610-01) was used as control siRNA. Briefly, 5×104 HO-1/luc cells were plated in each well of the 24-well dish one day prior to transfection. 100 nM of control and HO-1 siRNA were transfected into each well using Lipofectamine 2000 (Invitrogen) as transfection reagent. For each transfection, siRNA and Lipofectamine 2000 reagent were diluted with serum-free medium in separate tubes, followed by mixing and incubation at room temperature for 20 minutes. The mixture was added to each well of the HO-1/luc cells. After 72 hours of incubation, samples were assayed for HO-1 promoter activation and HO-1 protein levels.
Visualization of HO-1 promoter activation
After transfection or protein delivery, the 3T3-HO-1/luc cells were incubated with DMEM containing 1% luciferin for 5 min. The cultures were imaged using the IVIS camera system. (Xenogen, Alameda, CA). Pseudoimages of the photon emission were generated and light intensity was expressed as a ratio to cell counts, as previously described (17).
Determination of protein levels
Western blot analysis was performed as previously described (18) to evaluate HO-1 immunoreactive protein. Equal loading of 10µg of total cell lysate protein was verified with Coomassie blue staining and Western blot analysis using GAPDH or actin antibodies. Quantification of the protein signal was performed using densitometry (PDI, Sunnyvale, CA) and analyzed as HO-1to actin or HO-1 to GAPDH ratios.
HO activity assay
To determine whether HO activity was altered with delivery of HO-1 protein, CO production, as a marker of HO activity, was measured using gas chromatography as previously described (19).
Statistical Analysis
To allow for comparisons between treatment groups, the null hypothesis that there was no difference between treatment means was tested by a single factor analysis of variance for multiple groups or unpaired t test for two groups (Statview 4.02, Abacus Concepts, Inc., Berkeley, CA). Statistical significance (p < 0.05 or p<0.001) between and within groups was determined by means of the Fischer method of multiple comparisons.
RESULTS
HO activity inhibitor does not inhibit the HO-1 promoter activation by HO-1 protein
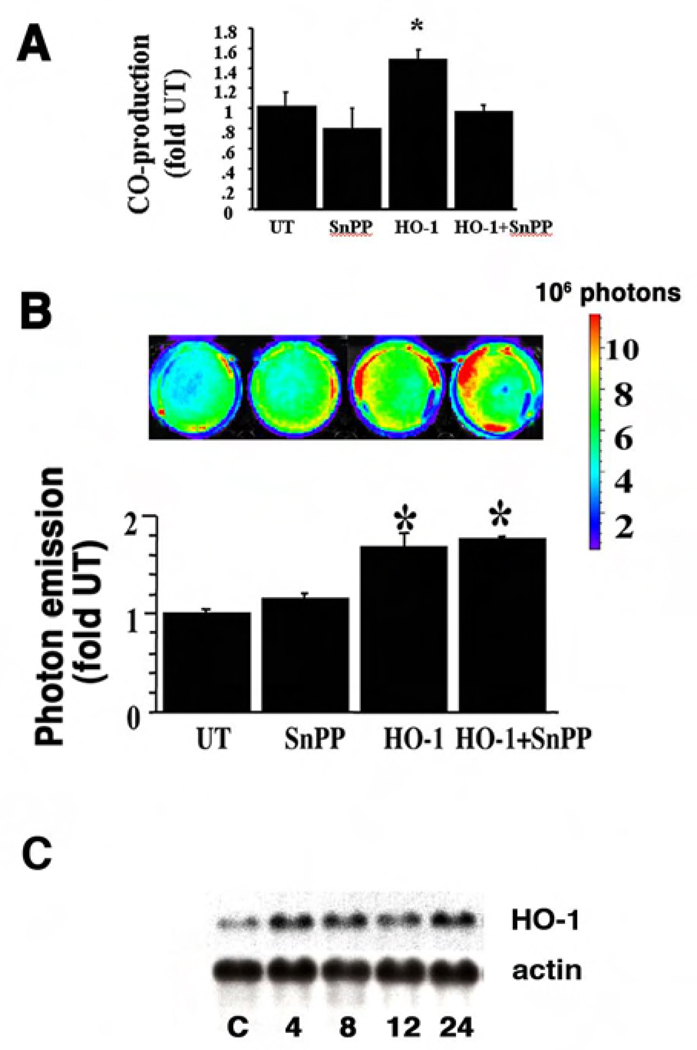
Previously we have published that under certain stress conditions such as hypoxia and hemin exposure, HO-1 protein can migrate into the nucleus and modulate gene transcription. HO-1 protein can also activate its own promoter in cultured cells (6). Many of the HO reaction byproducts have signaling effects (20–22). To understand whether the HO-1 byproducts or HO-1 protein itself activate its own transcription, cells were incubated with an inhibitor of HO-activity, tin protoporphyrin (SnPP, 20µM), prior to HO-1 protein delivery. We used NIH3T3 cells that were stably transfected with a luciferase reporter driven by a 15kb HO-1 promoter (3T3-HO-1/luc). Luciferase activity in these cells directly correlates with the HO-1 promoter activation and can be measured by the photon emission with In Vivo Imaging System (IVIS). Incubation of cells with SnPP resulted in a 30% inhibition in HO activity in both untransfected 3T3-HO-1/luc cells and after HO-1 protein delivery (Figure 1A).
Figure 1. Inhibition of HO activity did not affect the HO-1 promoter activation after HO-1 protein delivery.
Cells were pre-incubated with SnPP, a potent inhibitor of HO activity. (A) HO activity in the cells where HO-1 protein was delivered (HO-1) with or without SnPP. (B) A representative image of photon emission and quantitative assessment of light intensity is shown. Values are the mean ± standard error of four separate determinations. Values are expressed as a fold of untransfected (UT) controls. *P<0.05 vs. UT. (C) A representative Northern blot illustrating HO-1 mRNA steady state levels 4 to 24 h after delivery of HO-1 protein is shown. The housekeeping gene β-actin demonstrates equal loading. C: control cells receiving Pro-ject reagent alone.
We used Pro-ject, a lipid-based protein delivery system (Pierce), to deliver pure HO-1 protein intracytoplasmically into the 3T3-HO-1/luc cells and examined the effects on activation of the HO-1/luc reporter. A GST-HO-1 fusion protein was expressed and purified with a GST column followed by thrombin digestion to cleave the GST tag. Delivery of HO-1 protein was associated with increased HO-1 promoter activation in these cells. Compared to untreated cells, the maximum HO-1 promoter activation (1.7 fold) was observed 12 h after the HO-1 protein delivery (Figure 1B).
Despite the inhibition in HO activity, increased HO-1 promoter activation was observed after HO-1 protein delivery as in cells not pre-incubated with SnPP (Figure 1B). The loss of HO activity does not prevent HO-1 protein from activating its own promoter, suggesting that the HO-1 enzymatic activity is not required for this function.
Enzymatic inactive HO-1 mutant can activate its own promoter
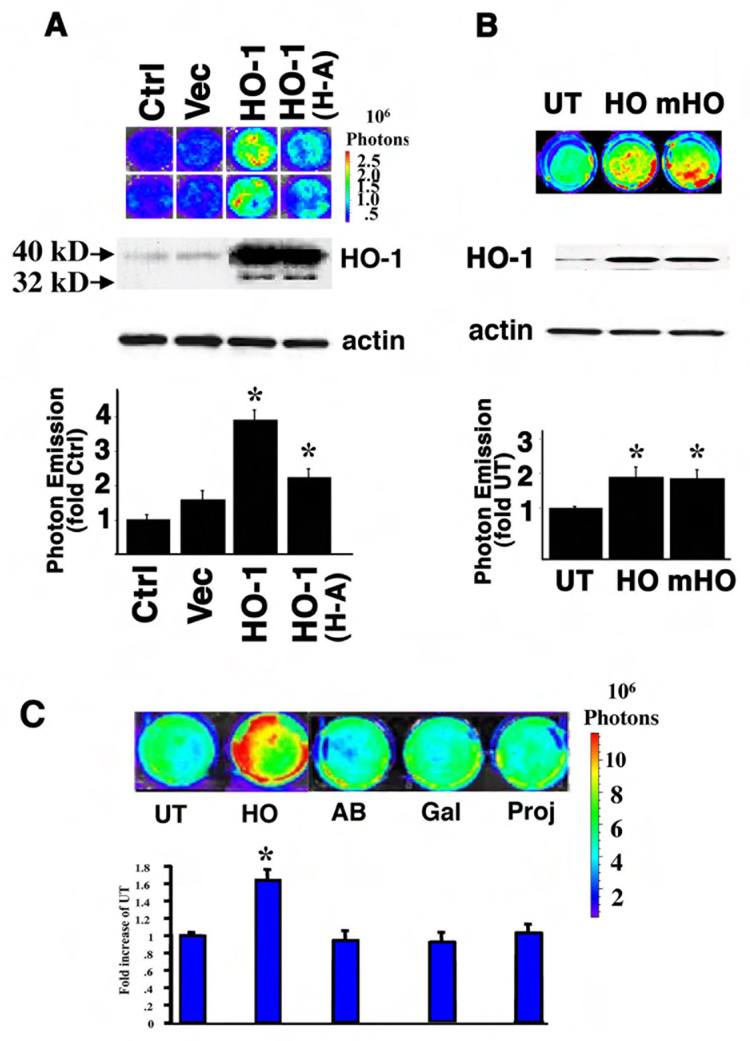
Full length HO-1 cDNA and 6XHis tag were cloned into expression vector with 3X C-terminal FLAG tag to generate HisHO-1FLAG. Histidine 25 is the essential amino acid for the enzymatic activity of HO-1 and mutation of this site completely abolishes the HO-1 catalytic activity(5). Using site-directed mutagenesis techniques, a mutant HO-1 cDNA construct in which Histidine 25 was changed to Alanine (HisHO-1mutFLAG), was generated, resulting in an enzymatically inactive HO-1. Expression of the wild type and mutant cDNAs in 3T3-HO-1/luc cells after transfection was verified by Western Blot analysis using anti-HO-1 and anti-His antibodies (data not shown). Both the wildtype and mutant HisHO-1Flag constructs as well as the expression vector alone were transiently transfected into 3T3-HO-1/luc cells. Twenty-four hours after transfection, luciferin was added into the culture medium and luciferase activity was measured with IVIS. Transfection of the 3T3-HO-1/luc cells with HO-1 cDNA was associated with a 3–5 fold increase in HO-1 promoter activation compared with cells transfected with transfection reagent or p3XFLAG vector, as demonstrated by increased photon emission (Figure 2A). When transfected into the 3T3-HO-1/luc cells, this inactive mutant construct was associated with a significant (2.2 fold) increase in HO-1 promoter activation (Figure 2A). Furthermore, intracytoplasmic delivery of enzymatically inactive HO-1 (His25Ala) mutant protein (gift from Dr. Ortiz de Montellano, USCF) also resulted in significant activation of the HO-1 promoter (Figure 2B). To demonstrated that the activation of the HO-1 promoter was not due to the project delivery procedure or non-specific protein effect, we included negative controls such as cells that did not receive any protein (UT) or cells where other proteins galatosidase (Gal), a FITC-labeled immunoglobulin (AB) or Pro-ject reagent alone (Proj) were delivered (Figure 2C).
Figure 2. Inactive HO-1 protein also increases the HO-1 promoter activity.
(A) 3T3-HO-1/luc cells were transiently transfected with pCMV vector, HO-1 cDNA (HO-1) and an enzymatically inactive HO-1 mutant in which Histidine 25 was substituted with Alanine (HO-1 H-A). A representative pseudoimage of photon emission is shown in the upper panel. Western blot analysis showed the 40 KD transfected HO-1 fusion proteins and the 32 KD endogenous HO-1 protein. Actin was used for loading control. Quantitation of the photon emission is shown in the lower panel. (B) Wildtype and inactive HO-1 proteins were delivered into 3T3-HO-1/luc cells. A representative pseudoimage of photon emission is shown in the upper panel. A representative Western blot of HO-1 immunoreactive protein is shown in the middle panel. Quantitation of the photon emission is shown in the lower panel. UT: untransfected; HO-1: Cells were HO-1 protein delivered; mutant HO-1: cells were an inactive mutant HO-1 (His25Ala) protein delivered. Values are expressed as a fold of untransfected controls and are the mean ± standard error of four separate determinations. *p < 0.05 vs. UT. (C) A pseudoimage of the photon emission from 3T3-HO-1/luc cells after delivery of HO-1 protein of 12 hours. Negative controls are cells that did not receive any protein (UT) or cells where other proteins such as galatosidase (Gal), a FITC-labeled immunoglobulin (AB) or Pro-ject alone (Proj) were delivered. Quantitative luminescence intensity is shown below. Values are the mean ± standard error of four independent measurements. *P<0.05 vs. UT.
HO-1 protein is required for hemin and cadmium chloride mediated induction of the HO-1 promoter
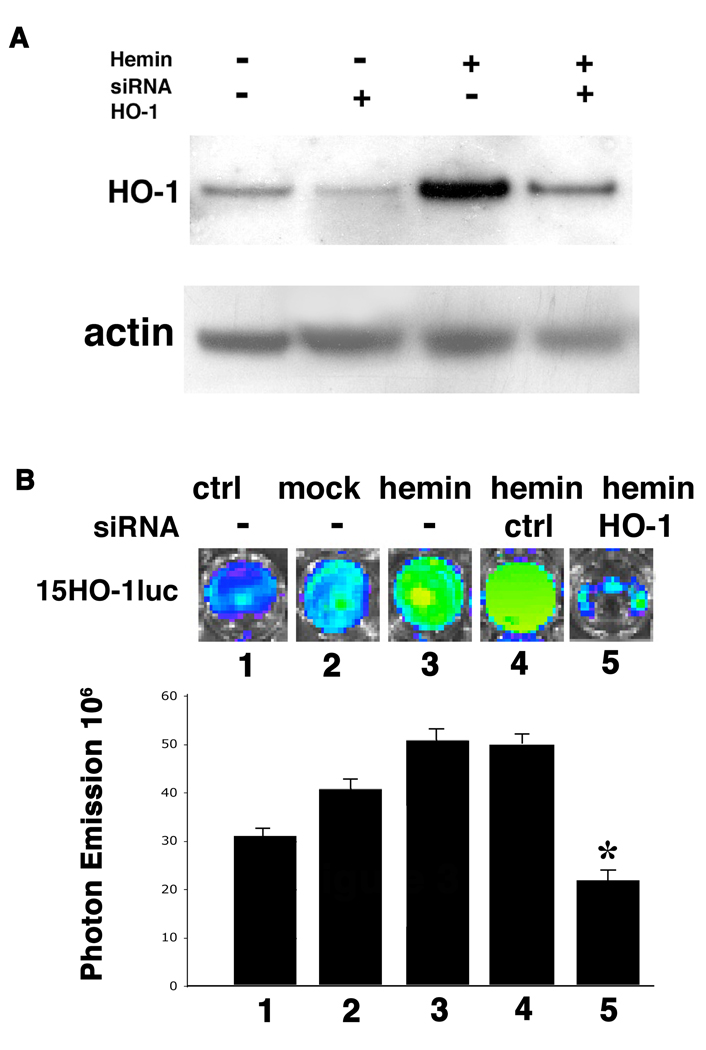
HO-1 gene can be induced by a variety of stress conditions and agents such as hyperoxia, hypoxia, UV radiation as well as hemin, inflammatory cytokines and heavy metals (1, 2, 3). To determine whether HO-1 protein is involved in the signaling pathway of any of these induction processes, siRNA mediated gene knockdown was used to disrupt the endogenous HO-1 expression during hemin and cadmium chloride mediated inductions. Transfection of HO-1 specific siRNA oligonucleotides into 3T3-HO-1/luc cells dramatically reduced HO-1 protein levels within 72 hours (Figure 3A). Expression levels of HO-2 and GAPDH protein were not affected by HO-1 siRNA transfection. In addition, control siRNA against Cyclophillin B did not change the expression of HO-1 protein, indicating the specificity of gene knock down with HO-1 siRNA (data not shown). Seventy-two hours after siRNA transfection, cells were incubated with 30µM hemin for 8 hours and the luciferase reporter assay was performed. Hemin incubation resulted in 1.6 fold induction of HO-1 promoter activity compared to control cells without hemin incubation (Figure 3B). Transfection with a control siRNA oligonucleotide specific to Cyclophilin B did not affect hemin-mediated induction of HO-1, however, siRNA against HO-1 significantly diminished the hemin-mediated activation of the HO-1 promoter (Figure 3B). These results suggest that HO-1 protein is involved in the hemin-mediated induction of the HO-1 gene.
Figure 3. HO-1 is required for hemin induced HO-1 promoter activation.
3T3-HO-1/luc cells were transfected with siRNA targeting HO-1 gene or Cyclophillin B (Dharmacon). (A) Western blot analysis showing the repression of HO-1 expression by HO-1 siRNA. 72 hrs after siRNA transfection, cells were treated with or without 30 µM hemin for 24 hrs before harvesting. Whole cell lysates were separated on SDS gels and HO-1 protein levels were examined by Western Blot analysis. Actin was used for loading control. (B) Luciferase activity visualized via the IVIS. Note the significant decrease of light emission in cells transfected with HO-1 siRNA. siRNA against Cyclophilin B was used as non-specific siRNA control. 1: control 15HO-1luc cells; 2: 15HO-1luc cells with mock transfection; 3: 15HO-1luc cells with hemin treatment; 4: 15HO-1luc cells with control Cyclophilin B siRNA followed by hemin treatment; and 5: 15HO-1luc cells with HO-1 siRNA followed by hemin treatment. Quantitative analysis was presented at the lower panel as mean ± standard error. *P<0.05 vs. hemin with no siRNA (lane 3) or control siRNA (lane 4).
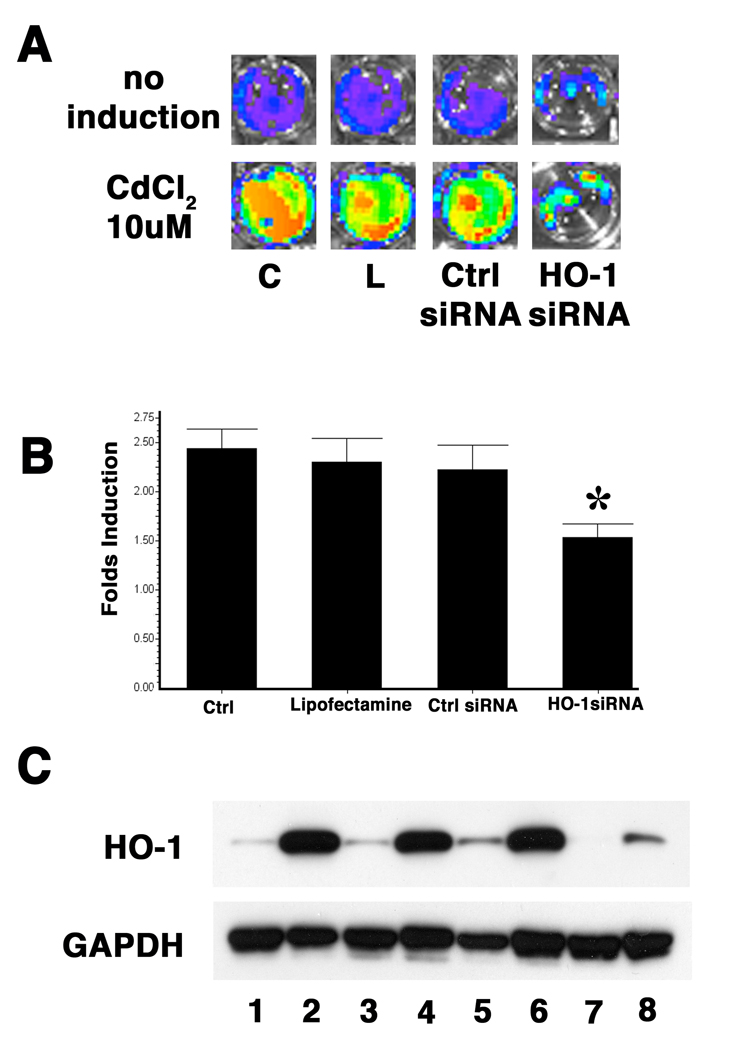
Similarly, transfection with a siRNA against HO-1 significantly diminished the cadmium chloride mediated activation of the HO-1 promoter as compared to transfection with a control siRNA oligonucleotide specific to Cyclophilin B (Figure 4). This suggests that HO-1 protein also plays a role in regulating cadmium chloride mediated HO-1 gene induction. Overall, these findings suggest that HO-1 protein may modulate factors involved in HO-1 induction by various stresses.
Figure 4. HO-1 is involved in Cadmium mediated HO-1 promoter induction.
3T3-HO-1/luc cells were transfected with siRNA targeting HO-1 gene or Cyclophillin B (Darmacon). 72 hours after siRNA transfection, cells were treated with 10µM CdCl2 for 24 hours followed by analysis. (A) A representative pseudoimage of photon emission 24 hours after CdCl2 induction. C: control cells that were not transfected. L: cells transfected with lipofectamine 2000 only. Ctrl siRNA: cells transfected with Cyclophillin B siRNA. (B) Comparison of fold change of photon emissions after CdCl2 induction. C: control cells that were not transfected. L: cells transfected with lipofectamine 2000 only. Ctrl siRNA: cells transfected with Cyclophillin B siRNA. Values are expressed as a fold of untransfected controls and are the mean ± standard error of three separate experiments. *P<0.05 vs. cells transfected with control siRNA. (C) Western Blot analysis with anti-HO-1 antibody showing that the cadmium induction of HO-1 protein was blocked by HO-1 siRNA transfection. GAPDH expression was used as loading control. Lane 1, 2: control cells that were not transfected; lane 3, 4: cells transfected with lipofectamine 2000 only; lane 5, 6: cells transfected with Cyclophillin B siRNA; and land 7,8: cells transfected with HO-1 siRNA. Lane 1, 3, 5, 7: cells with no induction. Lane 2, 4, 6, 8: cells were induced with CdCl2.
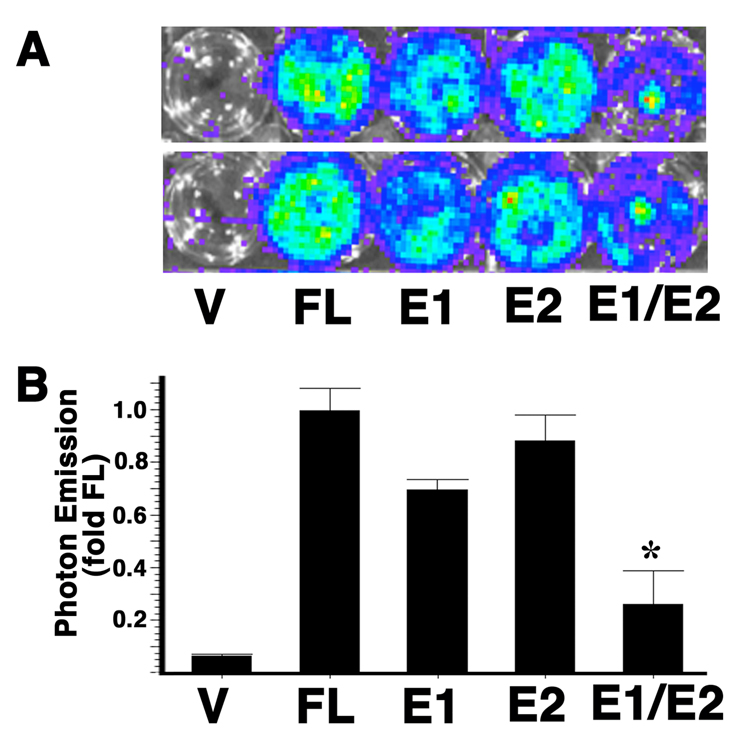
HO-1-mediated promoter activation is regulated via the distal enhancers E1 and E2
To determine the genomic regions within the HO-1 upstream promoter that are regulated by HO-1 protein, we analyzed luciferase reporter constructs containing mutations in the distal enhancer regions. The distal enhancer (DE) 1 and 2 are important regulatory regions that are important for most situations of HO-1 induction (1, 11, 13). Multiple transcription factor binding sites have been identified within each of these enhancer regions. We therefore co-transfected HO-1 cDNA with HO-1/luc mutants containing DE1 and DE2 deletions into NIH3T3 cells. The luciferase expression was analyzed by photon emission with IVIS. Compared with the wildtype full length 15kb HO-1 promoter, DE1 mutation was associated in a 31 % decrease whereas the DE2 mutation was associated with a 13% decrease in promoter activation. When both the enhancers are deleted, 74% of the promoter activation was inhibited (Figure 3). These data indicate that HO-1 auto-regulation is synergistically mediated via the DE1 and DE2 regions.
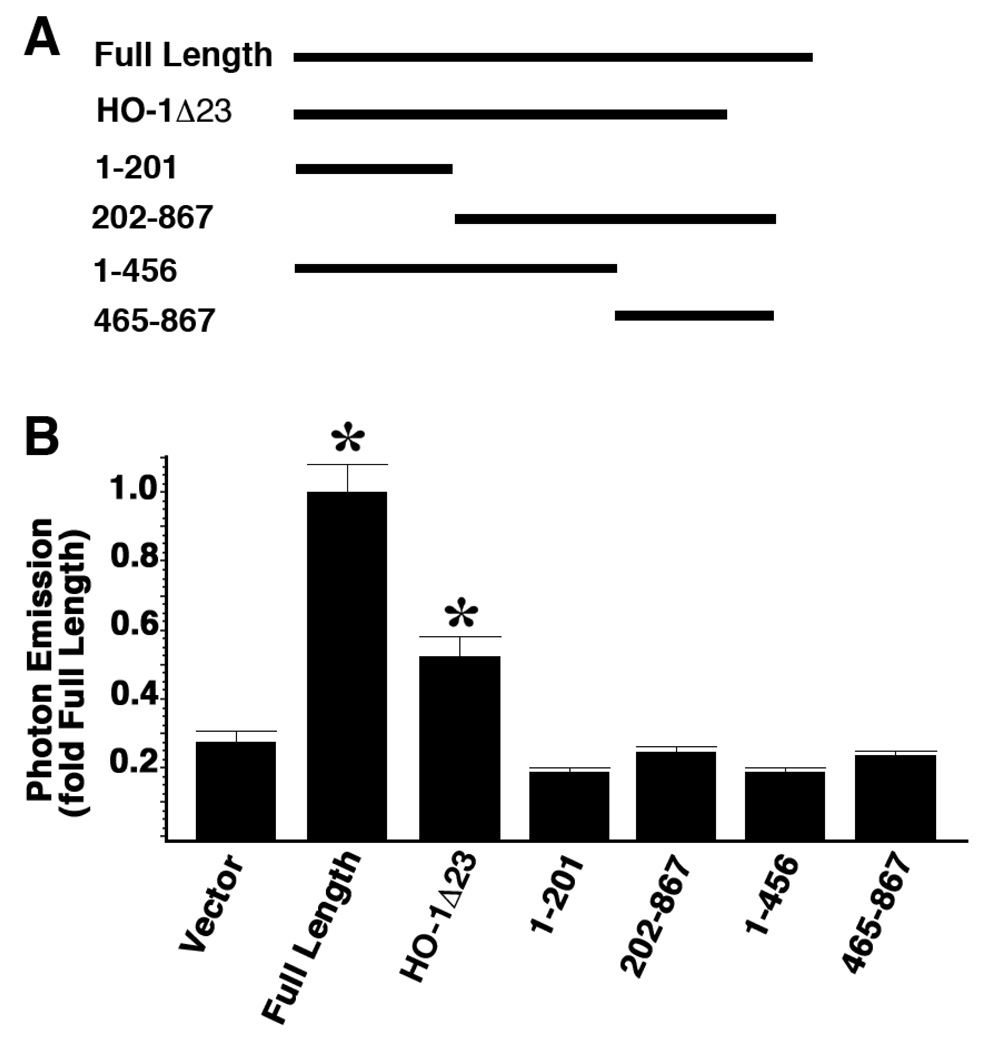
HO-1 protein with C-terminal truncation can also activate the promoter
To determine the domains within HO-1 protein that are required for HO-1 promoter activation, we compared the promoter activation activities of HO-1 cDNAs with different truncations with that of the full length HO-1 cDNA (Figure 6). Previous studies in our group (6) identified that HO-1 protein with a C-terminal truncation is the major isoform found in the nucleus. When introduced into the cells, a C-terminal truncated protein lacking the last 23 amino acids (HO-1Λ23) increased the HO-1 promoter activity to 1.8 fold compared to vector control. This was less than the degree of promoter activation achieved with transfection of the full length HO-1 cDNA construct. The latter activated the HO-1 promoter by 3.58 fold compared to the vector control. We also tested HO-1 with various deletions from either the N- or the C-terminus (Figure 6A, 1–201, 202–867, 1–456, 465–867). None of these constructs activated the HO-1 promoter. These data indicate that the intact HO-1 protein is required for full HO-1 promoter activation; however, C-terminal truncated isoform can also facilitate the promoter activation.
Figure 6. Intact HO-1 protein is required for maximal promoter activation but truncated HO-1 protein can also active the promoter.
3T3-HO-1/luc cells were transfected with full length HO-1 cDNA as well as cDNAs with various truncations. Luciferase activities were measured with IVIS 48 hours after transfection. A: Schematic drawings of the cDNA constructs. Numbers represent the DNA base pairs in HO-1 coding region. B: Data represents fold of full length value and are mean ± standard error of three experiments with duplicate samples. Full Length: full length HO-1 cDNA; HO-1Λ23: HO-1 cDNA with 23 amino acids deleted from the C-terminal; 1–201, 202–867, 1–456, 465–867 represent different truncation isoforms. *p < 0.05 vs. vector.
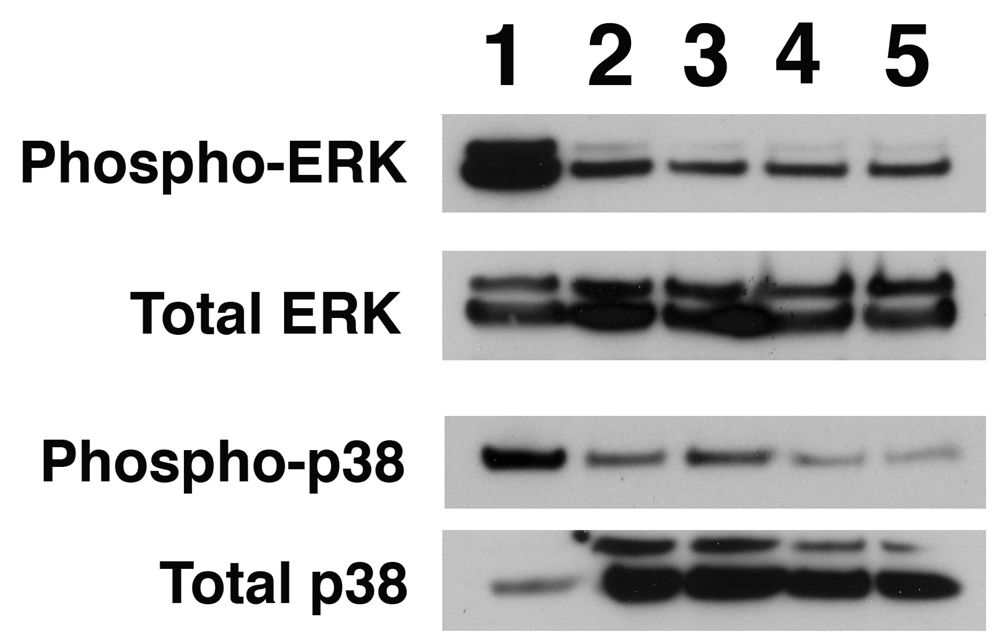
Activation of MAPK pathways is not required in HO-1 self-regulation
MAP kinase pathways can be activated by oxidative stress (23–25). Specifically, p38 MAP kinase pathway can be activated by CO and mediates the anti-inflammatory effects in LPS induced inflammation (23) and ischemia-reperfusion lung injury (16, 24). Perhaps HO-1 protein could mediate activation of the HO-1 promoter, via CO induced p38 MAP kinase or ERK activation. We therefore examined the activation of p38 and ERK pathways by analyzing the phosphorylation status of both p38 and ERK after HO-1 transfection. Compared to untransfected cells or cells transfected with the cloning vector alone, we found no increase in either phosphorylated p38 or phosphorylated ERK (Fig. 7). These results indicated that the p38 and ERK MAPK pathways are not required for HO-1 self-regulation.
Figure 7. HO-1 self-regulation does not require activation of p38 and ERK MAPK pathways.
3T3-HO-1/luc cells were transfected with expression vector alone and full-length cDNA encoding wildtype and catalytic inactive forms of HO-1. 48 hours after transfection, cells were harvested and assayed for total p38, phosphorylated p38, total ERK and phosphorylated ERK by Western Blot analysis. Lane1: Cells treated with TNF-a, as positive control for p38 activation; lane 2: untreated 3TS-HO-1/luc cells; lane 3: cells transfected with vector alone; lane 4: cells transfected with wildtype full length HO-1 cDNA; Lane 5: cells transfected with enzymatically inactive HO-1 mutant in which Histidine 25 was substituted with Alanine (HO-1 H-A).
DISCUSSION
In this paper, we demonstrate that HO-1 protein can regulate its own promoter independent of its enzymatic activity. We document that this self-activation is involved in the HO-1 up-regulation via the oxidants cadmium chloride and hemin. In addition, we have identified the genomic regions in the HO-1 promoter that respond to HO-1 protein and the essential domains within the HO-1 protein required for the self-activation.
Auto-regulation of the HO-1 gene is a novel observation. This process could facilitate maintenance of basal levels of HO-1 protein even when substrate is limited. It would also permit enhanced and prolonged activation of HO-1 expression after induction with various stimuli. Nonetheless, expression of HO-1 would also need to be intermittently down-regulated. Despite cytoprotection with low (less then five fold) HO activity induction, high levels of HO-1 expression (greater than 15-fold) were associated with significant oxygen cytotoxicity (26). The Bach-1 mediated negative regulation of HO-1 expression is one of the possible mechanisms to turn off the positive “feed forward” stimulation of HO-1 gene to maintain a stable expression level of HO-1. There are many examples of positive and negative self-regulation of gene expression including the early growth response factor (Egr-1) (27), human chorionic gonadotropin (hCG) in the placenta (28), and the heat shock protein Hsp70 under certain circumstances (29).
Although most previous studies have directly linked functions of HO-1 with its enzymatic activity to degrade heme and to generate CO and bilirubin, we have found that HO-1 activity inhibitor SnPP does not affect the HO-1 self-regulation. Furthermore, an enzymatically inactive mutant HO-1 protein can also activate the HO-1 promoter to the same extent as the wildtype form. We have previously documented that HO-1 can migrate into the nucleus under certain stress conditions, such as exposure to hypoxia and incubation with hemin or heme-hemopexin and this nuclear form of HO-1 is enzymatically inactive (6). When delivered into the cells, both the wildtype and inactive HO-1 can modulate DNA binding activity of transcription factors including AP-1 (6). Together, these data suggest that in addition to heme degradation HO-1 may have other cellular functions including regulating the expression of oxidative stress response genes.
Self-regulation of HO-1 could be mediated by direct protein-DNA interaction akin to the action of other transcription factors. However, the structure of HO-1 does not reveal traditional DNA binding motifs, a characteristic of most transcription factors. Direct HO-1 mediated transcriptional activation is less likely without a DNA binding motif as seen with transcription factors (reviewed by (30)). Using EMSA and ChIP assays, we were not able to detect direct binding of HO-1 protein to DE1, DE2 and PE regions of the HO-1 promoter. Another potential mechanism could involve binding of HO-1 to a transcription factor or a protein complex resulting in activation of the HO-1 promoter. Although HO-1 can bind to its isoenzyme HO-2 (31), no reports demonstrate binding of HO-1 to known transcription factors. In addition, preliminary co-immunoprecipitation experiments using FLAG-tagged HO-1 protein did not reveal binding to Nrf2 nor to Bach-1, known HO-1 promoter regulatory proteins. Another way by which the autoregulation could be mediated is through the binding of HO-1 to other proteins, serving as the non-DNA binding protein of a transcriptional factor complex as with the non-DNA-binding CBFβ ubunit of the core binding factor, which increases the affinity of the DNA-binding domain of CBFα for DNA while making no direct contacts to the DNA (32).
Alternatively, increased HO-1 protein could perturb the cell and alter transcription factors known to regulate the HO-1 promoter. The HO-1 promoter has consensus binding sequences for many of the transcription factors. Therefore it is conceivable that activation of one of these transcription factors by over-expression of HO-1 protein could result in downstream effects on HO-1 transcriptional activation itself. DNA binding activity of AP-1, a key transcription factor that can bind to HO-1 promoter, is increased after HO-1 is delivered into the cells. Previous studies showed that during endoplasmic reticulum (ER) stress AP-1 is activated via chaperone glucose-regulated protein 78 (Grp78) binding status to ER stress sensor proteins such as IRE1 and ATF6 (33, 34). Despite the clear role of the byproducts of the HO reaction including CO and bile pigments, in modulating transcription factors such as NF-kB and STAT (35, 36), delivery of either catalytically active or inactive HO-1 also modulates these transcription factors (6). Therefore, these effects cannot be strictly attributed to the byproducts of the HO reaction. How the inactive HO-1 protein mediates its effects on transcription factors is not fully understood.
In the current work, we also demonstrate the lack of involvement of p38 MAP kinase and ERK signaling in this auto-regulatory process. Others have clearly shown that CO released from the HO reaction activated p38 MAP kinase, thereby altering many downstream pathways (23, 24). ERK signaling is a common oxidative stress mediated response. In lung epithelial cells, hyperoxia causes activation of the ERK1/2 MAPK pathway which leads to caspase activation and cell death (37). However, levels of both phosphorylated p38 and phosphorylated ERK remain unchanged after HO-1 over-expression, indicating that neither pathway is involved in the HO-1 self-regulation process.
It is well documented that HO-1 can be induced by a variety of oxidant agents such as hemin and cadmium. Using siRNA gene knockdown techniques to specifically reduce HO-1 expression, we have demonstrated that the HO-1 protein itself is involved in both the hemin and cadmium induced HO-1 up-regulation (Fig 4, 5), suggesting that the HO-1 protein is important to amplify its own levels during oxidative stress. Because HO-1 can migrate to the nucleus during oxidative stress, it may be able to quickly modify the nuclear environment and trigger increased gene expression including its own. This remains to be explored.
Figure 5. HO-1 activates its own promoter through the distal E1 and E2 enhancers.
NIH3T3 cells were co-transfected with HO-1 cDNA and different luciferase reporter constructs. V: vector containing only the luciferase reporter. FL: luciferase reporter controlled by the 15 kb full length HO-1 promoter. E1: luciferase reporter controlled by the 15 kb full length HO-1 promoter with mutation on the E1 enhancer. E2: luciferase reporter controlled by the 15 kb full length HO-1 promoter with mutation on the E2 enhancer. E1/E2: luciferase reporter controlled by the 15 kb full length HO-1 promoter with mutations on both the E1 and E2 enhancers. (A) A representative pseudoimage of photon emission 24 hours after transfections. (B) Quantitation of the photon emissions. Values are expressed as the mean ± standard error of three independent experiments with duplicate samples. *P<0.05 vs. FL
The regulation of HO-1 gene expression has been well characterized. The proximal enhancer region as well as two 5' distal enhancer regions, DE1 and DE2, mediates transcriptional activation of the HO-1 gene in response to various stimuli. Each enhancer region contains multiple copies of the cis-acting stress responsive element (StRE), similar to the Maf response element and binding sites of AP-1 class of transcription factors. Key regulators through DE1 and DE2 include the Nrf2 transcription factor which can heterodimerize to Maf and activate HO-1 gene transcription and Bach-1, a heme-regulated and hypoxia-inducible basic leucine zipper protein to represses HO-1 gene expression by binding to the multiple Maf recognition elements (MAREs) on the HO-1 gene (38). Here we demonstrate that HO-1 auto-regulation is also mediated through the DE1 and DE2 regions in the HO-1s promoter. It remains to be determined which specific site(s) on the promoter mediate the regulation. Our experiments demonstrate that a C-terminally truncated inactive form of HO-1 can also regulate HO-1 expression. This suggests that the protein itself is also a regulatory molecule that does not rely on the HO-1 reaction byproducts to mediate its effects. Nonetheless, HO activity can also mediate this process, as the full length HO-1 protein was most effective at auto-regulation.
In summary, we document a critical role for the HO-1 protein itself in a feed forward transcriptional regulation of its own gene and also demonstrate that HO-1 mediated transcriptional activation plays a role in HO-1 induction by oxidants. The former may be a mechanism by which basal levels of HO-1 are maintained in a substrate poor environment and the latter may serve to prolong the induction of HO-1 with various stresses.
ACKNOWLEDGMENTS
This work was funded by grants from the National Institute of Health (HL-58752, P.A.D.) and the Hess and Mary L Johnson funds from Stanford University (P.A.D.). We thank Jessica Bordner for her invaluable technical assistance. We are grateful to Jennifer McIntyre and Cheryn Jarvis for their administrative assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Alam J, Killeen E, Gong P, Naquin R, Hu B, Stewart D, Ingelfinger JR, Nath KA. Heme activates the heme oxygenase-1 gene in renal epithelial cells by stabilizing Nrf2. Am J Physiol Renal Physiol. 2003;284:F743–F752. doi: 10.1152/ajprenal.00376.2002. [DOI] [PubMed] [Google Scholar]
- 2.Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991;51:974–978. [PubMed] [Google Scholar]
- 3.Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricchetti GA, Williams LM, Foxwell BM. Heme oxygenase 1 expression induced by IL-10 requires STAT-3 and phosphoinositol-3 kinase and is inhibited by lipopolysaccharide. J Leukoc Biol. 2004;76:719–726. doi: 10.1189/jlb.0104046. [DOI] [PubMed] [Google Scholar]
- 5.Hori R, Kashiba M, Toma T, Yachie A, Goda N, Makino N, Soejima A, Nagasawa T, Nakabayashi K, Suematsu M. Gene transfection of H25A mutant heme oxygenase-1 protects cells against hydroperoxide-induced cytotoxicity. J Biol Chem. 2002;277:10712–10718. doi: 10.1074/jbc.M107749200. [DOI] [PubMed] [Google Scholar]
- 6.Lin Q, Weis S, Yang G, Weng YH, Helston R, Rish K, Smith A, Bordner J, Polte T, Gaunitz F, Dennery PA. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem. 2007;282:20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- 7.Sheftel AD, Kim SF, Ponka P. Non-heme induction of heme oxygenase-1 does not alter cellular iron metabolism. J Biol Chem. 2007;282:10480–10486. doi: 10.1074/jbc.M700240200. [DOI] [PubMed] [Google Scholar]
- 8.Djiane J, Durand P. Prolactin-progesterone antagonism in self regulation of prolactin receptors in the mammary gland. Nature. 1977;266:641–643. doi: 10.1038/266641a0. [DOI] [PubMed] [Google Scholar]
- 9.Sanz-Ezquerro JJ, Tickle C. Autoregulation of Shh expression and Shh induction of cell death suggest a mechanism for modulating polarising activity during chick limb development. Development. 2000;127:4811–4823. doi: 10.1242/dev.127.22.4811. [DOI] [PubMed] [Google Scholar]
- 10.Kravets A, Hu Z, Miralem T, Torno MD, Maines MD. Biliverdin reductase, a novel regulator for induction of activating transcription factor-2 and heme oxygenase-1. J Biol Chem. 2004;279:19916–19923. doi: 10.1074/jbc.M314251200. [DOI] [PubMed] [Google Scholar]
- 11.Alam J. Multiple elements within the 5' distal enhancer of the mouse heme oxygenase-1 gene mediate induction by heavy metals. J Biol Chem. 1994;269:25049–25056. [PubMed] [Google Scholar]
- 12.Alam J, Cook JL. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr Pharm Des. 2003;9:2499–2511. doi: 10.2174/1381612033453730. [DOI] [PubMed] [Google Scholar]
- 13.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 14.Kitamuro T, Takahashi K, Ogawa K, Udono-Fujimori R, Takeda K, Furuyama K, Nakayama M, Sun J, Fujita H, Hida W, Hattori T, Shirato K, Igarashi K, Shibahara S. Bach1 functions as a hypoxia-inducible repressor for the heme oxygenase-1 gene in human cells. J Biol Chem. 2003;278:9125–9133. doi: 10.1074/jbc.M209939200. [DOI] [PubMed] [Google Scholar]
- 15.Sun J, Brand M, Zenke Y, Tashiro S, Groudine M, Igarashi K. Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network. Proc Natl Acad Sci U S A. 2004;101:1461–1466. doi: 10.1073/pnas.0308083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Bedard EL, Potter R, Zhong R, Alam J, Choi AM, Lee PJ. Mitogen-activated protein kinases regulate HO-1 gene transcription after ischemia-reperfusion lung injury. Am J Physiol Lung Cell Mol Physiol. 2002;283:L815–L829. doi: 10.1152/ajplung.00485.2001. [DOI] [PubMed] [Google Scholar]
- 17.Yang G, Abate A, George AG, Weng YH, Dennery PA. Maturational differences in lung NF-kappaB activation and their role in tolerance to hyperoxia. J Clin Invest. 2004;114:669–678. doi: 10.1172/JCI19300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dennery PA, Sridhar KJ, Lee CS, Wong HE, Shokoohi V, Rodgers PA, Spitz DR. Heme oxygenase-mediated resistance to oxygen toxicity in hamster fibroblasts. J Biol Chem. 1997;272:14937–14942. doi: 10.1074/jbc.272.23.14937. [DOI] [PubMed] [Google Scholar]
- 19.Vreman HJ, Stevenson DK. Heme oxygenase activity as measured by carbon monoxide production. Anal Biochem. 1988;168:31–38. doi: 10.1016/0003-2697(88)90006-1. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Shan P, Alam J, Fu XY, Lee PJ. Carbon Monoxide Differentially Modulates STAT1 and STAT3 and Inhibits Apoptosis via a Phosphatidylinositol 3-Kinase/Akt and p38 Kinase-dependent STAT3 Pathway during Anoxia-Reoxygenation Injury. J Biol Chem. 2005;280:8714–8721. doi: 10.1074/jbc.M408092200. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita K, McDaid J, Ollinger R, Tsui TY, Berberat PO, Usheva A, Csizmadia E, Smith RN, Soares MP, Bach FH. Biliverdin, a natural product of heme catabolism, induces tolerance to cardiac allografts. Faseb J. 2004;18:765–767. doi: 10.1096/fj.03-0839fje. [DOI] [PubMed] [Google Scholar]
- 22.Ryter SW, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem. 2002;234–235:249–263. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Shan P, Alam J, Davis RJ, Flavell RA, Lee PJ. Carbon monoxide modulates Fas/Fas ligand, caspases, and Bcl-2 family proteins via the p38alpha mitogen-activated protein kinase pathway during ischemia-reperfusion lung injury. J Biol Chem. 2003;278:22061–22070. doi: 10.1074/jbc.M301858200. [DOI] [PubMed] [Google Scholar]
- 25.Lee PJ, Zhang X, Shan P, Ma B, Lee CG, Homer RJ, Zhu Z, Rincon M, Mossman BT, Elias JA. ERK1/2 mitogen-activated protein kinase selectively mediates IL-13-induced lung inflammation and remodeling in vivo. J Clin Invest. 2006;116:163–173. doi: 10.1172/JCI25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. Faseb J. 1999;13:1800–1809. doi: 10.1096/fasebj.13.13.1800. [DOI] [PubMed] [Google Scholar]
- 27.Gosslar U, Schmid RM, Holzmann B. Regulation of Egr-1-dependent gene expression by the C-terminal activation domain. Biochem Biophys Res Commun. 1999;255:208–215. doi: 10.1006/bbrc.1999.0182. [DOI] [PubMed] [Google Scholar]
- 28.Licht P, Cao H, Lei ZM, Rao CV, Merz WE. Novel self-regulation of human chorionic gonadotropin biosynthesis in term pregnancy human placenta. Endocrinology. 1993;133:3014–3025. doi: 10.1210/endo.133.6.8243330. [DOI] [PubMed] [Google Scholar]
- 29.Craig EA, Gross CA. Is hsp70 the cellular thermometer? Trends Biochem Sci. 1991;16:135–140. doi: 10.1016/0968-0004(91)90055-z. [DOI] [PubMed] [Google Scholar]
- 30.Struhl K. Helix-turn-helix, zinc-finger, and leucine-zipper motifs for eukaryotic transcriptional regulatory proteins. Trends Biochem Sci. 1989;14:137–140. doi: 10.1016/0968-0004(89)90145-X. [DOI] [PubMed] [Google Scholar]
- 31.Weng YH, Yang G, Weiss S, Dennery PA. Interaction between heme oxygenase-1 and -2 proteins. J Biol Chem. 2003;278:50999–51005. doi: 10.1074/jbc.M307644200. [DOI] [PubMed] [Google Scholar]
- 32.Yan J, Liu Y, Lukasik SM, Speck NA, Bushweller JH. CBFbeta allosterically regulates the Runx1 Runt domain via a dynamic conformational equilibrium. Nat Struct Mol Biol. 2004;11:901–906. doi: 10.1038/nsmb819. [DOI] [PubMed] [Google Scholar]
- 33.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 34.Liu CY, Schroder M, Kaufman RJ. Ligand-independent dimerization activates the stress response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J Biol Chem. 2000;275:24881–24885. doi: 10.1074/jbc.M004454200. [DOI] [PubMed] [Google Scholar]
- 35.Brouard S, Berberat PO, Tobiasch E, Seldon MP, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kappa B to protect endothelial cells from tumor necrosis factor-alpha-mediated apoptosis. J Biol Chem. 2002;277:17950–17961. doi: 10.1074/jbc.M108317200. [DOI] [PubMed] [Google Scholar]
- 36.Wu J, Ma J, Fan ST, Schlitt HJ, Tsui TY. Bilirubin derived from heme degradation suppresses MHC class II expression in endothelial cells. Biochem Biophys Res Commun. 2005;338:890–896. doi: 10.1016/j.bbrc.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Shan P, Sasidhar M, Chupp GL, Flavell RA, Choi AM, Lee PJ. Reactive oxygen species and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase mediate hyperoxia-induced cell death in lung epithelium. Am J Respir Cell Mol Biol. 2003;28:305–315. doi: 10.1165/rcmb.2002-0156OC. [DOI] [PubMed] [Google Scholar]
- 38.Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, Taketo MM, Yamamoto M, Igarashi K. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. Embo J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]