Abstract
BACKGROUND AND OBJECTIVE:
An increased risk of febrile seizure (FS) was identified with concomitant administration of trivalent inactivated influenza vaccine (IIV3) and pneumococcal conjugate vaccine (PCV) 13-valent during the 2010–2011 influenza season. Our objective was to determine whether concomitant administration of IIV3 with other vaccines affects the FS risk.
METHODS:
We examined the risk of FS 0 to 1 day postvaccination for all routinely recommended vaccines among children aged 6 through 23 months during a period encompassing 5 influenza seasons (2006–2007 through 2010–2011). We used a population-based self-controlled risk interval analysis with a control interval of 14 to 20 days postvaccination. We used multivariable regression to control for receipt of concomitant vaccines and test for interaction between vaccines.
RESULTS:
Only PCV 7-valent had an independent FS risk (incidence rate ratio [IRR], 1.98; 95% confidence interval [CI], 1.00 to 3.91). IIV3 had no independent risk (IRR, 0.46; 95% CI, 0.21 to 1.02), but risk was increased when IIV3 was given with either PCV (IRR, 3.50; 95% CI, 1.13 to 10.85) or a diphtheria-tetanus-acellular-pertussis (DTaP)-containing vaccine (IRR, 3.50; 95% CI, 1.52 to 8.07). The maximum estimated absolute excess risk due to concomitant administration of IIV3, PCV, and DTaP-containing vaccines compared with administration on separate days was 30 FS per 100 000 persons vaccinated.
CONCLUSIONS:
The administration of IIV3 on the same day as either PCV or a DTaP-containing vaccine was associated with a greater risk of FS than when IIV3 was given on a separate day. The absolute risk of postvaccination FS with these vaccine combinations was small.
Febrile seizures (FS) are seizures that occur in febrile children between the ages of 6 and 60 months who do not have an intracranial infection, metabolic disturbance, or history of afebrile seizures.1 About 5% of children experience a FS.2 FS can occur with various infections, including influenza.3,4 The risk of FS is also temporarily increased for several days after certain vaccines (diphtheria, tetanus toxoids, whole-cell pertussis vaccine, measles, mumps, rubella vaccine [MMR], and MMR, varicella combination vaccine [MMRV]).5,6 Before 2010, no increased FS risk had been observed after trivalent inactivated influenza vaccine (IIV3).7,8 Then, an increased risk of FS was detected in Australia for the IIV3 manufactured by CSL Limited (Victoria, Australia), with most FS occurring within 12 hours of vaccination.9 Subsequent vaccine safety monitoring in the United States during the 2010–2011 influenza season also detected an increased risk of FS for the IIV3 manufactured by Sanofi Pasteur.7 Pneumococcal conjugate vaccine 13-valent (PCV13) had been introduced in the United States in 2010, and it was hypothesized that concomitant vaccine administration might have played a role in the US findings.10 Additional epidemiologic investigation found an independent risk of FS in 2010–2011 with IIV3 and also PCV13, and the greatest risk when both vaccines were given together.10 The effect of other vaccines was not evaluated in that study. Our objective was to determine whether other vaccines given concomitantly with IIV3 affected the risk of FS after vaccination in 2010–2011 or previous influenza seasons. This required examining the FS risk after the 10 vaccines recommend for all children in the United States.11
METHODS
The Vaccine Safety Datalink (VSD) is a collaboration between the US Centers for Disease Control and Prevention and several integrated health care organizations (sites) in the United States that performs vaccine safety research and surveillance.12 Ten sites with a combined annual population of ~9.8 million members contributed data to this study. We identified VSD members aged 6 through 23 months who had an FS during prespecified time intervals after receipt of ≥1 vaccines of any type. We focused on the 6–23-month age range because FS occur most commonly at these ages, and this is when most vaccine doses recommended for children ages 6 to 60 months are given. FS was defined as a clinical diagnosis of seizure with a recorded temperature ≥38°C or caregiver-reported fever within 24 hours, or a clinician’s diagnosis of FS, excluding patients with intracranial infection, metabolic disturbance, or a history of afebrile seizure. We defined the day of vaccination as day 0. We identified potential FS cases by emergency department visits or inpatient admissions coded with International Classification of Diseases, Ninth Revision (ICD-9) diagnoses 780.3 through 780.39 (convulsions) that occurred on postvaccination days 0 to 1 or 14 to 20 from July 1, 2006 through June 30, 2011.13 We excluded visits that occurred ≤42 days after a previous visit with a convulsion ICD-9 code because those are less likely to represent a new event.10 We abstracted medical records for all eligible visits at 9 sites and a random sample at 1 large site (due to abstraction capacity limitations) to confirm the FS diagnosis and time of onset and to obtain additional descriptive information.
We used the self-controlled risk interval method to compare the incidence of FS during the risk interval (0–1 days postvaccination) to the control interval (14–20 days postvaccination).10 The risk interval represents the biologically plausible time period during which inactivated vaccines can induce fever.14 The control interval represents a time period during which neither inactivated nor live-attenuated vaccines induce fever and the risk of FS is at baseline. Comparing 2 different time intervals for the same individual inherently controls for factors that do not change over time. Choosing a control interval that is relatively short and close in time to the risk interval implicitly controls for factors that change over time, such as age and season.
We modeled the incidence rate ratio (IRR) of FS using conditional Poisson regression with an offset term to account for the different interval lengths. Separate dichotomous variables indicated whether each vaccine type was given on day 0 or not. Different vaccine products that have antigens in common (such as the various diphtheria-tetanus-acellular-pertussis vaccine [DTaP]-containing vaccines) were grouped into 1 variable for certain models. An influenza season was defined as July 1 of 1 year through June 30 of the following year. We first examined the 2006–2007 through 2009–2010 seasons, then replicated the analysis for the 2010–2011 season, which was examined separately because an increased risk of FS had previously been observed only with the 2010–2011 IIV3, and PCV13 replaced PCV 7-valent (PCV7) in the United States during 2010. Findings were similar for the 2 time periods, so all study time was then pooled together. We selected a final multivariable model by starting with all vaccines in the model and performing a manual sequential elimination process, first removing vaccines with an IRR <1 and then in decreasing order of variance. Vaccines with an independent effect, IIV3, and any vaccines that affected the relationship of IIV3 with FS were retained in the model to examine effect–measure modification (interaction) involving IIV3 using product terms, which were also retained if the likelihood ratio statistic P value was < .10. We calculated model-based estimates of the IRR for each unique combination of vaccines included in the final model and further assessed multiplicative and additive measures of interaction between these vaccines using the ratio of IRRs and the relative excess risk due to interaction (RERI), respectively.15 We calculated 95% confidence intervals (CIs) for the ratio of IRRs based on the IRR SEs and for RERI using the bootstrap percentile interval method with 10 000 samples.16,17 We created additional models to obtain adjusted IRR estimates for the vaccines not found to interact with IIV3.
We estimated the attributable risk (AR) as: (IRR – 1) × (the baseline incidence rate of FS in the VSD population per person-day) × (2 person-days in the risk interval). The baseline incidence was determined using the ICD-9 code criteria described above regardless of previous vaccination and without chart confirmation during 2000 to 2011.
Analysis was performed by using SAS 9.3 (SAS Institute, Inc, Cary, NC). Institutional review boards at US Centers for Disease Control and Prevention and each site approved this study and determined that informed consent was not required.
RESULTS
We identified 596 potential FS cases during the specified intervals (risk interval, n = 183; control interval, n = 413) after 1 915 108 vaccination events. We requested charts for 468 of the potential cases, of which 428 had records available. FS was chart confirmed in 348 cases (all unique individuals), from which 13 were excluded because the seizure occurred outside of the specified intervals and 2 because vaccines were also given during the specified intervals after day 0, resulting in 333 chart-confirmed FS cases for analysis.
Approximately half of the case-patients had experienced a previous FS (Table 1). Patients whose FS occurred during the risk interval had received a greater median number of vaccines on day 0 than patients whose FS occurred during the control interval and were more likely to have received an antipyretic medication before the FS, although it is unknown whether this difference was due to prophylactic use or in response to fever. At the medical visit for the FS, an infection was documented in 77% of control interval cases but only 37% of risk interval cases. The most common type in both groups was upper respiratory tract infection.
TABLE 1.
Characteristics of Patients With a Chart-Confirmed FS That Occurred Postvaccination in Either the Risk Interval (0–1 Days) or the Control Interval (14–20 Days)
| Patients With an FS during the 0–1 d Postvaccination (N = 103) (%) |
Patients with an FS during the 14–20 d Postvaccination (N = 230) (%) |
P | |
|---|---|---|---|
| Age (mo) | .06 | ||
| 6–11 | 25 | 17 | |
| 12–23 | 75 | 83 | |
| Gender | .21 | ||
| Boys | 51 | 58 | |
| Girls | 49 | 42 | |
| Race | .19 | ||
| White | 34 | 32 | |
| Black | 16 | 10 | |
| American Indian/Alaska Native | 0 | 1 | |
| Asian | 11 | 17 | |
| Native Hawaiian/Pacific Islander | 1 | 1 | |
| Other | 21 | 15 | |
| ≥2 races | 6 | 6 | |
| Unknown | 11 | 18 | |
| Ethnicity | .60 | ||
| Hispanic | 34 | 36 | |
| Non-Hispanic | 66 | 64 | |
| Medical history | |||
| Premature birth | 11 | 8 | .40 |
| Previous FS | 48 | 51 | .80 |
| Family history of FS | 18 | 14 | .40 |
| Antipyretic given in 7 d before FS | 52 | 38 | .01 |
| Antibiotic given in 7 d before FS | 8 | 6 | .57 |
| Infection documented at visit for FS | 37 | 77 | <.001 |
| Number of vaccines received on day | Median, 3 (range, 1–6) | Median 2 (range, 1–7) | <.001 |
| Fever in 24 h before vaccination | 6 | 2 | .06 |
| Temperature >100.4°F at time of vaccination | 0 | 0 | n/a |
| Treatment | |||
| Admitted to hospital because of FS | 13 | 10 | .88 |
n/a, not applicable.
Other infections included otitis media, viral infection not otherwise specified, urinary tract infections, and lower respiratory tract infections. The 333 case-patients had received 129 unique vaccine combinations on day 0, with the most common being IIV3 alone (n = 45), hepatitis A (HepA) alone (n = 22), and DTaP + HepA (n = 14).
None of the IIV3 formulations for the 2006–2007 through 2009–2010 influenza seasons were associated with FS (IRR < 1), so these were combined into a single variable for subsequent models. The influenza A(H1N1)2009 pandemic monovalent inactivated vaccine (2009 H1N1 MIV) also had an IRR < 1. The IRR for the 2010–2011 IIV3 was 1.17 (95% CI, 0.24 to 5.78) (Table 2).
TABLE 2.
IRR of FS During the 0 to 1 Days After Vaccination for Each Type of Vaccine Estimated Using Self-controlled Risk Interval Analysis
| Vaccine | No. of Patients With FS in the Risk Interval (N = 103) |
No. of Patients With FS in the Control Interval (N = 230) |
IRR (95% CI) | IRR (95% CI) |
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| PCV (includes 7- and 13-valent) | 69 | 91 | 2.65 (1.94 to 3.63) | 1.81 (0.97 to 3.38)a |
| PCV7 | 52 | 68 | 2.68 (1.87 to 3.84) | 1.98 (1.00 to 3.91)b |
| PCV13 | 17 | 23 | 2.59 (1.38 to 4.84) | 1.40 (0.27 to 7.22)c |
| DTaP-containing | 67 | 94 | 2.49 (1.82 to 3.41) | 1.04 (0.47 to 2.28)a |
| DTaP | 40 | 63 | 2.22 (1.50 to 3.30) | 1.17 (0.52 to 2.60)d |
| DTaP-HepB-IPV | 22 | 19 | 4.05 (2.19 to 7.49) | 1.64 (0.62 to 4.35)e |
| DTaP-IPV/Hib | 5 | 12 | 1.46 (0.51 to 4.14) | 0.72 (0.13 to 3.85)f |
| Hib | 47 | 57 | 2.89 (1.96 to 4.25) | 1.53 (0.87 to 2.72)g |
| IIV3 (all study seasons) | 44 | 84 | 1.83 (1.27 to 2.64) | 0.46 (0.21 to 1.02)a |
| 2006–2007 through 2009–2010 seasons | 31 | 66 | 1.64 (1.07 to 2.52) | 0.37 (0.15 to 0.94)b |
| 2010–2011 season | 13 | 18 | 2.53 (1.24 to 5.16) | 1.17 (0.24 to 5.78)c |
| HepA | 44 | 104 | 1.48 (1.04 to 2.11) | 0.88 (0.56 to 1.38)g |
| MMR | 25 | 50 | 1.75 (1.08 to 2.83) | 0.78 (0.44 to 1.40)g |
| VAR | 25 | 51 | 1.72 (1.06 to 2.77) | 0.80 (0.45 to 1.42)g |
| RV5 | 13 | 12 | 3.79 (1.73 to 8.31) | 1.18 (0.47 to 2.99)g |
| MMRV | 12 | 19 | 2.21 (1.07 to 4.55) | 1.12 (0.49 to 2.54)g |
| HepB | 4 | 7 | 2.00 (0.59 to 6.83) | 1.17 (0.31 to 4.40)g |
| 2009 H1N1 MIV | 3 | 19 | 0.55 (0.16 to 1.87) | 0.28 (0.08 to 1.02)g |
| IPV | 1 | 1 | 3.50 (0.22 to 55.96) | 1.41 (0.08 to 24.53)g |
| PPSV23 | 1 | 0 | Undefined | n/a |
Because some vaccines are not given together at the same visit (eg, DTaP and DTaP-HepB-iPV) or were not used during the same time period (eg, PCV7 and PCV13), different subgroups and regression models were used to obtain the adjusted estimate for each vaccine. The models are listed below. PPSV23, pneumococcal polysaccharide vaccine 23-valent; RV5, rotavirus vaccine pentavalent.
Terms in model: IIV3, PCV, DTaP-containing, IIV3*DTaP-containing, PCV*DTaP-containing, IIV3*PCV, IIV3*PCV*DTaP-containing.
Terms in model: IIV3, PCV7, DTaP-containing, IIV3*DTaP-containing, PCV7*DTaP-containing, IIV3*PCV7, IIV3*PCV7*DTaP-containing (restricted to the 2006–2007 through 2009–2010 time period and excluding patients who received PCV13).
Terms in model: IIV3, PCV13, DTaP-containing, IIV3*DTaP-containing, PCV13*DTaP-containing, IIV3*PCV13, IIV3*PCV13*DTaP-containing (restricted to the 2010–2011 time period and excluding patients who received PCV7).
Terms in model: IIV3, PCV, DTaP, IIV3*PCV, PCV*DTaP, IIV3*DTaP, IIV3*PCV*DTaP (excluding patients who received DTaP-HepB-IPV or DTaP-IPV/Hib).
Terms in model: IIV3, PCV, DTaP-HepB-iPV, IIV3*PCV, IIV3*DTaP-HepB-IPV (excluding patients who received DTaP or DTaP-iPV/Hib; the resulting sample size for this model did not allow for assessment of the 3-way product term).
Terms in model: IIV3, PCV, DTaP-IPV/Hib, IIV3*PCV, IIV3*DTaP-IPV/Hib (excluding patients who received DTaP or DTaP-HepB-IPV; the resulting sample size for this model did not allow for assessment of the 3-way product term).
Terms in model: vaccine-X, IIV3, PCV, DTaP-containing, IIV3*DTaP-containing, PCV*DTaP-containing, IIV3*PCV, IIV3*PCV*DTaP-containing.
Multivariable modeling identified potential interaction between IIV3, PCV, and DTaP-containing vaccines, therefore the final model included these 3 vaccines and their interaction terms. After adjusting for receipt of concomitant vaccines, only PCV7 was associated with an independent increased risk of FS (IRR = 1.98, 95% CI, 1.00 to 3.91). There was no independent increased risk with either IIV3 or DTaP-containing vaccines (Table 2).
Evaluating the risk of concomitant administration of IIV3, PCV, and DTaP-containing vaccines revealed the risk of FS was increased when IIV3 was given with either PCV or a DTaP-containing vaccine or when all 3 were given together (Table 3). The RERI between IIV3 and DTaP-containing vaccines was statistically significant and greater than the interaction between IIV3 and PCV, which was increased but not statistically significant (Table 4). The AR estimates vary with age because the baseline FS incidence changes with age, so we show the AR estimates for 3 ages at which vaccines are recommended (Table 5).
TABLE 3.
IRR of FS during the 0 to 1 Day After Vaccination for Each Unique Combination of IIV3, PCV, and DTaP-Containing Vaccines Estimated Using Self-controlled Risk Interval Analysis
| Time Period | 2006–2007 to 2009–2010 (N = 263)a |
2010–2011 (N = 66) | 2006–2007 to 2010–2011 (N = 333) | ||||
|---|---|---|---|---|---|---|---|
| Vaccines Administered Separately or Together |
Nc | IRR (95% CI)d | nc | IRR (95% CI)e | nc | IRR (95% CI)d |
P Value for Test of Interactionb |
| IIV3 | 52 | 0.37 (0.15 to 0.94) | 8 | 1.17 (0.24 to 5.78) | 60 | 0.46 (0.21 to 1.02) | n/a |
| PCVe | 36 | 1.98 (1.00 to 3.91) | 7 | 1.40 (0.27 to 7.22) | 44 | 1.81 (0.97 to 3.38) | n/a |
| DTaPf | 29 | 1.11 (0.48 to 2.61) | 6 | 0.70 (0.08 to 5.99) | 35 | 1.04 (0.47 to 2.28) | n/a |
| PCV + DTaP | 55 | 2.16 (1.25 to 3.72) | 12 | 2.50 (0.79 to 7.88) | 70 | 2.33 (1.45 to 3.76) | .7003 |
| IIV3 + DTaP | 16 | 3.50 (1.31 to 9.33) | 6 | 3.50 (0.71 to 17.34) | 22 | 3.50 (1.52 to 8.07) | .0038 |
| IIV3 + PCV | 6 | 3.50 (0.71 to 17.34) | 6 | 3.50 (0.71 to 17.34) | 12 | 3.50 (1.13 to 10.85) | .0624 |
| IIV3 + PCV + DTaP | 23 | 6.56 (2.78 to 15.48) | 11 | 2.92 (0.89 to 9.56) | 34 | 5.00 (2.53 to 9.90) | .0735 |
| Any vaccination event not involving IIV3, PCV, or DTaP | 46 | 0.43 (0.17 to 1.08) | 10 | 1.50 (0.39 to 5.80) | 56 | 0.58 (0.28 to 1.23) | n/a |
Our study included 267 FS cases during this time period, but we excluded from this analysis 4 patients who received PCV13 during this time period to avoid any potential residual confounding when evaluating the effect of PCV7.
Each product term between ≥2 vaccine variables in the final multivariable regression model was tested using a likelihood ratio statistic.
n equals the sum of FS cases in the risk and control intervals who received the vaccine or combination of vaccines listed in each row.
IRR was calculated using the final multivariable regression model, which included the following terms: IIV3, PCV, DTaP-containing, IIV3*DTaP-containing, PCV*DTaP-containing, IIV3*PCV, IIV3*PCV*DTaP-containing (PCV represents PCV7, PCV13, or either depending on the time period).
PCV7 was evaluated during the 2006–2007 to 2009–2010 time period because it was the PCV product in use for the vast majority of this period; PCV13 was evaluated during the 2010–2011 time period.
In this table, the notation “DTaP” includes all of the different types of DTaP-containing vaccine products.
TABLE 4.
Measures of Effect-Measure Modification (Interaction) Between All Unique Combinations of IIV3, PCV, and DTaP-Containing Vaccines
| Comparing the IRR (95% CI)a for Concomitant Vaccination With X+Y |
To the IRR (95% CI)a for X and Y Given on Separate Days |
Ratio of IRRs (95% CI) | RERI (95% CI)b | |||||
|---|---|---|---|---|---|---|---|---|
| X+Y | PCV + DTaP | 2.33 (1.45 to 3.76) | X | PCV | 1.81 (0.97 to 3.38) | (X+Y)/X | 1.29 (0.59 to 2.83) | 0.49 (−1.54 to 2.31) |
| Y | DTaP | 1.04 (0.47 to 2.28) | (X+Y)/Y | 2.25 (0.89 to 5.66) | ||||
| X+Y | IIV3 + DTaP | 3.50 (1.52 to 8.07) | X | IIV3 | 0.46 (0.21 to 1.02) | (X+Y)/X | 7.57 (2.40 to 23.89) | 3.00 (0.57 to 8.23) |
| Y | DTaP | 1.04 (0.47 to 2.28) | (X+Y)/Y | 3.38 (1.07 to 10.65) | ||||
| X+Y | IIV3 + PCV | 3.50 (1.13 to 10.85) | X | IIV3 | 0.46 (0.21 to 1.02) | (X+Y)/X | 7.57 (1.91 to 30.07) | 2.23 (−0.87 to 12.76) |
| Y | PCV | 1.81 (0.97 to 3.38) | (X+Y)/Y | 1.93 (0.53 to 7.04) | ||||
| X+Y | IIV3 + PCV + DTaP | 5.00 (2.53 to 9.90) | X | PCV + DTaP | 2.33 (1.45 to 3.76) | (X+Y)/X | 2.14 (0.93 to 4.93) | 3.20 (0.26 to 8.75) |
| Y | IIV3 | 0.46 (0.21 to 1.02) | (X+Y)/Y | 10.82 (3.81 to 30.69) | ||||
| X | IIV3+ DTaP | 3.50 (1.52 to 8.07) | (X+Y)/X | 1.43 (0.49 to 4.20) | 0.69 (−5.11 to 6.48) | |||
| Y | PCV | 1.81 (0.97 to 3.38) | (X+Y)/Y | 2.76 (1.10 to 6.96) | ||||
| X | IIV3+ PCV | 3.50 (1.13 to 10.85) | (X+Y)/X | 1.43 (0.38 to 5.36) | 1.46 (−9.27 to 7.45) | |||
| Y | DTaP | 1.04 (0.47 to 2.28) | (X+Y)/Y | 4.82 (1.70 to 13.69) | ||||
IRR values are from the self-controlled risk interval final multivariable regression model using all study time (N = 333).
Relative excess risk due to interaction (RERI) = IRRX+Y – IRRX – IRRY + 1. For the RERI, a value of 0 indicates that there is no interaction.
TABLE 5.
Estimated AR of FS during the 0 to 1 Days After Vaccination for Each Unique Combination of IIV3, PCV, and DTaP-Containing Vaccines
| Vaccines Administered Separately or Together |
AR per 100 000 Persons Vaccinateda |
||
|---|---|---|---|
| Age 6 mo | Age 12 mo | Age 15 mo | |
| IIV3 | 0 | 0 | 0 |
| PCV | 2 | 5 | 8 |
| DTaPb | 0 | 0 | 0 |
| PCV + DTaP | 2c | 5c | 8c |
| IIV3 + DTaP | 6 | 15 | 24 |
| IIV3 + PCV | 6 | 15 | 24 |
| IIV3 + PCV + DTaP | 10 | 24 | 38 |
Calculated using the IRR estimates from the pooled data (ie, 2006–2007 to 2010–2011 [N = 333]).
In this table, the notation “DTaP” includes all of the different types of DTaP-containing vaccine products.
The IRR for the combination of PCV + DTaP was not statistically significantly different from the sum of the IRRs for PCV and DTaP given on separate days, therefore the AR value shown is the same as the independent risk associated with PCV, which had the greater independent risk of the 2 vaccines.
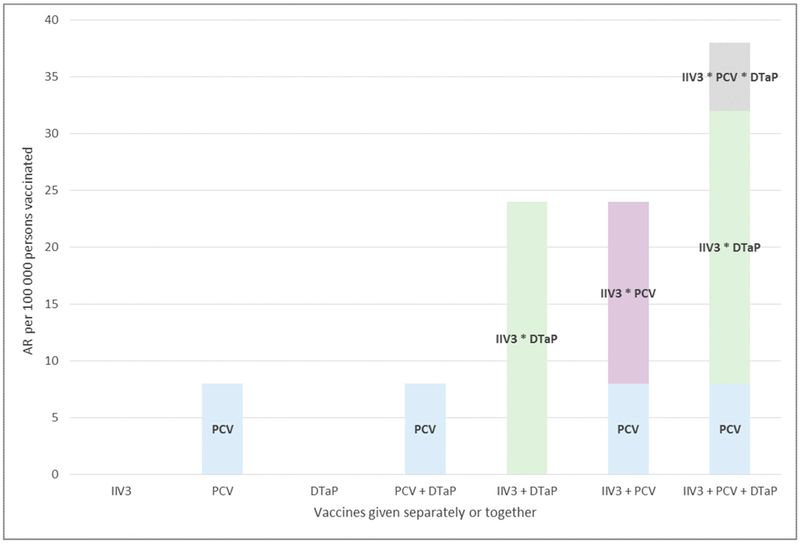
Subtracting the estimated AR for each vaccine from the AR for all 3 given together at age 15 months revealed that the maximum absolute excess risk of FS due to administration of these 3 vaccines together compared with receiving all 3 vaccines on separate days was 30 per 100 000 persons vaccinated. Compared with administration on separate days, the excess risk of receiving IIV3 with a DTaP-containing vaccine was 24 per 100 000 persons vaccinated and was 16 per 100 000 persons vaccinated for IIV3 with PCV. The absolute excess risk due to interaction is illustrated in Fig 1.
FIGURE 1.
Comparison of the estimated AR of FS associated with each unique combination of IIV3, PCV, and DTaP-containing vaccines when administered on separate days or together The AR estimates shown are for vaccine administration at 15 months of age. The x-axis denotes the vaccine or vaccine combination (indicated by a + between vaccine names). The total height of each column represents the AR for the vaccine or vaccine combination named on the x-axis. The individual blocks within each column indicate the estimated portion of the total risk that would be expected to be contributed by each individual vaccine (eg, PCV) or the excess risk contributed by the interaction between ≥2 vaccines (as indicated by a * [eg, IIV3*DTaP]). The AR for IIV3 or DTaP given alone is each 0. In this figure, the notation “DTaP” refers to any of the different types of DTaP-containing vaccines; “PCV” refers to either PCV7 or PCV13.
DISCUSSION
Among children aged 6 through 23 months who had a FS after vaccination, we found an increased risk of FS when IIV3 was given at the same time as either PCV or a DTaP-containing vaccine, but no increased risk of FS when IIV3 was given alone. Influenza vaccine has been recommended for all children ages 6 to 23 months since the 2004–2005 influenza season.18 IIV3 includes 3 different influenza virus strains, which may be changed each year to match influenza viruses expected in the upcoming season. During the 5 influenza seasons we studied, at least 1 of the 3 strains was changed each year. The 2010–2011 season formulation (the last year included in our study) was used again in the 2011–2012 season, and epidemiologic vaccine safety surveillance detected an increased risk of FS in both seasons.10,19 The IIV3 formulation was changed for the 2012–2013 season, and no increased risk of FS was observed in that season.19 In our study, IIV3 in the seasons before the 2010–2011 season appeared to have a protective effect against FS in the 0 to 1 days postvaccination. A true protective effect during this time period does not seem biologically plausible because it takes about 2 weeks for antibodies to develop postvaccination. This finding is likely due to the “healthy vaccinee effect,” in which the incidence of adverse events in the first few days after vaccination is less than the baseline incidence, because vaccination may be deferred for children who are acutely ill.20,21 For the 2010–2011 IIV3 formulation, we observed an IRR >1, which was not statistically significant but suggests a higher risk of FS than the previous seasons.
Studies conducted by CSL Limited using in vitro assays showed that the combination of the influenza B and A/H1N1 strains included in the 2010–2011 vaccine formulation elicited greater bioactivity than the strain combinations used in seasons going back to the 2005–2006 season, although it is unknown if this would also apply to the Sanofi Pasteur vaccines used in the United States.22,23
Our results indicate that PCV7 increases the risk of FS in the 2 days postvaccination by itself regardless of concomitant vaccination. During our study period, there were fewer doses of PCV13 given than PCV7, and our adjusted risk estimate for PCV13 had wide CIs, but our results do not suggest a difference in FS risk between PCV13 and PCV7. A previous study found no difference in FS risk for PCV13 compared with PCV7 in the 0 to 7 days postvaccination.24
Concerns about adverse events, including FS, with whole-cell pertussis vaccines prompted the development of the acellular pertussis vaccines currently used in the United States.25 In our study, the FS case-patients received 5 different DTaP-containing products. Most common were the DTaP-only products (64%), of which 83% were Infanrix (GlaxoSmithKline, Philadelphia, PA). The DTaP hepatitis b, inactivated poliovirus combination vaccine (DTaP-HepB-IPV; Pediarix, GlaxoSmithKline) was more common than the DTaP-IPV, Haemophilus influenza type b combination vaccine (DTaP-IPV/Hib; Pentacel, Sanofi Pasteur, Lyon, France). Comparing the risk of FS between the different types of DTaP-containing vaccines was not a primary aim of this study. The point estimate for DTaP-HepB-IPV was somewhat greater than for the other DTaP-containing products, but was not statistically significantly different. In clinical trials, DTaP-HepB-IPV had higher rates of fever compared with its separately administered component vaccines whereas DTaP-IPV/Hib did not.26,27 Because the various DTaP-containing vaccines are not given together, we categorized them as a single variable in our primary model for the purposes of testing effect-measure modification between vaccines given at the same time.
After controlling for the receipt of concomitant vaccines, we did not observe an independent risk of FS in the 0 to 1 days postvaccination for any vaccines other than PCV. The 0- to 1-day risk interval is likely only biologically plausible for inactivated vaccines (ie, IIV3, PCV, DTaP, HepA, HepB, Hib, IPV, and 2009 H1N1 MIV were assessed in this study). We also examined the risk in the 0- to 1-day interval for live vaccines for the sake of completeness. As expected, we observed no independent risk with rotavirus vaccine pentavalent, MMR, varicella vaccine (VAR), or MMRV. A previous study examined MMRV, MMR, and VAR using an appropriate 7- to 10-day risk interval.6 We did not have any FS cases who received either rotavirus vaccine monovalent or live attenuated influenza vaccine because neither is recommended in the 6- to 23-month-old age group.
Our main question was whether the risk of FS after IIV3 given at the same time as other vaccines was greater than the risk after administration of IIV3 and the other vaccines on separate days. We found that the concomitant administration of IIV3 with a DTaP-containing vaccine was associated with a risk of FS that was statistically significantly greater than the sum of the independent risks of each vaccine given separately. The relative excess risk due to the interaction between IIV3 and PCV was similarly elevated but not statistically significant, perhaps due to the smaller sample size for this combination. The clinical significance of the IIV3–PCV interaction is, however, supported by a study of fever incidence after IIV3 and PCV13 during the 2011–2012 influenza season that revealed a synergistic effect with concomitant administration of those 2 vaccines.28 Elucidating the biologic mechanism of these synergistic responses is a potential area for additional research. In our study, the concomitant administration of IIV3, PCV, and a DTaP-containing vaccine was associated with the greatest relative risk estimate, corresponding to an excess risk of 30 FS cases per 100 000 persons vaccinated compared with the administration of each vaccine on a separate day. This is similar to the AR of FS previously identified for the MMR vaccine of 25 to 34 FS per 100 000 persons vaccinated.5 This absolute risk may be outweighed by the benefits of timely vaccination that can be achieved by giving IIV3 on the same day as other vaccines when needed. We did not observe an excess risk when PCV and DTaP were given together without IIV3. The Advisory Committee on Immunization Practices does not recommend separating any of these vaccines to different days.29
Approximately 30% of children who have 1 FS will have more, and several risk factors for recurrence have been identified.30 Evidence is lacking for potential strategies to reduce the risk of FS. Giving prophylactic antipyretics before or at the time of vaccination is not recommended.31 Antipyretics given after a fever started did not prevent recurrent FS in a clinical trial.32 Oral diazepam given at the onset of febrile illness may be effective in preventing recurrent FS, but is not recommended because of the medication’s potential adverse effects.1
There are several challenges to studying the risk of concomitant vaccination using observational data. In practice, most childhood vaccines are administered concomitantly with other vaccines. There are 10 types of vaccines routinely recommended for all children between the ages of 6 and 23 months in the United States. When different formulations of the same product that change over time (eg, PCV13 vs PCV7, or IIV3 for each influenza season) are considered, the resulting number of unique combinations is large compared with the small number of FS cases that occur after vaccination. This means that every unique combination cannot be studied separately and all possible vaccine interactions cannot be examined at once. The original concern about an increased risk of FS with concomitant IIV3 and PCV13 vaccination was identified in a single influenza season. By pooling data from 5 seasons, we had a larger sample size, which enabled us to examine 2-way and 3-way interactions between IIV3 and other vaccines. Despite our large source population, we did not have enough FS cases to support regression models with more than 3-way interaction terms. The aim of this study was to assess the risk of concomitant administration of IIV3 with other vaccines, and our multivariable model building approach focused on vaccine combinations that included IIV3. We therefore cannot rule out the possibility that vaccine combinations other than those described above might also be associated with an increased risk of FS. We were not able to examine other risk windows in this study because we prespecified the 0- to 1-day interval and only reviewed charts for those cases; however, based on previous studies of postvaccination fever and FS, this is the most appropriate interval to examine for inactivated vaccines.
CONCLUSIONS
Our results suggest that the risk of FS is increased after certain combinations of vaccines, but the absolute risk of FS after these combinations is small. The risk of FS must be weighed against the benefits of timely vaccination as recommended. IIV3, PCV, and DTaP vaccines each have the potential to prevent multiple episodes of infection, including fevers and FS caused by those infections. The potential benefit of vaccination to prevent FS over longer periods is less readily apparent than the short-term risk of FS in the first few days immediately after vaccination. Over the long-term, vaccination might reduce the risk of FS, though additional study would be needed to quantify this for each type of vaccine. Additional research will be needed to identify evidence-based strategies to mitigate the short-term risk of FS postvaccination among individuals at high risk of FS.
WHAT’S KNOWN ON THIS SUBJECT:
Febrile seizures are mostly benign, but potentially alarming. Previous studies have found an increased risk of fever and febrile seizure on postvaccination days 0 and 1 when inactivated influenza vaccine and pneumococcal conjugate vaccine were given on the same day.
WHAT THIS STUDY ADDS:
Administration of an inactivated influenza vaccine and a diphtheria-tetanus-acellular pertussis vaccine on the same day was also associated with an increased risk of febrile seizure. The excess risk of giving these 3 vaccines together was 30 per 100 000 persons vaccinated.
ACKNOWLEDGMENTS
We thank the following individuals for their assistance with this study: Felicia Bixler, Kate Burniece, James Donahue, Karen Forsen, Sungching Glenn, Theresa Im, Galina Inzhakova, Stephanie Irving, Nancy Canul jauriga, Tara Johnson, Alison Kawai, Jennifer King, Ben Kruskal, Leslie Kuckler, Martin Kulldorff, Cat Magallon, Margarita Magallon, Natalie McCarthy, Michael McNeil, Jill Mesa, Sharyn M. Nuha, Valentyna Pishchalenko, Jackie Porcel, Melisa Rett, Virginia Robinson, Pat Ross, Denison Ryan, Ana Espinosa Rydman, Mark M. Schmidt, Laura Sirikulvadhana, Zendi Solano, Sandy Strey, Lina Sy, Aileen R. Uchida, Laurie VanArman, Vinutha X. Vijayadeva, and Carmen P. Wong.
We also thank the following organizations for contributing data to this study: Group Health, Harvard Vanguard Medical Associates and Harvard Pilgrim Health Care, HealthPartners, Kaiser Permanente Colorado, Kaiser Permanente Georgia, Kaiser Permanente Hawaii, Kaiser Permanente Northern California, Kaiser Permanente Northwest, Kaiser Permanente Southern California, and Marshfield Clinic.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names and commercial sources are for identification only and do not imply endorsement by the Centers for Disease Control and Prevention, the US Department of Health and Human Services, or the US Government.
FINANCIAL DISCLOSURE: Dr Naleway reports receiving research funding from GlaxoSmithKline and Pfizer for unrelated studies. Dr Klein reports receiving research support from GlaxoSmithKline, Sanofi-Pasteur, Pfizer, Merck & Co., Novartis, Protein Science, Nuron Biotech, and MedImmune for unrelated studies. The remaining authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This study was funded by the Centers for Disease Control and Prevention. No external funding was obtained for this study.
ABBREVIATIONS
- 2009 H1N1 MIV
influenza A(H1N1)2009 pandemic monovalent inactivated vaccine
- AR
attributable risk
- CI
confidence interval
- DTaP
diphtheria-tetanus-acellular-pertussis vaccine
- FS
febrile seizure
- HepA
hepatitis A vaccine
- HepB
Hepatitis B vaccine
- Hib
Haemophilus influenza type b vaccine
- ICD-9
International Classification of Diseases, Ninth Revision
- IIV3
trivalent inactivated influenza vaccine
- IPV
inactivated poliovirus vaccine
- IRR
incidence rate ratio
- MMR
measles, mumps, rubella vaccine
- MMRV
measles, mumps, rubella, varicella combination vaccine
- PCV
pneumococcal conjugate vaccine
- PCV7
pneumococcal conjugate vaccine 7-valent
- PCV13
pneumococcal conjugate vaccine 13-valent
- RERI
relative excess risk due to interaction
- VAR
varicella vaccine
- VSD
Vaccine Safety Datalink
Footnotes
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2016-0976.
REFERENCES
- 1.Steering Committee on Quality Improvement and Management, Subcommittee on Febrile Seizures American Academy of Pediatrics. Febrile seizures: clinical practice guideline for the long-term management of the child with simple febrile seizures. Pediatrics. 2008;121(6)1281–1286 [DOI] [PubMed] [Google Scholar]
- 2.Nelson KB, Ellenberg JH. Predictors of epilepsy in children who have experienced febrile seizures. N Engl J Med. 1976;295(19):1029–1033 [DOI] [PubMed] [Google Scholar]
- 3.Chung B, Wong V. Relationship between five common viruses and febrile seizure in children. Arch Dis Child. 2007;92(7):589–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu SS, Tse CY, Lau YL, Peiris M. Influenza A infection is an important cause of febrile seizures. Pediatrics. 2001;108(4). Available at: http://pediatrics.aappublications.org/content/108/4/e63 [DOI] [PubMed] [Google Scholar]
- 5.Barlow WE, Davis RL, Glasser JW, et al. ; Centers for Disease Control and Prevention Vaccine Safety Datalink Working Group. The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. N Engl J Med. 2001;345(9):656–661 [DOI] [PubMed] [Google Scholar]
- 6.Klein NP, Fireman B, Yih WK, et al. ; Vaccine Safety Datalink. Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizures. Pediatrics. 2010;126(1). Available at: http://pediatrics.aappublications.org/content/126/1/e1 [DOI] [PubMed] [Google Scholar]
- 7.Broder KR, Martin DB, Vellozzi C. In the heat of a signal: responding to a vaccine safety signal for febrile seizures after 2010–11 influenza vaccine in young children, United States. Vaccine 2012;30(11):2032–2034 [DOI] [PubMed] [Google Scholar]
- 8.Hambidge SJ, Glanz JM, France EK, et al. ; Vaccine Safety Datalink Team. Safety of trivalent inactivated influenza vaccine in children 6 to 23 months old. JAMA. 2006;296(16):1990–1997 [DOI] [PubMed] [Google Scholar]
- 9.Armstrong PK, Dowse GK, Effler PV, et al. Epidemiological study of severe febrile reactions in young children in Western Australia caused by a 2010 trivalent inactivated influenza vaccine. BMJ Open. 2011. ;1 (1):e000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tse A, Tseng HF, Greene SK, Vellozzi C, Lee G; VSD Rapid Cycle Analysis Influenza Working Group. Signal identification and evaluation for risk of febrile seizures in children following trivalent inactivated influenza vaccine in the Vaccine Safety Datalink Project, 2010–2011. Vaccine. 2012;30(11):2024–2031 [DOI] [PubMed] [Google Scholar]
- 11.US Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP). Available at: www.cdc.gov/vaccines/acip/index.html. Accessed March 17, 2016
- 12.McNeil MM, Gee J, Weintraub ES, et al. The Vaccine Safety Datalink: successes and challenges monitoring vaccine safety. Vaccine. 2014;32(42):5390–5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shui IM, Shi P, Dutta-Linn MM, et al. ; Vaccine Safety Datalink Research Team. Predictive value of seizure ICD-9 codes for vaccine safety research. Vaccine. 2009;27(39):5307–5312 [DOI] [PubMed] [Google Scholar]
- 14.Rowhani-Rahbar A, Klein NP, Dekker CL, et al. ; Risk Interval Working Group of the Clinical Immunization Safety Assessment Network. Biologically plausible and evidence-based risk intervals in immunization safety research. Vaccine. 2012;31(1):271–277 [DOI] [PubMed] [Google Scholar]
- 15.Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41(2):514–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Assmann SF, Hosmer DW, Lemeshow S, Mundt KA. Confidence intervals for measures of interaction. Epidemiology. 1996;7(3):286–290 [DOI] [PubMed] [Google Scholar]
- 18.Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB; Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2004;53(RR-6):1–40 [PubMed] [Google Scholar]
- 19.Kawai AT, Li L, Kulldorff M, et al. Absence of associations between influenza vaccines and increased risks of seizures, Guillain-Barré syndrome, encephalitis, or anaphylaxis in the 2012–2013 season. Pharmacoepidemiol Drug Saf. 2014;23(5):548–553 [DOI] [PubMed] [Google Scholar]
- 20.Virtanen M, Peltola H, Paunio M, Heinonen OP. Day-to-day reactogenicity and the healthy vaccinee effect of measles-mumps-rubella vaccination. Pediatrics. 2000;106(5). Available at: http://pediatrics.aappublications.org/content/106/5/e62 [DOI] [PubMed] [Google Scholar]
- 21.Wilson K, Hawken S, Kwong JC, et al. Adverse events following 12 and 18 month vaccinations: a population-based, self-controlled case series analysis. PLoS One. 2011;6(12):e27897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rockman S, Becher D, Dyson A, et al. Role of viral RNA and lipid in the adverse events associated with the 2010 Southern Hemisphere trivalent influenza vaccine. Vaccine 2014;32(30):3869–3876 [DOI] [PubMed] [Google Scholar]
- 23.Rockman S, Dyson A, Koernig S, et al. Evaluation of the bioactivity of influenza vaccine strains in vitro suggests that the introduction of new strains in the 2010 Southern Hemisphere trivalent influenza vaccine is associated with adverse events. Vaccine. 2014;32(30):3861–3868 [DOI] [PubMed] [Google Scholar]
- 24.Tseng HF, Sy LS, Liu IL, et al. Postlicensure surveillance for prespecified adverse events following the 13-valent pneumococcal conjugate vaccine in children. Vaccine. 2013;31(22):2578–2583 [DOI] [PubMed] [Google Scholar]
- 25.US Centers for Disease Control and Prevention. Pertussis vaccination: use of acellular pertussis vaccines among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1997;46(RR-7):1–25 [PubMed] [Google Scholar]
- 26.US Food and Drug Administration. Package Insert - Pentacel. Available at: www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm172502.htm. Accessed March 17, 2016 [Google Scholar]
- 27.US Food and Drug Administration. Package Insert - Pediarix. Available at: www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm146759.htm. Accessed March 17, 2016 [Google Scholar]
- 28.Stockwell MS, Broder K, LaRussa P, et al. Risk of fever after pediatric trivalent inactivated influenza vaccine and 13-valent pneumococcal conjugate vaccine. JAMA Pediatr. 2014;168(3):211–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Centers for Disease Control and Prevention. Childhood vaccines and febrile seizures. Available at: www.cdc.gov/vaccinesafety/concerns/febrile-seizures.html. Accessed March 17, 2016 [Google Scholar]
- 30.Sadleir LG, Scheffer IE. Febrile seizures. BMJ. 2007;334(7588):307–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Center for Immunization and Respiratory Diseases. General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(2):1–64 [PubMed] [Google Scholar]
- 32.Strengell T, Uhari M, Tarkka R, et al. Antipyretic agents for preventing recurrences of febrile seizures: randomized controlled trial. Arch Pediatr Adolesc Med. 2009;163(9):799–804 [DOI] [PubMed] [Google Scholar]