Abstract
The majority of the Yucatán State, México, presents subtropical climate that is suitable for many species of mosquitoes that are known to be vectors of diseases, including those from the genera Aedes and Culex. The objective of this study is to identify the geographic distribution of five species from these two genera and estimate the human population at risk of coming in contact with them. We compiled distributional data for Aedes aegypti (L.), Aedes (Howardina) cozumelensis (Diaz Najera), Culex coronator Dyar and Knab, Culex quinquefasciatus Say, and Culex thriambus Dyar from several entomological studies in Yucatán between March 2010 and September 2014. Based on these data, we constructed ecological niche models to predict the spatial distribution of each species using the MaxEnt algorithm. Our models identified areas with suitable environments for Ae. aegypti in most of Yucatán. A similar percentage of urban (97.1%) and rural (96.5%) populations were contained in areas of highest suitability for Ae. aegypti, and no spatial pattern was found (Moran’s I = 0.33, P=0.38); however, we found an association of abundance of immature forms of this species with annual mean temperature (r = 0.19, P≤0.001) and annual precipitation (r = 0.21, P≤0.001). Aedes cozumelensis is also distributed in most areas of the Yucatán State; Cx. quinquefasciatus, Cx. coronator, and Cx. thriambus are restricted to the northwest. The information generated in this study can inform decision-making to address control measures in priority areas with presence of these vectors.
Keywords: Aedes aegypti, ecological niche model, spatial distribution, Yucatán State
Several mosquito species of medical and veterinary importance have been identified in Yucatán state, México, and contribute to the transmission of a variety of human diseases (Farfán-Ale et al. 2009, 2010; Baak-Baak et al. 2014b). Aedes (Stegomyia) aegypti (L.), a primary vector of dengue, yellow fever, chikungunya, and Zika viruses, is the principal urban vector of dengue virus (Halstead 2008). Aedes aegypti’s efficiency as a vector is in part owing to its close association with humans (Cigarroa-Toledo et al. 2016); immature stages can be found in a wide range of water-holding containers, while females often feed and rest indoors (García-Rejón et al. 2008, Baak-Baak et al. 2014a). Aedes aegypti is known to occur in 66 communities in Yucatán State (Nájera-Vázquez et al. 2004, García-Rejín et al. 2012). In January 2016, the U.S. Centers for Disease Control and Prevention (CDC) advised pregnant women to postpone travel to what is now >30 countries and territories in the Caribbean, Central and South America (including México), where the agency has identified active transmission of the mosquito-borne Zika virus (http://www.cdc.gov/).
Dengue fever is the most important viral vector-borne disease in the world; 390 million of dengue infections are reported annually, of which, nearly 100 million manifest any level of disease severity (Bhatt et al. 2013). This disease is capable of collapsing health services when epidemics occur and represent enormous economic burden owing to hospitalizations, patient care, and vector surveillance (Halasa et al. 2012). México contributes to the high number of dengue cases in the Americas, with high incidence observed in Yucatán State in recent years (Dantes et al. 2014). Currently, there is a licensed vaccine for the dengue virus named Dengvaxia (developed by Sanofi Pasteur laboratories). However, dengue control focuses mainly on reducing vector populations owing to the high cost of the vaccine. Understanding the factors that determine the spatial and temporal distribution of Ae. aegypti could help improve dengue control programs.
Aedes (Howardina) cozumelensis (Diaz Najera) is also distributed in the Yucatán Peninsula; it overlaps the distribution of Ae. aegypti and Aedes albopictus (Skuse) and is also found in peridomestic settings; its ability of Ae. cozumelensis to transmit arboviruses or other pathogens is currently unknown but worth investigating owing to its close relatedness to known vectors and its behavioral characteristics (García-Rejón et al. 2012).
Several species of mosquitoes of the genus Culex are important vectors of arboviruses in North America, including West Nile virus (WNV), St. Louis encephalitis virus, and Western equine encephalitis virus, among others. Culex coronator Dyar and Knab, Culex lactator Dyar and Knab, Culex quinquefasciatus Say, and Culex thriambus Dyar are commonly present in Yucatán State (Nájera-Vázquez et al. 2004, García-Rejón et al. 2011, Baak-Baak et al. 2014b). Culex quinquefasciatus and Cx. thriambus are capable of transmitting WNV (Goddard et al. 2002, Reisen et al. 2006). Although this virus has not yet been detected from these mosquito species in Yucatán State, WNV was detected from Cx. quinquefasciatus in northern México (Elizondo-Quiroga et al. 2005). Recent studies revealed the presence of a novel virus in Cx. quinquefasciatus collected from Mérida City, THo virus, with still unknown pathogenicity to humans, and Culex flavivirus (CxFV), an insect-specific virus (Farfán-Ale et al. 2009, 2010). In Veracruz, Venezuelan equine encephalomyelitis virus was detected in Cx. coronator (Scherer et al. 1971).
Ecological niche modeling (ENM) algorithms have been extensively used in the study of the distribution and ecology of a variety of infectious diseases, their vectors, and hosts (Peterson et al. 2002, 2005, Fuller et al. 2011). These models are used to identify the geographic distribution of suitable environments for the transmission of diseases, vectors, or hosts and measure areas overlapping human populations (Moo-Llanes et al. 2013, Nakazawa et al. 2013), which in turn can be useful for planning effective surveillance programs and mitigation activities. The study of the associations between the distribution of mosquitoes and their biological or ecological characteristics enables further understanding of disease incidence (Morin et al. 2013) and contributes to explaining the spatial relationships of mosquitoes with climate change effects on distribution of diseases (Hales et al. 2002, Cummings et al. 2004).
To ensure effective prevention of dengue and other mosquito-borne diseases at a local level, it is important to understand the potential distribution of the vectors and their ecology. Here, we perform ENM to estimate the spatial distribution of mosquitoes and measure the human population at risk of contact with these species of mosquitoes, and to determine their associations with environmental variables.
Materials and Methods
Study Area
Yucatán State was chosen as a study site because it has been involved in the national history of dengue disease and other mosquito-borne diseases are known to circulate in this state. Yucatán has a subtropical climate with seasonal heavy rainfall from June–October (typically >100mm rainfall per month) and sporadic rainfall during the remaining, drier part of the year (García-Rejón et al. 2012). The classification of communities were carried out as described in previous studies based on the traditional settlements in Yucatán, where small urban homes (100 m2) have water drains and scarce vegetation, while bigger rural houses (300 m2) have dense vegetation (García-Rejón et al. 2012, Arana-Guardia et al. 2014); communities were classified based on the number of inhabitants: >10,000 for urban and <10,000 for rural (www.censo2010.org.mx/; last accessed Dec 2014). Urban environments were subjectively classified as homes with small yards, streets or sidewalks with storm-water drains and catch basins, or cemeteries located within densely populated areas, whereas rural environments were classified as homes in rural settings with larger yards and more vegetation or cemeteries located on the edge of a populated area (García-Rejón et al. 2012).
Mosquito Database
The database was constructed from immature mosquito collections performed from January 2014 to September 2014 and from published literature (García-Rejón et al. 2012, Arana-Guardia et al. 2014, Baak-Baak et al. 2014b). We visited 106 municipalities in Yucatán State. Each municipality was visited two times a year except for the city of Mérida, where collection was more frequent, about 10 collections per month. The database includes five species of mosquitoes: Ae. aegypti, Cx. quinquefasciatus, Cx. thriambus, Ae. (Howardina) cozumelensis (Diaz Najera), Cx. coronator (Table 1). Sampling for immature mosquitoes was performed two times a month using nets, turkey basters, or pipettes. Immature mosquitoes were collected in several water-holding containers such as disposable containers, tires, animal water bowls, buckets, discarded toilets, flower pots, vases, and storm-water drains and catch basins in a wide range of urban and rural environments, including residential premises, vacant lots, parking lots, cemeteries, and streets or sidewalks. When sampling storm-water drains and catch basins, long-handled zooplankton nets (20 by 10 cm, 100-μm mesh) were used as described previously by García-Rejón et al. (2011) and Arana-Guardia et al. (2014). The geographic coordinates of mosquito collection locations were collected using a global positioning system receiver (Garmin). Species identification was performed using stereomicroscopes and published keys (Carpenter and LaCasse 1955, Darsie and Ward 2005). The database included occurrence and total number of mosquitoes. The occurrence records were used to predict the spatial distribution of each species of mosquito, as well as to estimate the human population at risk for coming in contact with them, and the abundance of immatures mosquitoes was used to estimate the correlation of variables and spatial distribution.
Table 1.
Percentage of total area of Yucatán occupied by immature mosquitoes (sorted by species and environmental suitability); total area=34,100 km2
| Species | No. of localities in which species was collected | AUC value | Categories |
||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Ae. aegypti | 1,530 | 0.93 | 2.1 | 4.2 | 5.4 | 8.0 | 79.1 |
| Ae. cozumelensis | 11 | 0.92 | 18.5 | 14.2 | 10.8 | 6.9 | 3.4 |
| Cx. coronator | 11 | 0.98 | 1.4 | 0.9 | 0.6 | 1.1 | 2.2 |
| Cx. quinquefasciatus1 | 112 | 0.98 | 0.7 | 0.7 | 0.8 | 0.8 | 3.5 |
| Cx. thriambus | 12 | 0.96 | 2.6 | 2.9 | 0.7 | 1.3 | 3.4 |
AUC value of the ROC test. Environmental suitability: very low (1), low (2), medium (3), high (4), and very high (5)
Ecological Niche Models
Ecological niche models (ENMs) were constructed with mosquito occurrence data (n = 1,530) from all 106 municipalities that integrate Yucatán State, as well as abundance of immatures (n = 111,976). Thirteen environmental layers were used for the construction of ENM (Moo-Llanes et al. 2013): nine bioclimatic data layers (Bio1, Bio4, Bio5, Bio6, Bio7, Bio12, Bio13, Bio14, and Bio15: http://idrisi.uaemex.mx/index.php/ligas/geodatos/306-superficies-climaticas-paramexico; Cuervo-Robayo et al. 2014) and four topographic layers (aspect, slope, topographic index, and elevation; Hydro 1k data set-Earth Resources Observations and Science http://eros.usgs.gov/products/elevation/gtopo30/gtopo30.html). All environmental variables were selected based on intercorrelation values from a multicollinearity analysis (r<0.75) and have a spatial resolution of 1 km2 (Moo-Llanes et al. 2013). We used the maximum entropy (MaxEnt) algorithm to construct ENMs (Phillips et al. 2004). MaxEnt characterizes known occurrences in the context of the broader background to estimate a probability surface that maximizes entropy subject to constraints imposed by the occurrence data. Occurrence points were randomly divided by the algorithm into training data for model building (70%) and test data for model testing (30%; Anderson et al. 2003, Owens et al. 2013). The parameters used to build the ENMs were features (linear, product, threshold, and hinge), random test percentage (70%), replicated run type (bootstrap), maximum iterations (500), and threshold rule (minimum training presence). The models were evaluated using AUC (area under curve) value of the receiver operating characteristic (ROC) curve (Anderson et al. 2003, Moo-Llanes et al. 2013). For each species, 10 runs were performed with sets of randomly chosen points; a binary map (1 = suitable and 0 = unsuitable) of each model was obtained based on the probability threshold that allows 5% omission of training localities (Peterson et al. 2008). Finally, the 10 models were added to obtain a map of a scale of 0–10, where 10 represent the area where the 10 models agree.
Data Analysis
Ecological niche models for each mosquito species were reclassified into five risk categories: very low (1) = 1–2, low (2) = 3–4, medium (3) = 5–6, high (4) = 7–8, and very high (5) = 9–10, that represent suitable habitat for the distribution of mosquitoes. 1) The proportion of area occupied by each of the described categories was calculated using the entire area of Yucatan State for each mosquito species. 2) According to the 2010 census, the population of Yucatán State was 1,953,169 inhabitants, divided into rural (n = 676,943) and urban (n = 1,276,226) areas (www.censo2010.org.mx; last accessed December 2014). The rural communities are defined as those with <10,000 inhabitants, while urban communities have >10,000 inhabitants (Moo-Llanes et al. 2013). Potential population at risk was calculated as the proportion of the population within predicted suitable areas for each of the five species of mosquitoes in rural and urban settings. 3) Correlation between abundance of Ae. aegypti and annual temperature (Bio1), annual precipitation (Bio12), and elevation (E) was evaluated for the entire dataset and each year separately (Reiskind and Lounibos 2013), using the Spearman correlation in JMP statistical package version 7.0.0. 4) Spatial autocorrelation: Moran’s I was used to test whether the abundance of mosquitoes within Yucatán State is spatially correlated or not. Moran’s I measures spatial autocorrelation and is usually applied to area units where numerical ratio or interval data are available. It ranges from −1 for strong negative spatial autocorrelation to +1 for strong positive spatial autocorrelation. A value near 0 would indicate a spatially random pattern (Er et al. 2010). Analyses were performed with the total abundance of five species of mosquitoes from March 2010 to June 2013.
Results
Risk Category
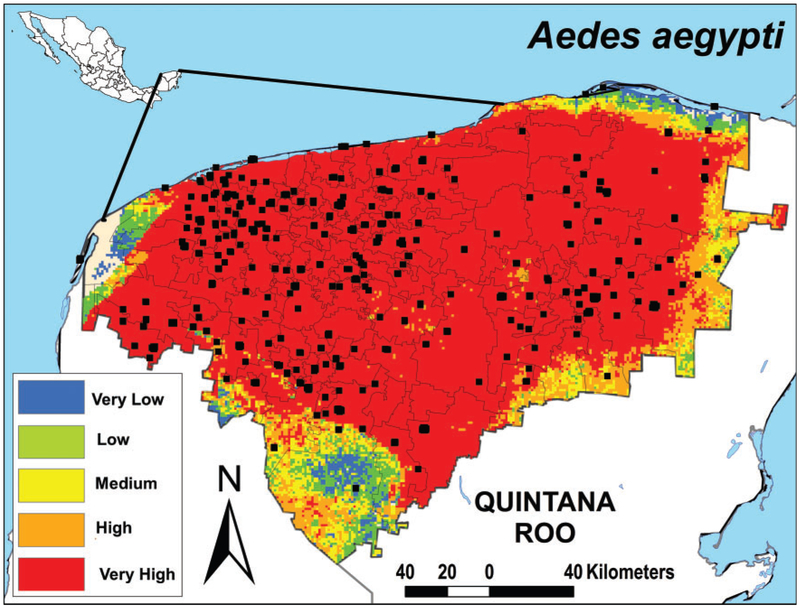
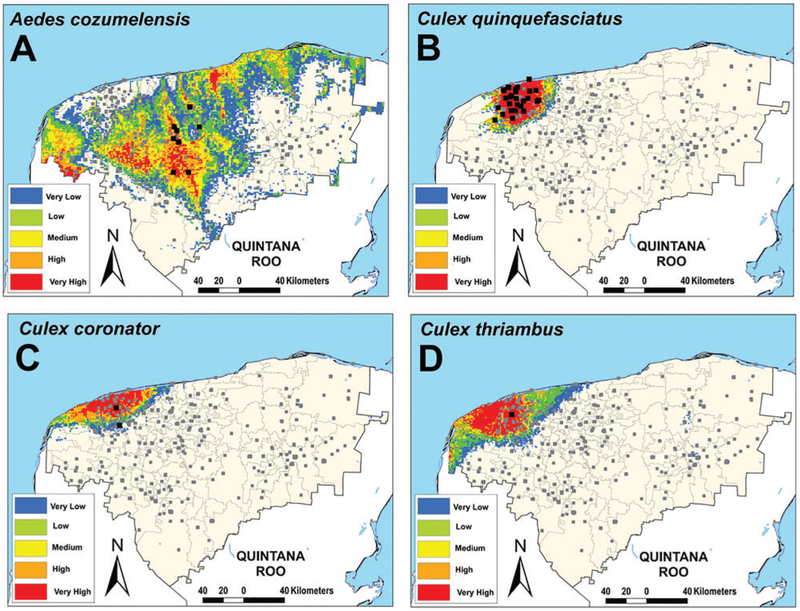
Our model predicted suitable areas with high exposure risk for Ae. aegypti (Table 1) throughout the state (AUC value = 0.93); Fig. 1 shows that the majority of Yucatán State was identified as suitable for Ae. aegypti. Many areas along the borders with Campeche and Quintana Roo states, as well as the coastline were identified as highly suitable environments for Ae. aegypti (Fig. 1). Currently, the involvement of Ae. cozumelensis as a vector of arboviruses in Yucatán is unknown. However, its distribution covered 53.8% of the state (Fig. 2A). The other mosquitoes were more geographically restricted; Cx. quinquefasciatus (Fig. 2B), Cx. coronator (Fig. 2C), and Cx. thriambus (Fig. 2D) were mainly distributed in the Northwest of the Yucatán state (Table 1).
Fig. 1.
Ecological niche models for spatial distribution of Ae. aegypti using MaxEnt and divided in five categories for risk. Black dots are sampling locations where Ae. aegypti was found to occur.
Fig. 2.
Ecological niche models of other mosquitoes in Yucatán State using MaxEnt and divided in five categories for risk. (A) Ae. cozumelensis; (B) Cx. quinquefasciatus; (C) Cx. coronator; and D) Cx. thriambus. Dots represent sampling locations where each species was found (black) and not found (gray).
At-Risk Human Population for Exposure to Mosquitoes
Similar proportions of the total urban (97.1%) and rural (96.5%) populations of Yucatán state were included in the areas identified as suitable for Ae. aegypti, but the very high risk category is the only one that includes urban population (Table 2). Sixty-six percent of the urban population of Yucatán was included in the very high risk category for Cx. quinquefasciatus and 68.3% was included in the very low risk category for Ae. cozumelensis, whereas 15.1% of the rural population is contained in categories 1–4 for Cx. thriambus and 9.3% for Cx. coronator (Table 2).
Table 2.
Percentage of the human population at risk for exposure to mosquitoes in Yucatán
| Species | Type | Categories |
||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Ae. aegypti | R | 0.1 | 0.2 | 0.9 | 3.8 | 91.6 |
| U | – | – | – | – | 97.1 | |
| Ae. cozumelensis | R | 18.9 | 19.3 | 14.3 | 8.5 | 5.9 |
| U | 68.3 | 4.4 | 8.7 | – | – | |
| Cx. coronator | R | 3.3 | 3.1 | 0.1 | 1.0 | 1.8 |
| U | – | 2.0 | – | – | – | |
| Cx. quinquefasciatus | R | 0.8 | 0.3 | 2.6 | 2.6 | 7.4 |
| U | – | – | – | – | 66.0 | |
| Cx. thriambus | R | 4.6 | 4.3 | 1.5 | 2.4 | 2.3 |
| U | – | 3.1 | – | – | 2.0 | |
The percentages are based on total population for Yucatán State and divided into five categories according to environmental suitability: very low (1), low (2), medium (3), high (4), and very high (5). Two types of populations: rural (R) and urban (U).
Correlation of Environmental Variables for Ae. aegypti
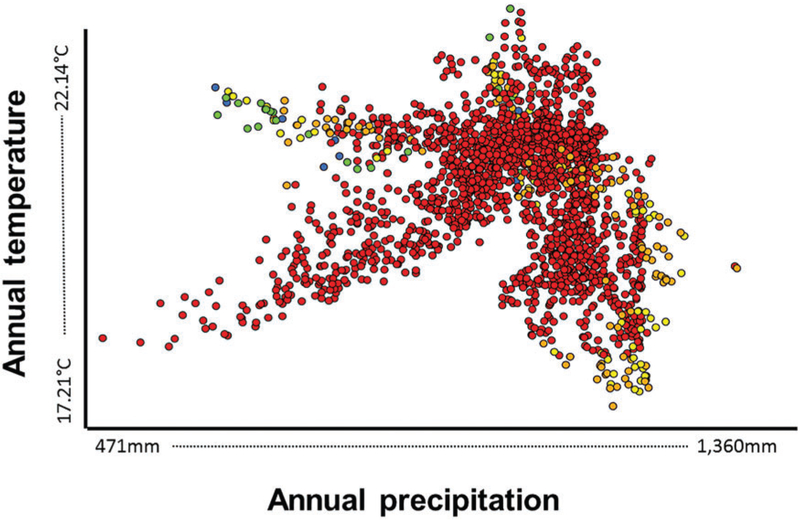
Abundance of immature Ae. aegypti from March 2010 to June 2013 was associated with annual mean temperature (r=0.19, P≤0.001), annual precipitation (r = 0.21, P≤0.001), and elevation (r = 0.15, P = 0.02). When the analysis was performed per year, association of abundance of Ae. aegypti with annual mean temperature (r=0.54, P<0.001) and annual precipitation (r=0.52, P<0.001) was only found in 2011. Based on the ENM, the presence of immature Ae. aegypti in Yucatán was found to be directly proportional to annual precipitation (>1,000mm) and annual mean temperature (~20 °C; Fig. 3).
Fig. 3.
Scatterplot showing the distribution of Ae. aegypti occurrences in an environmental space built with annual precipitation (X axis) and mean annual temperature (Y axis). Colors represent the five categories of environmental suitability: very low (blue), low (green), medium (yellow), high (orange), and very high (red).
Spatial Distribution
Spatial autocorrelation analyses were not significant for all species: Ae. aegypti (Moran’s I = 0.33, P=0.38), Cx. coronator (Moran’s I = −0.368, P=0.11), Cx. quinquefasciatus (Moran’s I = −0.007, P=0.45), Cx. thriambus (Moran’s I = −0.121, P=0.61), and Ae. cozumelensis (Moran’s I = 0.001, P=0.06); thus, it is not possible to reject the null hypothesis of random distribution of their occurrences throughout Yucatán.
Discussion
In México, dengue is one major public health problem; particularly, Yucatán State has had major outbreaks in the past seven years (Dantes et al. 2014). In the present study, we determined that Ae. aegypti is broadly distributed throughout the state of Yucatán, including the border with the Campeche and Quintana Roo states, and the coastline. Our results are consistent with the wide pattern of Ae. aegypti distribution previously reported in Yucatán (Nájera-Vázquez et al. 2004, García-Rejón et al. 2012).
The mosquito Ae. cozumelensis is not known to be a vector for any pathogens of medical or veterinary importance. However, the present study highlights areas in which environmental conditions are suitable for its presence and that rural population could have higher risk of exposure to this species. Previously, these were reported only in small rural communities (<6,000 inhabitants) in the north-central part of Yucatán State (García-Rejón et al. 2012). Many Culex species are potential vectors of WNV in North America (Goddard et al. 2002, Reisen et al. 2006). Formerly, this virus was detected in Cx. quinquefasciatus in Nuevo León in the northern México (Elizondo-Quiroga et al. 2005). Likewise, Cx. thriambus is susceptible to oral infection with WNV. In Yucatán, several birds (black vulture, great horned owl, peacock, plain chachalaca, and ruddy ground dove) and mammals (jaguar and coyote) have been seropositive for WNV by plaque reduction neutralization test (PRNT; Farfán-Ale et al. 2006). On the other hand, in Veracruz, Venezuelan equine encephalitis virus was isolated from Cx. coronator (Scherer et al. 1971). In our work, suitable environments for Culex species were restricted to the northwest region of Yucatán; only Cx. quinquefasciatus identifies a high proportion of the urban population within its potential distribution, which could represent a high-risk area for transmission of WNV by this vector.
Interestingly, urban and rural populations had similar risk for exposure to Ae. aegypti. This model is in accordance with the biology of this mosquito, because Ae. aegypti is found in urban (Halstead 2008; Baak-Baak et al. 2014a,b) and rural environments (Nájera-Vázquez et al. 2004, García-Rejón et al. 2012). Therefore, it is essential to carry out control measures and surveillance of Ae. aegypti not only in urban areas; including rural areas is crucial.
The abundance of Ae. aegypti is determined by adult emergence and the presence of immature stages. This cycle is influenced by climate, mainly by rainfall and temperature (Barrera et al. 2011). In our model, high annual precipitation (>1,000mm) and moderate annual temperature (~20 °C) was correlated with the presence of immature Ae. aegypti. In previous studies carried out in Mérida City, monthly average temperature, rainfall, and abundance of Ae. aegypti adults were positively correlated (Eisen et al. 2014), with higher presence in the rainy season between July and October (García-Rejón et al. 2008, Baak-Baak et al. 2014b). Likewise, a meta-analysis found the environmental factor of temperature was sufficient to explain development rate variability in Ae. aegypti (Couret and Benedict 2014). It is worth noting that the influence of climatic variables on the presence of Ae. aegypti is not always linear and stationary. For example, great abundance of adults is related to rainfall in Puerto Rico and maximum temperature in Thailand (Chaves et al. 2012). Moreover, hot environments are associated to decreased larval survival and increased fecundity as compensatory factor the populations (Chaves et al. 2014). In addition, climatic variables also influence the presence of diseases. The increase in weekly minimum temperature and rainfall were also found to be a significant factor in the increase of reported cases of dengue in Veracruz (Hurtado-Diaz et al. 2007). Similarly, weather was found to influence dengue incidence in México with nonlinear associations (Colon-Gonzalez et al. 2013).
Our analyses did not show spatial autocorrelation in the distribution of Ae. aegypti throughout Yucatán State. Previous studies in the Americas found immature infestations to be nonuniformly present throughout homes in urban environments, mainly found in heterogeneous habitats as cemeteries, vacant lots, markets, and public places (Morrison et al. 2006, Baak-Baak et al. 2014a). Random distributions of dengue cases has been described in Malaysia (Er et al. 2010), and in Thailand, the distribution of dengue fever, dengue hemorrhagic fever, and dengue shock syndrome had spatial associations (Jeefoo 2012). A likely explanation for the difference in spatial distribution of Ae. aegypti reported in the present study compared with studies performed with dengue cases could be that our sample population included immature mosquitoes collected in a wide variety of environmental settings, including residential premises, vacant lots, and cemeteries, as opposed to other studies focused on the distribution of dengue cases in homes.
Mérida, the capital of Yucatán, is considered the main hotspot of Ae. aegypti and dengue in the state (Loroño Pino et al. 1993; García-Rejón et al. 2008, 2011; Eisen et al. 2014). Some studies have highlighted the importance of hotspot detection of dengue and Ae. aegypti in Argentina (Rotela et al. 2007), Malaysia (Er et al. 2010, Dom et al. 2013), and Thailand (Jeefoo 2012). Other studies found that residents of dengue hotspots carry out more mosquito-breeding control measures compared with residents of nondengue hotspots (Ong et al. 2010).
One limitation of our study is that the risk of exposure of humans to mosquitoes was estimated using larval data and it is known that not all immatures reach the adult stage. However, we provide data on the presence of mosquitoes near humans in rural and urban environment. Ecological niche model estimated that Ae. aegypti has potential distribution throughout the state of Yucatán, so it is difficult to focus their control. Models generated for other species identified more restricted geographic distributions, which could help ensure more effective and efficient use of available resources for vector control and mitigation of risk exposure. The information generated during this study can inform decision-makers to address control measures in priority areas with presence of mosquito species of the genus Aedes and Culex.
Acknowledgments
We thank the laboratory staff Arbovirology of Universidad Autónoma de Yucatán for assistance with fieldwork and mosquito identification, and the involved home owners for granting us permission to collect mosquitoes. The study was supported partially by the Consejo Nacional de Ciencia y Tecnología de México grant INFR-2014-01-225046. We also thank the Secretaría de Investigación, Innovación y Educación Superior of the Government of Yucatan, for funding this publication,especially to Dr. Raúl Humberto Godoy-Montañez, Secretary.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References Cited
- Anderson RP, Lew D, and Peterson AT. 2003. Evaluating predictive models of species’ distributions: criteria for selecting optimal models. Ecol. Model. 162: 211–232. [Google Scholar]
- Arana-Guardia R, Baak-Baak CM, Loroño-Pino MA, Machain-Williams C, Beaty BJ, Eisen L, and García-Rejón JE. 2014. Stormwater drains and catch basins as sources for production of Aedes aegypti and Culex quinquefasciatus. Acta Trop. 134: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baak-Baak CM, Arana-Guardia R, Cigarroa-Toledo N, Loroño-Pino MA, Reyes-Solís G, Machain-Williams C, Beaty BJ, Eisen L, and García-Rejón JE. 2014a. Vacant lots: productive sites for Aedes (Stegomyia) aegypti (Diptera: Culicidae) in Mérida City, México. J. Med. Entomol. 51: 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baak-Baak CM, Arana-Guardia R, Cigarroa-Toledo N, Puc-Tinal M, Coba-Tun C, Rivero-Osorno V, Lavalle-Kantun D, Alba Lorono-Pino M, Machain-Williams C, Reyes-Solis GC, et al. 2014b. Urban mosquito fauna in Merida City, Mexico: immatures collected from containers and storm-water drains/catch basins. Southwest. Entomol. 39: 291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera R, Amador M, and MacKay AJ. 2011. Population dynamics of Aedes aegypti and dengue as influenced by weather and human behavior in San Juan, Puerto Rico. PLoS Negl. Trop. Dis. 5: e1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. 2013. The global distribution and burden of dengue. Nature 496: 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter SJ, and LaCasse WJ. 1955. Mosquitoes of North America (North of Mexico). University of California Press, Berkeley, CA. [Google Scholar]
- Chaves LF, Morrison AC, Kitron UD, and Scott TW. 2012. Nonlinear impacts of climatic variability on the density-dependent regulation of an insect vector of disease. Glob. Change Biol. 18: 457–468. [Google Scholar]
- Chaves LF, Scott TW, Morrison AC, and Takada T. 2014. Hot temperatures can force delayed mosquito outbreaks via sequential changes in Aedes aegypti demographic parameters in autocorrelated environments. Acta Trop. 129: 15–24. [DOI] [PubMed] [Google Scholar]
- Cigarroa-Toledo N, Blitvich BJ, Cetina-Trejo RC, Talavera-Aguilar LG, Baak-Baak CM, Torres-Chable OM, Hamid MN, Friedberg I, Gonzalez-Martinez P, Alonzo-Salomon G, et al. 2016. Chikungunya virus in febrile humans and Aedes aegypti mosquitoes, Yucatan, Mexico. Emerg. Infect. Dis. 22: 1804–1087. doi: 10.3201/eid2210.152087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon-Gonzalez FJ, Fezzi C, Lake IR, and Hunter PR. 2013. The effects of weather and climate change on dengue. PLoS Negl. Trop. Dis. 7: e2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couret J, and Benedict MQ. 2014. A meta-analysis of the factors influencing development rate variation in Aedes aegypti (Diptera: Culicidae). BMC Ecol. 14: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo-Robayo AP, Tellez-Valdes O, Gomez-Albores MA, Venegas-Barrera CS, Manjarrez J, and Martinez-Meyer E. 2014. An update of high-resolution monthly climate surfaces for Mexico. Int. J. Climatol. 34: 2427–2437. [Google Scholar]
- Cummings DA, Irizarry RA, Huang NE, Endy TP, Nisalak A, Ungchusak K, and Burke DS. 2004. Travelling waves in the occurrence of dengue hemorrhagic fever in Thailand. Nature 427: 344–347. [DOI] [PubMed] [Google Scholar]
- Dantes HG, Farfán-Ale JA, and Sarti E. 2014. Epidemiological trends of dengue disease in México (2000-2011): A systematic literature search and analysis. PLoS Negl. Trop. Dis. 8: e3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsie R, and Ward RA. 2005. Identification and geographical distribution of the mosquitoes of North America, North of Mexico. University Press of Florida, Gainesville, FL. [Google Scholar]
- Dom NC, Ahmad AH, Latif ZA, and Ismail R. 2013. Measurement of dengue epidemic spreading pattern using density analysis method: Retrospective spatial statistical study of dengue in Subang Jaya, Malaysia, 2006-2010. Trans. R. Soc. Trop. Med. Hyg. 107: 715–722. [DOI] [PubMed] [Google Scholar]
- Eisen L, Garcia-Rejon JE, Gomez-Carro S, Najera Vazquez MDR, Keefe TJ, Beaty BJ, and Lorono-Pino MA. 2014. Temporal correlations between mosquito-based dengue virus surveillance measures or indoor mosquito abundance and dengue case numbers in Merida City, Mexico. J. Med. Entomol. 51: 885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizondo-Quiroga D, Davis CT, Fernandez-Salas I, Escobar-Lopez R, Velasco Olmos D, Soto Gastalum LC, Aviles Acosta M, Elizondo-Quiroga A, Gonzalez-Rojas JI, Contreras Cordero JF, et al. 2005. West Nile Virus isolation in human and mosquitoes, Mexico. Emerg. Infect. Dis. 11: 1449–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Er AC, Rosli MH, Asmahani A, Mohamad NMR, and Harsuzilawati M. 2010. Spatial mapping of Dengue incidence: A case study in Hulu Langat District, Selangor, Malaysia. Int. J. Humanit. Soc. Sci. 4: 1–5. [Google Scholar]
- Farfán-Ale JA, Blitvich BJ, Marlenee NL, Lorono-Pino MA, Puerto-Manzano F, Garcia-Rejon JE, Rosado-Paredes EP, Flores-Flores LF, Ortega-Salazar A, Chavez-Medina J, et al. 2006. Antibodies to West Nile virus in asymptomatic mammals, birds, and reptiles in the Yucatan Peninsula of Mexico. Am. J. Trop. Med. Hyg. 74: 908–914. [PubMed] [Google Scholar]
- Farfán-Ale JA, Loroño-Pino MA, García-Rejón JE, Hovav E, Powers AM, Lin M, Dorman KS, Platt KB, Bartholomay LC, Soto V, et al. 2009. Detection of RNA from a novel West Nile-like virus and high prevalence of an insect-specific flavivirus in mosquitoes in the Yucatán Peninsula of México. Am. J. Trop. Med. Hyg. 80: 85–95. [PMC free article] [PubMed] [Google Scholar]
- Farfán-Ale JA, Loroño-Pino MA, García-Rejón JE, Soto V, Lin M, Staley M, Dorman KS, Bartholomay LC, Hovav E, and Blitvich BJ. 2010. Detection of flaviviruses and orthobunyaviruses in mosquitoes in the Yucatán Peninsula of México in 2008. Vector Borne Zoonotic Dis. 10: 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller T, Thomassen HA, Mulembakani PM, Johnston SC, Lloyd-Smith JO, Kisalu NK, Lutete TK, Blumberg S, Fair JN, and Wolfe ND. 2011. Using remote sensing to map the risk of human monkeypox virus in the Congo Basin. EcoHealth 8: 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rejón J, Loroño-Pino MA, Farfán-Ale JA, Flores-Flores L, Del Pilar Rosado-Paredes E, Rivero-Cardenas N, Nájera-Vázquez R, Gómez-Carro S, Lira-Zumbardo V, Gónzalez-Martínez P, et al. 2008. Dengue virus-infected Aedes aegypti in the home environment. Am. J. Trop. Med. Hyg. 79: 940–950. [PubMed] [Google Scholar]
- García-Rejón JE, Loroño-Pino MA, Farfán-Ale JA, Flores-Flores LF, López-Uribe MP, Nájera-Vázquez Mdel R, Nunez-Ayala G, Beaty BJ, and Eisen L. 2011. Mosquito infestation and dengue virus infection in Aedes aegypti females in schools in Mérida, México. Am. J. Trop. Med. Hyg. 84: 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rejóm JE, López-Uribe MP, Loroño-Pino MA, Arana-Guardia R, Puc-Tinal M, López-Uribe GM, Coba-Tun C, Baak-Baak CM, Machain-Williams C, Reyes-Solís GC, et al. 2012. Aedes (Stegomyia) aegypti and Aedes (Howardina) cozumelensis in Yucatán State, México, with a summary of published collection records for Ae. cozumelensis. J. Vector Ecol. 37: 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard LB, Roth AE, Reisen WK, and Scott TW. 2002. Vector competence of California mosquitoes for West Nile virus. Emerg. Infect. Dis. 8: 1385–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasa YA, Shepard DS, and Zeng W. 2012. Economic cost of dengue in Puerto Rico. Am. J. Trop. Med. Hyg. 86: 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales S, de Wet N, Maindonald J, and Woodward A. 2002. Potential effect of population and climate changes on global distribution of dengue fever: An empirical model. Lancet 360: 830–834. [DOI] [PubMed] [Google Scholar]
- Halstead SB 2008. Dengue virus-mosquito interactions. Annu. Rev. Entomol. 53: 273–291. [DOI] [PubMed] [Google Scholar]
- Hurtado-Diaz M, Riojas-Rodriguez H, Rothenberg SJ, Gomez-Dantes H, and Cifuentes E. 2007. Short communication: Impact of climate variability on the incidence of dengue in Mexico. Trop. Med. Int. Health. 12: 1327–1337. [DOI] [PubMed] [Google Scholar]
- Jeefoo P 2012. Spatial temporal dynamics and risk zonation of dengue fever, dengue hemorrhagic fever, and dengue shock syndrome in Thailand. I. J. Mod. Educ. Comp. Sci. 4: 58–68. [Google Scholar]
- Loroño Pino MA, Farfán Ale JA, Rosado Paredes E. d P., Kuno G, and Gubler DJ. 1993. Epidemic dengue 4 in the Yucatán, México, 1984. Inst. Med. Trop. Sao Paulo 35: 449–455. [DOI] [PubMed] [Google Scholar]
- Moo-Llanes D, Ibarra-Cerdena CN, Rebollar-Tellez EA, Ibanez-Bernal S, Gonzalez C, and Ramsey JM. 2013. Current and future niche of North and Central American sand flies (Diptera: Psychodidae) in climate change scenarios. PLoS Negl. Trop. Dis. 7: e2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CW, Comrie AC, and Ernst K. 2013. Climate and dengue transmission: Evidence and implications. Environ. Health Perspect. 121: 1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison AC, Sihuincha M, Stancil JD, Zamora E, Astete H, Olson JG, Vidal-Ore C, and Scott TW. 2006. Aedes aegypti (Diptera: Culicidae) production from non-residential sites in the Amazonian city of Iquitos, Peru. Ann. Trop. Med. Parasitol. 100: S73–S86. [DOI] [PubMed] [Google Scholar]
- Nájera-Vázquez R, Dzul F, Sabido M, Tun-Ku E, and Manrique-Saide P. 2004. New distribution records of mosquitoes (Diptera: Culicidae) for Yucatan, Mexico. Entomol. News 115: 181–190. [Google Scholar]
- Nakazawa Y, Lash RR, Carroll DS, Damon IK, Karem KL, Reynolds MG, Osorio JE, Rocke TE, Malekani JM, and Muyembe JJ. 2013. Mapping monkeypox transmission risk through time and space in the Congo Basin. PLoS Negl. Trop. Dis. 8: e74816.doi: 10.1371/journal.pone.0074816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong DQ, Sitaram N, Rajakulendran M, Koh GC, Seow AL, Ong ES, and Pang FY. 2010. Knowledge and practice of household mosquito breeding control measures between a dengue hotspot and non-hotspot in Singapore. Ann. Acad. Med. Singapore 39: 146–149. [PubMed] [Google Scholar]
- Owens HL, Campbell LP, Dornak LL, Saupe EE, Barve N, Soberon J, Ingenloff K, Lira-Noriega A, Hensz CM, Myers CE, et al. 2013. Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol. Model. 263: 10–18. [Google Scholar]
- Peterson AT, Sánchez-Cordero V, Beard CB, and Ramsey JM. 2002. Ecologic niche modeling and potential reservoirs for Chagas disease, México. Emerg. Infect. Dis. 8: 662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AT, Martínez-Campos C, Nakazawa Y, and Martínez-Meyer E. 2004. Time-specific ecological niche modeling predicts spatial dynamics of vector insects and human dengue cases. Trans. R. Soc. Trop. Med. Hyg. 99: 647–655. [DOI] [PubMed] [Google Scholar]
- Peterson AT, Papeş M, and Soberón J. 2008. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol. Model. 213: 63–72. [Google Scholar]
- Phillips S, Dudík M, and Schapire R. 2004. A maximum entropy approach to species distribution modeling. In Proceedings of the 21st International Conference on Machine Learning, Banff, Canada. [Google Scholar]
- Reisen WK, Fang Y, and Martinez VM. 2006. Vector competence of Culiseta incidens and Culex thriambus for West Nile virus. J. Am. Mosq. Control Assoc. 22: 662–665. [DOI] [PubMed] [Google Scholar]
- Reiskind MH, and Lounibos LP. 2013. Spatial and temporal patterns of abundance of Aedes aegypti L. (Stegomyia aegypti) and Aedes albopictus (Skuse) [Stegomyia albopictus (Skuse)] in southern Florida. Med. Vet. Entomol. 27: 421–429. [DOI] [PubMed] [Google Scholar]
- Rotela C, Fouque F, Lamfri M, Sabatier P, Introini V, Zaidenberg M, and Scavuzzo C. 2007. Space-time analysis of the dengue spreading dynamics in the 2004 Tartagal outbreak, Northern Argentina. Acta Trop. 103: 1–13. [DOI] [PubMed] [Google Scholar]
- Scherer WF, Dickerman RW, Diaz-Najera A, Ward BA, Miller MH, and Schaffer PA. 1971. Ecologic studies of Venezuelan encephalitis virus in southeastern Mexico. Infection of mosquitoes. Am. J. Trop. Med. Hyg. 20: 969–979. [DOI] [PubMed] [Google Scholar]