Abstract
Stroke is one of the most feared complications of aortic valve replacement. Although the outcomes of transcatheter aortic valve implantation (TAVI) improved substantially over time, concerns remained about a potentially higher incidence of stroke with TAVI compared with surgical replacement (SAVR). However, comparative data are sparse. We performed a meta-analysis comparing the incidence of stroke among patients undergoing TAVI versus SAVR. Of the 5067 studies screened, 28 eligible studies (22 propensity-score matched studies and 6 randomized trials) were analyzed. Primary endpoints were 30-day stroke and disabling stroke. Secondary endpoints were 1-year stroke and disabling stroke. A total of 23,587 patients were included, of whom 47.27% underwent TAVI and 52.72% underwent SAVR. For each endpoint, pooled estimates of odds ratio (OR) with 95% confidence interval (CI) were calculated. The pooled estimates for stroke (2.7% vs 3.1%, OR 0.86; 95% CI 0.72 to 1.02; p=0.08) and disabling stroke (2.5% vs 2.9%, OR 0.96; 95% CI 0.57 to 1.62; p=0.89) were comparable following TAVI versus SAVR at 30 days. Similarly, the pooled estimates for stroke (5.0% vs 4.6%, OR 1.01; 95% CI 0.79 to 1.28; p=0.96) and disabling stroke (4.1% vs 4.5%, OR 0.92; 95% CI 0.92 to 1.39; p=0.71) were similar at 1 year. A sensitivity analysis including only RCTs yielded similar results. Our meta-analysis documents comparable rates of strokes and disabling strokes following TAVI or SAVR both at 30 days and 1 year.
The introduction of transcatheter aortic valve implantation (TAVI) and the continuous improvement in the outcomes of surgical aortic valve replacement (SAVR) have revolutionized the treatment of severe aortic stenosis in the last decade.1 However, stroke remains one of the most feared and unresolved devastating complications of TAVI and SAVR.2,3 Although the interest in postprocedural stroke in patients undergoing TAVI or SAVR is growing, comparative studies between the two modalities are sparse.2,4 We performed a comprehensive systematic review and a meta-analysis of the published studies to compare the incidence of stroke and disabling stroke at 30 days and 1 year among patients undergoing TAVI and SAVR.
Methods
Our review protocol was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) reporting guidelines (Supplementary Protocol). We conducted a literature search in PUBMED, MEDLINE, EMBASE, EBSCO, CINAHL, Web of Science, and Cochrane (January 2, 2018) to identify eligible studies using the Medical Subject Headings search terms and text word search. The data were independently extracted by authors (T.B. and K.S.). Disagreements were resolved through consensus and arbitration by author (M.A.). Studies were included if they were (1) randomized controlled trials (RCTs) and propensity-matched prospective (PSM) observational studies comparing TAVI and SAVR, (2) published in peer-reviewed journals, (3) had follow-up of at least 30 days, and (4) reported stroke and/or disabling stroke as a clinical endpoint. Exclusion criteria included observational studies reporting nonpropensity matched populations and nonpublished studies (abstracts). The study characteristics extracted were the year of publication, study design, the number of patients, clinical characteristics, confounding factors, comparability between groups at baseline, outcomes, and study follow-up. The main outcomes of interest between the two interventions in this study included 30-day stroke and disabling stroke and 1-year stroke and disabling stroke.
The meta-analyses were performed using Comprehensive Meta-Analysis version 2.0 (Biostat, www.meta-analysis.com). For each clinical endpoint, pooled estimates of odds ratio (OR) with 95% confidence interval (CI) were calculated using the random effects model with the Mantel-Haenszel (MH) method. Heterogeneity among individual study effect sizes was examined using the I2 index, tau-squared, and the Q-test p value. Publication bias was assessed using funnel plots and Egger’s linear regression test of funnel plot asymmetry (Supplementary eFigure 1). Pooled estimates were displayed with 95% CI values and were considered statistically significant at p<0.05. A subanalysis was performed comparing stroke rates after TAVI or SAVR in subgroups of patients (low-to-inter-mediate risk and high risk). A sensitivity analysis was performed including RCT only.
Results
A total of 5067 potentially relevant citations were screened (Figure 1). After removal of duplicate and nonrelevant studies, we retrieved 76 full-text articles for evaluation, of which 28 satisfied the selection criteria. A total of 22 PSM observational studies and 6 RCTs were included in the meta-analysis (Table 1).3,5–31 All eligible studies were in the English language. Table 1 summarizes the baseline characteristics of the patients in the included studies. The 28 studies enrolled a total of 23,587 patients; 11,150 (47.27%) in the TAVI group and 12,437 (52.72%) in the SAVR group. Sample sizes ranged from 28 to 4732 patients. Although most studies involved patients at a high surgical risk, 2 RCTs and 6 PSM observational studies included patients at low-to-intermediate surgical risk. Detailed baseline characteristics of individual studies included in our meta-analysis are illustrated in Supplementary eTable 1.
Figure 1.
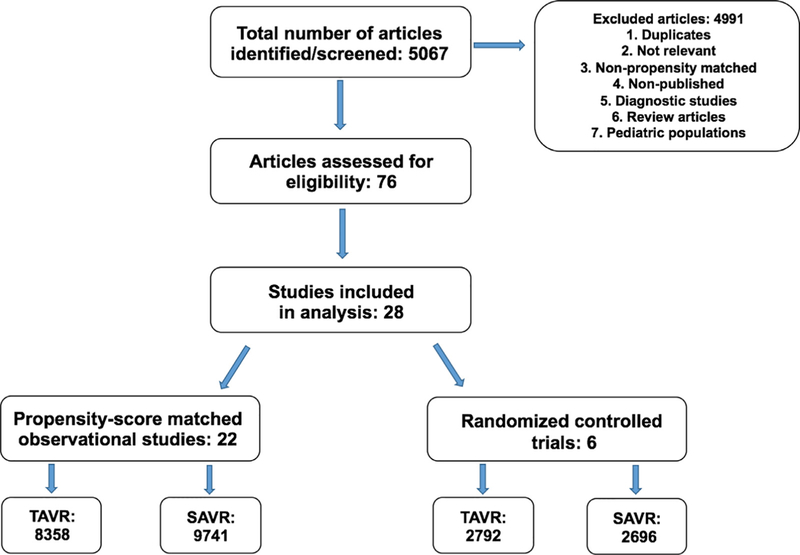
Flow chart of the meta-analysis.
Table 1.
Baseline characteristics of the patients included in the meta-analysis
| Baseline characteristics | TAVI (N=11,150) |
SAVR (N=12,437) |
p Value |
|---|---|---|---|
| Age (years) | 80.7 ± 1.8 | 80.2± 2.8 | 0.37 |
| Men | 47.6% | 47.2% | 0.89 |
| Coronary artery disease | 51.7% | 51.1% | 0.94 |
| Chronic kidney disease | 19% | 17.5% | 0.81 |
| (GFR<60 mL/min) | |||
| Diabetes mellitus | 27.4% | 27.8% | 0.89 |
| Atrial fibrillation | 29.3% | 29.4% | 0.98 |
| Chronic obstructive | 22.2% | 21.5% | 0.81 |
| pulmonary disease | |||
| Hypertension | 78.1% | 78.1% | 0.99 |
| Frailty | 25.9% | 26.8% | 0.96 |
| Hypercholesterolemia | 55.0% | 51.6% | 0.72 |
| Left ventricular ejection | 56.1±6.8 | 54.9±9.3 | 0.69 |
| fraction | |||
| Liver disease | 6.7% | 4.6% | 0.55 |
| Pulmonary hypertension | 21.9% | 20.9% | 0.88 |
| Peripheral vascular disease | 22.5% | 22.2% | 0.95 |
| Prior stroke or transient | 15.3% | 15.5% | 0.96 |
| ischemic attack | |||
| NYHA III or IV | 71.7% | 71.3% | 0.93 |
| Prior myocardial infarction | 15.9% | 15.3% | 0.84 |
| Prior coronary artery bypass | 31.7% | 23.0% | 0.37 |
| graft | |||
| Prior percutaneous coronary | 24.1% | 19.3% | 0.21 |
| intervention | |||
| STS score | 7.2±3.6 | 6.2±2.5 | 0.38 |
| Euro score | 17.0±8.3 | 15.5±7.1 | 0.52 |
GFR = glomerular filtration rate; NYHA = New York Heart Association; STS = Society of Thoracic Surgeons; SAVR = surgical aortic valve replacement; TAVI = transcatheter aortic valve implantation.
Meta-analysis of RCT and PSM studies
There was no statistically significant difference in 30-day stroke between TAVI and SAVR (2.7% vs 3.1%, OR 0.86; 95% CI 0.72 to 1.02; p=0.08; I2=3.019%; Figure 2). Of the total 28 studies included in the analysis, 8 studies (4 RCTs and 4 PSM observational studies; 3086 TAVI patients; 2998 SAVR patients) reported the rate of disabling stroke at 30 days, which was not statistically different following TAVI versus SAVR (2.5% vs 2.9%, OR 0.96; 95% CI 0.57 to 1.62; p=0.89; I2=42.81%; Figure 3). The incidence of all strokes at 1 year was similar in patients who underwent TAVI or SAvR (9 studies [5 RCtS and 4 PSM studies]; 16,544 total patients; 5.0% vs 4.6%, OR 1.01; 95% CI, 0.79 to 1.28; p=0.96; I2=46.94%; Figure 4). There were no PSM studies that reported disabling stroke at 1 year, and hence, only RCTs were included in the analysis. In a secondary analysis of low-to-intermediate risk patients and high-risk patients, there was still no difference in the incidence of stroke or disabling strokes at 30 days and 1 year between TAVI and SAVR in both cohorts (Supplementary eFigures 2-5).
Figure 2.
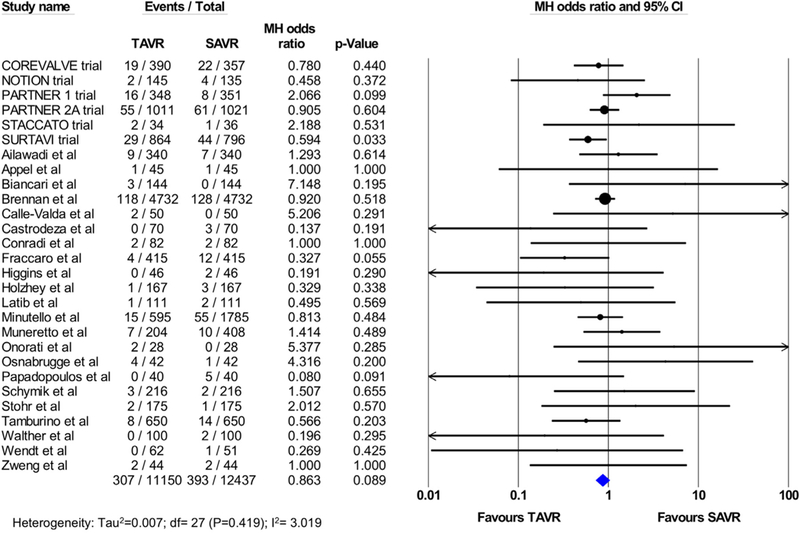
Pooled effect estimates for the risk of stroke at 30-day follow-up according to the type of aortic valve replacement procedure.
Figure 3.
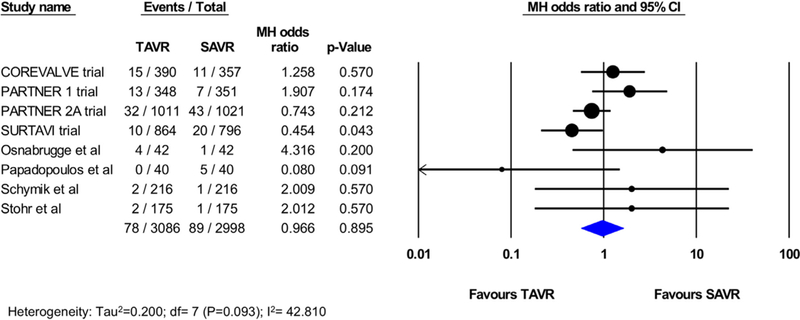
Pooled effect estimates for the risk of disabling stroke at 30-day follow-up according to the type of aortic valve replacement procedure.
Figure 4.
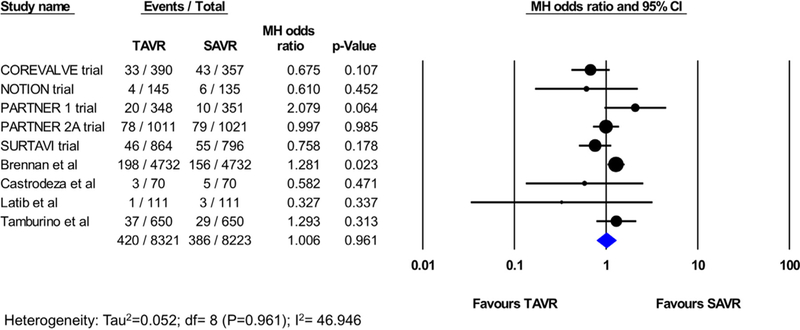
Pooled effect estimates for the risk of stroke at 1-year follow-up according to the type of aortic valve replacement procedure.
Meta-analysis of RCT only
In a sensitivity analysis excluding PSM studies and including RCTs only, similar rates of stroke were observed at 30-day follow-up after TAVI vs SAVR (6 RCTs, 5488 patients, 4.4% vs 5.2%, OR 0.86; 95% CI 0.61 to 1.2; p=0.41; I2=33.2%; Figure 5). Rates of disabling stroke at 30 days were also similar (4 RCTs, 5138 patients, 2.7% vs 3.2%, OR 0.90; 95% CI 0.52 to 1.53; p=0.684; I2=55.3%; Figure 5). Similarly, rates of stroke at 1 year (5 studies; 5418 patients; 6.6% vs 7.3%, OR 0.90; 95% CI 0.66 to 1.2; p=0.53; I2=45.3%) and disabling stroke (4 studies; 5138 patients; 4.1% vs 4.6%, OR 0.93; 95% CI 0.62 to 1.4; p=0.71; I2=48.5%) were not different between patients undergoing TAVI and those undergoing SAVR (Figure 6).
Figure 5.
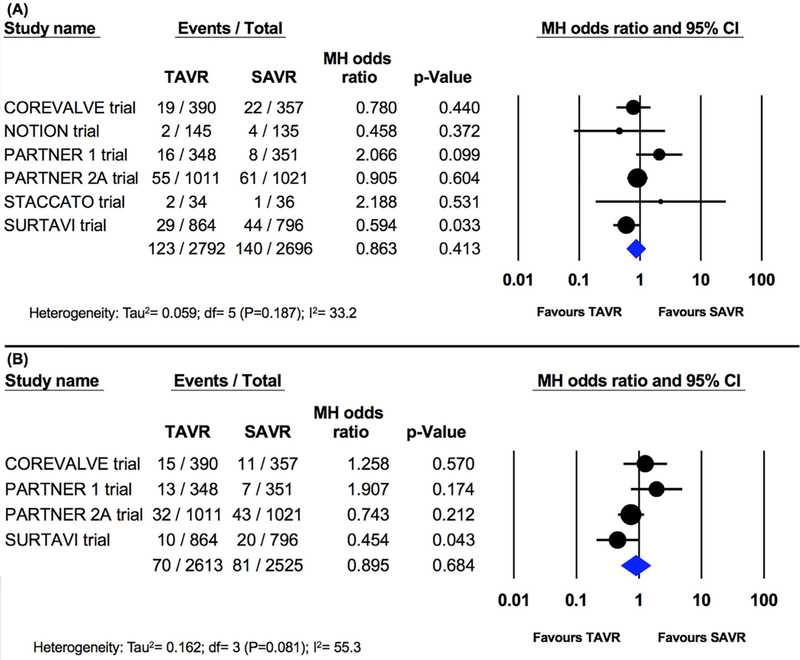
Pooled effect estimates for the risk of stroke at 30-day and 1-year follow-up according to the type of aortic valve replacement procedure in the randomized controlled trials.
Figure 6.
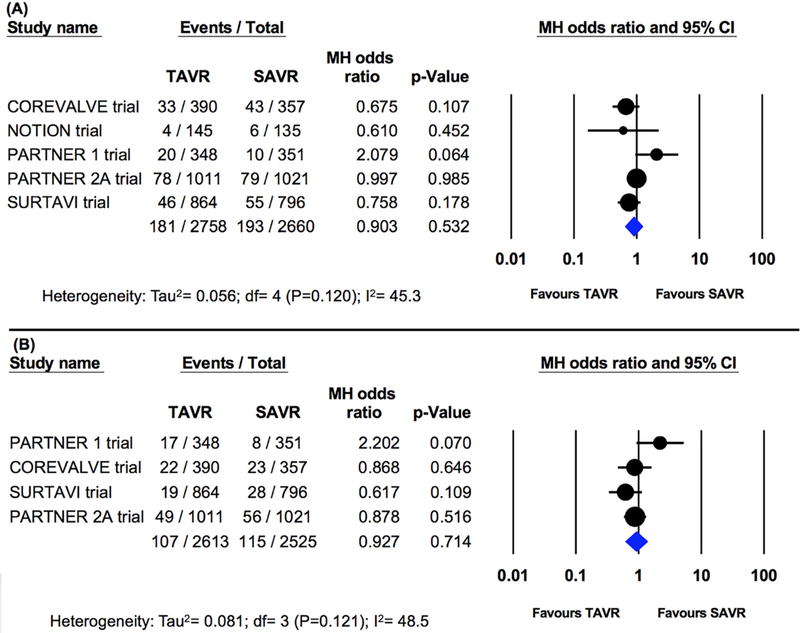
Pooled effect estimates for the risk of disabling stroke at 30-day and 1-year follow-up according to the type of aortic valve replacement procedure in the randomized controlled trials.
Discussion
Stroke is a potentially devastating consequence of aortic valve replacement, regardless of the replacement method. Postoperative stroke has been associated with a significantly increased risk of morbidity, mortality, and resource utilization following both transcatheter and SAVR.32 In the early experience with TAVI, concerns were raised about an excess rate of stroke with this technology compared with the traditional surgical aortic valve replacement; in the PARTNER trial (cohort A), there was a twofold higher stroke rate in the TAVI group compared with the SAVR group (4.6% vs 2.4%, p=0.07).5 However, neurologic outcomes were ascertained by a Clinical Events Committee chart review and not with neurologist-adjudicated testing. In the Pivotal CoreValve trial, no differences between TAVI and SAVR with regard to 30-day incidence of stroke were seen.7 Although both trials utilized first-generation transcatheter heart valves, these differences in neurologic outcomes were attributed to (1) prospective ascertainment of neurologic events with neurologist-adjudicated testing for all patients and (2) the inclusion of generally lower risk cohorts in the CoreValve trial compared with the PARTNER trial.7
Subsequent to the publication of these two pivotal trials, several RCTs and a large number of cohort studies have been published with variable reported incidence of stroke following surgical or transcatheter aortic valve replacement (see Supplementary Materials). However, uncertainties persisted regarding the differential impact of the replacement approach (transcatheter vs surgical) due to the variable definitions and reporting methodologies of stroke across the studies. Therefore, in this meta-analysis, we sought to assess the pooled incidence of two hard endpoints (stroke and disabling stroke) following SAVR or TAVI. To our knowledge, this is the largest study to date (23,587 patients) aiming to synthesize the best available evidence on stroke following aortic valve replacement in contemporary practice.
Our analysis reveals several intriguing findings. First, we found no significant difference between SAVR and TAVI with regards to the incidence of stroke at 30-day and 1-year follow-up. These findings were persistent in the overall cohort including RCTs and PSM studies, and when only RCTs were included. These findings confirm that in both controlled study and real-world settings, neither SAVR nor TAVI has been found to be a superior approach with regards to early or late stroke events. Whether this will persist in future studies including the latest generation transcatheter heart valves, those involving low-risk patients, and those utilizing cerebral embolic protection devices remain to be seen. Second, likewise, no differences were found between SAVR and TAVI in the 30-day and 1-year incidence of disabling stroke, confirming the equivalence between the two treatment modalities across a wide spectrum of stroke severity. Third, the reported incidences of stroke and disabling stroke were persistently greater in RCT than in PSM analyses, likely due to the protocoled assessment and adjudication of neurologic events in most RCTs. Last, there was a variable but persistent incremental increase in both stroke and disabling stroke events between 30-day and 1-year follow-up. There is a growing interest in improving post-TAVI neurologic outcomes, but most efforts are focused on strategies to minimize the short-term risk of stroke including testing various cerebral embolic protection devices, and optimizing peri-procedural antithrombotic management. However, this finding emphasizes the equally important knowledge gap surrounding preventative strategies that minimize the risk of stroke beyond the 30-day mark. This is no small task as establishing the definitive etiology of late strokes is rather complex in patients with typical risk factors for stroke (hypertension, diabetes, atrial fibrillation, carotid disease, and so on), and post-procedural factors that are often missed or are difficult to diagnose (new onset atrial fibrillation, leaflet thrombosis, and so on).
This is a study-level meta-analysis, and hence, the effect of individual baseline characteristics on the outcomes cannot be thoroughly assessed. Also, significant variability in the definition and ascertainment of stroke were noted. However, we only included RCTs and PSM analyses with similar intrastudy definitions of stroke and disabling stroke. In addition, the results of our meta-analysis persisted in a sensitivity analysis including RCTs only, and in subanalyses of low-to-intermediate risk and high-risk patients, further confirming the validity of our results.
Postoperative stroke remains one of the most clinically detrimental consequences of both TAVI and SAVR. Based on this meta-analysis, there was no difference in early or late stroke or disabling stroke rates in patients undergoing either TAVI or SAVR. Neuroprotective strategies newer generation devices and improved long-term secondary stroke prevention may help improve cerebral complication rates.
Supplementary Material
Footnotes
Disclosures
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.amjcard.2018.06.032.
The authors have no conflicts of interest to disclose.
References
- 1.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD. American College of Cardiology/American Heart Association Task Force on Practice G. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57–185. [DOI] [PubMed] [Google Scholar]
- 2.Kahlert P, Al-Rashid F, Dottger P, Mori K, Plicht B, Wendt D, Bergmann L, Kottenberg E, Schlamann M, Mummel P, Holle D, Thielmann M, Jakob HG, Konorza T, Heusch G, Erbel R, Eggebrecht H. Cerebral embolization during transcatheter aortic valve implantation: a transcranial Doppler study. Circulation 2012;126:1245–1255. [DOI] [PubMed] [Google Scholar]
- 3.Fraccaro C, Tarantini G, Rosato S, Tellaroli P, D’Errigo P, Tamburino C, Onorati F, Ranucci M, Barbanti M, Grossi C, Santoro G, Santini F, Covello RD, Fusco D, Seccareccia F, Group OR. Early and midterm outcome of propensity-matched intermediate-risk patients aged >/=80 years with aortic stenosis undergoing surgical or transcatheter aortic valve replacement (from the Italian Multicenter OBSERVANT study). Am J Cardiol 2016;117:1494–1501. [DOI] [PubMed] [Google Scholar]
- 4.Abdul-Jawad Altisent O, Ferreira-Gonzalez I, Marsal JR, Ribera A, Auger C, Ortega G, Cascant P, Urena M, Del Blanco BG, Serra V, Sureda C, Igual A, Rovira A, Gonzalez-Alujas MT, Gonzalez A, Puri R, Cuellar H, Tornos P, Rodes-Cabau J, Garcia-Dorado D. Neurological damage after transcatheter aortic valve implantation compared with surgical aortic valve replacement in intermediate risk patients. Clin Res Cardiol 2016;105:508–517. [DOI] [PubMed] [Google Scholar]
- 5.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. Investigators PT. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl JMed 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen HH, Klaaborg KE, Nissen H, Terp K, Mortensen PE, Kjeldsen BJ, Jakobsen CJ, Andersen HR, Egeblad H, Krusell LR, Thuesen L, Hjortdal VE. A prospective, randomised trial of transapical transcatheter aortic valve implantation vs. surgical aortic valve replacement in operable elderly patients with aortic stenosis: the STACCATO trial. EuroIntervention 2012;8:383–389. [DOI] [PubMed] [Google Scholar]
- 7.Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J Jr., Kleiman NS, Chet-cuti S, Heiser J, Merhi W, Zorn G, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte J, Maini B, Mumtaz M, Chenoweth S, Oh JK Investigators USCC. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N EnglJMed 2014;370:1790–1798. [DOI] [PubMed] [Google Scholar]
- 8.Thyregod HG, Steinbruchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, Chang Y, Franzen OW, Engstrom T, Clemmensen P, Hansen PB, Andersen LW, Olsen PS, Sondergaard L. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the All-Comers NOTION randomized clinical trial. JAm Coll Cardiol 2015;65:2184–2194. [DOI] [PubMed] [Google Scholar]
- 9.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG, Investigators P. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N EnglJMed 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 10.Reardon MJ, Van Mieghem NM Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PW, Kappetein AP Investigators S. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]
- 11.Walther T, Schuler G, Borger MA, Kempfert J, Seeburger J, Ruckert Y, Ender J, Linke A, Scholz M, Falk V, Mohr FW. Transapical aortic valve implantation in 100 consecutive patients: comparison to propensity-matched conventional aortic valve replacement. Eur Heart J 2010;31:1398–1403. [DOI] [PubMed] [Google Scholar]
- 12.Higgins J, Ye J, Humphries KH, Cheung A, Wood DA, Webb JG, Lichtenstein SV. Early clinical outcomes after transapical aortic valve implantation: a propensity-matched comparison with conventional aortic valve replacement. J Thorac Cardiovasc Surg 2011;142:e47–e52. [DOI] [PubMed] [Google Scholar]
- 13.Stohr R, Dohmen G, Herpertz R, Brehmer K, Aktug O, Koos R, Altiok E, Stegemann E, Autschbach R, Marx N, Hoffmann R. Thirty-day outcome after transcatheter aortic valve implantation compared with surgical valve replacement in patients with high-risk aortic stenosis: a matched comparison. Coron Artery Dis 2011;22:595–600. [DOI] [PubMed] [Google Scholar]
- 14.Holzhey DM, Shi W, Rastan A, Borger MA, Hansig M, Mohr FW. Transapical versus conventional aortic valve replacement – a propensity-matched comparison. Heart Surg Forum 2012;15:E4–E8. [DOI] [PubMed] [Google Scholar]
- 15.Appel CF, Hultkvist H, Nylander E, Ahn H, Nielsen NE, Freter W, Vanky F. Transcatheter versus surgical treatment for aortic stenosis: patient selection and early outcome. Scand Cardiovasc J 2012;46:301–307. [DOI] [PubMed] [Google Scholar]
- 16.Latib A, Maisano F, Bertoldi L, Giacomini A, Shannon J, Cioni M, Ielasi A, Figini F, Tagaki K, Franco A, Covello RD, Grimaldi A, Spagnolo P, Buchannan GL, Carlino M, Chieffo A, Montorfano M, Alfieri O, Colombo A. Transcatheter vs surgical aortic valve replacement in intermediate-surgical-risk patients with aortic stenosis: a propensity score-matched case-control study. Am Heart J 2012;164:910–917. [DOI] [PubMed] [Google Scholar]
- 17.Osnabrugge RL, Head SJ, Genders TS, Van Mieghem NM, De Jaegere PP, van der Boon RM, Kerkvliet JM, Kalesan B, Bogers AJ, Kappetein AP, Hunink MG. Costs of transcatheter versus surgical aortic valve replacement in intermediate-risk patients. Ann Thorac Surg 2012;94:1954–1960. [DOI] [PubMed] [Google Scholar]
- 18.Conradi L, Seiffert M, Treede H, Silaschi M, Baldus S, Schirmer J, Kersten JF, Meinertz T, Reichenspurner H. Transcatheter aortic valve implantation versus surgical aortic valve replacement: a propensity score analysis in patients at high surgical risk. J Thorac Cardiovasc Surg 2012;143:64–71. [DOI] [PubMed] [Google Scholar]
- 19.Papadopoulos N, Schiller N, Fichtlscherer S, Lehmann R, Weber CF, Moritz A, Doss M, Zierer A. Propensity matched analysis of longterm outcomes following transcatheter based aortic valve implantation versus classic aortic valve replacement in patients with previous cardiac surgery. J Cardiothorac Surg 2014;9:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamburino C, Barbanti M, D’Errigo P, Ranucci M, Onorati F, Covello RD, Santini F, Rosato S, Santoro G, Fusco D, Grossi C, Seccareccia F, Group OR. 1-year outcomes after transfemoral transcatheter or surgical aortic valve replacement: results from the Italian OBSERVANT study. J Am Coll Cardiol 2015;66:804–812. [DOI] [PubMed] [Google Scholar]
- 21.Minutello RM, Wong SC, Swaminathan RV, Feldman DN, Kaple RK, Horn EM, Devereux RB, Salemi A, Sun X, Singh H, Bergman G, Kim LK. Costs and in-hospital outcomes of transcatheter aortic valve implantation versus surgical aortic valve replacement in commercial cases using a propensity score matched model. Am J Cardiol 2015;115:1443–1447. [DOI] [PubMed] [Google Scholar]
- 22.Schymik G, Heimeshoff M, Bramlage P, Herbinger T, Wurth A, Pilz L, Schymik JS, Wondraschek R, Suselbeck T, Gerhardus J, Luik A, Gonska BD, Tzamalis P, Posival H, Schmitt C, Schrofel H. A comparison of transcatheter aortic valve implantation and surgical aortic valve replacement in 1,141 patients with severe symptomatic aortic stenosis and less than high risk. Catheter Cardiovasc Interv 2015;86:738–744. [DOI] [PubMed] [Google Scholar]
- 23.Muneretto C, Alfieri O, Cesana BM, Bisleri G, De Bonis M DiBartolomeo R, Savini C, Folesani G, Di Bacco L, Rambaldini M, Maureira JP, Laborde F, Tespili M, Repossini A, Folliguet T A comparison of conventional surgery, transcatheter aortic valve replacement, and sutureless valves in “real-world” patients with aortic stenosis and intermediate- to high-risk profile. J Thorac Cardiovasc Surg 2015; 150:1570–1577. discussion 1577_1579. [DOI] [PubMed] [Google Scholar]
- 24.Wendt D, Al-Rashid F, Kahlert P, El-Chilali K, Demircioglu E, Neuhauser M, Liakopoulos O, Sebastian Dohle D, Erbel R, Jakob H, Thielmann M. Conventional aortic valve replacement or transcatheter aortic valve implantation in patients with previous cardiac surgery. J Cardiol 2015;66:292–297. [DOI] [PubMed] [Google Scholar]
- 25.Zweng I, Shi WY, Palmer S, MacIsaac A, Whitbourn R, Davis P, Newcomb AE. Transcatheter versus surgical aortic valve replacement in high-risk patients: a propensity-score matched analysis. Heart Lung Circ 2016;25:661–667. [DOI] [PubMed] [Google Scholar]
- 26.Biancari F, Barbanti M, Santarpino G, Deste W, Tamburino C, Gulino S, Imme S, Di Simone E, Todaro D, Pollari F, Fischlein T, Kasama K, Meuris B, Dalen M, Sartipy U, Svenarud P, Lahtinen J, Heikkinen J, Juvonen T, Gatti G, Pappalardo A, Mignosa C, Rubino AS. Immediate outcome after sutureless versus transcatheter aortic valve replacement. Heart Vessels 2016;31:427–433. [DOI] [PubMed] [Google Scholar]
- 27.Castrodeza J, Amat-Santos IJ, Blanco M, Cortes C, Tobar J, Martin- Morquecho I, Lopez J, Di Stefano S, Rojas P, Varela-Falcon LH, Gomez I, San Roman JA. Propensity score matched comparison of transcatheter aortic valve implantation versus conventional surgery in intermediate and low risk aortic stenosis patients: a hint of real-world. Cardiol J 2016;23:541–551. [DOI] [PubMed] [Google Scholar]
- 28.Onorati F, D’Onofrio A, Biancari F, Salizzoni S, De Feo M, Agrifoglio M, Mariscalco G, Lucchetti V, Messina A, Musumeci F, Santarpino G, Esposito G, Santini F, Magagna P, Beghi C, Aiello M, Ratta ED, Savini C, Troise G, Cassese M, Fischlein T, Glauber M, Passerone G, Punta G, Juvonen T, Alfieri O, Gabbieri D, Mangino D, Agostinelli A, Livi U, Di Gregorio O, Minati A, Rinaldi M, Gerosa G, Faggian G. Investigators ITA Record. Results of surgical aortic valve replacement and transapical transcatheter aortic valve replacement in patients with previous coronary artery bypass grafting. Interact Cardiovasc Thorac Surg 2016;22:806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ailawadi G, LaPar DJ, Speir AM, Ghanta RK, Yarboro LT, Crosby IK, Lim DS, Quader MA, Rich JB. Contemporary costs associated with transcatheter aortic valve replacement: a propensity-matched cost analysis. Ann Thorac Surg 2016;101:154–160. [DOI] [PubMed] [Google Scholar]
- 30.Calle-Valda CM, Aguilar R, Benedicto A, Sarraj A, Monguio E, Munoz D, De Antonio N, Reyes G. Outcomes of aortic valve replacement according to surgical approach in intermediate and low risk patients: a propensity score analysis. Heart Lung Circ 2018;27:885–892. [DOI] [PubMed] [Google Scholar]
- 31.Brennan JM, Thomas L, Cohen DJ, Shahian D, Wang A, Mack MJ, Holmes DR, Edwards FH, Frankel NZ, Baron SJ, Carroll J, Thourani V, Tuzcu EM, Arnold SV, Cohn R, Maser T, Schawe B, Strong S, Stickfort A, Patrick-Lake E, Graham FL, Dai D, Li F, Matsouaka RA, O’Brien S, Li F, Pencina MJ, Peterson ED. Transcatheter versus surgical aortic valve replacement: propensity-matched comparison. J Am Coll Cardiol 2017;70:439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agarwal S, Garg A, Parashar A, Svensson LG, Tuzcu EM, Navia JL, Mick S, Kapadia SR. In-hospital mortality and stroke after surgical aortic valve replacement: a nationwide perspective. J Thorac Cardiovasc Surg 2015;150:571–578.e8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


