Abstract
Background:
Vascular age is an emerging health indicator and predictor of end-organ damage to the heart, brain, and kidney. Although there have been many review publications concerning risk factors for vascular aging, most include cross-sectional epidemiological studies, limiting inferences about temporality. There is a need for a review of longitudinal epidemiological studies with repeat measures of vascular structure and function to allow for a systematic examination of determinants of vascular age and the association of vascular aging with outcomes.
Content:
Arterial stiffness is the most frequently used measure of vascular aging. We report here results of an extensive literature review of longitudinal cohort studies with repeat measures of arterial stiffness to characterize determinants of vascular age. Additionally, we summarize population-based studies which have focused on the association of arterial stiffness with end-organ damage and adverse cardiovascular outcomes.
Summary:
Changes in arterial stiffness are evident in early childhood. In adults, arterial stiffness has been observed to progress at the average rate of 0.2–0.7 meters per second for every five years of life. The state of the science is limited by the small number of studies with repeat measures of arterial stiffness and determinants of arterial stiffness progression, as well as limited studies in children and diverse race/ethnic groups. Several extant studies suggest that beyond age, cardiometabolic risk factors and adverse lifestyle behaviors contribute to arterial stiffening. Arterial stiffness is therefore, important in the assessment of healthy vascular aging and a possible target for the prevention of subclinical and clinical disease.
Keywords: vascular age, arterial stiffness, aging, cardiovascular disease, risk factors
Vascular aging
Chronic disease, functional limitations, and mortality increase exponentially with age, yet significant heterogeneity exists among individuals in the extent to which age affects outcomes. Thus, there is a need to define aging in a way that encompasses the complex processes occurring with age at the organ, tissue, and cellular levels. Compared with chronological age, the concept of biological age is a proposed measure that would more accurately reflect structural and functional changes taking place in the body as it ages and predict health outcomes.(1)
In the vasculature, biological aging manifests as impairments in endothelial function, reduced vascular elasticity, and increased stiffness.(2) In addition to age, vascular aging may be accelerated by the cumulative effect of risk factors, including blood pressure,(3) impaired glucose homeostasis,(4) adiposity,(5) and hypercholesterolemia.(6) Age and vascular risk factors lead to biochemical, molecular, and epigenetic alterations that decrease the ratio of the elastic to load bearing components of the arterial wall.(7) Consequently, the vessel wall stiffens and is less able to accommodate blood pressure pulsatility. The increased arterial stiffness leads to excessive blood pressure and pulsatility observed in microvasculature of organs such as the brain, kidney, and heart, resulting in small vessel injury and functional impairment of those organ systems.
Although arterial stiffening is not synonymous with chronological aging of the blood vessels, it is a phenotypic expression of heterogeneous set of biological processes occurring in the vessel with age and provides insight into vascular function and its impact on other tissues. Importantly, vascular aging, defined as an increase in arterial stiffness, is associated with a decrease in physical function event after adjustment for chronological age (8). In this review, we discuss epidemiological evidence from prospective cohort studies with repeat measurements of arterial stiffness to identify determinants of vascular aging. We provide a summary of evidence from population-based studies on arterial stiffness progression that occur with age and how various risk factors may increase such progression, leading to early vascular aging. We conclude by providing suggestions for future research to increase our understanding of the role of age and risk factors in vascular structure and function. This review aims to provide the context for considering vascular aging as a distinct subclinical physiological process, which may be targeted in interventions to reduce the burden of end-organ damage.
Mechanisms associated with vascular aging
To maintain both cyclical pressure and load demands of blood flow, the extracellular matrix of the vascular wall undergoes continuous remodeling.(9) This remodeling is achieved through a complex interplay of enzymatic and non-enzymatic modification of matrix components,(10) de-novo matrix synthesis,(11) as well as changes in smooth muscle cell phenotype and organization (12) (Figure 1) Age and disease disrupt the highly regulated homeostasis of the arterial extracellular matrix through imbalances in regulatory pathways, one of which is the renin-angiotensin system.(13) Upregulation of the renin-angiotensin leads to activation of the pro-inflammatory pathways and alters the ratio of reactive oxygen species relative to antioxidant defenses. The resulting increase in inflammatory cytokines and in oxidative stress disrupt the balance of synthesis and degradation of the components of the extracellular matrix of the vascular wall. With age and disease, the vascular elastin fibers undergo fragmentation due to fatigue, calcification, and enzymatic degradation. At the same time, de-novo synthesis of vascular wall collagens increases, shifting the arterial wall load bearing to these stiffer structural matrix components. Vascular smooth muscle cells, which are the primary cell type responsible for the secretion of the major components of the vascular extracellular matrix, play a pivotal role in vascular remodeling by adapting their phenotype to the increased mechanical load on the vessel that occurs with age.(12)
Figure 1.
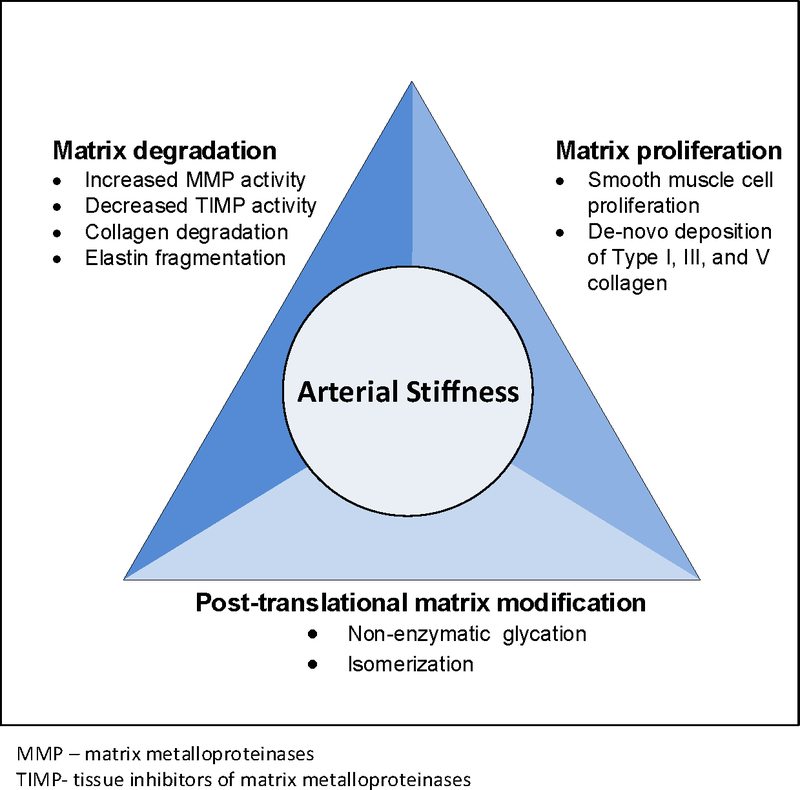
Conceptual framework of vessel wall remodeling that contributes to increase in arterial stiffness
Arterial aging adversely affects pulsatile hemodynamics. Following systole, forward-traveling pulse waves are transmitted through conduit vessels and are partly reflected along the vasculature at sites of impedance mismatch. Such sites occur at points of arterial branching and changes in arterial diameter and wall properties. The reflected waves merge to form a net backward traveling pulse wave to the heart. With increasing arterial stiffness, the reflected wave returns progressively earlier in systole rather than in diastole.(14) This augments central systolic blood pressure (cSBP) at the expense of central diastolic blood pressure (cDBP),(15) and widens the central pulse pressure (cPP), estimated as cSBP minus cDBP.
Arterial stiffening occurs along the length of the arterial tree, but is most evident in the aorto-illiac pathway, the highly elastic segment of the central artery (16, 17) and the one most proximal to the heart; less marked age-related changes are observed in peripheral arteries.(18)
Estimating arterial stiffness
Arterial stiffness cannot be easily assessed directly; however, central arterial pressure waveform parameters provide important indirect information regarding systemic arterial structure and function allowing for the assessment of wave reflection and amplification.(19) Pulse wave velocity (PWV), the most commonly used method for the assessment of arterial stiffness, is measured as the transit time of the forward-traveling pulse wave between two arterial sites.(15) Carotid-femoral PWV (cfPWV), a measurement based on blood pressures recorded at the carotid and femoral arteries, is considered the referent standard in the assessment of central arterial stiffness. The value of measuring cfPWV to predict cardiovascular health outcomes and longevity has been established in laboratory and population-based longitudinal studies.(20) International reference norms for cfPWV have been established for population, age, and risk factor strata.(21) PWV can be assessed using dedicated equipment, including oscillometric, tonometric, volume plethysmographic, and photo-plethysmographic devices, of which applanation tonometry is considered the gold-standard (Table 1). A focused assessment of central arterial stiffness, which allows for measurement of segmental PWV, including that of the aortic arch, can be obtained from magnetic resonance imaging (MRI).(22) Ultrasonographic devices can be used to measure properties of the superficial arteries, such as the carotid artery.(23)
Table 1.
Measurement of Central Pulse Wave Velocity
| Technology | Arterial Segment | Example Studies |
|---|---|---|
| Magnetic Resonance Imaging (MRI) | Aorta, including the aortic arch | The Multi-Ethnic Study of Atherosclerosis (34) |
| Tonometry | Carotid-Femoral, Carotid-Radial | The Atherosclerosis Risk in Communities Study,(68) Framingham Heart Study, (Investigations Préventives et Cliniques,(3) Cardiovascular health in adolescents with type 1 diabetes,(29) Whitehall-II (8) |
| Ultrasound | Aorta-Femoral, Carotid-Femoral, Carotid (single-point) | Bogalusa Heart Study ,(39) Baltimore Longitudinal Study of Aging ,(69) SardiNIA,(25) The Study of Women’s Health Across the Nation Heart (27) |
| Oscillometry | Brachial-Ankle, Carotid-Femoral, Cardio-ankle vascular index (CAVI), Brachial (single-point) | The Atherosclerosis Risk in Communities Study (68) |
| Photoplethysmography | Carotid-Radial, Carotid-Ankle | Northern Ireland Young Hearts Project (70) |
| Impedance Cardiography | Heart-Popliteal | Young Finns Study (71) |
Progression of arterial stiffness with age
Longitudinal epidemiological studies with repeat measures of arterial stiffness provide an important contribution to understanding changes in arterial stiffness that occur with age. Those studies, conducted in Europe, Asia, and the Americas provide a wide age range and include children as young as 12.5 years of age (24) and the oldest old (the SardiNIA Study) (25). Most studies include both men and women, with the exception of the electron-beam tomography, risk factor assessment among Japanese and U.S. men in the post-World War II birth cohort (the ERA JUMP Study),(26) which enrolled Japanese men, and the Study of Women’s Health Across the Nation (the SWAN) Heart Study,(27) which enrolled African American and Caucasian women. Differences in study populations and in the assessment of arterial stiffness limit direct comparisons; however, the studies provide important information on age-related vascular changes and its determinants. Below, we discuss salient studies selected from those listed in Tables 2 (children) and 3 (adults) to highlight the age-related progression of arterial stiffness.
Table 2.
Longitudinal studies reporting progression of arterial stiffness in children
| Study (or lead author), reference | Country | Years of Study Initiation | Study population | Years between arterial stiffness measures | Arterial Stiffness Measure |
Change in Arterial Stiffness |
||
|---|---|---|---|---|---|---|---|---|
| Number of study participants | Sex (% female) | Mean age baseline (SD) | ||||||
| Determinants of Macrovascular Disease in Adolescents with type 1 diabetes (28) | United States | 2008–2010 | 297 | 49.1% | 15.4 (2.1) | 2 | Carotid-femoral, tonometric | 0.25 m/s/yr for those who achieved type 1 diabetes control goals |
| Cardiovascular health in adolescents with type 1 diabetes (29) | United States | 2004–2005 | 298 | 46.3% | 14.5 (2.8) | 5 | Carotid-femoral, tonometric | 0.15 m/s/yr |
| Chen et al.,(30) | Sweden | 2006 | 162 | 57.7% | 14.5 (12.5–16.7) | 3 | Carotid femoral tonometric | Boys: 0.27 m/s/yr; Girls: 0.06 m/s/yr |
| Fujiwara et al.,(24) | Japan | 2006 | 1729 | 46.2% | 12.5 (0.5) | 2 | Brachial-ankle, oscillometry | Boys: 8.7 to 9.5 m/s (0.8m/s/yr) Girls: 8.6 to 9.2m/s (0.6m/s/yr) |
Table 3.
Longitudinal studies reporting progression of arterial stiffness in adults
| Study population |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study, reference | Country | Years of Study initiation | # study participants | Sex (% female) | Mean age baseline | Years between assessment of arterial stiffness | Arterial Stiffness Measure | Change in Arterial Stiffness |
|
| Baseline | Follow-up | ||||||||
| Asymptomatic Polyvascular Abnormalities Community Study (72) | China | 2010–2011 | 7000 | 40.1% | 55.2 | 2 | Brachial-ankle, Oscillometry | Men: 15.8 m/s |
16.7 m/s |
| Women: 14.4 m/s |
15.2 m/s |
||||||||
| Total: 15.2 m/s |
16.0 m/s |
||||||||
| Baltimore Longitudinal Study of Aging(69) | United States | 1988 | 449 | 55.2% | 55.8 | 4.9 | Carotid-femoral, tonometric | Change per decade: −0.2–1.3 m/s in normotensives | |
| Bogalusa Heart Study(39) | United States | 2000–2002 | 446 | 58.1% | 43.3 | 6.8 | Aortic-femoral, ultrasound | White: 5.1 m/s |
6.9 m/s |
| Black: 5.3 m/s |
7.0 m/s |
||||||||
| Total: 6.1 m/s |
6.9 m/s |
||||||||
| Investigations Préventives et Cliniques (3) | France | 1992–1993 | 483 | 36% | 51.2 | 6 | Carotid-femoral, tonometric | Normotensive: 9.8 m/s |
10.4 m/s |
| Treated hypertensive: 11.4 m/s |
12.2 m/s |
||||||||
| Kaohsiung Medical University Hospital(36) | Taiwan | 2006–2010 | 577 | 61% | 54.5 | 4.2 | Single point pulse, ultrasound | Change per year: | |
| Men: 0.20 m/s | |||||||||
| Women: 0.18 m/s | |||||||||
| Electron-Beam Tomography, Risk Factor Assessment Among Japanese and U.S. Men in the Post-World War II Birth Cohort (26) | United States | 2000–2006 | 240 | 0% | 45.0 | 4.6 | Carotid-femoral, tonometric |
8.4 m/s |
annual change: 0.3% |
| Framingham Heart Study (38) | United States | 1998–2001 | 1759 from the Offspring cohort | 55% | 60.0 | 6.5 | Carotid-femoral, tonometric | 9.6 m/s | 10.4 m/s |
| Harbin, China study(73) | China | 2006–2007 | 2297 | 40.6% | 51.2 | 5 | Brachial-ankle, oscillometry | With NAFLD: 12.3 m/s |
13.3 m/s |
| Without NAFLD: 11.7 m/s |
12.3 m/s |
||||||||
| Japan Multi-Institutional Collaborative Cohort Study (74) | Japan | 2008–2010 | 8004 | 67.6% | 53.1 | 5 | CAVI, oscillometry | 7.4 m/s | 7.9 ms |
| The Multi-Ethnic Study of Atherosclerosis (34) | United States | 2000–2002 | 1160 | 55% | 60.0 | 10 | Magnetic resonance imaging | 6.7 m/s | 8.1 m/s |
| SardiNIA Study (25) | Italy | 4358 | 58.5% | 43.7 | 5.4 | Carotid-femoral, ultrasound | 6.7 m/s | NR | |
| Community-based follow-up cohort study in Shijingshan district (75) | China | 2007–2009 | 1680 | 60% | 61 | 5 | CAVI | 7.38 | 7.85 |
| The Study of Women’s Health Across the Nation Heart (27) | United States | 2001–2003 | 608 | 100% | 42–52 | 2.3 | Carotid-femoral, ultrasound | White: 7.9 m/s |
8.4 m/s |
| Black: 8.2 m/s |
9.3 m/s |
||||||||
| Total: 8.0 m/s |
8.7 m/s |
||||||||
| Taichung Community Health Study (40) | Taiwan | 2004–2005 | 1,648 | 49% | 56 | 3 | Brachial-ankle, oscillometry | No MetS: 9.5 cm/s 3 yr change | |
| MetS: 31.1 cm/s 3 yr change | |||||||||
| Whitehall-II study (8) | United Kingdom | 2008–2009 | 5196 | 26% | 65 | 4 | Carotid-femoral, tonometric | 8.4 m/s | |
| Atherosclerosis Risk in Communities Study (68) | United States | 2011–2013 | 6,538 | 58.8% | 75.8 | 5 | Carotid-femoral, oscillometry | Repeat measures in progress (2016–2018) | |
NR: Not Recorded
CAVI: Cardio-Ankle Vascular Index
NAFLD: Non-Alcoholic Fatty Liver Disease
MetS: Metabolic Syndrome
Central PWV is of most interest to research regarding arterial stiffening. It can be assessed directly at the level of the aorta, or between the carotid-femoral or brachial-ankle arterial sites. A limited number of longitudinal studies have incorporated central PWV assessments in children, including three studies which measured cfPWV in adolescents (mean age 14.5 to 15.4 years),(28–30) and one which measured brachial-ankle PWV (baPWV) in children (mean age 12.4 years; Table 2).(24) Collectively, the studies reported a cfPWV increase of 0.15–0.18 m/s per year and baPWV increase of 0.70 m/s per year in adolescents aged 12.5 to 17.5 years. These values are in line with the 0.02–0.18 m/s per year increase reported in cross-sectional studies, which have provided cfPWV reference values for children aged 6–22.(31) The cross-sectional evidence also suggests that arterial stiffness progression is non-linear in children and differs by sex, with the progression being flat between age 3–8 years, and the first major progression occurring at age 12.1 years in boys and 10.4 years in girls.(32) Further, one of the longitudinal studies in children aged 12.5–16.7 years reported that PWV increased by 0.26 m/s per year in boys and 0.09 m/s per year in girls.(30) The cross-sectional cfPWV reference studies support the later findings, indicating an increase of 0.10–0.19 m/s per year in boys and 0.04–0.16 m/s per year in girls.
In adults, the Baltimore Longitudinal Study of Aging (BLSA) conducted serial cfPWV measures among 775 men and women 21–94 years of age and free of clinically overt cardiovascular disease.(33) Study investigators observed a progressive change in cfPWV that was steeper among men as compared to women. Differences by sex in the rate of change in PWV per decade of age were pronounced at ages greater than 60 years, with little difference observed at younger ages. Additionally, study investigators observed a trend of greater, although not statistically significant, progression of cfPWV in non-white, as compared to white, study participants.
An increase in the median PWV at the aortic arch, the highly elastic segment of the aorta, from 6.7 m/s to 8.1 m/s was observed during 10 years of follow-up among 1160 Multi-Ethnic Study of Atherosclerosis (MESA) participants, aged 60 years at baseline.(34) In line with findings from the BLSA, a greater progression in aortic arch PWV was observed during this follow-up period in black as compared to white MESA participants free of cardiovascular events.
Evidence for increasing progression of arterial stiffness with age was reinforced by a larger study, the Whitehall II study, which measured cfPWV 4 years apart in 3789 men and 1383 women with an average age of 65.5 years at baseline.(5, 8) Study investigators observed a gradient in the 5-year progression in cfPWV with age. Among study participants 55–59 years old at baseline, the 5-year change in cfPWV was 0.18 m/s, increasing to 0.36 m/s in those 60–64 years at baseline, 0.80 m/s in those 65–69 years old at baseline, and finally 0.99 m/s in those 70 years or older. All estimates were adjusted for mean arterial pressure (MAP). Similar trajectories showing more pronounced cfPWV progression with advancing age were seen for men and women in the SardiNIA study, which followed 1810 men and 2548 women aged 20–101 years old for 2–3 visits across an average 5.4 years of follow-up.(25)
Along with additional studies listed in Table 3, these longitudinal studies show that the progression of arterial stiffness occurs at approximately 0.2–0.7 m/s per 5 years in adults, with accelerated progression observed among those 60 years of age or older.(3) Due to methodological differences between existing studies, such estimates cannot yet be provided for children and adolescents. Additionally, similar to what has been observed among children, there is evidence of accelerated progression of arterial stiffness in men versus women (3) that increases after age 60 years.(33) Studies have shown higher progression among those of non-white race compared with whites,(34, 35) although findings were not statistically significant in some studies.(27, 33)
The bidirectional association of arterial stiffness with blood pressure
In addition to age, blood pressure is a major determinant of progression of arterial stiffness. Although animal studies strongly suggest that increases in arterial stiffness precede increases in blood pressure, several longitudinal population studies provide evidence of a bidirectional association between blood pressure and arterial stiffness. Further, longitudinal studies consistently show greater arterial stiffness in adults with hypertension than in adults with blood pressure in the normal range,(3) suggesting an acceleration in arterial aging in hypertension.
In the BLSA, steeper cfPWV progression was reported in men and women with peripheral systolic blood pressure (SBP) values 120 mm Hg and higher as compared to those with SBP below 120 mm Hg.(33) In men, there was a strong dose-response association, whereby those with a SBP of ≥140 mm Hg had the highest cfPWV progression. In MESA, higher baseline SBP was associated with aortic PWV progression within 10 years of follow-up.(34) Both higher baseline and increases in MAP and DBP over time were also associated with aortic PWV progression. MESA participants who reduced their blood pressure from ≥140 mmHg at baseline to <140 mmHg at the 10-year follow-up had less aortic PWV progression compared to those with a consistently high SBP. Other studies have shown that baseline SBP and annual increases in SBP were associated with an annual increase in cfPWV in men,(26) baseline SBP was positively associated with cfPWV progression in women,(27) and baseline and increases in MAP were positively associated with cfPWV progression.(36)
In contrast, several longitudinal studies have questioned the temporality of the association of blood pressure with arterial stiffness. During a mean 5.4-year follow-up period, the SardiNIA study showed no association between change in blood pressure and PWV progression.(25) Interestingly, during the study follow-up period, blood pressure decreased in men, but increased in women; while cfPWV increased over time in both men and women. Similarly, among 1759 participants from the Framingham Offspring study during a mean 6.5 years follow-up period, baseline SBP was associated with an increase in the augmentation index, a measure of wave reflection,(37) but not with cfPWV progression.(38) Additionally, in this study, baseline cfPWV was associated with higher SBP, pulse pressure, and incident hypertension at follow-up, suggesting that arterial stiffness is a precursor to the development of hypertension.
To address the question of the bidirectional association of blood pressure and arterial stiffness, investigators from the Bogalusa Heart Study used a cross-lagged panel design, a method that allows for the analysis of data that have reciprocal relationships over time, to investigate the association of blood pressure with arterial stiffness over a mean 6.8 years of follow-up.(39) Based on this method, baseline SBP and DBP had a stronger association with aortic-femoral PWV at follow-up compared with the association of aortic-femoral PWV with SBP and DBP at follow-up, suggesting that blood pressure may be a precursor to arterial stiffening in middle-aged adults.
Factors associated with accelerated progression of arterial stiffness
Beyond age and blood pressure, several metabolic and lifestyle factors contribute to vascular aging. Here we summarize the evidence from longitudinal studies regarding the association of adiposity, cardiometabolic factors, and lifestyle factors with vascular aging characterized as arterial stiffness progression. As we described above, the average expected cfPWV progression is around 0.2–0.7 m/s per 5 years in healthy adults.
Adiposity
Adiposity has been consistently associated with PWV progression. In the Whitehall II study, each standard deviation increase in body mass index (BMI), waist circumference, waist to hip ratio, and fat mass percent independently predicted cfPWV progression (range in increase in cfPWV: 0.14–0.18 m/s per 5-year).(5) The BLSA also demonstrated an association between higher waist circumference and greater PWV progression that was more pronounced with age in women.(33) Similarly among a cohort of women, SWAN Heart found waist circumference to be a strong predictor of cfPWV progression.(27) When considering change in adiposity, a study of 152 black and white adults 20–40 years old showed that increases in BMI and weight were associated with 0.053 m/s and 0.056 m/s greater annual cfPWV progression, respectively.(35) Baseline waist circumference, but not change, was associated with cfPWV progression.
Cardiometabolic factors
Longitudinal studies with repeat measures of arterial stiffness have shown that cardiometabolic factors, including impaired glucose homeostasis and the metabolic syndrome (MetS) are associated with PWV progression. Among participants without type 2 diabetes, the Whitehall II study reported a standard deviation increase in hemoglobin A1c (HbA1c) and the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) was associated with a 0.11 m/s and 0.09 m/s greater 5-year progression of cfPWV, respectively, representing ~12–14% of the mean 5-year cfPWV progression.(4) HOMA-IR was not statistically associated with greater cfPWV progression after adjusting for BMI. Other longitudinal studies did not show an association between glucose(3, 27, 35), insulin, or HOMA-IR (26) and arterial stiffness progression in multivariable analyses. Discrepancies may be due to differences in covariate adjustment, study population, and PWV assessment methods.
A few longitudinal studies have investigated other cardiometabolic conditions, such as the metabolic syndrome (MetS), with arterial stiffness progression. The Taichung Community Health Study measured baPWV 3 years apart in 1518 adults (mean age 56 years; 49% women).(40) After 3 years, participants with MetS had a 0.36 m/s higher baPWV compared to those without MetS. A greater baPWV progression was observed with an increasing number of MetS components. In a multivariable model, where the individual MetS components were compared with their normal levels, only high blood pressure and high fasting glucose were associated with baPWV progression. Similar results were obtained in the Investigations Préventives et Cliniques study of 675 participants, in which the 6-year cfPWV progression was higher for those with 3 or more MetS compared to those without any MetS components.(41)
Social and Lifestyle factors
Among men and women civil servants in London from the Whitehall II study, low education, income, and employment grade were associated with a 0.30 to 0.58 m/s higher 5-year change in cfPWV compared to the respective high category.(42) Also in the Whitehall II study, men who were former drinkers had a 0.11 m/s greater 5-year change in cfPWV compared to stable moderate drinkers.(43) The association between alcohol intake and cfPWV was not statistically significant among women. Among men 40–49 years old in the ERA Jump study, drinking ≥2 drinks/week was associated with a 1.6% greater relative annual change in cfPWV.(26) Smoking is another lifestyle factor associated with arterial stiffness. Continuous smoking, defined by current smoking at baseline and 10 years, was associated with aortic PWV progression over 10 years compared with non-smokers in MESA.(34)
Only one longitudinal study has investigated the role of physical activity in arterial stiffness progression. The Whitehall II study measured cfPWV in 5196 adults at age 65 and 70 and evaluated self-reported physical activity measured at age 65 as well as 6 and 11 years before.(8) Compared to the average 5-year cfPWV progression, each hour/week of sports activity, moderate-to-vigorous activity, or cycling was associated with a 0.02 m/s lower 5-year cfPWV progression, whereas each hour of sedentary time was associated with a 0.007 m/s greater 5-year cfPWV progression. Additionally, increasing physical activity was associated with a 0.16 m/s lower 5-year cfPWV progression.
Longitudinal studies of risk factors associated with the progression of arterial stiffness point to modifiable cardiometabolic and lifestyle factors in the pathogenesis of arterial stiffness, offering opportunities for preventing accelerated vascular aging. However, evidence regarding determinants of arterial stiffness is limited due to the few studies with repeat measures of arterial stiffness.
Importance of arterial stiffening – effects on end-organs
Large elastic arteries, such as the aorta, absorb and dampen pulsatile flow, creating a steady flow to the smaller resistance vessels that are incapable of absorbing pulsatile energy. With arterial stiffening due to age and the cumulative effect of vascular risk factors, increased pulsatility reaches the smaller resistance vessels in the brain, kidney, and the heart, causing end-organ damage (Figure 2).
Figure 2.
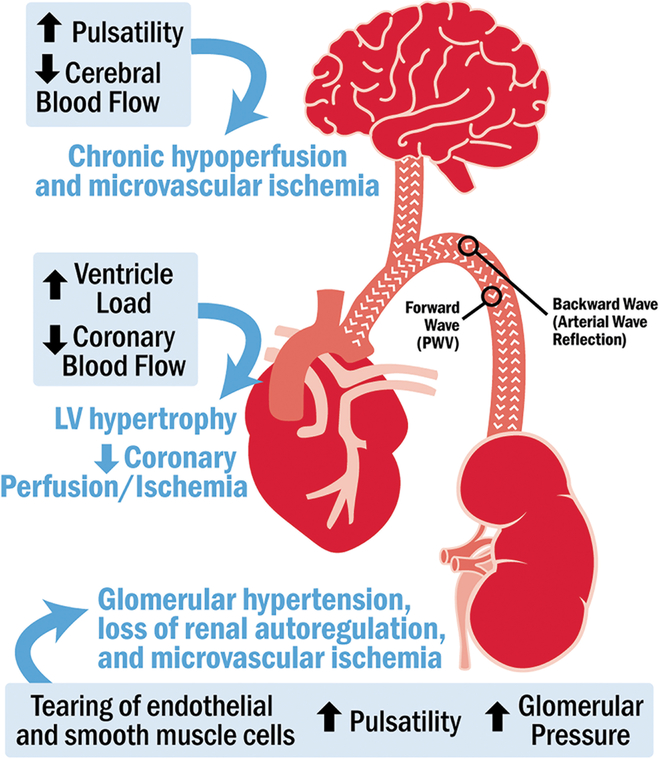
Effect of an increase in arterial stiffness on end-organ structure and function
Arterial stiffness is an emerging risk factor for structural and functional changes in the brain.(44) Arterial stiffness is hypothesized to contribute to cerebral microvascular damage, cognitive impairment, and dementia by reducing mean cerebral blood flow and increasing pulsatile stress in the brain. Chronic cerebral hypoperfusion and repeated occurrences of microvascular ischemia may lead to structural brain changes and tissue damage,(45) manifested in the brain as white matter hyperintensities, brain infarcts, and brain atrophy (46) that are associated with cognitive decline and dementia.(47) PWV (48) and cPP (49) are associated with these hemodynamic alterations in the middle cerebral artery, supporting the link between PWV and cerebral small vessel disease, cognitive impairment, and dementia.
Measures of vascular aging are also strongly associated with kidney dysfunction. Investigators from the Health, Aging and Body Composition (Health ABC) study observed a positive association of PWV with the development of chronic kidney disease during a median 8.9-year follow-up among 2129 men and women (aged 74 years) free of kidney disease at baseline.(50) Measures of arterial stiffness were also positively associated with a decline in kidney function in the Rotterdam study (51) and in studies conducted in populations of patients from Korea and China.(52, 53)
Lastly, arterial stiffness has been consistently associated with cardiovascular disease. Although earlier studies of the association of arterial stiffness with heart failure were negative, recent data from MESA (54, 55) and the Framingham Heart Study (56) have demonstrated an independent association of PWV with incident heart failure. Currently, no definitive data exist to compare the associations of PWV and heart failure with preserved ejection fraction versus reduced ejection fraction.
Vascular stiffness has been shown to be a long-term predictor of downstream cardiovascular events and mortality in various populations. A review and meta-analysis of 17 longitudinal studies found that a 1 m/s increase in cfPWV was associated with a 14% increased risk of a cardiovascular event (cardiovascular death and non-fatal events), 15% increased risk of cardiovascular mortality, and 15% increased risk for all-cause mortality.(57) Similarly, a study of 1678 Danish adults 40–70 years old found the risk of a fatal or non-fatal cardiovascular event increased 17% for every 3.4 m/s increase in cfPWV (95% CI: 1.04–1.32).(58) Among elderly adults in The Health ABC study, the highest cfPWV quartile was associated with 2.6 times the risk of stroke (95% CI: 1.19–5.64) compared with the lowest quartile.(59) Extant studies of baPWV are limited and primarily apply to Japanese populations. The highest tertile of baPWV (≥17m/s) was associated with 6.8 times the risk of all-cause mortality (95% CI: 1.4–32.8) in 2,480 Japanese adults.(60)
Estimates from studies referenced above were adequately adjusted for age, prevalent disease status, blood pressure (SBP or mean arterial pressure), use of anti-hypertensive medication or hypertension, and lifestyle and metabolic factors.
Conclusion and perspectives for future research
Arterial stiffness has been shown to progress at the rate of approximately 0.2–0.7 m/s per 5 years in healthy adults. The range in arterial stiffness progression reflects the heterogeneous set of processes occurring in the arteries with age, normal physiological variation, differences in population characteristics, including age, sex and ethnicity, and technological considerations. As we outline in Table 1, several different methodologies to assess PWV have been used in large population studies. The most commonly assessed arterial segment, and one recommended by a Scientific Statement from the American Heart Association,(61) is the carotid-femoral arterial segment. However, a wide range of arterial segments have been investigated, and a wide range of technologies have been used, making comparisons between the studies difficult. Additionally, there is a lack of standardization for measurement of wave path length, which has been demonstrated to account for some of the discrepancy in PWV values from different devices (62) and not every study considers MAP, which is an important physiological variable affecting arterial stiffness.(61) Despite these technical considerations, the evidence clearly indicates that: (a) PWV is a marker of “arterial aging”; (b) cardiometabolic and lifestyle risk factors contribute to the acceleration of normal vascular aging; and (c) increased arterial stiffness has a direct and pronounced effect on the pulsatility at end-organs such as the heart, brain, and kidney, leading to subclinical damage and adverse outcomes.
From a clinical perspective, arterial de-stiffening is an active area of research in blood pressure control.(63) Lifestyle interventions, such as habitual physical activity(64) weight loss, smoking cessation, salt reduction, (65) and dietary interventions (64) have also been shown to reduce cfPWV. Beyond the management of these modifiable risk factors, several therapeutic modalities have been shown to reduce vascular aging through regulation of the vascular smooth muscle cell growth and proliferation, (66) and by inducing changes in the vessel wall structure.(67) However, targeting structural components of the vascular wall in pharmacological approaches aimed at attenuating arterial stiffness remains largely unexplored. Arterial stiffness may thus be a possible target for interventions intended to prevent and reduce end-organ damage.
Studies of determinants of arterial stiffness are limited, warranting more research. Additionally, prior studies of risk factors associated with arterial stiffening have been conducted primarily in Caucasian, Asian, and, to a more limited extent, also African American populations, with little representation from a range of ethnic groups. It will be important to examine and understand processes of vascular aging across multiple populations and in younger age groups, thus providing information on the development of vascular sequelae across the lifespan and prevention of end-organ damage later in life.
Acknowledgements
MLM was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) 5K12HD001441.
Footnotes
Publisher's Disclaimer: Disclaimer: This is an un-copyedited authored manuscript copyrighted by the American Association for Clinical Chemistry (AACC). This may not be duplicated or reproduced, other than for personal use or within the rule of ‘Fair Use of Copyrighted Materials’ (section 107, Title 17, U.S. Code) without permission of the copyright owner, AACC. The AACC disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The final publisher-authenticated version of the article is available at http://www.clinchem.org
REFERENCES
- 1.Belsky DW, Caspi A, Houts R, Cohen HJ, Corcoran DL, Danese A, et al. Quantification of biological aging in young adults. Proceedings of the National Academy of Sciences of the United States of America 2015;112:E4104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harvey A, Montezano AC, Lopes RA, Rios F, Touyz RM. Vascular fibrosis in aging and hypertension: Molecular mechanisms and clinical implications. The Canadian journal of cardiology 2016;32:659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benetos A, Adamopoulos C, Bureau JM, Temmar M, Labat C, Bean K, et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation 2002;105:1202–7. [DOI] [PubMed] [Google Scholar]
- 4.McEniery CM, Wilkinson IB, Johansen NB, Witte DR, Singh-Manoux A, Kivimaki M, et al. Nondiabetic glucometabolic status and progression of aortic stiffness: The whitehall ii study. Diabetes care 2017;40:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunner EJ, Shipley MJ, Ahmadi-Abhari S, Tabak AG, McEniery CM, Wilkinson IB, et al. Adiposity, obesity, and arterial aging: Longitudinal study of aortic stiffness in the whitehall ii cohort. Hypertension 2015;66:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEniery CM, Yasmin, McDonnell B, Munnery M, Wallace SM, Rowe CV, et al. Central pressure: Variability and impact of cardiovascular risk factors: The anglo-cardiff collaborative trial ii. Hypertension 2008;51:1476–82. [DOI] [PubMed] [Google Scholar]
- 7.Osborne-Pellegrin M, Labat C, Mercier N, Challande P, Lacolley P. Changes in aortic stiffness related to elastic fiber network anomalies in the brown norway rat during maturation and aging. American journal of physiology Heart and circulatory physiology 2010;299:H144–52. [DOI] [PubMed] [Google Scholar]
- 8.Ahmadi-Abhari S, Sabia S, Shipley MJ, Kivimaki M, Singh-Manoux A, Tabak A, et al. Physical activity, sedentary behavior, and long-term changes in aortic stiffness: The whitehall ii study. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed]
- 9.Wight T The vascular extracellular matrix Philadelphia: Lippincott-Raven Publishers, 1996. [Google Scholar]
- 10.Peeters SA, Engelen L, Buijs J, Chaturvedi N, Fuller JH, Jorsal A, et al. Circulating matrix metalloproteinases are associated with arterial stiffness in patients with type 1 diabetes: Pooled analysis of three cohort studies. Cardiovascular diabetology 2017;16:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlatmann TJ, Becker AE. Histologic changes in the normal aging aorta: Implications for dissecting aortic aneurysm. The American journal of cardiology 1977;39:13–20. [DOI] [PubMed] [Google Scholar]
- 12.Lacolley P, Regnault V, Avolio AP. Smooth muscle cell and arterial aging: Basic and clinical aspects. Cardiovascular research 2018;114:513–28. [DOI] [PubMed] [Google Scholar]
- 13.Aroor AR, Demarco VG, Jia G, Sun Z, Nistala R, Meininger GA, Sowers JR. The role of tissue renin-angiotensin-aldosterone system in the development of endothelial dysfunction and arterial stiffness. Front Endocrinol (Lausanne) 2013;4:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension 2005;46:200–4. [DOI] [PubMed] [Google Scholar]
- 15.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J 2006;27:2588–605. [DOI] [PubMed] [Google Scholar]
- 16.Fortier C, Agharazii M. Arterial stiffness gradient. Pulse (Basel) 2016;3:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cameron JD, Bulpitt CJ, Pinto ES, Rajkumar C. The aging of elastic and muscular arteries: A comparison of diabetic and nondiabetic subjects. Diabetes care 2003;26:2133–8. [DOI] [PubMed] [Google Scholar]
- 18.Boutouyrie P, Laurent S, Benetos A, Girerd XJ, Hoeks AP, Safar ME. Opposing effects of ageing on distal and proximal large arteries in hypertensives. J Hypertens Suppl 1992;10:S87–91. [PubMed] [Google Scholar]
- 19.Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Aortic input impedance in normal man: Relationship to pressure wave forms. Circulation 1980;62:105–16. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. Journal of the American College of Cardiology 2014;63:636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reference Values for Arterial Stiffness C. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘Establishing normal and reference values’. European heart journal 2010;31:2338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wentland AL, Grist TM, Wieben O. Review of mri-based measurements of pulse wave velocity: A biomarker of arterial stiffness. Cardiovasc Diagn Ther 2014;4:193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calabia J, Torguet P, Garcia M, Garcia I, Martin N, Guasch B, et al. Doppler ultrasound in the measurement of pulse wave velocity: Agreement with the complior method. Cardiovasc Ultrasound 2011;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujiwara H, Nakajima H, Inoue F, Kosaka K, Asano H, Yoshii K. Arterial stiffness in junior high school students: Longitudinal observations. Pediatr Int 2018;60:127–35. [DOI] [PubMed] [Google Scholar]
- 25.Scuteri A, Morrell CH, Orru M, Strait JB, Tarasov KV, Ferreli LA, et al. Longitudinal perspective on the conundrum of central arterial stiffness, blood pressure, and aging. Hypertension 2014;64:1219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Khoudary SR, Barinas-Mitchell E, White J, Sutton-Tyrrell K, Kuller LH, Curb JD, et al. Adiponectin, systolic blood pressure, and alcohol consumption are associated with more aortic stiffness progression among apparently healthy men. Atherosclerosis 2012;225:475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birru MS, Matthews KA, Thurston RC, Brooks MM, Ibrahim S, Barinas-Mitchell E, et al. African-american ethnicity and cardiovascular risk factors are related to aortic pulse-wave velocity progression. American journal of hypertension 2011;24:809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjornstad P, Pyle L, Nguyen N, Snell-Bergeon JK, Bishop FK, Wadwa RP, Maahs DM. Achieving international society for pediatric and adolescent diabetes and american diabetes association clinical guidelines offers cardiorenal protection for youth with type 1 diabetes. Pediatric diabetes 2015;16:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dabelea D, Talton JW, D’Agostino R Jr., Wadwa RP, Urbina EM, Dolan LM, et al. Cardiovascular risk factors are associated with increased arterial stiffness in youth with type 1 diabetes: The search cvd study. Diabetes care 2013;36:3938–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Dangardt F, Osika W, Berggren K, Gronowitz E, Friberg P. Age- and sex-related differences in vascular function and vascular response to mental stress. Longitudinal and cross-sectional studies in a cohort of healthy children and adolescents. Atherosclerosis 2012;220:269–74. [DOI] [PubMed] [Google Scholar]
- 31.Alejandro D, Yanina Z, Daniel B, Franco S, Victoria R, Edmundo CF. Reference intervals of aortic pulse wave velocity assessed with an oscillometric device in healthy children and adolescents from argentina. Clin Exp Hypertens 2018:1–12. [DOI] [PubMed]
- 32.Hidvegi EV, Illyes M, Benczur B, Bocskei RM, Ratgeber L, Lenkey Z, et al. Reference values of aortic pulse wave velocity in a large healthy population aged between 3 and 18 years. Journal of hypertension 2012;30:2314–21. [DOI] [PubMed] [Google Scholar]
- 33.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: The baltimore longitudinal study of aging. Hypertension 2013;62:934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohyama Y, Teixido-Tura G, Ambale-Venkatesh B, Noda C, Chugh AR, Liu CY, et al. Ten-year longitudinal change in aortic stiffness assessed by cardiac mri in the second half of the human lifespan: The multi-ethnic study of atherosclerosis. Eur Heart J Cardiovasc Imaging 2016;17:1044–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wildman RP, Farhat GN, Patel AS, Mackey RH, Brockwell S, Thompson T, Sutton-Tyrrell K. Weight change is associated with change in arterial stiffness among healthy young adults. Hypertension 2005;45:187–92. [DOI] [PubMed] [Google Scholar]
- 36.Lin LY, Liao YC, Lin HF, Lee YS, Lin RT, Hsu CY, Juo SH. Determinants of arterial stiffness progression in a han-chinese population in taiwan: A 4-year longitudinal follow-up. BMC cardiovascular disorders 2015;15:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Rourke MF, Pauca AL. Augmentation of the aortic and central arterial pressure waveform. Blood Press Monit 2004;9:179–85. [DOI] [PubMed] [Google Scholar]
- 38.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA : the journal of the American Medical Association 2012;308:875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen W, Li S, Fernandez C, Sun D, Lai CC, Zhang T, et al. Temporal relationship between elevated blood pressure and arterial stiffening among middle-aged black and white adults: The bogalusa heart study. American journal of epidemiology 2016;183:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li CI, Kardia SL, Liu CS, Lin WY, Lin CH, Lee YD, et al. Metabolic syndrome is associated with change in subclinical arterial stiffness: A community-based taichung community health study. BMC public health 2011;11:808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Safar ME, Thomas F, Blacher J, Nzietchueng R, Bureau JM, Pannier B, Benetos A. Metabolic syndrome and age-related progression of aortic stiffness. Journal of the American College of Cardiology 2006;47:72–5. [DOI] [PubMed] [Google Scholar]
- 42.Trudel X, Shipley MJ, McEniery CM, Wilkinson IB, Brunner EJ. Socioeconomic status, education, and aortic stiffness progression over 5 years: The whitehall ii prospective cohort study. J Hypertens 2016;34:2038–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Neill D, Britton A, Brunner EJ, Bell S. Twenty-five-year alcohol consumption trajectories and their association with arterial aging: A prospective cohort study. Journal of the American Heart Association 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Sloten TT, Protogerou AD, Henry RM, Schram MT, Launer LJ, Stehouwer CD. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: A systematic review and meta-analysis. Neurosci Biobehav Rev 2015;53:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henskens LH, Kroon AA, van Oostenbrugge RJ, Gronenschild EH, Fuss-Lejeune MM, Hofman PA, et al. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension 2008;52:1120–6. [DOI] [PubMed] [Google Scholar]
- 46.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: A review. Stroke; a journal of cerebral circulation 1997;28:652–9. [DOI] [PubMed] [Google Scholar]
- 47.Eckerstrom C, Olsson E, Klasson N, Bjerke M, Gothlin M, Jonsson M, et al. High white matter lesion load is associated with hippocampal atrophy in mild cognitive impairment. Dement Geriatr Cogn Disord 2011;31:132–8. [DOI] [PubMed] [Google Scholar]
- 48.Kwater A, Gasowski J, Gryglewska B, Wizner B, Grodzicki T. Is blood flow in the middle cerebral artery determined by systemic arterial stiffness? Blood pressure 2009;18:130–4. [DOI] [PubMed] [Google Scholar]
- 49.Pase MP, Grima NA, Stough C, Scholey A, Pipingas A. Association of pulsatile and mean cerebral blood flow velocity with age and neuropsychological performance. Physiology & behavior 2014;130:23–7. [DOI] [PubMed] [Google Scholar]
- 50.Madero M, Peralta C, Katz R, Canada R, Fried L, Najjar S, et al. Association of arterial rigidity with incident kidney disease and kidney function decline: The health abc study. Clin J Am Soc Nephrol 2013;8:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sedaghat S, Mattace-Raso FU, Hoorn EJ, Uitterlinden AG, Hofman A, Ikram MA, et al. Arterial stiffness and decline in kidney function. Clinical journal of the American Society of Nephrology : CJASN 2015;10:2190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim CS, Kim HY, Kang YU, Choi JS, Bae EH, Ma SK, Kim SW. Association of pulse wave velocity and pulse pressure with decline in kidney function. J Clin Hypertens (Greenwich) 2014;16:372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong X, Ma X, Tang L, Wang Z, Li W, Cui M, Xu D. Arterial stiffness evaluated by carotid-femoral pulse wave velocity increases the risk of chronic kidney disease in a chinese population-based cohort. Nephrology (Carlton) 2017;22:205–12. [DOI] [PubMed] [Google Scholar]
- 54.Chester RC, Gornbein JA, Hundley WG, Srikanthan P, Watson KE, Horwich T. Reflection magnitude, a measure of arterial stiffness, predicts incident heart failure in men but not women: Multi-ethnic study of atherosclerosis (mesa). Journal of cardiac failure 2017;23:353–62. [DOI] [PubMed] [Google Scholar]
- 55.Chow B, Rabkin SW. The relationship between arterial stiffness and heart failure with preserved ejection fraction: A systemic meta-analysis. Heart failure reviews 2015;20:291–303. [DOI] [PubMed] [Google Scholar]
- 56.Tsao CW, Lyass A, Larson MG, Levy D, Hamburg NM, Vita JA, et al. Relation of central arterial stiffness to incident heart failure in the community. J Am Heart Assoc 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. Journal of the American College of Cardiology 2010;55:1318–27. [DOI] [PubMed] [Google Scholar]
- 58.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 2006;113:664–70. [DOI] [PubMed] [Google Scholar]
- 59.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 2005;111:3384–90. [DOI] [PubMed] [Google Scholar]
- 60.Turin TC, Kita Y, Rumana N, Takashima N, Kadota A, Matsui K, et al. Brachial-ankle pulse wave velocity predicts all-cause mortality in the general population: Findings from the takashima study, japan. Hypertension research : official journal of the Japanese Society of Hypertension 2010;33:922–5. [DOI] [PubMed] [Google Scholar]
- 61.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the american heart association. Hypertension 2015;66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weber T, Ammer M, Rammer M, Adji A, O’Rourke MF, Wassertheurer S, et al. Noninvasive determination of carotid-femoral pulse wave velocity depends critically on assessment of travel distance: A comparison with invasive measurement. J Hypertens 2009;27:1624–30. [DOI] [PubMed] [Google Scholar]
- 63.Koumaras C, Tzimou M, Stavrinou E, Griva T, Gossios TD, Katsiki N, et al. Role of antihypertensive drugs in arterial ‘de-stiffening’ and central pulsatile hemodynamics. Am J Cardiovasc Drugs 2012;12:143–56. [DOI] [PubMed] [Google Scholar]
- 64.Sacre JW, Jennings GL, Kingwell BA. Exercise and dietary influences on arterial stiffness in cardiometabolic disease. Hypertension 2014;63:888–93. [DOI] [PubMed] [Google Scholar]
- 65.Pucci G, Battista F, Schillaci G. Aerobic physical exercise and arterial de-stiffening: A recipe for vascular rejuvenation? Hypertens Res 2012;35:964–6. [DOI] [PubMed] [Google Scholar]
- 66.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol 2001;21:1712–9. [DOI] [PubMed] [Google Scholar]
- 67.London GM, Asmar RG, O’Rourke MF, Safar ME, Investigators RP. Mechanism(s) of selective systolic blood pressure reduction after a low-dose combination of perindopril/indapamide in hypertensive subjects: Comparison with atenolol. Journal of the American College of Cardiology 2004;43:92–9. [DOI] [PubMed] [Google Scholar]
- 68.Meyer ML, Tanaka H, Palta P, Cheng S, Gouskova N, Aguilar D, Heiss G. Correlates of segmental pulse wave velocity in older adults: The atherosclerosis risk in communities (aric) study. American journal of hypertension 2016;29:114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the baltimore longitudinal study of aging. Journal of the American College of Cardiology 2008;51:1377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van de Laar RJ, Stehouwer CD, Boreham CA, Murray LM, Schalkwijk CG, Prins MH, et al. Continuing smoking between adolescence and young adulthood is associated with higher arterial stiffness in young adults: The northern ireland young hearts project. Journal of hypertension 2011;29:2201–9. [DOI] [PubMed] [Google Scholar]
- 71.Aatola H, Koivistoinen T, Tuominen H, Juonala M, Lehtimaki T, Viikari JSA, et al. Influence of child and adult elevated blood pressure on adult arterial stiffness: The cardiovascular risk in young finns study. Hypertension 2017;70:531–6. [DOI] [PubMed] [Google Scholar]
- 72.Zhou Y, Li Y, Xu L, Xu J, Wang A, Gao X, et al. Asymptomatic polyvascular abnormalities in community (apac) study in china: Objectives, design and baseline characteristics. PloS one 2013;8:e84685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li N, Zhang GW, Zhang JR, Jin D, Li Y, Liu T, Wang RT. Non-alcoholic fatty liver disease is associated with progression of arterial stiffness. Nutr Metab Cardiovasc Dis 2015;25:218–23. [DOI] [PubMed] [Google Scholar]
- 74.Tabara Y, Setoh K, Kawaguchi T, Takahashi Y, Kosugi S, Nakayama T, Matsuda F. Factors affecting longitudinal changes in cardio-ankle vascular index in a large general population: The nagahama study. Journal of hypertension 2018;36:1147–53. [DOI] [PubMed] [Google Scholar]
- 75.Ding XH, Wang X, Cao R, Yang X, Xiao W, Zhang Y, et al. A higher baseline plasma uric acid level is an independent predictor of arterial stiffness: A community-based prospective study. Medicine 2017;96:e5957. [DOI] [PMC free article] [PubMed] [Google Scholar]


