Abstract
Background.
Prevention of GVHD without malignant relapse reflects the overall goal of allogeneic hematopoietic cell transplantation (HCT). Novel regimens utilizing either maraviroc (MVC), bortezomib (BOR), or post-transplant cyclophosphamide (PTCy) have been reported to modulate GVHD and were tested against commonly used combination of tacrolimus (TAC) and methotrexate (MTX). The study aim was to evaluate each of these novel approaches for GvHD prophylaxis compared to contemporary controls using a novel composite primary endpoint to determine the most promising intervention to be further tested on a phase III clinical trial.
Methods.
This completed prospective multicenter phase II trial randomly assigned adult patients, aged 18–75, who received reduced intensity conditioning HCT to TAC/MMF/PTCy (Cy 50mg/kg on days +3 and +4, followed by TAC starting on day +5 and MMF starting on day +5 at 15mg/kg every 8 hours from day +5 to day +35); TAC/MTX/BOR (BOR 1.3mg/m2 IV on days +1, +4 and +7 post HCT); or TAC/MTX/MVC (MVC 300 mg PO twice daily from day –3 to day +30 post HCT). MTX was administered at 15 mg/m2 IV bolus on day +1, and 10 mg/m2 IV bolus on days +3, +6 and +11 post HCT; TAC was given intravenously at a dose of 0.05 mg/kg twice daily (or oral equivalent) starting day –3 (except the PTCy as indicated), with a target level of 5–15 ng/mL. TAC continued at least until day +90 and was tapered off by day 180. Each compared separately to a contemporary nonrandomized prospective cohort of patients who fulfilled the same eligibility criteria as the trial but treated with TAC/MTX at centers not participating in the trial. The primary endpoint (GFRS) was measured as the time from HCT to first of four events: onset of grade III-IV acute GVHD (aGVHD), chronic GVHD (cGVHD) requiring systemic immunosuppression (IS), disease relapse, or death. Randomization was performed in a 1:1:1 ratio using random block sizes for the three arms. The study was analyzed as a modified intent to treat. The study is closed to accrual and this is the study planned analysis. (ClinicalTrials.gov NCT#02208037).
Findings.
273 patients were randomized between the 3 study arms; 224 controls received Tac/MTX. Controls were generally well balanced except for more frequent comorbidities and type of conditioning regimens utilized. Compared to controls, hazard ratio (HR) for GRFS were 0.72 (90% CI 0.54, 0.94) (p=0.04), 0.98 (90% CI 0.76, 1.27) (p=0.92) and 1.11 (90% CI 0.86, 1.41)(p=0.45) for TAC/MMF/PTCy, TAC/MTX/BOR and TAC/MTX/MVC, respectively. Overall, 238 patients experienced grades 3 and 4 toxicities: TAC/MMF/PTCy 12 (13%) and 67 (72.8%), TAC/MTX/BOR 10 (11.2%) and 68 (76.4%), and TAC/MTX/MVC 18 (19.6%) and 63 (68.5%) respectively. The most common toxicities where hematological: TAC/MMF/PTCy 77(83.7%), TAC/MTX/BOR 73 (82%), TAC/MTX/MVC 78 (84.8%) and cardiac: TAC/MMF/PTCy 43(46.7%), TAC/MTX/BOR 44 (49.4%), TAC/MTX/MVC 43 (46.7%).Interpretation. TAC/MMF/PTCy was the most promising intervention yielding the best GRFS; and the best to prospectively compare to TAC/MTX in a phase III randomized trial.
Introduction
Graft-versus-host disease (GVHD) is a frequent cause of morbidity and mortality after allogenic hematopoietic cell transplantation (HCT) (1–3). Over the last few decades, the combination of methotrexate (MTX) and a calcineurin inhibitor, have been the cornerstone for GVHD prevention(4). However, despite prophylaxis over 50% of patients undergoing HCT will suffer from acute, chronic GVHD, or both(1, 5–7). Unfortunately, these outcomes have changed little despite the introduction of agents such as mycophenolate mofetil (MMF) or sirolimus(8, 9). Moreover, in patients who develop GVHD and failed to respond to treatment, the survival is poor due to infectious complications, organ failure and toxicity of immunosuppressive agents(10). Therefore, a strategy that minimizes not just the incidence of GVHD, but other adverse events, should translate into better outcomes after HCT.
Novel agents that have demonstrated promising results in the prevention of GVHD include bortezomib (BOR), maraviroc (MVC), and post-transplant cyclophosphamide (PTCy). BOR resulted in significant protection from acute GVHD in murine models with no adverse effects on long-term donor reconstitution(11). The drug was effective in single center studies of mismatched unrelated donor reduced intensity HCT(12, 13). CCR5 is a chemokine receptor that is important in GVHD pathogenesis in murine models (14, 15). MVC, a CCR5 antagonist, inhibits lymphocyte chemotaxis without impairing T-cell function, and appeared promising in a study of reduced intensity HCT, primarily through reduction of severe acute GVHD in the liver and gut (16, 17). Lastly, PTCy allows transplantation between matched and mismatched donor-recipient pairs with low rates of chronic GVHD presumably via killing of activated effector T-cells and up-regulation of regulatory T-cells (18–21).
The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) analyzed several single center GVHD prevention approaches and selected three promising interventions to be analyzed prospectively in a multi-center setting, focusing on recipients of reduced intensity conditioning regimens(22). This phase II trial randomized patients to receive tacrolimus TAC/MTX/BOR, TAC/MTX/MVC, or TAC/MMF/PTCy, and outcomes were compared to TAC/MTX contemporary controls. Moreover, the BMT CTN developed a composite endpoint for GVHD prevention trials to account not only for a reduction in GVHD but to include disease relapse and death, resulting in a more comprehensive assessment of overall transplant success(22). The study hypothesis was to evaluate novel approaches for GvHD prophylaxis compared to contemporary controls using a novel composite primary endpoint to determine the most promising intervention to be further tested on a phase III clinical trial.
Methods
Study design and participants
This was a BMT CTN randomized phase II, open label, multicenter trial comparing each of TAC/MTX/BOR, TAC/MTX/MVC, and TAC/MMF/PTCy to a nonrandomized prospective contemporary TAC/MTX control. HCT centers not participating in the clinical trial were recruited to participated in the control arm using data collected by the Center for International Blood and Marrow Transplant Research® (CIBMTR®). The CIBMTR® is a research collaboration between the National Marrow Donor Program® (NMDP)/Be The Match® and the Medical College of Wisconsin. The CIBMTR® represents an international network of transplant centers that submit transplant-related outcomes data. It has been collecting HCT outcomes data for >40 years and has an extensive prospectively collected longitudinal database of detailed patient-, transplant- and disease-related information(23). The CIBMTR® data were collected in compliance with HIPAA regulations and with all applicable federal regulations pertaining to the protection of human research participants, as determined by a continuous review by the NMDP Institutional Review Board and the Medical College of Wisconsin. Centers participating in the control cohort provided supplemental information specific for this trial, including date of Grades III and IV aGVHD and use of corticosteroids, in additional to standard CIBMTR® data collection forms.
Eligible patients were 18–75 year-old candidates for a reduced intensity conditioning with a related 6/6 match for HLA-A and -B at intermediate resolution, and –DRB1 at high resolution; or unrelated donor who is HLA-matched 7/8 or 8/8 HLA-A, -B, -C and –DRB1 at high resolution using DNA-based typing. Eligible diseases were acute leukemia, chronic myelogenous leukemia and myelodysplasia with no circulating blasts and with less than 5% blasts in the bone marrow, chronic lymphocytic leukemia/small lymphocytic lymphoma, as well as follicular, marginal zone, diffuse large-B cell, Hodgkin, or mantle cell lymphoma with chemosensitive disease at time of transplant.
Exclusion criteria included prior allogeneic transplant, ejection fraction <45%, hepatitis B or C, HIV infection, transformed lymphoma, other cancer diagnosis, planned post-BMT therapy including tyrosine kinase inhibitors, uncontrolled bacterial or fungal infections, poor performance status, creatinine clearance <50 mL/minute, DLCO <40% or FEV1 <50%, and inability to withhold agents that interact with hepatic cytochrome P450 enzymes or glutathione S-transferases involved in BOR and/or busulfan (BU) metabolism from day −5 to +7. The final control cohort had the same eligibility criteria as patients enrolled in the clinical trial, which was applied in two stages to reach the final population. The first stage included centers that agreed to participate in the control cohort, age, diseases, conditioning regimens and TAC based GVHD prophylaxis. All consecutive patients who were transplanted from August 2014 to July 2016 at participating centers were included in the pool of potential controls. The second phase assessed all reported data for consistencies and applied additional eligibility criteria, not included in the earlier entry forms, e.g. excluding transformed lymphoma and presence of any viral infections prior to transplant. Most cases excluded in the controls were related to use of TAC/MMF among other GVHD prevention approaches (Figure 1). Acute GVHD was graded according to Keystone consensus(24). Chronic GVHD was diagnosed and evaluated per NIH Consensus Conference(25). The study was approved by the Institutional Review Board at each institution and all patients provided written informed consent before being admitted to the clinical study.
Figure 1.
CONSORT diagram.

*Randomized trial patients who were replaced and removed from the trial due to: disease progression after randomization and prior to transplant (N=3), donor refusal to donate peripheral blood stem cells (N=2), and physician withdrew patient from the trial (N=1). Prospective cohort patients not eligible to be used as controls: AML not in complete remission at time of transplant (n=5), bone marrow graft recipient (n=1), patients performance score confirmed to be <70% (n=4) and recipients of myeloablative regimen (n=5).
Randomization and masking.
Subjects were approached for this study after the decision to proceed with transplantation was made and a suitable HLA-matched donor, identified. Transplant physicians evaluated the patient eligibility for randomization onto this study. Eligibility criteria was verified and ineligible patients were off study and no further follow-up was obtained. Transplant center personnel registered the patient in EMMES AdvantageEDCSM (Electronic Data Capture, an Internet-based data entry system) which upon completion of registration will inform of the results of randomization to the study center. All patients were randomized within 7 days prior to the initiation of conditioning therapy. Randomization was performed in a 1:1:1 ratio using permuted blocks with a random block sizes, stratified by donor type (HLA-matched sibling vs. matched unrelated vs. mismatched unrelated) and disease risk (high vs. low). Disease risk was modeled based on the risk for GRFS and patients with CLL and MDS were considered high risk and all the others were standard risk based on these criteria.
Procedures
Conditioning regimens allowed in the randomized clinical trial included fludarabine (FLU) (120–180 mg/m2)/BU (≤8 mg/kg PO or 6.4 mg/kg IV); FLU (90–120 mg/m2)/ Cy (120 mg/kg or 2250mg/m2); FLU (120–180 mg/m2)/ melphalan (≤150 mg/m2); FLU (90 mg/m2)/ TBI 200cGy, and fludarabine (150 mg/m2)/cyclophosphamide (29 mg/Kg) /TBI (200cGy). Donors were mobilized with filgrastim and collected by leukapheresis to a target stem cell dose between 2×106 and 10×106 CD34+/kg based on actual recipient body weight. Cells were administered on day 0 per institutional standards. Patients in the clinical trial were randomized to one of three GVHD prophylaxis regimens: TAC/MTX with BOR 1.3mg/m2 IV on days +1, +4 and +7 post HCT; TAC/MTX with MVC 300 mg PO twice daily from day –3 to day +30 post HCT; or PTCy 50mg/kg day +3 and +4 followed by TAC/MMF 15mg/kg TID not to exceed 1g TID starting on day+5, MMF was stopped at day +35. In patients receiving MTX, the drug was administered at doses of 15 mg/m2 IV bolus on day +1, and 10 mg/m2 IV bolus on days +3, +6 and +11 after post HCT; TAC was given intravenously at a dose of 0.05 mg/kg twice daily (or oral equivalent) starting day –3, with a target level of 5–15 ng/mL. TAC was recommended to continue at least until day +90 and to be completely tapered off by day 180. Drugs for prophylaxis against P. jiroveci, fungal, and herpetic infections, use of growth factors, IVIG, blood products, and other supportive care, were per institutional standards. Dose adjustments were allowed. Tacrolimus dose reductions were made if toxicity was present or whole blood levels are above the recommended range (15 ng/mL) in the absence of toxicity. Patients with severe intolerance of tacrolimus could be placed on cyclosporine (trough level of 200–400 ng/mL) or sirolimus (trough level of 3–8 ng/mL). MTX dose reductions due to worsening creatinine clearance after initiation of conditioning regimen, high serum levels or development of oral mucositis were done according to institutional practices. Maraviroc dose reduction of 50% took place if Grade 3 or higher liver toxicity not attributable to other causes such as infection, GVHD, toxicity from other drugs or sinusoid obstructive syndrome/veno-occlusive disease was present or if severe mucositis, nausea or other complications that precludes administration of an oral medication also was seen. For coadministration of CYP3 inhibitors with or without a potent CYP3A inducer)(ketoconazole, itraconazole, clarithromycin) the dose of MVC was 150 mg twice daily. For potent CYP3A inducers (without a potent CYP3A inhibitor)(rifampin, carbamazepine, phenobarbital, and phenytoin) the dose of MVC was 600 mg twice daily. Bortezomib dose reductions occurred in the presence of grade 1 or 2 neuropathy, the drug was reduced to 1 mg/m2. For grade 2 with pain or grade 3, the drug was held until resolution. For grade 4, it was discontinued. For PTCy no dose adjustments were made, but no immunosuppressants were allowed until after 24 hours after completion of the drug.
Outcomes
The primary endpoint was the composite endpoint of GVHD-free, relapse-free survival (GRFS) defined as time to onset of any of the following events from time of HCT: grade III-IV acute GVHD, chronic GVHD requiring systemic immunosuppressive (IS) treatment, disease relapse or progression, death from any cause, loss to follow-up or end of 1 year, whichever came first(22). Systemic IS was defined as continuation of drugs in patients with chronic GVHD or addition of new drugs to treat this complication, which included non-topical IS medications, extracorporeal photopheresis or prednisone doses of 10 mg and higher or equivalent. Secondary endpoints included cumulative incidence rates of grades II-IV and III-IV acute GVHD, cumulative incidence rate of chronic GVHD, 1-year IS-free survival, neutrophil and platelet recovery, donor cell engraftment, disease relapse or progression, transplant-related mortality (TRM), toxicities, infections, disease-free survival (DFS), GVHD-free survival, overall survival (OS) and causes of death.
To accommodate the unique design of comparing the arms within a randomized phase II trial to contemporary control, we compared each arm with the control, with the exception of donor cell engraftment, toxicities, infections and causes of death, which were compared across the three randomized arms.
Acute GVHD was graded according to the Consensus Criteria(26). Chronic GVHD was defined by NIH Consensus(25). Outcomes including IS were defined as use of any systemic IS. Corticosteroids were included as systemic IS when patients were receiving doses higher than 10 mg of prednisone or equivalent. IS-free survival included patients who were off systemic IS as defined above at 1 year (±15 days) after transplant. Neutrophil engraftment was defined as the first of 3 consecutive measurements with an absolute neutrophil count of 500 cells/µL or greater. Platelet engraftment was defined as the first day of a sustained platelet count of >20,000/µL, with no platelet transfusion in the preceding 7 days. Donor cell engraftment was assessed by chimerism studies in the blood or bone marrow and defined based on proportion of donor chimerism as full (> 95%) mixed (5–95%) or graft failure (<5%). Disease relapse was defined by either morphological or cytogenetic evidence of acute leukemia or MDS, or radiological or clinical evidence of lymphoma confirmed histologically. Disease progression applied to lymphoproliferative diseases not in remission at time of transplant and was defined as increase in the size of disease at prior sites of disease or at new sites. Toxicities were defined as the development of grade >3 according to the Common Terminology Criteria for Adverse events (CTCAE v4). Infections included the number of events, grade and organism group (bacteria, virus or fungus). DFS included disease relapse or progression and death as events. GVHD-free survival included grades III-IV acute GVHD, chronic GVHD requiring IS and death as events.
Statistical Analysis
The trial was designed to randomize 270 patients across three novel GVHD prophylaxis groups (n=90 each), and also collect comparable data on a concurrent nonrandomized CIBMTR control cohort of approximately 270 patients. The planned sample size of 540 patients had at least 80% power to identify a treatment as promising (HR relative to concurrent nonrandomized control group significant at one-sided 5% significance level) when its GRFS at one year was 15% better than control, based on a simulation study which also accounted for a futility stopping rule. The incidence rates of acute and chronic GVHD, GVHD requiring IS, relapse/progression, TRM and hematologic recovery were calculated for each group using the cumulative incidence estimator, along with 90% confidence intervals. GRFS, IS-free survival, DFS, GVHD-free survival and OS were estimated using the Kaplan-Meier method along with 90% confidence intervals.
Because the control group was nonrandomized, multivariate analyses were done using Cox proportional hazards regression models for GRFS, acute and chronic GVHD, chronic GVHD requiring IS, relapse, treatment-related mortality, DFS, GVHD-free and overall mortality (1-OS) after adjusting for age, disease and donor type/HLA matching. Additional variables including sex, race/ethnicity, conditioning regimen, Karnofsky performance score, HCT comorbidity index (HCT CI), disease status, donor/recipient CMV status, time to transplant, donor/recipient sex match, and donor/recipient ABO match, were also considered in each model using a stepwise model building strategy with a significance level of 10%. Ninety percent confidence intervals for each hazard ratio compared to the control group were constructed. Adjusted survival curves were estimated for each treatment group as well as the controls. SAS/STAT software, Version 9.4 of the SAS System for Windows was used for all the analyses. Comparisons to the control group used a modified intent to treat analysis, where randomized patients were analysed according to their randomized group, but which only included patients who were transplanted. This was because the control group was a non-randomized cohort enrolled at the time of transplant. Planned interim analyses for futility were conducted after the first 30 evaluable patients in each arm based on anticipated 6 month GRFS in the control group of 45–50%. Additional safety monitoring was conducted for transplant related mortality at 100 days post HCT.
Results
Patients
From November 17, 2014 through May 18, 2016, 279 patients (89 to TAC/MTX/BOR, 92 to TAC/MTX/MVC, 92 to TAC/MMF/PTCy) from 31 US centers; and from August 1, 2014 through September 14, 2016, 403 controls from 32 US centers, were enrolled. The final control population used to compare to each arm in the randomized trial was 224 control patients (Figure 1). Median age was 64 years (IQR 59–68) for all arms and controls. Patient characteristics were balanced in subjects enrolled in the randomized trial. Comparing each treatment arm with controls, patients in the control had a higher proportion of higher HCT CI, different distribution of types of RIC conditioning regimens and minor differences in the distribution of diseases (Table 1).
Table 1:
Demographic characteristics among patients enrolled in the clinical trial by treatment arm and controls. P=values comparisons are between each arm and controls
| Controls | TAC/MMF/PTCY | Pairwise p-value | TAC/MTX/BOR | Pairwise p-value | TAC/MTX/MVC | Pairwise p-value | |
|---|---|---|---|---|---|---|---|
| N=224(%) | N=92(%) | Control vs. TAC/MMF/PTCY | N=89(%) | Control vs. TAC/MTX/BOR | N=92(%) | Control vs. TAC/MTX/MVC | |
| Female | 95(42) | 30(33) | 0.13 | 31(35) | 0.25 | 37(40) | 0.80 |
| White | 203(91) | 82(89) | 0.68 | 79(89) | 0.68 | 81(88) | 0.54 |
| Age (years), Median(IQR) | 64 (55–73) | 64 (56–72) | 0.99 | 64 (55–73) | 0.99 | 64 (54–73) | 0.98 |
| HTC comorbidity index:> 3 | 139(62) | 39(42) | 0.0018 | 34(38) | 0.00021 | 37(40) | 0.00049 |
| HLA matched sibling | 89(40) | 29(32) | 0.20 | 29(33) | 0.25 | 33(36) | 0.61 |
| HLA matched other relative | 3(1) | 4(4) | 0.20 | 1(1) | 1.00 | 1(1) | 1.00 |
| Matched unrelated 8/8 | 119(53) | 50(54) | 0.90 | 53(60) | 0.32 | 48(52) | 0.90 |
| Mismatched unrelated 7/8 | 13(6) | 9(10) | 0.23 | 6(7) | 0.79 | 10(11) | 0.15 |
| Disease Risk Index high | 50(22) | 17(18) | 0.54 | 17(19) | 0.65 | 19(21) | 0.88 |
| Disease Risk Index very high | 0 | 2(2) | 0.084 | 1(1) | 0.28 | 1(1) | 0.29 |
| Fludarabine/Busulfan | 135 (60) | 42(46) | 0.018 | 45(51) | 0.13 | 48(52) | 0.21 |
| Fudarabine/Melphalan | 65(29) | 39(42) | 0.025 | 35(39) | 0.082 | 32(35) | 0.89 |
| Fludarabine/Cyclophosphamide | 14 (6) | 1(1) | 0.076 | 0 | 0.013 | 0 | 0.013 |
| Fludarabine/Total Body Irradiation | 9(4) | 3(3) | 1.00 | 2(2) | 0.73 | 3(3) | 1.00 |
| Fludarabine/Cyclophosphamide/Total Body Irradiation | 1 (<1) | 7(8) | 0.00092 | 7(8) | 0.00081 | 9 (10) | 0.00012 |
| Acute Myeloid Leukemia | 117 (52) | 49(53) | 0.90 | 46(52) | 1.00 | 49(53) | 0.90 |
| Acute Lymphoblastic Leukemia | 14 (6) | 8(9) | 0.47 | 12(13) | 0.043 | 11(12) | 0.11 |
| Chronic Myelogenous Leukemia | 1 (<1) | 3(3) | 0.076 | 2(2) | 0.20 | 2(2) | 0.20 |
| Chronic Lymphocytic Leukemia | 9(4) | 0 | 0.063 | 2(2) | 0.73 | 3(3) | 1.00 |
| Myelodysplastic Syndrome | 66(29) | 17(18) | 0.049 | 16(18) | 0.046 | 15(16) | 0.0038 |
| Follicular Lymphoma | 3(1) | 5(5) | 0.0491 | 3(3) | 0.36 | 6(7) | 0.020 |
| Diffuse Large B-Cell Lymphoma | 9(4) | 7(8) | 0.26 | 4(4) | 1.00 | 1(1) | 0.29 |
| Median time to transplant from diagnosis in months (IQR) | 7.2 (4–18.4) | 7.4 (2.4–17.2) | 0.85 | 7.1 (6–20.1) | 0.98 | 6.3 (7.3–19.9) | 0.99 |
Abbreviations: BOR, bortezomib; IQR, interquartile range; MMF, mycophenolate mofetil; MTX, methotrexate; MVC, maraviroc; PTCY, post-transplant cyclophosphamide; TAC, tacrolimus.
Primary endpoint
Adjusted one-year Kaplan-Meier estimates for GFRS were 34% (90% Confidence Intervals [CI] 28–40%) for TAC/MTX and 43% (90% CI 34–54%), 35% (90% CI 27–47%) and 28% (90% CI 20–38%) for TAC/MMF/PTCy, TAC/MTX/BOR and TAC/MTX/MVC, respectively (Table 2 and Figure 2A).
Table 2:
Summary of outcomes by treatment arms and controls. Pointwise probabilities or cumulative incidences and results of the multivariated regression models for each outcome.
| Outcome | Controls (n=224) | TAC/MMF/PTCY (n=92) | TAC/MTX/BOR (n=89) | TAC/MTX/MVC (n=92) | |
|---|---|---|---|---|---|
| GRFS | Adjusted 1 yr estimates (90% CI) | 34% (28–40%) | 43% (34–54%) | 35% (27–47%) | 28%(20–38%) |
| HR (90% CI) | 1.00 | 0.72(0.54–0.94) | 0.98(0.76–1.27) | 1.10(0.86–1.41) | |
| p-value | 0.044 | 0.92 | 0.49 | ||
| aGVHD II-IV | Cum. Inc. at 180 days (90% CI) | 30 (25–36%) | 27(20–35%) | 26(19–34%) | 32(24–40%) |
| HR (90% CI) | 1.00 | 0.85(0.58–1.24) | 0.81(0.54–1.22) | 1.004(0.69–1.45) | |
| p-value | 0.48 | 0.41 | 0.98 | ||
| aGVHD III-IV | Cum. Inc. 180 days (90% CI) | 13% (9–16%) | 2% (0–5%) | 8% (4–13)% | 9%(4–14)% |
| HR (90% CI) | 1.00 | 0.13(0.03–0.46) | 0.53(0.26–1.08) | 0.60(0.30–1.19) | |
| p-value | 0.0082 | 0.14 | 0.23 | ||
| cGVHD | Cum. Inc. at 1 yr (90% CI) | 38% (33–43%) | 28% (20–36)% | 39% (30–48%) | 43%(35–52%) |
| HR (90% CI) | 1.00 | 0.66(0.45–0.96) | 1.07(0.76–1.51) | 1.19(0.86–1.62) | |
| p-value | 0.069 | 0.72 | 0.36 | ||
| cGVHD requiring immunosuppression | Cum. Inc. at 1 yr (90% CI) | 37% (31–42%) | 22% (15–30%) | 29% (22–38%) | 33%(25–41%) |
| HR (90% CI) | 1.00 | 0.59(0.39–0.89) | 0.89(0.60–1.32) | 0.92(0.64–1.31) | |
| p-value | 0.037 | 0.63 | 0.71 | ||
| Relapse/progression | Cum. Inc. at 1 yr (90% CI) | 25% (20–29%) | 28% (21–37%) | 24% (17–32%) | 31%(23–39%) |
| HR (90% CI) | 1.00 | 1.23(0.83–1.83) | 1.01(0.65–1.57) | 1.36 (0.92−−2) | |
| p-value | 0.37 | 0.95 | 0.18 | ||
| Treatment related mortality | Cum. Inc. at 1 yr (90% CI) | 16% (12–21%) | 11% (6–17%) | 17% (11–24%) | 16%(10–23%) |
| HR (90% CI) | 1.00 | 0.64(0.36–1.15) | 1.09(0.65–1.83) | 0.99(0.58–1.68) | |
| p-value | 0.21 | 0.77 | 0.98 | ||
| Disease-free survival | Adjusted 1 yr estimates (90% CI) | 56% (51–62%) | 60% (51–68%) | 58% (49–66%) | 56% (47–64%) |
| HR (90% CI) | 1.00 | 0.92 (0.66–1.27) | 0.98 (0.71–1.37) | 1.21 (0.89–1.65) | |
| p-value | 0.68 | 0.95 | 0.28 | ||
| GVHD-free survival | Adjusted 1 yr estimates (90% CI) | 37% (31–42%) | 53% (44–61%) | 43% (34–52%) | 34% (26–42%) |
| HR (90% CI) | 1.00 | 0.63 (0.47–0.84) | 0.95 (0.72–1.25) | 1.05 (0.81–1.36) | |
| p-value | 0.011 | 0.76 | 0.75 | ||
| Overall Survival | Adjusted 1 yr estimates (90% CI) | 71% (66–76%) | 71% (63–78%) | 68% (59–76%) | 66% (57–74%) |
| HR (90% CI) | 1.00 | 0.98 (0.67–1.44) | 1.18 (0.80–169) | 1.27 (0.88–1.82) | |
| p-value | 0.94 | 0.49 | 0.28 |
Abbreviations: aGVHD, acute graft versus host disease; BOR, bortezomib; cGVHD, chronic GVHD; CI, confidence interval; Cy, cyclophosphamide, HR, hazard ratio; MMF, mycophenolate mofetil’ MTX, methotrexate; TAC, tacrolimus
Figure 2A:
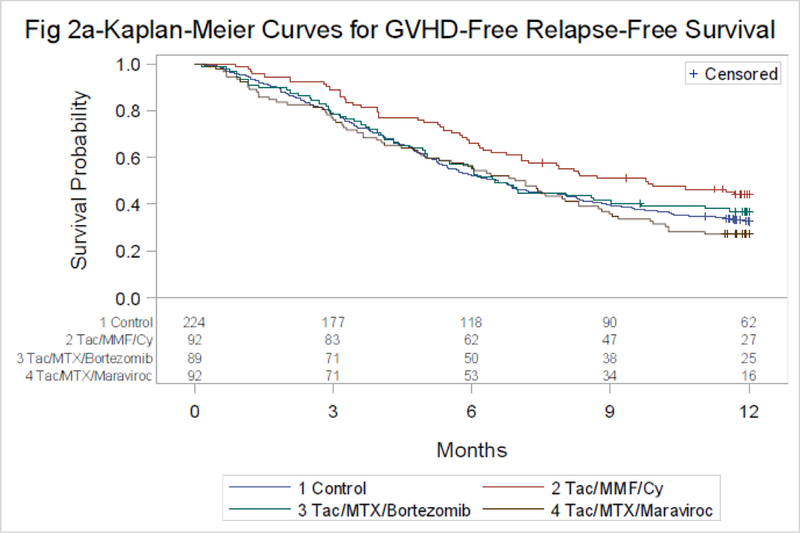
GRFS by treatment group
P-value by Log-rank
Control vs. Tac/MTX/Bortezomib-p=0.92*
Control vs. Tac/MTX/Maraviroc-p=0.45*
Control vs. Tac/MMF/Cy-p=0.044*
Corresponding Hazard Ratios (HR) compared to TAC/MTX were 0.72 (p=0.044), 0.98 (p=0.92) and 1.1 (p=0.49) (Table 2). Additional covariates in the GRFS model include age, disease, donor type and conditioning regimen (Supplemental material page 5).
Secondary endpoints.
Hematologic Recovery and Engraftment
Neutrophil recovery at day 28 and platelet recovery at day 60 did not differ in any arm compared with controls (Supplemental material page 6). Among the randomized arms the proportion of patients who were full donor chimeras at day 100 were 75% (n=62), 73% (n=63) and 65% (n=56) (p=0.36) for TAC/MMF/PTCy, TAC/MTX/BOR and TAC/MTX/MVC, respectively. Corresponding rates of graft failure at day 100 were 3.6% (n=3), 5.8% (n=5) and 3.5% (n=3) (p=0.65) (Supplemental material page 7).
GVHD
The cumulative incidence of acute GVHD grades II-IV at day 180 was 30% (90% CI 25–36%) for TAC/MTX and 27% (90% CI 20–35%), 26% (90% CI 19–34%) and 32% (90% CI 24–40%) for TAC/MMF/PTCy, TAC/MTX/BOR and TAC/MTX/MVC, respectively. Corresponding rates of acute GVHD grades III-IV was 13% (90% CI 9–16%) for TAC/MTX and 2% (90% CI 0–5%), 8% (90% CI 4–13%) and 9% (90% CI 4–14%) (Figure 2B, Table 2).
Figure 2B:
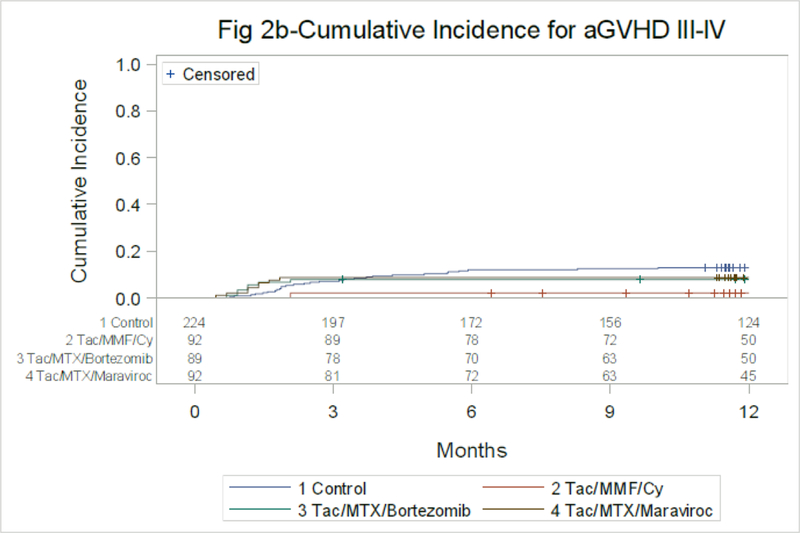
Acute GVHD, Grades III-IV through Day 180
Control vs. Tac/MTX/Bortezomib at 6 mo-p=0.25
Control vs. Tac/MTX/Maraviroc at 6 mo-p=0.36
Control vs. Tac/MMF/Cy at 6 mo-p=0.00021
The cumulative incidence of chronic GVHD at 1 year was 38% (90% CI 33–43%) for TAC/MTX and 28% (90% CI 20–36%), 39% (90% CI 30–48%) and 43% (90% CI 35–52%) for TAC/MMF/PTCy, TAC/MTX/BOR and TAC/MTX/MVC, respectively. Corresponding rates of chronic GVHD requiring IS was 37% (90% CI 31–42%) for TAC/MTX and 22% (90% CI 15–30%), 29% (90% CI 22–38%) and 33% (90% CI 25–41%)(Figure 2C, Table 2). Multivariate analysis of GVHD models are shown in Table 2.
Figure 2C:
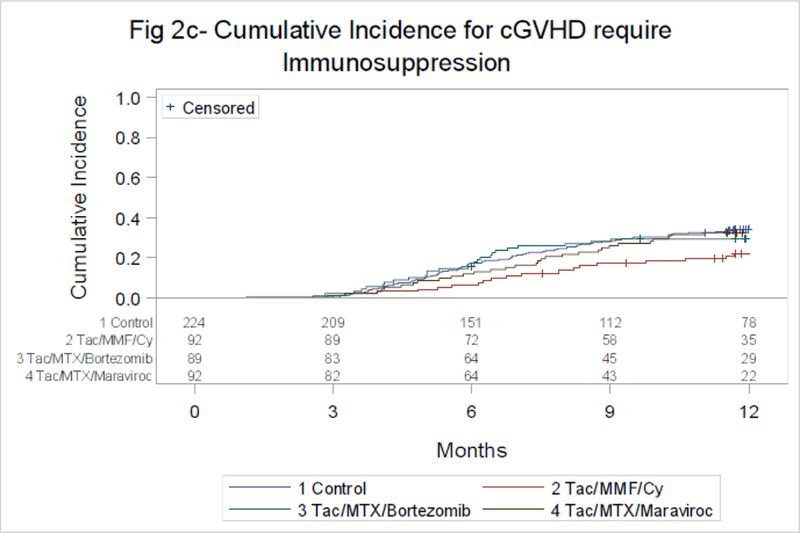
Chronic GVHD Requiring Systemic Immunosuppression
Control vs. Tac/MTX/Bortezomib at 12 mo-p=0.23
Control vs. Tac/MTX/Maraviroc at 12 mo-p=0.50
Control vs. Tac/MMF/Cy at 12 mo-p=0.0081
Toxicities and Infections
Toxicities appear similar within the experimental arms. Overall, 238 patients experienced grades 3 and 4 toxicities: TAC/MMF/PTCy 12 (13%) and 67 (72.8%), TAC/MTX/BOR 10 (11.2%) and 68 (76.4%), and TAC/MTX/MVC 18 (19.6%) and 63 (68.5%) respectively. The most common toxicities where hematological: TAC/MMF/PTCy 77(83.7%), TAC/MTX/BOR 73 (82%), TAC/MTX/MVC 78 (84.8%) and cardiac: TAC/MMF/PTCy 43(46.7%), TAC/MTX/BOR 44 (49.4%), TAC/MTX/MVC 43 (46.7%). Other toxicities with an incidence of over 30% in all arms included metabolic, gastrointestinal, and pulmonary. Toxicities and infections occurred among patients enrolled in the clinical trial are summarized in Table 3 (as well as in Supplemental material page 8). Detailed causes of death are listed in the Supplemental material (Supplemental material page 8).
Table 3:
Summary of all toxicities and infections by randomized arms.
| Treatment Arm | ||||
|---|---|---|---|---|
| System Organ | TAC/MMF/PTCY (N=92) | TAC/MTX/BOR (N=89) | TAC/MTX/MVC (N=92) | Total (N=273) |
| N (%) | N (%) | N (%) | N (%) | |
| Blood/Lymphatic Toxicity | 77(83.7%) | 73(82.0%) | 78(84.8%) | 228(83.5%) |
| Cardiac Toxicity | 43(46.7%) | 44(49.4%) | 43(46.7%) | 130(47.6%) |
| Metabolic Toxicity | 38(41.3%) | 40(44.9%) | 43(46.7%) | 121(44.3%) |
| Gastrointestinal Toxicity | 29(31.5%) | 36(40.4%) | 46(50.0%) | 111(40.7%) |
| Intestinal Obstruction1 | 2(2.2%) | 3(3.4%) | 5(5.4%) | 10(3.7%) |
| Pulmonary Toxicity | 31(33.7%) | 29(32.6%) | 29(31.5%) | 89(32.6%) |
| Neurologic Toxicity | 20(21.7%) | 33(37.1%) | 30(32.6%) | 83(30.4%) |
| Hepatobiliary/Pancreas Toxicity | 18(19.6%) | 23(25.8%) | 29(31.5%) | 70(25.6%) |
| Liver Failure1 | 2(2.2%) | 3(3.4%) | 4(4.3%) | 9(3.3%) |
| Dermatologic Toxicity | 14(15.2%) | 22(24.7%) | 27(29.3%) | 63(23.1%) |
| Musculoskeletal Toxicity | 17(18.5%) | 19(21.3%) | 24(26.1%) | 60(22.0%) |
| Renal Toxicity | 10(10.9%) | 21(23.6%) | 18(19.6%) | 49(17.9%) |
| Received Dialysis1 | 4(4.3%) | 5(5.6%) | 7(7.6%) | 16(5.9%) |
| Fatigue | 14(15.2%) | 17(19.1%) | 17(18.5%) | 48(17.6%) |
| Encephalopathy | 6(6.5%) | 5(5.6%) | 11(12.0%) | 22(8.1%) |
| Maximum Grade toxicities: | ||||
| 3 | 12(13.0%) | 10(11.2%) | 18(19.6%) | 40 (14.7%) |
| 4 | 67(72.8%) | 68(76.4%) | 63(68.5%) | 198 (72.5%) |
| 5 | 5(5.4%) | 6(6.7%) | 9(9.8%) | 20 (7.3%) |
| Infection Type [# of Patients] | ||||
| Bacterial | 74[38] | 61[32] | 69[37] | 204[107] |
| Viral | 59[32] | 51[20] | 31[22] | 141 [74] |
| Fungal | 6[5] | 7[7] | 8[6] | 21[18] |
| Protozoal | 0[0] | 0[0] | 0[0] | 0[0] |
| Other | 2 [2] | 2 [2] | 1[1] | 5[5] |
| Infections of Unknown Etiology [# of Patients] | 17 [16] | 21 [15] | 11 [8] | 49 [39] |
Abbreviations: BOR, bortezomib; MMF, mycophenolate mofetil; MTX, methotrexate; MVC, maraviroc; PTCY, post-transplant cyclophosphamide; TAC, tacrolimus.
Disease Relapse and Survival Outcomes
One-year cumulative incidence of disease relapse or progression, TRM and probabilities of DFS and OS are similar across groups and shown in Table 2 (as well as Supplemental material page 2 and 3. The proportion of patients who were alive, free of IS at one year were 56% for TAC/MTX and 71% (n=75, p=0.041), 67% (n=35, p=0.14) and 57% (n=28, p=0.85) for TAC/MMF/PTCy, TAC/MTX/BOR and TAC/MTX/MVC. Corresponding rates of GVHD-free survival are shown in Table 2 and Figure 2D.
Figure 2D:
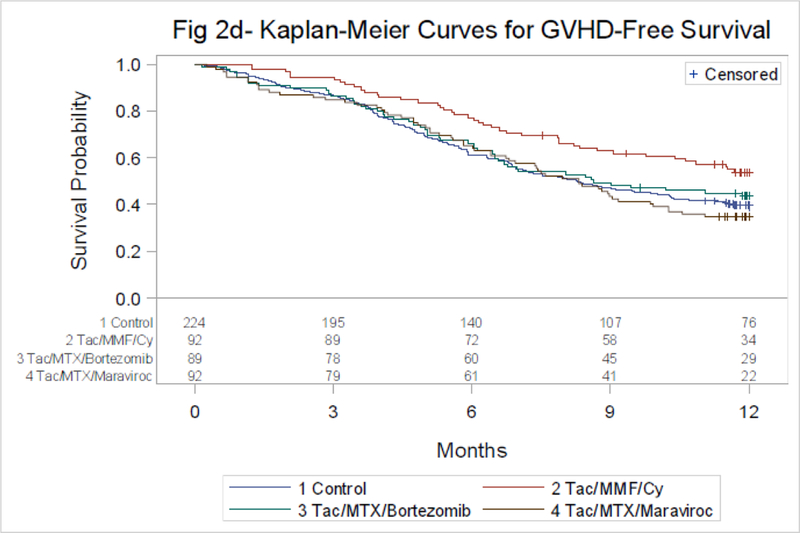
GVHD-Free Survival by Treatment Groups
P-value by Log-rank
Control vs. Tac/MTX/Bortezomib-p=0.77*
Control vs. Tac/MTX/Maraviroc-p=0.75*
Control vs. Tac/MMF/Cy-p=0.0066*
Discussion.
This phase II randomized trial tested three approaches for GVHD prophylaxis comparing each to a nonrandomized contemporary cohort using a novel endpoint assessing GVHD relapse and survival. TAC/MMF/PTCy was the only intervention shown to have better GRFS compared with controls. This benefit was mainly driven by lower rates of severe acute GVHD and chronic GVHD requiring IS, with comparable relapse and survival rates to controls.
In studies leading to this trial, all three agents showed promising activity with respect to preventing GVHD and its complications. Koreth et al. published a phase I study with TAC/MTX/BOR for GVHD prophylaxis after reduced-intensity BMT using human leukocyte antigen-mismatched unrelated donors(12). Twenty-three patients were enrolled. BOR toxicity was minimal. With a 12-month median follow-up, grade II-IV acute GVHD occurred in 3 out of 23 patients. Chronic GVHD occurred in 9 patients. At 1-year, the nonrelapse mortality was zero, cumulative incidence of relapse/progression was 29%, and overall, progression-free, and event-free survival were 75%, 64%, and 59%, respectively. In a similar study on 45 patients receiving peripheral blood grafts that were HLA-mismatched(13), the 180-day cumulative incidence of grade II-IV acute GVHD was 22% and the one-year cumulative incidence of chronic GVHD was 29%. Two-year cumulative incidences of NRM and relapse were 11% and 38% respectively. Two-year progression-free survival and overall survival were 51% and 64%. This BOR-based regimen provided similar outcomes utilizing HLA-mismatched donors to those observed in HLA-matched transplants. Reshef et al. hypothesized that CCR5 blockade with MVC would inhibit visceral GVHD(17). In 35 patients the cumulative incidence rate of grade II-IV acute GVHD was low at 14.7% on day 100 and 23.6% on day 180. Acute liver and gut GVHD were not observed before day 100 and remained uncommon before day 180. Interestingly as hypothesized, serum from patients receiving MVC prevented CCR5 internalization by CCL5 and blocked T-cell chemotaxis in vitro, providing evidence of antichemotactic activity(17). Finally, PTCy has been effective preventing GVHD in both the matched and mismatched, as well as in the non-myeloablative and myeloablative settings(20, 21, 27, 28). The mechanism of action suggests an upregulation of T-reg lymphocytes(18). Moreover, PTCy has been associated with very low rates of post-transplant lymphoproliferative disorder(29), donor derived leukemia(30), and a low immunosuppressive burden after BMT(31). This approach has consistently been associated with low rates of grades III-IV acute GVHD and chronic GVHD. Therefore, these 3 different approaches were ideal to be compared against TAC/MTX as GVHD preventing agents. For the control arm, the combination of MTX and TAC has been used for decades as standard GVHD prophylaxis since proven superior to MTX and cyclosporine, with rates of grade II-IV acute between 32–56% and chronic GvHD between 56–76%(6, 7).
Other MTX free schemas have effective preventing GVHD. Sirolimus based combinations have been effective in both, the reduced intensity as well as in the myeloablative settings, preventing GVHD but not more effective than MTX based combinations(9, 32). Also, the use of anti-thymocyte globulin has shown to decrease GVHD when compared to MXT and cyclosporine after myeloablative conditioning regimens(33). Non-pharmacologic strategies such as the use of bone marrow grafts instead of peripheral blood grafts can also decrease the incidence and severity of GvHD(34). Moreover, the incorporation of anti-thymocyte globulin to PTCy and bone marrow in a small cohort produced very low rates of GVHD(35). The results of this study suggests that PTCy could become an effective approach against GVHD.
The selection of the primary endpoint was an issue of intense analysis by the BMT CTN(22). In GVHD, several competing events can be influenced by a single intervention making the composite endpoint of great interest. Excessive immunosuppression, for instance, could be effective in preventing GVHD but could lead to relapse or life-threatening infections thereby making the intervention ineffective. Conversely, having a patient alive, in remission, with no GVHD and off IS is the ultimate goal of transplantation. Moreover, these composite endpoints improve efficiency reducing sample size in a clinical trial(22).
The results also demonstrated interesting patterns. Chronic GVHD requiring IS in the controls was not different than overall chronic GVHD. Patients enrolled in the clinical trial had mandated IS taper by day 180, whereas in the controls, clinicians followed standard of care practices. Controls tended to continue IS beyond day 180 more frequently and through the development of cGVHD, reflecting the higher rate of use of these agents among these patients.
The unique design of this study combining a randomized clinical trial with a prospective contemporary registry based cohort required strategies to minimize bias. The rationale of using a nonrandomized control was to avoid having a fourth arm in a phase 2 trial, which would be associated with unique challenges and costs, and to improve power and efficiency of the comparisons to the control group for the purpose of identifying promising treatments. This trial design however introduces potential for bias in the comparison to the control group from a number of sources. Lack of randomization may lead to selection bias or confounding. Selecting centers for the control group which are different from the ones participating in the clinical trial, and enrolling all of their eligible patients, may reduce selection bias and generate a “real world” comparison, but it also introduces potential confounding between center effects and treatment effects. The final composition of the control cohort demonstrated patients with more comorbidities; this difference between the study arms and the controls could represent a tendency to enroll healthier patients in clinical trials. Another challenge was to match the clinical assessments from the trial and from the outcomes database to minimize bias from outcome reporting. We addressed this issue by adding supplemental questions to the CIBMTR forms for the control group. After completion of the trial all the data from the trial and control cohorts were combined and randomly selected for review by an Adjudication Review Committee, which was blinded from treatment assignment. The review was performed on a subset of all cases following a review plan approved by the Data and Safety Monitoring Board. If concordance on the first subset was greater than 95%, review of all cases was not necessary. Despite the non-randomized comparison, the groups were comparable except for a few variables which were adjusted for the final models. However, given the nonrandomized nature of the treatment vs. control comparisons, these should be interpreted cautiously. Ultimately this novel clinical trial design combining a randomized phase II trial with a non-randomized concurrent control proved successful in efficiently identifying promising interventions prior to phase III trial development.
The clinical trial design combining a randomized phase II trial with a non-randomized concurrent control proved successful to recognize promising interventions prior to phase III trial development. The study has limitations: its phase II nature and non-randomized control arm prevent this from being a definitive trial. Therefore, the BMT CTN is launching a comparative phase III trial as a follow on to this study to further explore the PTCY platform as a substitute to CNI based GVHD prophylaxis.
Research in context
Evidence before this study
Since 1986 when it was published that the combination of methotrexate and cyclosporine was superior to cyclosporine alone preventing graft versus host disease in patients undergoing HLA-matched bone marrow transplants, this became the standard of care. Ever since, it has been used continuously after both, myeloablative transplants as well as reduced intensity, with bone marrow and with peripheral blood stem cell grafts. While other strategies have been developed to decrease the frequency of severe graft versus host disease, none has been shown superior to methotrexate and a calcineurin inhibitor.
We searched MEDLINE for articles published in English until October 12, 2018. The terms searched were “cyclophosphamide for graft versus host prophylaxis”, “maraviroc for graft versus host prophylaxis”, and “bortezomib for graft versus host prophylaxis”. We found that while cyclophosphamide has been used for prevention of graft versus host disease since the 1970s, it was not until Luznik et al. published their work on haploidentical transplantations that the drug proved effective and started to be used in HLA matched allografts. Reshef et al. published that maraviroc was effective blocking lymphocyte chemotaxis and preventing visceral graft versus host disease. Lastly, Koreth et al. as well as others, published that bortezomib based combinations were a promising strategy to prevent graft versus host disease after mismatched transplants and unrelated donor transplants. We did not find any study comparing these agents either against each other or against methotrexate and cyclosporine during the time the study was open and recruiting patients.
Added value of this study
In this randomized phase II study, a cohort receiving high-dose post-transplant cyclophosphamide was promising when compared a non-randomized CIBMTR contemporary control group receiving methotrexate and a calcineurin inhibitor when using a novel composite endpoint that combines severe acute graft versus host disease, chronic graft versus host disease requiring systemic immunosuppression, disease relapse, or death. Patients randomized to receive maraviroc or bortezomib experienced the similar outcomes compared to patients who received methotrexate and calcineurin inhibitor. Given the nature of the study, no direct comparison between the experimental arms was performed.
Implications of all the available evidence
To our knowledge, this study is the first randomized clinical trial to show promising outcomes using a novel composite endpoint as described for patients who receive high-dose post-transplant cyclophosphamide-based regimen versus the standard combination of methotrexate/calcineurin inhibitor for graft versus prophylaxis. The study has limitations. It is a phase II, compared to a non-randomized contemporary control and no direct comparison between the experimental arms occurred. However, based on the design of the clinical trial, results with high-dose post-transplant cyclophosphamide demonstrated to be sufficiently promising to warrant further study. Therefore, based on these findings, a phase III clinical study will be launched to compare high-dose post-transplant cyclophosphamide with methotrexate and calcineurin inhibitor for graft versus host disease prophylaxis (BMT CTN 1703).
Supplementary Material
Acknowledgments
Participating Centers in the Clinical Trial: Blood and Marrow Transplant Program at Northside Hospital, City of Hope National Medical Center, Cleveland Clinic Foundation, Columbia University Medical Center, Dana Farber Cancer Institute/Brigham & Women’s Hospital, Massachusetts General Hospital, Emory University, H. Lee Moffitt Cancer Center, Sidney Kimmel Comprehensive Cancers Center at Johns Hopkins, Karmanos Cancer Institute, Loyola University Medical Center, Mayo Clinic – Rochester, Medical University of South Carolina, Memorial Sloan Kettering Cancer Center, Ohio State University, Oregon Health and Science University, Roswell Park Cancer Institute, Stanford Hospital and Clinics, Texas Transplant Institute, University Hospital of Cleveland/Case Western University, University of Florida College of Medicine, University of Iowa Hospital and Clinics, University of Kansas Hospital, University of Nebraska Medical Center, University of North Carolina Hospital at Chapel Hill, University of Pennsylvania Cancer Center, University of Texas/MD Anderson Cancer Center, University of Utah Medical School, Virginia Commonwealth University, Washington University. Centers participating as controls: Cancer Transplant Institute at Virginia G. Piper Cancer Center, Cancer Treatment Centers of America, Cedars-Sinai Medical Center, Dartmouth Hitchcock Medical Center, Froedtert Memorial Lutheran Hospital, Greenebaum Cancer Center U M of MD, Henry Ford Hospital Bone Marrow Transplant Program, Indiana Blood & Marrow Transplantation (IBMT), Jewish Hospital Blood and Marrow Transplant Center, LDS Hospital, Mayo Clinic Arizona and Phoenix Children’s Hospital
North Shore University Hospital, Northwestern Memorial Hospital, Penn State Hershey Medical Center, Roger Williams Medical Center, Strong Memorial Hospital - Univ. of Rochester Med Ctr, The Nebraska Medical Center, Tufts Medical Center, Tulane University Medical Center, UCLA Center for Health Sciences, University of Arizona Medical Center, University of California San Francisco Medical Center, University of California, San Diego Medical Center, University of Chicago Hospitals, University of Illinois Medical Center at Chicago, University of Kentucky Chandler Medical Center, University of Mississippi Medical Center, University of Pittsburgh Medical Center, University of Wisconsin Hospital and Clinics, UT Southwestern Medical Center, Vanderbilt University Medical Center, West Virginia University Hospitals.
Funding:
US National Health, Lung, and Blood Institute; National Cancer Institute; National Institute of Allergy and Infectious Disease; and by Millennium Pharmaceuticals.
This study was registered with ClinicalTrials.gov, number NCT02208037
Role of funding source
Support for this study was provided by grant #U10HL069294 to the Blood and Marrow Transplant Clinical Trials Network from the National Heart, Lung, and Blood Institute and the National Cancer Institute along with contributions by Millennium Pharmaceuticals, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the above mentioned parties. The CIBMTR is supported primarily by the U24-CA76518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases and from HHSH234200637015C (HRSA/DHHS). RF and MF had access to the raw data. The funders had no role in the study design, data collection, data analysis, interpretation of results, or writing of the report. All authors approved the manuscript. The corresponding author had the final responsibility to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest:
Ms. Bickett reports grants from NHLBI, during the conduct of the study.
Dr. Bolaños-Meade reports DSMB fees from Incyte Corporation, outside the submitted work.
Dr. Chen reports personal fees from Takeda, personal fees from Magenta, personal fees from Incyte, personal fees from Regimmune, personal fees from Kiadis, outside the submitted work.
Dr. Giralt reports grants from Blood and Marrow Transplant Clinical Trials Network, during the conduct of the study; grants and personal fees from CELGENE, grants and personal fees from TAKEDA, personal fees from JAZZ, grants from MITENYI, grants and personal fees from AMGEN, personal fees from Novartis, personal fees from KITE, grants from CSL Behring, personal fees from Bristol Myers Squibb, grants and personal fees from SANOFI, outside the submitted work. Dr. Holtan reports consulting fees from Incyte, outside the submitted work.
Dr. Koreth reports grants and non-financial support from Prometheus Labs, non-financial support from BMS, non-financial support from Novartis, non-financial support from Miltenyi Biotec, grants and non-financial support from Millennium, personal fees from Amgen, personal fees from Equillium Biotech, from Fortress Biotech, outside the submitted work.
Dr. Noel reports spousal income from Novartis Pharmaceutical, outside the submitted work.
Dr. Pasquini reports personal fees from Pfizer, personal fees from Medigene, outside the submitted work.
Dr. Reshef reports personal fees from Kite, personal fees from Bristol Myers, personal fees from Incyte, personal fees from Pfizer, personal fees from Pharmacyclics, personal fees from Exelixis, personal fees from Takeda, personal fees from Jazz Pharmaceuticals, outside the submitted work. Dr. Weisdorf reports grants from Incyte, outside the submitted work.
The other authors declared no conflict of interests.
Data Sharing Statements:
De-identified participant data for BMT CTN 1203 will be deposited in the NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) (https://biolincc.nhlbi.nih.gov/home/); a publicly available database. Study documents including the study protocol, informed consent form, data dictionary, case report forms for data collection are also available via the repository. Data will become accessible 3 years after the end of clinical activity and 2 years after the primary publication, as anticipated in 2020. Instructions on specimen or data requests and contact information for BioLINCC are also available.
References:
- 1.Zeiser R, Blazar BR. Acute Graft-versus-Host Disease - Biologic Process, Prevention, and Therapy. N Engl J Med 2017;377(22):2167–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolaños-Meade J, Vogelsang GB. Acute graft-versus-host disease. Clin Adv Hematol Oncol 2004;2(10):672–82. [PubMed] [Google Scholar]
- 3.Bolaños-Meade J, Vogelsang GB. Chronic graft-versus-host disease. Curr Pharm Des 2008;14(20):1974–86. [DOI] [PubMed] [Google Scholar]
- 4.Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. New England Journal of Medicine 1986;314:729–35. [DOI] [PubMed] [Google Scholar]
- 5.Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood 2012;119(1):296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood 1998;92(7):2303–14. [PubMed] [Google Scholar]
- 7.Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood 2000;96(6):2062–8. [PubMed] [Google Scholar]
- 8.Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 100 adults with hematologic disease. Blood 2007;110(8):3064–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutler C, Logan B, Nakamura R, Johnston L, Choi S, Porter D, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood 2014;124(8):1372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin PJ, Schoch G, Fisher L, Byers V, Appelbaum FR, McDonald GB, et al. A retrospective analysis of therapy for acute graft-versus-host disease: secondary treatment. Blood 1991;77(8):1821–8. [PubMed] [Google Scholar]
- 11.Sun K, Welniak LA, Panoskaltsis-Mortari A, O’Shaughnessy MJ, Liu H, Barao I, et al. Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proc Natl Acad Sci U S A 2004;101(21):8120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koreth J, Stevenson KE, Kim HT, Garcia M, Ho VT, Armand P, et al. Bortezomib, tacrolimus, and methotrexate for prophylaxis of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation from HLA-mismatched unrelated donors. Blood 2009;114(18):3956–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koreth J, Stevenson KE, Kim HT, McDonough SM, Bindra B, Armand P, et al. Bortezomib-based graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor transplantation. J Clin Oncol 2012;30(26):3202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murai M, Yoneyama H, Ezaki T, Suematsu M, Terashima Y, Harada A, et al. Peyer’s patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat Immunol 2003;4(2):154–60. [DOI] [PubMed] [Google Scholar]
- 15.Murai M, Yoneyama H, Harada A, Yi Z, Vestergaard C, Guo B, et al. Active participation of CCR5(+)CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J Clin Invest 1999;104(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moy RH, Huffman AP, Richman LP, Crisalli L, Wang XK, Hoxie JA, et al. Clinical and immunologic impact of CCR5 blockade in graft-versus-host disease prophylaxis. Blood 2017;129(7):906–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reshef R, Luger S, Hexner EO, Loren A, Frey N, Nasta S, et al. Blockade of Lymphocyte Chemotaxis in Visceral Graft-versus-Host Disease. N Engl J Med 2012;367(2):29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanakry CG, Ganguly S, Zahurak M, Bolanos-Meade J, Thoburn C, Perkins B, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med 2013;5(211):211ra157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasamon YL, Ambinder RF, Fuchs EJ, Zahurak M, Rosner GL, Bolanos-Meade J, et al. Prospective study of nonmyeloablative, HLA-mismatched unrelated BMT with high-dose posttransplantation cyclophosphamide. Blood Adv 2017;1(4):288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luznik L, Bolanos-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood 2010;115(16):3224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2008;14(6):641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasquini MC, Logan B, Jones RJ, Alousi AM, Appelbaum FR, Bolanos-Meade J, et al. Blood and Marrow Transplant Clinical Trials Network Report on the Development of Novel Endpoints and Selection of Promising Approaches for Graft-versus-Host Disease Prevention Trials. Biol Blood Marrow Transplant 2018;24(6):1274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasquini MC, Wang Z, Horowitz M, Gale RP. 2013 Report from the Center for International Blood and Marrow Transplant Research (CIBMTR): Current Uses and Outcomes of Hematopoietic Cell Transplant for Blood and Bone Marrow Disorders. In: Everly JE, Terazaki PI, editors. Clinical Transplants 2013 Los Angeles: The Terasaki Foundation Laboratory; 2014. p. 187–98. [PubMed] [Google Scholar]
- 24.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995;15(6):825–8. [PubMed] [Google Scholar]
- 25.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005;11(12):945–56. [DOI] [PubMed] [Google Scholar]
- 26.Przepiorka D, Devine S, Fay J, Uberti J, Wingard J. Practical considerations in the use of tacrolimus for allogeneic marrow transplantation. Bone Marrow Transplant 1999;24(10):1053–6. [DOI] [PubMed] [Google Scholar]
- 27.Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood 2011;118(2):282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanakry CG, Tsai HL, Bolanos-Meade J, Smith BD, Gojo I, Kanakry JA, et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood 2014;124(25):3817–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanakry JA, Kasamon YL, Bolanos-Meade J, Borrello IM, Brodsky RA, Fuchs EJ, et al. Absence of post-transplantation lymphoproliferative disorder after allogeneic blood or marrow transplantation using post-transplantation cyclophosphamide as graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant 2013;19(10):1514–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanakry CG, Bolanos-Meade J, Kasamon YL, Zahurak M, Durakovic N, Furlong T, et al. Low immunosuppressive burden after HLA-matched related or unrelated BMT using posttransplantation cyclophosphamide. Blood 2017;129(10):1389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majzner RG, Mogri H, Varadhan R, Brown P, Cooke KR, Bolanos-Meade J, et al. Post-Transplantation Cyclophosphamide after Bone Marrow Transplantation Is Not Associated with an Increased Risk of Donor-Derived Malignancy. Biol Blood Marrow Transplant 2017;23(4):612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho VT, Aldridge J, Kim HT, Cutler C, Koreth J, Armand P, et al. Comparison of Tacrolimus and Sirolimus (Tac/Sir) versus Tacrolimus, Sirolimus, and mini-methotrexate (Tac/Sir/MTX) as acute graft-versus-host disease prophylaxis after reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2009;15(7):844–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol 2009;10(9):855–64. [DOI] [PubMed] [Google Scholar]
- 34.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med 2012;367(16):1487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolaños-Meade J, Fuchs EJ, Luznik L, Lanzkron SM, Gamper CJ, Jones RJ, et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood 2012;120(22):4285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


