ABSTRACT
C. elegans hermaphrodites display dramatic age-related decline of reproduction early in life, while somatic functions are still robust. To understand reproductive aging, we analyzed the assembly line of oocyte production that generates fertilized eggs. Aging germlines displayed both sporadic and population-wide changes. A small fraction of aging animals displayed endomitotic oocytes in the germline and other defects. By contrast, all animals displayed age-related decreases in germline size and function. As early as day 3 of adulthood, animals displayed fewer stem cells and a slower cell cycle, which combine to substantially decrease progenitor zone output. The C. elegans germline is the only adult tissue that contains stem cells, allowing the analysis of stem cells in aging. To investigate the mechanism of the decrease in stem cell number, we analyzed the Notch signaling pathway. The Notch effectors LST-1 and SYGL-1 displayed age-related decreases in expression domains, suggesting a role for Notch signaling in germline aging. The results indicate that although sporadic defects account for the sterility of some animals, population-wide changes account for the overall pattern of reproductive aging.
KEY WORDS: Germline, Reproductive aging, Caenorhabditis, Stem cells, Notch, Cell cycle, Endomitotic oocytes, Meiotic development
Highlighted Article: Age-related reproductive decline in C. elegans results from sporadic defects in some animals, but primarily from population-wide processes affecting stem cell number, Notch signaling, cell cycle timing, and meiotic entry and progression.
INTRODUCTION
Aging is characterized by progressive degenerative changes of tissue structure and function that impair physiology and ultimately lead to death. A crucial first step in understanding these changes is characterizing age-related changes in wild-type tissues, which constitutes the starting point for uncovering genes and pathways that modulate age-related decline. Most aging research focuses on somatic aging and lifespan. By contrast, much less is understood about aging of the reproductive system. Reproductive aging, which we define as the progressive, age-related decline in the ability of the reproductive system to produce offspring, is important for human health, as infertility is an increasing concern for women who wait until middle age to start families. The Caenhorabditis elegans hermaphrodite is an important model because the ability to produce oocytes displays rapid age-related decline and ceases entirely while the animals are all still alive, moving and feeding (Hughes et al., 2007). Furthermore, the C. elegans germline is the only adult tissue that contains stem cells, allowing the study of stem cells in aging (Luo and Murphy, 2011; Pazdernik and Schedl, 2013). Extensive studies of reproductive function have been conducted in young adults, where germline stem cells differentiate and mature in a linear assembly line-like pattern as they progress away from the somatic distal tip cell (DTC) towards the spermatheca and uterus over the course of about 2 days (Fig. 1C). The ∼20 cell-diameter long region of the germline that is capped by the DTC niche is called the progenitor zone (PZ); it includes the mitotically cycling germline stem cells, progenitor cells and meiotic S-phase cells, and is followed by stages of meiotic prophase and gametogenesis (Crittenden et al., 2006; Fox et al., 2011; Hansen and Schedl, 2013; Kimble and Seidel, 2013; Pazdernik and Schedl, 2013). At the proximal end, oocytes mature, are ovulated and fertilized, and begin embryogenesis in the uterus (Hirsh et al., 1976; Pazdernik and Schedl, 2013). Measures of egg laying are a convenient surrogate for measures of ovulation, because ∼100% of ovulations in sperm-replete animals results in live progeny (McCarter et al., 1999).
Fig. 1.
The female reproductive system displays rapid age-related decline in sperm-replete C. elegans. (A) Number of progeny produced in 24 h intervals by a wild-type self-fertile (gray) and mated (red) hermaphrodite (n=11, 12). Day 0 is defined as L4 stage. Black points indicate the percentage survival of wild-type mated hermaphrodites (n=54); reproduced, with permission, from Pickett et al. (2013). (See Table S1 for statistics.) (B) Diagram of young adult germ cell mitotic cell cycle and meiotic entry. Percentages represent the proportion of the cell cycle spent in each phase (Fox et al., 2011). (C) One of two gonad arms of the young adult hermaphrodite. Cells progress from mitotic cycling to meiotic prophase to meiotic maturation before being fertilized by sperm in the spermatheca (yellow). The progenitor zone (red, defined by WAPL-1 staining) contains mitotically cycling stem cells. The distal tip cell (nucleus in red, as are other somatic gonad cells) provides GLP-1/Notch signal to maintain the germline stem cell fate.
Although the age-related decline of reproductive output has been well documented for many years (de la Guardia et al., 2016; Garigan et al., 2002; Hughes et al., 2007; Hughes et al., 2011), it remains unclear which processes in the assembly line of oocyte production begin and sustain this decline. Previous studies have examined aging mechanisms in sperm-depleted and/or older animals (Luo et al., 2010; Narbonne et al., 2015; Qin and Hubbard, 2015). To begin to understand why the germline declines early in adult life, we applied state-of-the-art techniques to characterize the cellular and molecular changes that underlie age-related functional decline using sperm-replete animals before and during the decline in reproduction. Here, we report that aging germlines displayed both sporadic and population-wide changes. A small fraction of aging animals displayed endomitotic oocytes in the germline and a shifted DTC nucleus; longitudinal studies indicate that the sporadic endomitotic oocyte phenotype contributed to reduced progeny production, but only in a subset of aging animals. By contrast, there was a population-wide decrease in germline and PZ size during aging. The PZ mitotic cell cycle slowed, the number of germ cells entering meiosis decreased and the rate of meiotic prophase progression decreased. The domain of expression of GLP-1/Notch signaling effectors SYGL-1 and LST-1 decreased, indicating that stem cell number declined and suggesting there was an age-related downregulation of GLP-1/Notch signaling. These population-wide changes in the distal germline began as early as day 3 of adulthood, when reproductive output was at its peak. An important theoretical issue in aging research is the role of sporadic ‘stochastic’ damage as a cause of age-related degenerative change versus the role of population-wide ‘programmed’ decline. For example, Herndon et al. (2002) reported on the heterogeneity among aging individuals and described the stochastic nature of somatic aging. Our results highlight the importance of population-wide changes in driving the age-related decline of germline function and suggest that decreased number and activity of germline stem cells may be a root cause of reproductive aging.
RESULTS
Rapid population-wide reproductive aging preceded somatic aging
An age-related decline in progeny production occurs in both self-fertile and mated hermaphrodites (Hughes et al., 2007). Wild-type hermaphrodites produced on average ∼150 progeny in 24 h at the peak of their reproductive ability on adult day 2 (Fig. 1A). In self-fertile hermaphrodites, progeny number decreased to ∼60 by day 3 (2-fold decrease), to ∼8 by day 5 (20-fold decrease) and was negligible after that. This rapid decline was due to sperm depletion, complicating interpretation of these data relative to reproductive aging. To monitor reproductive aging without the confounding variable of sperm depletion (Angeles-Albores et al., 2017), we analyzed mated hermaphrodites. Mating for 24 h beginning at the L4 stage provides sufficient sperm to avoid sperm depletion, evidenced by production of male progeny until the cessation of reproduction and no increase in brood size after re-mating at day 5 (Cinquin et al., 2016; Hughes et al., 2007; Pickett et al., 2013; Ward and Carrel, 1979). The presence of sperm was confirmed by immunostaining for major sperm protein when possible, and sperm-depleted animals were excluded from analyses. In mated hermaphrodites, progeny number is increased and the reproductive span is extended compared with self-fertile animals. Nevertheless, progeny production declines to ∼40 by day 5 (4-fold decrease) and to ∼12 by day 7 (14-fold decrease) due to aging of the reproductive tract, consistent with previous findings (Hughes et al., 2007). In contrast to the rapid decline of progeny production, the decline of survival probability was slower; 100% of mated hermaphrodites were alive at day 10 and half remained alive until day 16 (Fig. 1A). Thus, the age-related decline of progeny production occurs in animals with relatively healthy somatic tissue.
Endomitotic oocytes were a sporadic low-frequency age-related defect that negatively affected reproduction
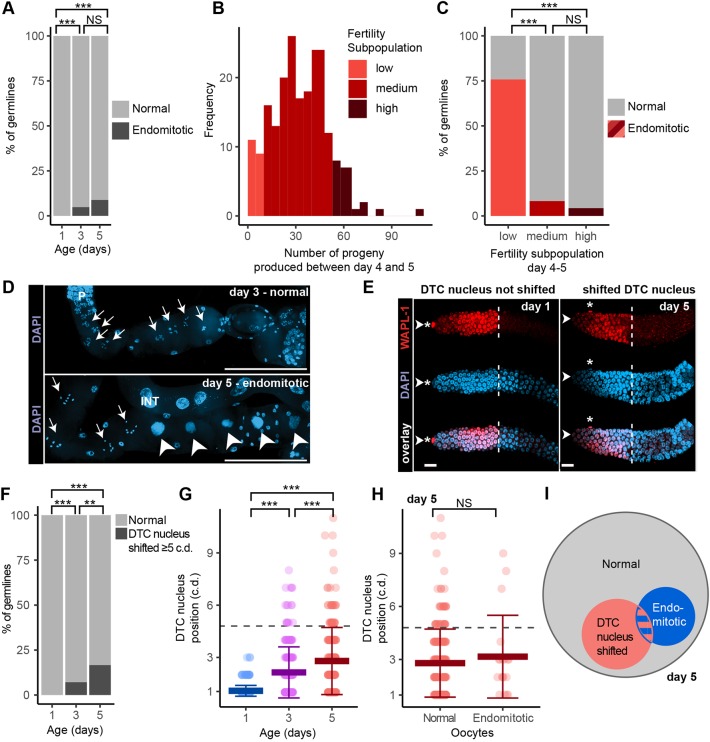
To investigate the basis for reproductive aging in mated hermaphrodites, we analyzed the morphology of dissected germlines in day 1, 3 and 5 adults. The ability to withstand dissection is unlikely to affect these comparisons, because gonads dissect reliably at these ages. DAPI staining was used to analyze nuclear morphology and WAPL-1 antibody staining was used to measure the size of the PZ and the position of the DTC and somatic gonad nuclei (Fig. S1). Some abnormalities were observed at such a low frequency (<2% of germlines) that they could not be effectively studied with our sample size. These included premature meiotic entry of all PZ cells (Glp phenotype), short and narrow PZ, enlarged PZ nuclei, ectopic proliferation, and gaps and oocyte-like formations in the middle of the pachytene region (Fig. S2). Two abnormalities were observed at a low but consistent frequency that supported statistical analysis: the presence of endomitotic oocytes and a shifted DTC nucleus.
Endomitotic oocytes within the proximal gonad arm result from miscoordination of meiotic maturation and ovulation, and can be visualized with DAPI staining (Fig. 2D). Mutations of at least 21 genes are known to result in endomitotic oocytes (Greenstein, 2005; Iwasaki et al., 1996; McCarter et al., 1997; Wormbase, www.wormbase.org/species/all/phenotype/WBPhenotype:0000668#0-10). Endomitotic oocytes can negatively affect reproduction, as several endomitotic oocytes in a gonad block productive maturation and ovulation. In mated hermaphrodites, endomitotic oocytes were extremely rare in day 1 adults. There was an age-related increase in frequency; about 5% of day 3 adults displayed single endomitotic oocytes, usually in only one gonad arm, positioned distal to the spermatheca and not in the uterus (Fig. 2A). At least 9% of germlines of day 5 adults displayed endomitotic oocytes, and affected animals typically displayed multiple endomitotic oocytes with large nuclei, often in both gonad arms.
Fig. 2.
Endomitotic oocytes and a shifted DTC nucleus occurred at a low frequency. (A) Day 1, 3 and 5 mated wild-type hermaphrodites were analyzed for endomitotic oocytes in dissected germlines. Gray indicates no detectable endomitotic oocytes (normal); black indicates one or more endomitotic oocytes (PC test). (B) The number of mated hermaphrodites (frequency) that produced the indicated number of progeny between days 4 and 5. We defined three fertility subpopulations: low (bottom 10%), medium (middle 80%) and high (top 10%). (C) Proportion of endomitotic germlines at day 5 in fertility subpopulations. (D) Representative fluorescence micrographs of normal diakinesis oocytes (arrows) in a day 3 adult (top) and of endomitotic oocytes (arrowheads) in a day 5 adult (bottom), with DNA stained using DAPI. P, pachytene. INT, intestine. Scale bars: 100 μm. (E) Representative fluorescence micrographs of day 1 and 5 adult germlines stained for WAPL-1 (red, top), DAPI (blue, middle) and WAPL-1+DAPI (bottom). Arrowheads indicate the distal tip of the gonad; asterisks indicate the position of the DTC nucleus. The DTC nucleus is at cell diameter positions 1 (left) and 5 (right). Dashed lines indicate the boundary of WAPL-1-positive cells. Scale bars: 10 μm. (F,G) DTC nucleus position was determined by counting the number of c.d. from the gonad tip in day 1, 3 and 5 mated hermaphrodites. (F) Light gray indicates that the DTC nucleus was four or fewer c.d. (normal) from the tip. Dark gray indicates that the DTC nucleus was five or more c.d. (shifted) from the tip (PC test). (G) Each data point indicates the position of one DTC nucleus. Position 1 means the DTC nucleus is at the distal tip (KW test). Dashed line represents cutoff for F. (H) We categorized day 5 adult mated hermaphrodite germlines as non-endomitotic (normal) or endomitotic, and each data point indicates the position of one DTC nucleus (KW test). (I) Venn diagram illustrating the proportion of day 5 mated hermaphrodite germlines showing endomitotic nuclei (blue), shifted DTC nucleus (red), both (blue/red) or neither (gray). All data are mean±s.d. (See Table S2 for statistics; NS indicates P>0.05, **P<0.001, ***P<0.0001.)
To investigate the model that endomitotic oocytes cause an age-related decrease in progeny production, we performed a longitudinal analysis. Progeny production by mated wild-type hermaphrodites is quite variable in the 24 h between days 4 and 5, averaging 32±18 and ranging from 0 to 108 (Fig. 2B). We measured progeny production in this interval for 186 hermaphrodites, then sacrificed the animals and measured endomitotic oocytes. The subpopulation with the lowest progeny production exhibited significantly more endomitotic germlines than the other subpopulations (Fig. 2C). There was no significant correlation between the least-fertile subpopulation and distal germline phenotypes (Fig. S3E,F). The strong correlation between the presence of endomitotic oocytes and low progeny production suggests these two processes are causally linked.
The somatic DTC caps the end of the germline and intercalates between the distal germ cells. The DTC provides the niche for the germline stem cells by expressing LAG-2 (delta ligand) that is received by GLP-1 (Notch receptor) on the germline stem cells (Greenwald and Kovall, 2013; Kimble and Seidel, 2013; Kimble and Ward, 1988; Pazdernik and Schedl, 2013; Pepper et al., 2003). To monitor age-related changes, we determined the position of the DTC nucleus relative to the distal end of the germline (Fig. 2E). In day 1 adults, the DTC nucleus was always positioned within three cell diameters (c.d.) of the end of the germline. Day 3 and 5 adults displayed a shift of 5 c.d. or more in 7% and 17% of germlines, respectively (Fig. 2F,G; Fig. S3B). To analyze the functional consequences of the shifted DTC, we used a longitudinal approach to determine the correlation with progeny production between days 4 and 5. Although the least-fertile subpopulation displayed a higher extent of shifted DTC nucleus, the trend was not statistically significant with this sample size (Fig. S3E). Additionally, the shifted DTC nucleus and endomitotic oocyte phenotypes were not correlated (Fig. 2H,I; Fig. S3C,D). These results document an age-related increase in the frequency of a shifted DTC but do not rigorously establish the functional consequences of this anatomical change, and it is possible that it does not negatively influence reproduction.
If endomitotic oocytes or shifted DTC nuclei are caused by systemic factors, then the two gonad arms of an individual are predicted to display similar behavior. By contrast, if these abnormalities result from anatomically local causes, then the two gonad arms in an individual are predicted to behave independently. To investigate these possibilities, we took advantage of the subset of animals in which both germlines were visible after dissection and compared within-pair variation to between-pair variation using an intraclass correlation analysis. There was no significant correlation in the degree of DTC nucleus shift within a pair of germlines in the same animal (Fig. S3G-I). Thus, the shifted DTC nucleus appears to result from local rather than systemic conditions. By contrast, if one gonad arm displayed endomitotic oocytes, then the other arm was also more likely to display this defect, indicating there may be systemic conditions that promote this defect (Fig. S3A).
Population-wide age-related decline in the size of the germline and progenitor zone
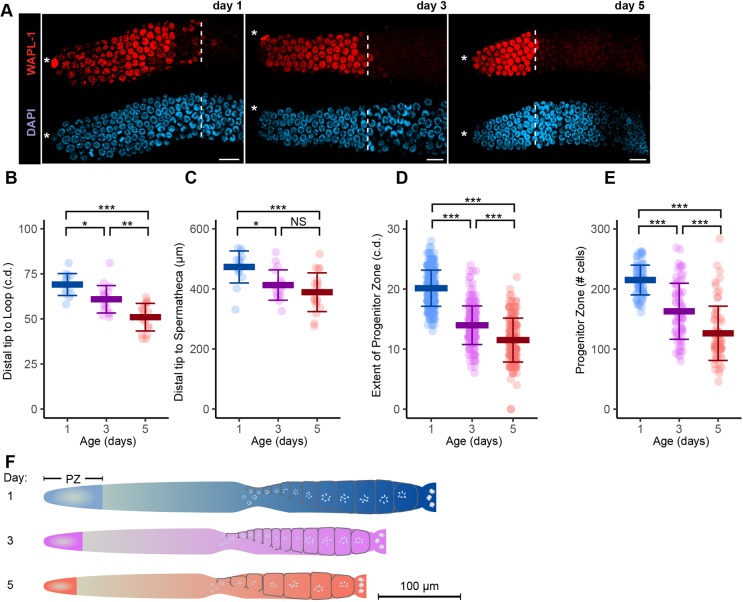
The majority of day 5 adults displayed neither endomitotic oocytes nor a shifted DTC nucleus, yet all these animals produced far fewer progeny than young adults. To explain the population-wide decline in progeny production, we searched for age-related changes in the germline that affect all animals. To measure germline size, we counted the number of c.d. from the distal tip to the loop (distal region of the germline) or the distance in μm from the distal tip to the spermatheca (entire germline). Both measurements revealed a significant decrease in overall size at days 3 and 5 (Fig. 3B,C). The size of the PZ measured in c.d. or total number of cells displayed a significant and progressive decrease (Fig. 3A,D,E). The age-related changes identified in this cross-sectional analysis appear to affect essentially the entire population, albeit within a normal distribution (Fig. 3B-F).
Fig. 3.
A population-wide, age-related decrease in the size of the germline and progenitor zone. (A) Representative fluorescence micrographs of one germline from day 1, 3 and 5 adults stained for WAPL-1 (top, red) and DAPI (bottom, blue). Asterisks indicate DTC nucleus position; white dashed line indicates proximal boundary of WAPL-1-positive cells. Scale bars: 10 μm. (B,C) Each data point is length from the distal tip to the loop (in cell diameters, c.d.) (B) or spermatheca (in μm, used because of the unequal size of cells in the proximal region) (C) in mated hermaphrodites at adult days 1, 3 or 5 (KW test). (D) Each data point is length of the PZ (in c.d.), defined by WAPL-1 antibody staining and measured from the distal tip to the last row, where at least half of the cells were WAPL-1 positive (KW test). (E) Each data point is the total number of PZ cells, defined as WAPL-1 positive (KW test). All data are mean±s.d. (See Table S3 for statistics.) (F) Diagram of the size of the entire germline and PZ in day 1, 3 and 5 adults.
If the decreased size of the PZ is caused by systemic factors, then a similar decrease is predicted to occur in both gonad arms of an individual. Indeed, a significant within-pair correlation of PZ size in day 3 and 5 adults was observed (Fig. S4A-C). The low relative variance within pairs of day 3 and 5 germlines is consistent with an age-related systemic process that affects both gonad arms in an individual animal.
To investigate the relationship between the DTC nucleus position and the size of the PZ, we analyzed the correlation between these features. Overall, the position of the DTC nucleus did not explain the decrease in the size of the PZ, indicating these two age-related changes do not share a common cause (Fig. S4D-F). Day 3 hermaphrodites that were self-fertile or mated to males displayed similar distal germline sizes and extents of the PZ, indicating male exposure did not cause these age-related changes (Fig. S5).
The duration of the mitotic cell cycle displayed population-wide age-related increase
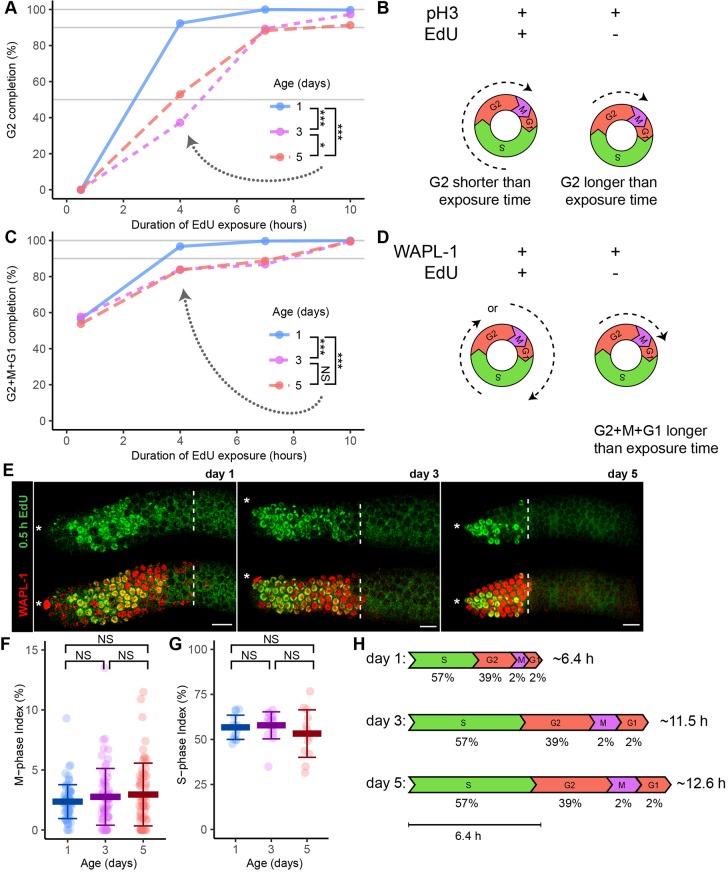
The reduction in size of the PZ may be the result of a decrease in the frequency of cell division and/or the size of the stem cell pool. To analyze the cell cycle, we measured multiple parameters that make it possible to calculate the overall duration of the cell cycle and the proportion of each phase (Fig. 1B, Fig. S1). In day 1 adult hermaphrodites, the mitotic cell cycle takes 6.5-8 h, germ cells do not undergo transit-amplifying divisions (Fox and Schedl, 2015) and quiescent cells are not observed (Crittenden et al., 2006). The cell cycle consists of the phases S, G2 and M, as the G1 phase is short or absent (Fox et al., 2011). To investigate age-related changes in the cell cycle, we measured the mean and maximum duration of the cell cycle in day 1, 3 and 5 adults.
To estimate the durations of cell cycle phases, we fed animals EdU continuously for 0.5, 4, 7 or 10 h and dissected them immediately. A significant fraction of day 5 animals failed to label following a 0.5 h EdU feed; the alternative method of soaking in EdU produced a similar result (see Materials and Methods; Fig. S6). Cinquin et al. (2016) reported a similar phenomenon. Some day 5 animals may have reduced ability to absorb and transport EdU. These animals were not informative about cell cycle duration and were excluded from data analysis.
The duration of G2 was estimated by analyzing the percentage of cells in M phase (pH3 immunoreactive) that were EdU positive; these cells must have been in S phase during the EdU pulse and then proceeded through G2 and entered M phase at the time of dissection (Fig. 4A,B). No M-phase cells were EdU positive after a 0.5 h pulse, indicating G2 always lasted longer than 0.5 h, whereas all M-phase cells were EdU positive after a 10 h pulse, indicating G2 was always less than 10 h. Using all four data points, we estimated the median G2 duration as ∼2.5, ∼4.5 and ∼4.9 h in day 1, 3 and 5 animals, respectively. Thus, there was a significant ∼100% increase in the duration of G2 between days 1 and 3.
Fig. 4.
A population-wide age-related increase in the duration of the cell cycle. Mated hermaphrodites at day 1 (blue), 3 (purple) or 5 (red) were exposed to EdU for 0.5, 4, 7 or 10 h, and germlines were dissected and stained with anti-pH3 antibody, anti-WAPL-1 antibody and/or EdU click chemistry. (A) Percentage of G2 completion was defined as the number of cells that were both pH3 positive (indicative of M phase) and EdU positive (indicative of cell in S phase sometime during EdU exposure), divided by the total number of pH3-positive cells. These data were used to estimate the median time taken to complete G2 phase (n=1655 cells, 366 germlines). Gray horizontal lines indicate the 50th, 90th and 100th percentiles. A PC test was used to compare the 4 h time point (dotted arrow). (B,D) Dashed arrows in diagrams illustrate inferred cell cycle stage at the beginning (S or G2) and end (M, G1 or S) of the experiment. (C) Percentage of G2+M+G1 completion was defined as the number of cells that were both WAPL-1 positive (interpreted as being in the PZ) and EdU positive, divided by the total number of WAPL-1-positive cells. These data were used to estimate the maximum time taken to complete G2+M+G1 phase (n=31,958 cells, 193 germlines). Gray horizontal lines indicate the 90th and 100th percentiles. A PC test was used to compare the 4 h time point. (E) Representative fluorescence micrographs of one germline from adults exposed to EdU for 0.5 h and stained for EdU (green, top), and EdU and WAPL-1 (red, yellow overlap, bottom). Asterisks indicate DTC nucleus position; white dashed lines indicate the proximal boundary of WAPL-1-positive cells. Scale bars: 10 μm. (F) Each data point indicates the M-phase index (number of cells that were pH3 positive divided by the number of PZ WAPL-1-positive cells). (G) Each data point indicates the S-phase index (number of EdU-positive cells divided by the number of PZ WAPL-1-positive cells) of adults following an EdU exposure of 0.5 h (KW test). All data are mean±s.d. (See Table S4 for statistics.) (H) Scale diagrams illustrate the inferred duration of each cell cycle phase. Values below indicate the percentage; values on the right indicate total duration. Bar: 6.4 h. NS indicates P>0.05, *P<0.05, ***P<0.0001.
The duration of G2+M+G1 was estimated from the percentage of all PZ cells (WAPL-1 immunoreactive) that were EdU negative (Fig. 4C,D). About 40% of cells were EdU negative following a 0.5 h pulse, indicating these cells were in G2+M+G1 during the entire pulse, whereas no cells were EdU negative following a 10 h pulse, indicating G2+M+G1 was always less than 10 h. These data were used to calculate maximum duration of these three phases, and the 90th percentile duration was determined by interpolation. We estimated the 90th percentile G2+M+G1 duration as ∼3.4, ∼7.6 and ∼7.4 h in day 1, 3 and 5 animals, respectively. Thus, there was a significant ∼100% increase in the duration of G2+M+G1 between days 1 and 3.
In principle, there are two explanations for the age-related increase in median and maximum cell cycle durations across the population of PZ cells at day 3: (1) in all cells, the duration of all phases of the cell cycle increased proportionately; or (2) in some cells, one phase of the cell cycle increased disproportionately, similar to cell cycle arrest that occurs during germline starvation, sperm depletion and during Drosophila germline aging (Angelo and Van Gilst, 2009; Kao et al., 2015; Narbonne et al., 2015; Seidel and Kimble, 2015). To distinguish these possibilities, we determined the fraction of cells in M phase using anti-pH3 antibody (M-phase index, Fig. 4F, Fig. S7A,B,D) and the fraction of cells in S phase using a 0.5 h pulse of EdU (S-phase index, Fig. 4E,G, Fig. S7C,E). The M-phase index was ∼2.5% in day 1, 3 and 5 animals, indicating there is not an age-related change in the fraction of cells in M phase. Similarly, the S-phase index did not display an age-related change but was ∼57% in day 1, 3 and 5 animals. These results indicate that adult germline stem cells do not become quiescent, at least up to day 3 of adulthood. These results indicate there is an age-related increase in cell cycle duration in all cells, whereas there is no age-related change in the proportion of cells in each cell cycle phase (Fig. 4H).
The rate of meiotic entry, a measure of the output of the progenitor zone, displayed population-wide age-related decline
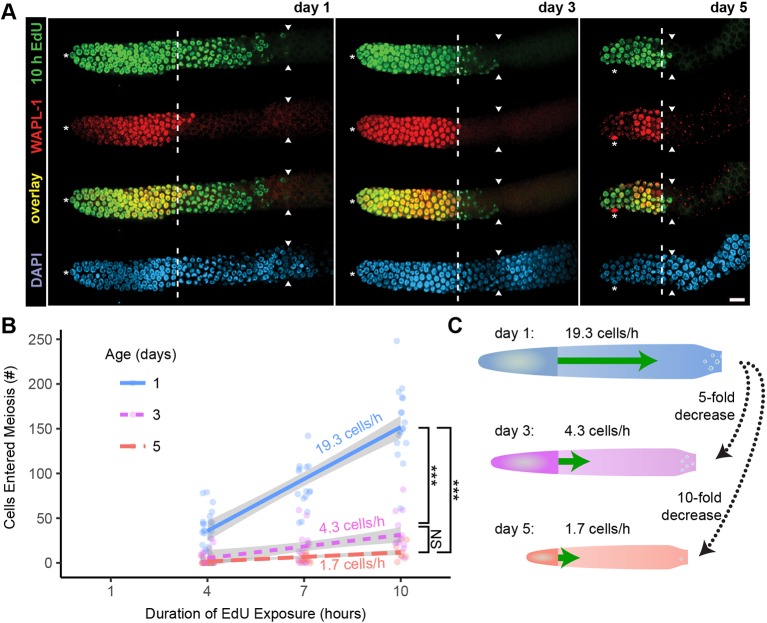
A crucial factor in germline function is the rate at which cells exit the PZ and enter meiosis. We predicted the output of the PZ would decrease in day 3 and 5 adults as a functional consequence of a slower cell cycle and a smaller stem cell pool (see below). To measure the rate of meiotic entry, we exposed animals to EdU for 4, 7 or 10 h, and counted the number of EdU-positive cells in the meiotic region, defined as cells that displayed EdU signal but no WAPL-1 signal. These cells must have resided in the PZ at the beginning of the experiment to become EdU labeled and then entered the meiotic region by the end of the experiment to become WAPL-1 negative (Fig. 5A).
Fig. 5.
A population-wide age-related decrease in the rate of meiotic entry. Mated hermaphrodites at day 1 (blue), 3 (purple) or 5 (red) were exposed to EdU for 4, 7 or 10 h, and germlines were dissected and stained using anti-WAPL-1 antibody and EdU click chemistry. (A) Representative fluorescence micrographs of one germline from day 1, 3 and 5 adults exposed to EdU for 10 h and stained for EdU (green, row 1), WAPL-1 (red, row 2), EdU+WAPL-1 (overlay, row 3) and DAPI (blue, row 4). Asterisk indicates DTC nucleus position; white dashed line indicates proximal boundary of WAPL-1-positive cells; white arrowheads indicate proximal boundary of EdU-positive cells. Scale bar: 10 μm. (B) The rate of meiotic entry (cells/hour) was calculated from the slope of the linear regression of the number of cells that entered meiosis (EdU positive, WAPL-1 negative) versus the duration of the EdU exposure. Gray range indicates 95% confidence interval on linear regression. KW test compared the number of cells that entered meiosis in 10 h. NS indicates P>0.05, ***P<0.0001. (See Table S5 for statistics.) (C) Diagram of day 1, 3 and 5 germlines highlighting the age-related decrease in the rate of meiotic entry (green arrows).
The rate of meiotic entry was calculated as the slope of the regression using three data points (Fig. 5B). Day 1 animals displayed 19.3 cells per hour entering meiosis, consistent with previous reports (Fox et al., 2011). Day 3 and 5 animals displayed a much lower rate, only ∼4 and ∼2 cells per hour entering meiosis, respectively. There was no significant correlation between the size of the PZ and the number of cells that entered meiosis in day 1, 3 and 5 animals (Fig. S8; Table S8). Thus, the rate of meiotic entry displayed a dramatic age-related decrease of ∼78% by day 3 and over 91% by day 5 (Fig. 5C).
A large proportion of germ cells that enter meiosis in the adult function as nurse cells that provide essential constituents to growing oocytes but do not become oocytes, as they undergo apoptosis in late pachytene (Gumienny et al., 1999; Wolke et al., 2007). We estimate that 84% of day 1 cells that enter meiosis become nurse cells, similar to other estimates (see Materials and Methods; Agarwal et al., 2018). The number of nurse cells did not change significantly at day 3 (79%) and day 5 (85%).
The assembly line-like progression from the progenitor zone to oocytes displayed population-wide age-related decline
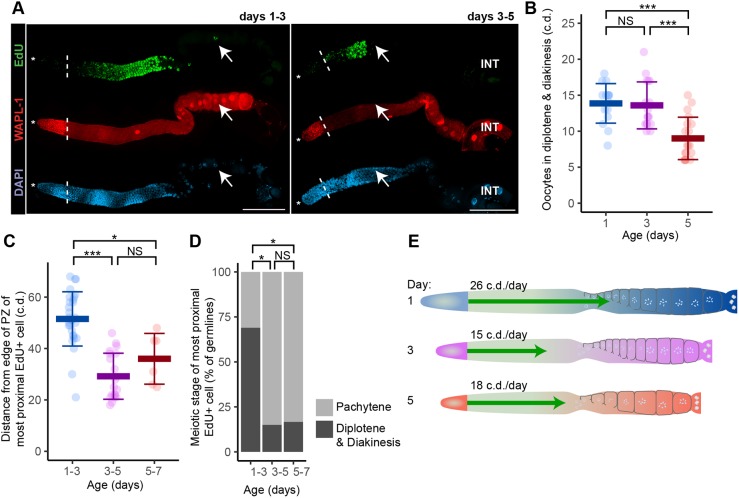
The rate at which cells progress through meiotic prophase is an important aspect of germline function. To determine how this value changes during aging, we used an EdU pulse-chase technique similar to that reported by Jaramillo-Lambert et al. (2007). The ‘pulse’ consisted of 4 h feeding with EdU-labeled bacteria, which mark the majority of PZ cells. The ‘chase’ consisted of transferring animals to unlabeled bacteria for 48 h, followed immediately by dissection. Cells labeled with EdU during the ‘pulse’ retain this label during the ‘chase’ as they progress through meiotic prophase and gametogenesis (Fig. 6A). Thus, the most proximal labeled cells provide a measure of the distance traveled from the PZ in 48 h. Because the distance is measured during a 2-day interval, the rate is an average during days 1-3, 3-5 or 5-7. We analyzed the length of the germline, length of the PZ and the position of the most-proximal cell of the population of EdU labeled cells (Fig. 6C, Fig. S9A-C). In addition, the meiotic prophase substage (mid-pachytene, late pachytene, diplotene or diakinesis) of the most proximal EdU-positive cell was determined (Fig. 6D, Fig. S9D,E).
Fig. 6.
A population-wide age-related decrease in the rate of meiotic progression. Mated hermaphrodites at day 1, 3 or 5 were fed EdU-labeled bacteria for 4 h (‘pulse’) to label all cells that underwent S phase and were then fed unlabeled bacteria for 48 h (‘chase’) to determine the extent of movement. Because the distance is measured during a 2-day interval, the rate is an average of the rate during days 1-3, 3-5 or 5-7. (A) Representative fluorescence micrographs of one germline labeled at day 1 and 3, and analyzed after 48 h by staining for EdU (green, top), WAPL-1 (red, middle) and DAPI (blue, bottom). Asterisk indicates the DTC nucleus position; white dashed line indicates proximal boundary of WAPL-1-positive cells; white arrows indicate the proximal boundary of EdU-positive cells. INT, intestine. Scale bars: 100 μm. (B) Each data point represents the number of cell diameters of diplotene and diakinesis oocytes in the proximal assembly line in mated hermaphrodites at adult day 1, 3 or 5 (KW test). (C) In each germline, the most proximal EdU-positive cell was identified, and each data point indicates its distance in c.d. from the proximal edge of the PZ. Because the most proximal EdU-positive cell was presumably near the proximal end of the PZ at the beginning of the experiment, this value estimates the distance moved over 48 h (KW test). (D) In each germline, the most proximal EdU-positive cell was categorized as pachytene or diplotene/diakinesis (PC test). All data are mean±s.d. (See Table S6 for statistics.) NS indicates P>0.05, *P<0.05, ***P<0.0001. (E) Diagram of day 1, 3 and 5 germlines highlighting the age-related decrease in the rate of meiotic progression (green arrows).
The rate of progression through meiotic prophase decreased on day 3 and day 5 compared with day 1. Starting with day 1 adults, after 48 h the most proximal cell progressed by ∼52 c.d. (Fig. 6C). It was most commonly in diplotene, with some in late pachytene and some in diakinesis (Fig. 6D, Fig. S9D,E). Day 3 animals displayed significantly less movement and maturity after 48 h. The most proximal cell progressed by ∼31 c.d., despite the entire germline decreasing in size, and was most commonly in late pachytene (Fig. 6C,D, Fig. S9D,E). Day 5 adults were not significantly different compared with day 3 adults. Thus, the rate that cells move through meiotic prophase during days 3-5 was dramatically slower than the rate during days 1-3 (Fig. 6E).
Age-related changes in the distal germline have a delayed effect on progeny production
Our results document that the distal gonad displays striking age-related declines by day 3, but at this time progeny production is still at or near its peak. We reasoned that the changes in the distal gonad would have a delayed effect on progeny production that might not be apparent until about 2.5 days later, given the time necessary for germ cells to transit through the germline (Fig. 6). Thus, progeny deposited into the environment at day 3 result from PZ cells on day 1 that transitioned into developing oocytes in the proximal arm of the germline. This model predicts that the number of developing oocytes would remain unchanged between days 1 and 3 but would decrease by day 5. To test this hypothesis, we counted the number of oocytes in diplotene and diakinesis in dissected DAPI-stained germlines. The number of oocytes was ∼14 at days 1 and 3, but decreased significantly to ∼9 at day 5 (Fig. 6B). Thus, the effect on progeny production caused by the slowing cell cycle and shrinking size of the PZ at day 3 only appears about 2 days later.
A population-wide, age-related decline in stem cell number and the domain of Notch signaling
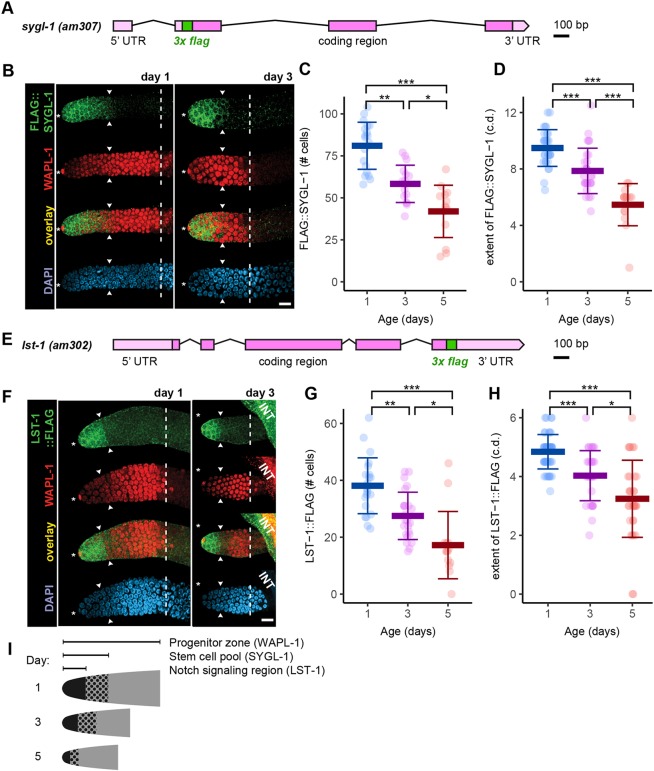
Our results document dramatic changes in the number and behavior of the PZ cells by days 3 and 5. To further investigate the mechanisms that promote these changes, we took a candidate approach by analyzing the Notch signaling pathway. The DTC expresses LAG-2 (Delta), which interacts with GLP-1 (Notch) receptor on germ cells. Following GLP-1 cleavage, the intracellular domain translocates to the nucleus and interacts with LAG-1 to activate the transcription of Notch effector genes (Greenwald and Kovall, 2013; Kershner et al., 2014; Kimble and Ward, 1988; Lee et al., 2016; Pazdernik and Schedl, 2013; Pepper et al., 2003; Shin et al., 2017). Notch signaling is crucial for the stem cell fate, as withdrawal of Notch signaling results in entry into meiosis. We hypothesized that an age-related decrease in GLP-1/Notch signaling contributes to age-related changes in stem cell fate specification. Two direct transcriptional targets of GLP-1 are the sygl-1 and lst-1 genes, which are redundantly necessary and each sufficient to promote germline stem cell fate and block differentiation (Kershner et al., 2014; Lee et al., 2016). To analyze sygl-1 and lst-1 expression, we engineered the FLAG epitope tag into the endogenous loci so that these genes express FLAG::SYGL-1 and LST-1::FLAG, respectively (Fig. 7A,E).
Fig. 7.
A population-wide age-related decrease in the stem cell pool and GLP-1 (Notch) signaling. Mated sygl-1(am307) and lst-1(am302) hermaphrodites expressing FLAG::SYGL-1 or LST-1::FLAG fusion proteins, respectively, were stained using anti-FLAG antibody. (A,E) Diagram of the sygl-1(am307) and lst-1(am302) genomic loci. DNA encoding 3xFLAG epitopes was inserted in-frame using CRISPR/Cas9 genome editing, resulting in an N-terminally (A, sygl-1) or a C-terminally (E, lst-1) tagged fusion protein expressed from the endogenous locus. (B,F) Representative fluorescence micrographs of one germline from day 1 and 3 adults stained for FLAG::SYGL-1 (B) or LST-1::FLAG (F) (green, row 1), WAPL-1 (red, row 2), FLAG+WAPL-1 (overlay, row 3) and DAPI (blue, row 4). Asterisks indicate DTC nuclear position; white arrowheads indicate proximal boundary of FLAG staining; white dashed lines indicate the boundary of WAPL-1-positive cells. INT, intestine. Scale bars: 10 μm. (C,D,G,H) Data points indicate the number of expressing cells (C,G) or the extent of these cells (c.d.) (D,H) (KW test). *P<0.05, **P<0.001, ***P<0.0001. All data are mean±s.d. (See Table S7 for statistics.) (I) The size decrease in the PZ, stem cell pool and Notch signaling region.
To investigate whether the number of germline stem cells decreases with age, we used FLAG::SYGL-1. We interpret the SYGL-1 expression zone to approximate the stem cell pool, as genetic analysis indicates SYGL-1 is sufficient for the stem cell fate (Shin et al., 2017). Dissected germlines of day 1 adults displayed expression of FLAG::SYGL-1 in the cytoplasm of the most distal ∼10 cell diameters of the germline; the FLAG::SYGL-1 region encompassed 81±14 cells (Fig. 7B-D, Fig. S10C). Day 3 adults displayed a significant ∼1.4-fold decrease in the number of FLAG::SYGL-1 cells, and day 5 adults displayed a significant ∼2-fold decrease (Fig. 7C,I). The expression zone measured in cell diameters displayed a similar age-related decline (Fig. 7D). Thus, the extent of SYGL-1 expression displayed age-related decline by day 3, indicating there is an age-related decline in the number of germline stem cells.
To investigate whether changes in GLP-1/Notch signaling are responsible for the age-related decrease in the size of the PZ, we used LST-1::FLAG. Dissected germlines of day 1 adults stained with anti-FLAG antibody displayed expression of LST-1::FLAG in the cytoplasm of the most distal ∼5 c.d. (Fig. 7F-H, Fig. S10A), which corresponded well with the localization of lst-1 pre-mRNA introns, and thus the region of Notch-mediated transcription (Lee et al., 2016). Consistent with this interpretation, when the DTC nucleus was shifted, the domain of LST-1::FLAG displayed a corresponding shift (Fig. S10B). The LST-1::FLAG region encompassed 38±10 cells at day 1 (Fig. 7G). Day 3 adults displayed a significant ∼1.4-fold decrease in the number of LST-1::FLAG cells, and day 5 adults displayed a further significant ∼2.2-fold decrease (Fig. 7G,I). The expression zone measured in cell diameters displayed a similar age-related decline (Fig. 7H). Thus, the extent of LST-1 expression displayed age-related decline by day 3, indicating there is an age-related decline in the GLP-1/Notch signaling system that maintains germline stem cells.
DISCUSSION
Sporadic changes contributed to an age-related reproductive decline in a subset of animals, but population-wide changes accounted for reproductive aging in the majority of animals
An important issue in understanding the biology of aging is distinguishing the roles of ‘stochastic’ versus ‘deterministic’ changes. Experimental data can be used to categorize changes as low frequency (sporadic) versus high frequency (pervasive, population-wide). The next layer of interpretation suggests that low-frequency or sporadic changes have a mechanism that is stochastic, probabilistic or unpredictable. In aging studies, the cause of such changes is inferred to be entropy, an energetic environment that damages biological systems in unpredictable ways that cause degeneration. By contrast, high-frequency or population-wide changes are inferred to have a mechanism that is programmed, deterministic or predictable, which arises from genetic programs, such as those that control development.
Here, we document both low-frequency sporadic changes as well as high-frequency population-wide changes. Some defects occurred so infrequently that we could not quantify them, whereas the appearance of endomitotic oocytes in the proximal germline and a shifted DTC nucleus could be quantified; by day 5, ∼9% and ∼17% of germlines displayed endomitotic oocytes and a shifted DTC nucleus, respectively. There was no correlation between the appearance of these two defects in individual animals, indicating that they do not share a common cause. A longitudinal study indicated that the presence of endomitotic oocytes reduced progeny production, consistent with previous studies of mutant strains that display high levels of endomitotic oocytes and are subfertile or sterile (Greenstein, 2005; Iwasaki et al., 1996; McCarter et al., 1997; Wormbase, www.wormbase.org/species/all/phenotype/WBPhenotype:0000668#0-10). These results suggest that low-frequency sporadic defects account for an age-related decrease in progeny production in a subset of animals (Fig. 8C). Similar to these results, sporadic defects of mispositioned niche cells and ectopic germ cell proliferation were reported in a subset of middle-aged Drosophila ovaries (Kao et al., 2015).
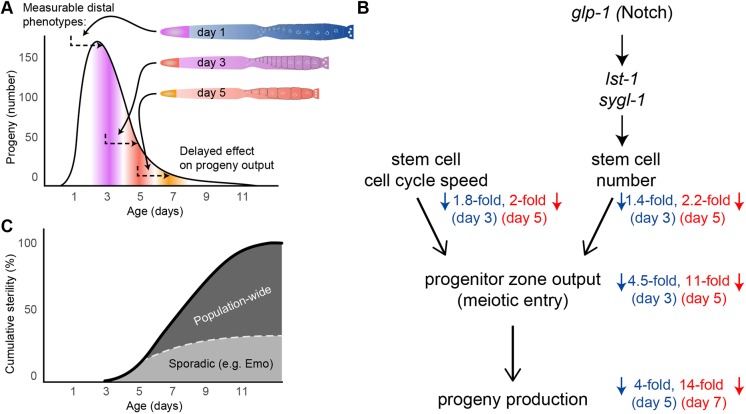
Fig. 8.
Model of age-related changes that drive reproductive decline in sperm-replete C. elegans. (A) Diagrams of day 1, 3 and 5 germlines are shown with a typical wild-type progeny production curve (like in Fig. 1A) to illustrate that PZ cells take 2 or more days to become oocytes and be laid as eggs. Thus, age-related changes in the PZ of day 3 and 5 adults affect egg laying on days 5 and 7, respectively. (B) A model relating glp-1 (Notch) activity and cell cycle dynamics to progeny production. Thick arrowheads indicate the molecular pathway; thin arrowheads indicate cell events from distal (top) to proximal (bottom). Blue and red numbers indicate fold declines in function between peak and days 3, 5 and 7. (C) The cumulative sterility of a population results from age-related changes that are both sporadic and population wide. The dotted line indicates a projection of the relative contribution of sporadic defects (e.g. endomitotic oocytes) as a cause of sterility. Population-wide declines in stem cell number and activity contribute to the quantitative decline in progeny production in all animals and are the cause of sterility in a fraction of the population.
A variety of high-frequency population-wide changes were observed by analyzing the stem cell pool and mitotic cell cycle. Striking declines in germline function were observed very early in life, at day 3 of adulthood, when each animal was still producing over 100 progeny per day, including a 25% decrease in PZ cell number, a 1.4-fold decrease in stem cell number, a doubling of the cell cycle duration and a 5-fold decrease in the rate of meiotic entry. Owing to the assembly-line organization of the germline, these declines in distal germline function were manifested at day 5, contributing at least in part to the 4-fold decrease in progeny production (Fig. 8A,B). Further distal germline declines on day 5 were manifested as decreased progeny production on day 7 and beyond. These age-related changes appeared to occur in all animals, and thus were quite different from the low-frequency changes. These studies suggest that population-wide changes are the predominant cause of the age-related decline in progeny production (Fig. 8C). Furthermore, we found a significant correlation between gonad arms in the decrease in PZ size, indicating that there is an age-related systemic change, likely cell non-autonomous, that results in a functional decline in both germlines.
Previous studies of C. elegans aging in the distal germline focused on sperm-depleted and/or older animals. The progenitor zone shrinks with age, and mating enhances this phenotype through promoting germ cell flux; TGF-β and insulin signaling pathways accelerate these changes (Luo et al., 2010; Narbonne et al., 2015; Qin and Hubbard, 2015). By contrast, we examined sperm-replete animals prior to and during the rapid decrease in reproductive output using mated hermaphrodites. Male exposure might have multiple effects on hermaphrodites, including male pheromones, seminal fluid and physical trauma (Maures et al., 2014; Shi and Murphy, 2014), and we cannot formally separate these possible effects from sperm transfer. However, self-fertile and mated day 3 hermaphrodites displayed no difference in the size of the progenitor zone, suggesting that the age-related decline in progenitor zone size was not caused by male exposure.
Age-related changes in Notch signaling and mitotic cell cycling were associated with reproductive decline
GLP-1/Notch signaling is necessary for the germline stem cell fate (Austin and Kimble, 1987). Based on our observation that the size of the PZ declined with age, we hypothesized that the extent of GLP-1/Notch signaling declines with age, leading to a decrease in germline stem cell number. We measured the extent of expression of two GLP-1/Notch effectors, sygl-1 and lst-1; these genes are direct transcriptional targets of the GLP-1/Notch intracellular domain transcription complex and are redundantly necessary and singly sufficient for the stem cell fate (Kershner et al., 2014; Lee et al., 2016). Expression of SYGL-1 encompassed ∼81 distal-most cells in day 1 adults, decreasing by ∼1.4-fold at day 3. As SYGL-1 expression is sufficient for the stem cell fate, the number of SYGL-1-expressing cells provides a measure of stem cell number (Shin et al., 2017). LST-1 expression was more limited, in the 38 distal-most cells in day 1 adults, decreasing ∼1.4-fold at day 3. Interestingly, single molecule fluorescent in situ hybridization showed that the transcription of introns for both lst-1 and sygl-1 extends only ∼5 c.d. from the DTC (Lee et al., 2016), which closely matches the region of LST-1 accumulation, but is significantly shorter than that of SYGL-1 accumulation, consistent with post-transcriptional mechanisms functioning in extending SYGL-1 expression (Shin et al., 2017). We suggest that LST-1 is a more direct readout of the amount of GLP-1/Notch signaling and thus propose that GLP-1/Notch signaling decreases in day 3 and 5 adults. This proposal is consistent with the age-dependent enhancement of the premature meiotic entry phenotype in a weak glp-1 loss-of-function mutant (Qin and Hubbard, 2015). One way to test the hypothesis that the age-related decline in Notch signaling is a cause of reproductive aging is to analyze mutant strains with altered Notch signaling. Chromosomal mutations that increase glp-1 activity and transgenic constructs that increase sygl-1 activity have been reported (Pepper et al., 2003; Shin et al., 2017), and if these manipulations delay reproductive aging it would provide direct support for our hypothesis.
The mitotic cell cycle duration doubled by day 3 of adulthood, with the proportions of the cell cycle phases unchanged and no cell cycle quiescence. Similarly, there is a slowing of cell cycle duration in Drosophila female germline aging (Kao et al., 2015). The doubling of cell cycle duration is not due to a decrease in GLP-1/Notch signaling, as cell cycle duration is unaffected in weak glp-1 loss-of-function mutants that decrease stem cell number (Lee et al., 2016; Fox and Schedl, 2015). In contrast to aging in day 3 mated adults, starvation at various life cycle stages (L1, L2 and mid-L4) results in germline quiescence, with a G2 cell cycle arrest (Baugh, 2013; Fukuyama et al., 2006; Narbonne et al., 2015; Seidel and Kimble, 2015). Likewise, PZ quiescence in the absence of sperm also appears to be in G2 phase (Narbonne et al., 2015; Qin and Hubbard, 2015). Thus, the mechanisms affecting cell cycle changes during aging in mated hermaphrodites appear to be distinct from those described to occur during starvation and the absence of sperm. The doubling of cell cycle duration and the decrease in stem cell number (GLP-1/Notch signaling) provide an informed point to further define the systemic molecular mechanisms that account for the rapid, population-wide changes in the distal germline.
The evolutionary biology of reproductive aging
Detailed measurements of reproductive aging raise the intriguing question: what selective forces during evolution sculpted the progeny production curve so that it displays such a dramatic decline early in life? Two long-standing theories are based on the premise that an organism achieves reproductive success by generating as many progeny as possible. According to this logic, reproductive aging is a deleterious trait because it decreases progeny production. Medawar (1952) proposed that extrinsic mortality creates a ‘shadow of selection’ that gradually prevents natural selection from favoring animals with a longer reproductive span. Williams (1957) proposed that selection for high levels of early reproduction causes a decline of late reproduction, as a result of antagonistically pleiotropic genes. By contrast, the optimal progeny number theory proposed by Hughes et al. (2007) is based on the premise that an organism achieves reproductive success by generating an optimal number of progeny – not too few and not too many. According to this logic, reproductive aging is an adaptive trait that contributes to sculpting the progeny production curve to achieve the optimal number. It is apparent that reproductive success is not as simple as the number of F1 progeny generated by the P0 parent – a more sophisticated perspective is the number of F1 progeny that mature to be reproductive adults and generate F2 progeny. The extension of this logic is that reproductive success must be judged based on an organism's long-term contribution to the gene pool measured after innumerable generations. Thus, reproductive success is related to long-term population dynamics. Individuals are part of populations that exist in ecological niches with finite resources. If the population exceeds the carrying capacity of the ecological niche, then there will be widespread deprivation and population instability characterized by cycles of boom and bust. Thus, reproductive success is fostered by individual reproductive patterns that promote stable population dynamics. By limiting progeny production, reproductive aging may be a cause of reproductive restraint that promotes the optimal progeny number and leads to adaptive population dynamics.
The results presented here do not directly test evolutionary theories, but they relate to these theories in two important ways. First, we demonstrate that reproductive aging in C. elegans is primarily caused by population-wide changes in stem cell number and activity, and only rarely caused by sporadic defects such as endomitotic oocytes. This pattern is suggestive of an evolved genetic program that controls the decline of reproduction, consistent with a prediction of the optimal progeny number theory. Second, our results document a very early decline in germline function, long before comparable declines in somatic function. This pattern suggests that, during evolution, somatic tissues were selected to be durable during the rise and fall of reproductive function, so that somatic aging does not limit reproduction. We speculate that this pattern facilitates selection to accelerate or delay reproductive aging as a way to manipulate progeny number, as somatic function is not the limiting factor. An important test of these evolutionary theories will be to experimentally determine how reproductive aging influences population dynamics over many generations. To achieve this, we are developing a laboratory ecosystem to measure population dynamics and a simulation model to determine how the progeny production curve and reproductive aging affect population dynamics.
MATERIALS AND METHODS
Strains and general methods
C. elegans strains were cultured at 20°C on 6 cm Petri dishes containing nematode growth media (NGM) agar and a lawn of E. coli strain OP50 unless otherwise noted. The wild-type C. elegans strain and parent of edited strains was Bristol N2 (Brenner, 1974). Mated wild-type N2 hermaphrodites were used except: unmated self-fertile N2 hermaphrodite data (Fig. 1A, Fig. S5) and mated hermaphrodites with the genotype lst-1(am302) and sgyl-1(am307), in which the endogenous loci encode the FLAG epitope (Fig. 7, Fig. S10).
Males of the strain CB4855 (‘Mr Vigorous’) were used to mate hermaphrodites because these males display a higher mating ability – they also deposit a copulatory plug after mating (Hodgkin and Doniach, 1997). Strains were obtained from the CGC unless otherwise noted.
Animals were synchronized by picking fourth-stage larvae (L4), defined as day 0, from populations that had not experienced starvation for a minimum of three generations. For mating experiments, 30-50 L4 hermaphrodites were cultured on a dish with 30-50 young adult males (at a 1:1 ratio) for 24 h, then the hermaphrodites were removed from the males. The presence of copulatory plugs on many of the hermaphrodites confirmed a high frequency of mating in the population; however, hermaphrodites without a copulatory plug were not excluded from the experiment. Hermaphrodites were moved to fresh NGM+E. coli OP50 dishes daily until they reached the desired age.
To measure progeny production of mated hermaphrodites, we placed 30 L4, wild-type hermaphrodites and 30 young adult, CB4855 males on a Petri dish with abundant food for 24 h. After 24 h (adult day 1), we placed each mated hermaphrodite on an individual dish, transferred the animal to a fresh dish daily, and 2 days later scored the number of live progeny produced daily. This method provides accurate and precise measurements of daily progeny production, consistent with previous findings (Hughes et al., 2007). Because animals were mated in groups, we did not obtain progeny counts for the first 24 h for the mated animals in Fig. 1A. To measure progeny production of self-fertile hermaphrodites, we placed each L4 hermaphrodite on an individual dish, transferred the animal to a fresh dish daily, and 2 days later scored the number of live progeny on the dish.
Previous studies have shown that exposure to a high concentration of males or male pheromone reduced longevity and caused shrinking of hermaphrodites (Maures et al., 2014; Shi and Murphy, 2014). The damage appears to be caused by functional sperm, but not seminal fluid (Shi and Murphy, 2014). In the experiments described here, male exposure was limited to the minimum required for sperm transfer. Under these conditions, day 3 adults that were mated and self-fertile displayed a similar size of the distal germline or extent of the PZ (Fig. S5); at day 3, most of the unmated hermaphrodites are still self-fertile, and thus the PZ has not become quiescent due to the absence of sperm (Narbonne et al., 2015; Qin and Hubbard, 2015). Previous work in our labs showed no effect of brief mating on the longevity of hermaphrodites (Pickett et al., 2013).
Generating alleles encoding the FLAG epitope using CRISPR/Cas9
To visualize the localization of LST-1 and SYGL-1, we engineered alleles that encode the FLAG epitope [C-terminal lst-1(am302[lst-1::flag])], in which all five predicted LST-1 isoforms are fused to FLAG and to N-terminal sygl-1(am307[flag::sygl-1]). Generally, the co-CRISPR approach was used to edit genomic loci (Arribere et al., 2014). Guides and repair templates were designed using ApE (jorgensen.biology.utah.edu/wayned/ape) (Table S10). Oligonucleotides and gblocks were purchased from IDT. gblocks were amplified by PCR, purified using an Invitrogen PCR cleanup kit, concentrated using ethanol precipitation and then resuspended in Tris-EDTA buffer. Guide oligonucleotides were ligated into Mike Nonet's derivative of pDR274 (previously digested with BsaI-HF and purified on a Quiagen column) to generate guide RNA expression plasmids, which were transformed into competent DH5-alpha cells. Plasmids were purified using a Quiagen miniprep column according to manufacturer's instructions, including the extra PB wash, and resuspended in Tris-EDTA buffer. All new plasmids were confirmed by sequencing.
Injection mixes were diluted into water and contained Cas9-expressing pDD162 (gift from Mike Nonet, Washington University School of Medicine, St Louis, MO, USA) at 50 ng/µl, dpy-10 guide plasmid (pMN3153) at 20 ng/µl, dpy-10(cn64) repair oligonucleotide AFZF827 at 500 nM, our gene of interest guide plasmids at 40 ng/µl for each plasmid, a ssDNA repair template at 600 nM or a dsDNA repair template at between 50 ng/µl and 500 ng/µl. Approximately 30 young adult P0 animals were injected in one or both gonads and recovered in recovery buffer [5 mm HEPES (pH 7.2), 3 mM CaCl2, 3 mM MgCl2, 66 mM NaCl, 2.4 mM KCl and 4% glucose (w/v)] on NGM dishes.
Young adult F1 hermaphrodites were screened for the Rol or Dpy phenotype, and mutant animals were picked singly or in groups of up to eight to fresh NGM dishes and allowed to produce progeny overnight. Next, animals were picked into 10 μl of 1×PCR buffer containing 0.1 mg/ml proteinase K, incubated at 65°C for 60 min and then at 95°C for 30 min to inactivate proteinase K. Using primers that hybridize outside of the homology template, PCR was used to detect insertions of 66 nucleotides (New England Biological). The young adult F2 progeny of homozygous or heterozygous edited F1 animals were picked to individual dishes and genotyped in a similar manner. The DNA sequence of the PCR product from homozygous edited F2 progeny was determined to confirm the in-frame insertion of DNA encoding the FLAG epitope. Strains were outcrossed to N2, resulting in WU1756 and WU1770 for lst-1 and sygl-1, respectively.
To address the possibility that insertion of DNA encoding the epitome tag disrupted the activity of the lst-1 or sygl-1 gene, we performed a functional test. Neither lst-1(null) nor sygl-1(null) single mutants display a visible phenotype, whereas lst-1(null);sygl-1(null) double mutants (or double RNAi knockdowns) display a sterile phenotype. We used RNAi to knock down the expression of sygl-1 in lst-1(am302) animals or lst-1 in sygl-1(am307) animals. We did not observe a sterile phenotype in either case, indicating that these alleles retain gene activity.
EdU labeling experiments
To make EdU dishes, we seeded M9 agar dishes (Stiernagle, 2006) with concentrated E. coli MG1693 Thy, which had been grown for 24 h at 37°C with shaking in minimal media containing 20 µM 5-ethynyl-2′-deoxyuridine (EdU, Invitrogen). The culture consisted of 100 ml M9, 4 ml overnight LB-grown MG1693 E. coli, 5 ml 20% glucose, 50 μl 1.25 mg/ml thiamine, 1.2 ml 0.5 mM thymidine, 100 μl 1 M MgSO4 and 200 μl 10 mM EdU (Fox et al., 2011).
Appropriately mated and aged animals were either washed with phosphate-buffered saline (PBS) or picked to EdU dishes and incubated for the appropriate time: 0.5, 4, 7 or 10 h. Cells with any amount of EdU signal overlapping with DAPI staining were scored as EdU positive. We defined the S-phase index as the proportion of all progenitor zone cells (WAPL-1 positive) that were also EdU positive. For the full detailed protocol, see Kocsisova et al. (2018a)
In day 5 hermaphrodites, a subpopulation displayed robust EdU labeling and a subpopulation displayed a complete lack of EdU labeling (Fig. S6A-C). When fed EdU for 0.5 h, 85% of day 5 animals showed no EdU-positive cells. In the remaining 15% of animals, the average S-phase index was 53% (range 31.5-76.7% of cells), which is not significantly different from the S-phase index for day 1 or 3 hermaphrodites (Fig. 4G). In 29 out of 33 day 5 animals fed EdU for 0.5 h where both germlines were visible, either both germlines displayed EdU incorporation or both germlines displayed no EdU incorporation (intraclass correlation coefficient=0.75, P<0.0001). In the remaining four out of 33 day 5 animals, two germlines were discordant for EdU labeling (Fig. S6D). Soaking day 5 animals in a solution of 500 μM EdU in M9, as described by Furuta et al. (2018), also resulted in a subpopulation that completely lacked EdU labeling. As the duration of EdU feeding increased, so did the proportion of animals showing labeling. When day 5 animals were fed EdU for 4 h, 81% of animals displayed EdU-positive cells (range 48.1% to 94.4% of cells). Our observation that older animals frequently displayed no EdU incorporation following a short duration of EdU exposure but only infrequently displayed this following a longer duration of EdU exposure is similar to findings reported by Cinquin et al. (2016).
We observed that both EdU-positive and EdU-negative animals displayed EdU-labeled bacteria in the intestinal lumen, indicating that both groups of animals successfully ingested EdU-labeled bacteria. One possible explanation for animals that do not incorporate EdU is that some day 5 hermaphrodites are inefficient in intestinal absorption of EdU, transport of EdU to the germline and/or incorporation of EdU into DNA. Alternatively, some day 5 hermaphrodites may be susceptible to trauma following transfer from one dish to another, resulting in a temporary block in EdU incorporation. Alternatively, in some day 5 hermaphrodites, all progenitor zone cells may be arrested in a cell cycle phase other than S phase. In this case, the arrest appears to be transient, because we observed: (1) scattered M-phase cells in germlines that lacked EdU labeling, indicating that in these germlines most cells were in a gap phase or arrested while others were in M phase; (2) longer durations of EdU exposure resulted in a significantly higher proportion of EdU labeled animals; and (3) in EdU pulse-chase experiments, the distal germline did not contain cells with intense EdU labeling, indicating that all stem cells underwent several cell divisions during the 48 h chase. To our knowledge, there is no method to unambiguously identify S-phase cells in the C. elegans germline without feeding or injecting nucleotide analogs (Kocsisova et al., 2018a,b; van den Heuvel and Kipreos, 2012).
Dissection and immunohistochemistry
The dissection and staining protocol follows the batch method (Francis et al., 1995), where all dissected tissues were incubated within small glass tubes rather than on a slide. Animals were washed with phosphate-buffered saline (PBS) into a dissecting watchglass (Carolina Biological, 742300), immobilized with levamisole (final concentration 200 μM), and dissected with a pair of 25 G 5/8″ needles (PrecisionGlide from BD) by cutting at the pharynx and/or at the tail. Dissected gonads were fixed in 2 ml 3% paraformaldehyde (PFA) (10 ml 16% PFA, EM Grade, Electron Microscopy Sciences, 15710) in phosphate-buffered saline for 10 min at room temperature and then post-fixed with 2 ml 100% methanol (Gold-label from Fisher) at −20°C for 1 h or longer (up to several days).
In the past there were discrepancies in the literature, possibly due to different analysis methods in very old worms: in situ analysis versus dissected gonads. To circumvent this issue, we performed dissections at time points when these discrepancies are less likely to be a problem.
Fixed gonads were rehydrated and washed three times in PBS+0.1% Tween-20 (PBSTw), then incubated in 100 µl of primary antibody at room temperature for 4-24 h, washed three times in PBSTw and incubated in 100 µl of secondary antibody at room temperature or 4°C for 2-24 h. Antibodies were diluted in 30% goat serum (Gibco, C16210-072) in PBS. Primary antibodies used were: rabbit-anti-WAPL-1 (Novus Biologicals, 49300002, Lot G3048-179A02) (1:2000), mouse-anti-MSP (major sperm protein) (Miller et al., 2001) (1:2000), mouse-anti-pH3 (Millipore clone 3H10, 05-806, Lot#2680533) (1:500) and mouse-anti-FLAG (SIGMA M2, purified in-house) (1:1000). Secondary antibodies (1:400) were: goat-anti-mouse IgG-conjugated Alexa Fluor 488/594/647, goat-anti-rabbit IgG-conjugated Alexa Fluor 488/594/647 (Invitrogen).
Following antibody staining, gonads were washed 3 times in PBSTw. An EdU Click-iT reaction was performed according to manufacturer's instructions (Invitrogen C10350), using 100 µl reagent for a 30 min incubation, followed by a quick wash in manufacturer-supplied rinse buffer and four ∼15 min washes in PBSTw. Stained gonads were resuspended in 1 drop of Vectashield containing 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Vector Laboratories, H-1200), applied to a large pre-made agarose pad on a glass slide, and covered with a 22 mm×40 mm #1 cover glass. The slide was allowed to settle overnight at room temperature, sealed with clear nail polish and stored at 4°C as needed. Images were acquired within 72 h when possible.
Confocal imaging
Images were captured using a Zeiss Plan Apo 63×1.4 oil-immersion objective lens on a PerkinElmer Ultraview Vox spinning disc confocal system on a Zeiss Observer Z1 microscope using Volocity software. Approximately 20 1 μm z-slice images were acquired for each gonad. Images were exported as hyperstack .tif files for further analysis.
Image analysis
Images were stitched either in Volocity or using the ImageJ plug-ins for pairwise stitching and Grid/Collection of sequential images (Preibisch et al., 2009). Images were rotated, cropped, arranged and annotated in Illustrator (Adobe).
Cells were manually counted in each z-slice where they occurred. The person performing counts was not blinded to experimental groups, because the differences were generally obvious to an experienced observer. Counts of cells were performed in Fiji/ImageJ (Schindelin et al., 2012) using the Cell Counter plug-in (De Vos, 2015; Rasband, 2016). To remove multiply-counted cells, a modified version of the R-script Marks-to-Cells was used (Seidel and Kimble, 2015). This script improved the precision of cell counts in the germline.
Nuclear morphology
DAPI staining was used to assess meiotic prophase stages, nuclear counts, row counts and progression of gametogenesis. Endomitotic oocytes in the proximal gonad arm, which is distal to the spermatheca, were recognized as large DAPI-stained shapes. Endomitotic oocytes are known to result from failure to coordinate meiotic maturation of diakinesis stage oocytes with ovulation, resulting in the unfertilized mature oocytes that are mitotic cell cycling without cytokinesis because of the absence of the sperm-derived centriole (Greenstein, 2005; Iwasaki et al., 1996; McCarter et al., 1997). This is distinct from endomitotic oocytes in the uterus, which naturally occur in older unmated hermaphrodites that have exhausted their self-sperm. In this case, the most proximal oocyte undergoes low-frequency spontaneous maturation and ovulation, but because there are no sperm in the spermatheca the matured but unfertilized oocyte begins endomitotic cycling (McCarter et al., 1999). Endomitotic oocytes were identified from dissected germlines; as the proximal germline was not always visible, this raises the possibility that the frequency was under-estimated.
Distal tip cell nucleus
The DTC nucleus was identified by its position at the exterior of the distal gonad and bright WAPL-1 staining. When analyzing the shift of the DTC nucleus, we chose a stringent cutoff of 5 c.d., because of a concern that a DTC nucleus might shift by 1 or 2 c.d. due to physical forces during dissection and staining. We did analyze our data with the cutoff set to 2 c.d., and even with this broad definition of a ‘shifted’ DTC nucleus, the majority of germlines were ‘normal’.
Progenitor zone
The PZ has been previously called the mitotic zone or the proliferative zone. We employed staining with the cohesin chaperone WAPL-1 to measure the size of the PZ (Mohammad et al., 2018). In mated hermaphrodites, the total number of PZ cells was 215±25 in day 1 adults, and it decreased to 163±47 in day 3 animals and to 126±45 in day 5 animals. A similar number of PZ cells, ∼230, was found for day 1 unmated hermaphrodites using a combination of the nucleoplasmic REC-8 staining PZ marker and the meiotic prophase marker HIM-3 (Fox and Schedl, 2015; Fox et al., 2011; Hansen et al., 2004). Other studies of unmated day 1 hermaphrodites used the crescent-shaped DAPI morphology of leptotene cells to approximate the proximal boundary of the PZ and found that the PZ contains ∼214 (Korta et al., 2012), ∼205 (Roy et al., 2016) or ∼225 cells (Seidel and Kimble, 2015). Our day 1 PZ cell numbers for mated hermaphrodites are therefore similar to PZ cell numbers for unmated day 1 hermaphrodites reported in other studies; therefore, mating did not have a significant effect on PZ cell numbers at day 1. Previous studies of changes in PZ cell number during aging with unmated hermaphrodites report the following: Hubbard and colleagues found ∼250 PZ cells in day 1 adults, ∼156 by day 3, ∼100 by day 6 and ∼50 by day 12 (Killian and Hubbard, 2005; Qin and Hubbard, 2015); Murphy and colleagues reported a decrease in PZ cell number from ∼220 at day 2 to ∼150 at day 6 in unmated hermaphrodites (Luo et al., 2010); and Kimble and colleagues reported that PZ cell number remained unchanged up to day 6 of adulthood (day 1, ∼243; day 2, ∼227; day 3, ∼214) (Crittenden et al., 2006). In mated hermaphrodites, Shi and Murphy (2014) found a decrease in the number of PZ cells with age, from ∼150 at day 1 to ∼100 at day 2 through day 4. However, under the conditions employed, the mated hermaphrodites displayed damage to the soma (body size shrinkage), which was not the case with the conditions employed in the experiments described here. The Hubbard and Narbonne groups reported that, starting at day 3 and older, when unmated hermaphrodites have become sperm depleted (or in younger adult genetic females), the absence of sperm, and thus flux through the germline, results in mitotic cell cycle arrest of PZ cells, likely in G2 phase (Narbonne et al., 2015; Qin and Hubbard, 2015). One interpretation of this finding is that the mitotic cell cycle quiescence of the PZ in the absence of sperm is a physiological mechanism of preserving the germline during aging, in anticipation of subsequent mating. To avoid this preservation mechanism in our studies of germline aging, we always used mated hermaphrodites.
M-phase cells and the presence of sperm
Cells in M-phase were detected by staining with mouse anti-phospho-histone 3 (pH3) antibody, while sperm were identified by staining with mouse anti-MSP (major sperm protein) antibody. In these co-staining experiments, M-phase cells and sperm were distinguished by: (1) position in the germline; and (2) DAPI morphology. M-phase cells were in the PZ at the distal end of the germline, whereas sperm were in the spermatheca at the proximal end of the gonad. Stages of M phase (prophase, metaphase and anaphase) were analyzed in cells detected using pH3 antibody; minimal differences were observed between days 1, 3 and 5 (Fig. S7).
SYGL-1 and LST-1
The length in cell diameters and the number of cells in the PZ that contain cytoplasmic SYGL-1 and LST-1 were assessed with anti-FLAG antibody staining using strains in which CRISPR genome editing was employed to insert DNA encoding a 3×FLAG epitope into the endogenous locus. In day 1 animals, we observed staining similar to that reported by Shin et al. (2017). However, we note that at day 3 and 5, the length of the SYGL-1 staining region could be similar to the length of the PZ, as assessed using WAPL-1 staining (i.e. only a few WAPL-1-positive SYGL-1-negative cells were observed).
Germline stem cell differentiation occurs through essentially direct differentiation and thus lacks transit-amplifying divisions; following loss of GLP-1 Notch signaling, germ cells complete their ongoing mitotic cell cycle and then begin meiotic S phase (Fox and Schedl, 2015). SYGL-1 activity is sufficient for the stem cell fate (Kershner et al., 2014; Lee et al., 2016; Shin et al., 2017). However, it is unknown whether all cells that are expressing endogenous levels of SYGL-1 are stem cells. There is a noticeable gradient of SYGL-1 staining between the highly expressing distal-most and lowly expressing proximal-most cells, raising the possibility that the level of SYGL-1 in the proximal-most cells is not sufficient to promote the stem cell fate and inhibit the meiotic fate. Additionally, Spike et al. (2018) have shown that RNA-binding proteins LIN-41 and GLD-1 are post-translationally inactivated prior to their degradation; similarly, proximal SYGL-1 may be inactivated prior to degradation. Thus, although the extent of SYGL-1 accumulation provides a readout that is correlated with stem cell identity, detectable SYGL-1 accumulation cannot currently be used to define the number of stem cells.
Meiotic entry and nurse cells
Most cells that exit the progenitor zone and enter meiosis in an adult hermaphrodite do not become oocytes. Instead, these cells function as nurse cells and undergo apoptosis. We calculated the nurse cell proportion following the method outlined by Agarwal et al. (2018). We compared the output of the progenitor zone on days 1, 3 and 5 (Fig. 5B, Table S5) with the number of embryos (and therefore oocytes) produced on days 3, 5 and 7, then divided by 2 to account for two gonads per animal (Fig. 1A, Table S1). The 2-day difference was used to account for the time required for a cell to progress through meiotic prophase (Fig. S9, Table S9). We defined the nurse cell proportion as 1−(0.5 × number of oocytes per hour/number of cells entered meiosis per hour in one gonad).
Statistical analysis
Statistical analyses were performed in R (R Core Team, 2013) using R studio (R Studio Team, 2015) and the following packages: ggplot2 (Wickham, 2009), svglite, plyr, gridExtra, grid, lattice, multcomp, car, broom, psych, FSA and fifer. Individual measurements and scripts used to analyze them are provided in the supplementary Materials and Methods. Depending on the type of variable, the following tests were performed and are indicated in the text and figure legends with the relevant test name abbreviation. KW test: for continuous measurement variables, the Kruskal-Wallis rank-sum test was used with a Dunn post-hoc and P-values adjusted with the Benjamini-Hochberg FDR method or using an ANOVA with a Tukey post-hoc test. PC test: for categorical measurement variables, the Pearson's Chi-squared test of independence was used with post-hoc P-values adjusted with the False Discovery Rate method. PIC test: to test for linear relationships between variables, we used the Pearson's interclass correlation. ICC test: used for intraclass correlations to compare the pooled variance between pairs of germlines from one animal and pooled variance within pairs (Shrout and Fleiss, 1979). Boolean variables were treated as TRUE=1, FALSE=0 for purposes of correlation analysis. All data represent the mean±s.d. NS indicates P>0.05, *P<0.05, **P<0.001 and ***P<0.0001.
All data were subjected to consistent exclusion criteria. Animals that displayed evidence of matricidal hatching or sperm depletion (as indicated by lack of major sperm protein immunofluorescence) were excluded from all analyses. Animals that displayed sporadic phenotypes, such as endomitotic oocytes, a shifted DTC nucleus or failure to exhibit any EdU staining, are reported, but were excluded from further analyses. Exact statistical methods and exclusion criteria are provided in the supplemental R script.
Supplementary Material
Acknowledgements
We are grateful to the E. coli stock center for MG1693; to Wormbase; to the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health Office of Research Infrastructure Programs (P40OD010440), for strains; to Mike Nonet and Scott Dour for advice, reagents and injection microscope use for CRISPR/Cas9 genome engineering; to Zach Pincus for statistical advice and injection microscope use; to Aiping Feng for reagents; to Luke Schneider, Andrea Scharf, Sandeep Kumar, Ariz Mohammad and John Brenner for training, advice, support, reagents and helpful discussion; and to the Kornfeld and Schedl labs for feedback on this manuscript. Finally, we are grateful to three anonymous reviewers for suggestions that greatly improved the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: Z.K., K.K., T.S.; Methodology: Z.K.; Software: Z.K.; Validation: Z.K.; Formal analysis: Z.K.; Investigation: Z.K.; Resources: K.K., T.S.; Data curation: Z.K.; Writing - original draft: Z.K., K.K.; Writing - review & editing: Z.K., K.K., T.S.; Visualization: Z.K.; Supervision: K.K., T.S.; Funding acquisition: Z.K., K.K., T.S.
Funding
This work was supported in part by the National Institutes of Health (R01 AG02656106A1 to K.K., R01 GM100756 to T.S.), by a National Science Foundation predoctoral fellowship (DGE-1143954 and DGE-1745038 to Z.K.) and by The Douglas Covey Fellowship to Z.K. The National Institutes of Health, the National Science Foundation and Douglas Covey had no role in the design of the study, collection, analysis and interpretation of data, or in writing the manuscript. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.173195.supplemental
References
- Agarwal I., Farnow C., Jiang J., Kim K.-S., Leet D. E., Solomon R. Z., Hale V. A. and Goutte C. (2018). HOP-1 presenilin deficiency causes a late-onset notch signaling phenotype that affects adult germline function in caenorhabditis elegans. Genetics 208, 745-762. 10.1534/genetics.117.300605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeles-Albores D., Leighton D. H. W., Tsou T., Khaw T. H., Antoshechkin I. and Sternberg P. W. (2017). The Caenorhabditis elegans female state: decoupling the transcriptomic effects of aging and sperm-status. G3 (Bethesda) 7, 2969-2977. 10.1534/g3.117.300080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo G. and Van Gilst M. R. (2009). Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science 326, 954-958. 10.1126/science.1178343 [DOI] [PubMed] [Google Scholar]
- Arribere J. A., Bell R. T., Fu B. X. H., Artiles K. L., Hartman P. S. and Fire A. Z. (2014). Efficient marker-free recovery of custom genetic modifications with CRISPR/Cas9 in Caenorhabditis elegans. Genetics 198, 837-846. 10.1534/genetics.114.169730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin J. and Kimble J. (1987). glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell 51, 589-599. 10.1016/0092-8674(87)90128-0 [DOI] [PubMed] [Google Scholar]
- Baugh L. R. (2013). To grow or not to grow: nutritional control of development during Caenorhabditis elegans L1 Arrest. Genetics 194, 539-555. 10.1534/genetics.113.150847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinquin A., Chiang M., Paz A., Hallman S., Yuan O., Vysniauskaite I., Fowlkes C. C. and Cinquin O. (2016). Intermittent stem cell cycling balances self-renewal and senescence of the C. elegans germ line . PLoS Genetics 12, e1005985 10.1371/journal.pgen.1005985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden S. L., Leonhard K. A., Byrd D. T. and Kimble J. (2006). Cellular analyses of the mitotic region in the Caenorhabditis elegans adult germ line. Mol. Biol. Cell 17, 3051-3061. 10.1091/mbc.e06-03-0170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Guardia Y., Gilliat A. F., Hellberg J. and Rennert P. (2016). Run-on of germline apoptosis promotes gonad senescence in C. elegans. Oncotarget 7, 39082-39096. 10.18632/oncotarget.9681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos K. (2015). Cell Counter Plugin. https://imagej.nih.gov/ij/plugins/cell-counter.htm.
- Fox P. M. and Schedl T. (2015). Analysis of germline stem cell differentiation following loss of GLP-1 notch activity in Caenorhabditis elegans. Genetics 201, 167-184. 10.1534/genetics.115.178061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P. M., Vought V. E., Hanazawa M., Lee M.-H. H., Maine E. M. and Schedl T. (2011). Cyclin E and CDK-2 regulate proliferative cell fate and cell cycle progression in the C. elegans germline. Development 138, 2223-2234. 10.1242/dev.059535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis R., Barton M. K., Kimble J. and Schedl T. (1995). gld-1, a tumor suppressor gene required for oocyte development in Caenorhabditis elegans. Genetics 139, 579-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama M., Rougvie A. E. and Rothman J. H. (2006). C. elegans DAF-18/PTEN mediates nutrient-dependent arrest of cell cycle and growth in the germline. Curr. Biol. 16, 773-779. 10.1016/j.cub.2006.02.073 [DOI] [PubMed] [Google Scholar]
- Furuta T., Joo H.-J., Chen S.-Y., Arur S., Trimmer K. A., Chen S.-Y. and Arur S. (2018). GSK-3 promotes S phase entry and progression in C. elegans germline stem cells to maintain tissue output. Development 145, dev.161042 10.1242/dev.161042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garigan D., Hsu A. L., Fraser A. G., Kamath R. S., Abringet J. and Kenyon C. (2002). Genetic analysis of tissue aging in Caenorhabditis elegans: A role for heat-shock factor and bacterial proliferation. Genetics 161, 1101-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein D. (2005). Control of oocyte meiotic maturation and fertilization. WormBook (ed. The C. elegans Research Community), WormBook, http://www.wormbook.org 10.1895/wormbook.1.53.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald I. and Kovall R. (2013). Notch signaling: genetics and structure. In WormBook (ed. The C. elegans Research Community), WormBook, http://www.wormbook.org 10.1895/wormbook.1.10.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumienny T. L., Lambie E., Hartweig E., Horvitz H. R. and Hengartner M. O. (1999). Genetic control of programmed cell death in Caenorhabditis elegans hermaphrodite germline. Development 126, 1011-1022. [DOI] [PubMed] [Google Scholar]
- Hansen D. and Schedl T. (2013). Stem cell proliferation versus meiotic fate decision in Caenorhabditis elegans. Adv. Exp. Med. Biol. 757, 71-99. 10.1007/978-1-4614-4015-4_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen D., Hubbard E. J. A. and Schedl T. (2004). Multi-pathway control of the proliferation versus meiotic development decision in the Caenorhabditis elegans germline. Dev. Biol. 268, 342-357. 10.1016/j.ydbio.2003.12.023 [DOI] [PubMed] [Google Scholar]
- Herndon L. A., Schmeissner P. J., Dudaronek J. M., Brown P. A., Listner K. M., Sakano Y., Paupard M. C., Hall D. H. and Driscoll M. (2002). Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 419, 808-814. 10.1038/nature01135 [DOI] [PubMed] [Google Scholar]
- Hirsh D., Oppenheim D. and Klass M. (1976). Development of the reproductive system of Caenorhabditis elegans. Dev. Biol. 49, 200-219. 10.1016/0012-1606(76)90267-0 [DOI] [PubMed] [Google Scholar]
- Hodgkin J. and Doniach T. (1997). Natural variation and copulatory plug formation in Caenorhabditis elegans. Genetics 146, 149-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. E., Evason K., Xiong C. and Kornfeld K. (2007). Genetic and pharmacological factors that influence reproductive aging in nematodes. PLoS Genet. 3, e25 10.1371/journal.pgen.0030025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. E., Huang C. and Kornfeld K. (2011). Identification of mutations that delay somatic or reproductive aging of Caenorhabditis elegans. Genetics 189, 341-356. 10.1534/genetics.111.130450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K., McCarter J., Francis R. and Schedl T. (1996). emo-1, a Caenorhabditis elegans Sec61p gamma homologue, is required for oocyte development and ovulation. J. Cell Biol. 134, 699-714. 10.1083/jcb.134.3.699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo-Lambert A., Ellefson M., Villeneuve A. M. and Engebrecht J. (2007). Differential timing of S phases, X chromosome replication, and meiotic prophase in the C. elegans germ line. Dev. Biol. 308, 206-221. 10.1016/j.ydbio.2007.05.019 [DOI] [PubMed] [Google Scholar]
- Kao S.-H., Tseng C.-Y., Wan C.-L., Su Y.-H., Hsieh C.-C., Pi H. and Hsu H.-J. (2015). Aging and insulin signaling differentially control normal and tumorous germline stem cells. Aging Cell 14, 25-34. 10.1111/acel.12288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershner A. M., Shin H., Hansen T. J. and Kimble J. (2014). Discovery of two GLP-1/Notch target genes that account for the role of GLP-1/Notch signaling in stem cell maintenance. Proc. Natl. Acad. Sci. USA 111, 3739-3744. 10.1073/pnas.1401861111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian D. J. and Hubbard E. J. A. (2005). Caenorhabditis elegans germline patterning requires coordinated development of the somatic gonadal sheath and the germ line. Dev. Biol. 279, 322-335. 10.1016/j.ydbio.2004.12.021 [DOI] [PubMed] [Google Scholar]
- Kimble J. and Seidel H. S. (2013). C. elegans germline stem cells and their niche. In StemBook, pp. 1-12. Harvard Stem Cell Institute. [PubMed] [Google Scholar]
- Kimble J. and Ward S. (1988). Germ-line development and fertilization. Cold Spring Harbor Monograph Archive 17, 191-213. [Google Scholar]
- Kocsisova Z., Mohammad A., Kornfeld K. and Schedl T. (2018a). Cell cycle analysis in the C. Elegans germline with the thymidine analog EdU. J. Vis. Exp. 140, e58339-e58339. 10.3791/58339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsisova Z., Kornfeld K. and Schedl T. (2018b). Cell cycle accumulation of the proliferating cell nuclear antigen PCN-1 transitions from continuous in the adult germline to intermittent in the early embryo of C. elegans. BMC Dev. Biol. 18, 1-12. 10.1186/s12861-018-0171-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korta D. Z., Tuck S. and Hubbard E. J. A. (2012). S6K links cell fate, cell cycle and nutrient response in C. elegans germline stem/progenitor cells. J. Cell Sci. 125, 859-870. 10.1242/jcs.109330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Sorensen E. B., Lynch T. R., Kimble J., Parrish S., Timmons L., Plasterk R., Fire A., Singh A., Raj A. et al. (2016). C. elegans GLP-1/Notch activates transcription in a probability gradient across the germline stem cell pool. eLife 5, 269-282. 10.7554/eLife.18370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S. and Murphy C. T. (2011). Caenorhabditis elegans reproductive aging: Regulation and underlying mechanisms. Genesis 49, 53-65. 10.1002/dvg.20694 [DOI] [PubMed] [Google Scholar]
- Luo S., Kleemann G. A., Ashraf J. M., Shaw W. M. and Murphy C. T. (2010). TGF-beta and insulin signaling regulate reproductive aging via oocyte and germline quality maintenance. Cell 143, 299-312. 10.1016/j.cell.2010.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maures T. J., Booth L. N., Benayoun B. A., Izrayelit Y., Schroeder F. C. and Brunet A. (2014). Males shorten the life span of C. elegans hermaphrodites via secreted compounds. Science 343, 541-544. 10.1126/science.1244160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter J., Bartlett B., Dang T. and Schedl T. (1997). Soma-germ cell interactions in Caenorhabditis elegans: multiple events of hermaphrodite germline development require the somatic sheath and spermathecal lineages. Dev. Biol. 181, 121-143. 10.1006/dbio.1996.8429 [DOI] [PubMed] [Google Scholar]
- McCarter J., Bartlett B., Dang T. and Schedl T. (1999). On the control of oocyte meiotic maturation and ovulation in caenorhabditis elegans. Dev. Biol. 205, 111-128. 10.1006/dbio.1998.9109 [DOI] [PubMed] [Google Scholar]
- Medawar P. B. (1952). An Unsolved Problem of Biology. London: Lewis. [Google Scholar]
- Miller M. A., Nguyen V. Q., Lee M. H., Kosinski M., Schedl T., Caprioli R. M. and Greenstein D. (2001). A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science 291, 2144-2147. 10.1126/science.1057586 [DOI] [PubMed] [Google Scholar]
- Mohammad A., Vanden Broek K., Wang C., Daryabeigi A., Jantsch V., Hansen D. and Schedl T. (2018). Initiation of meiotic development is controlled by three post-transcriptional pathways in caenorhabditis elegans. Genetics 209, 1197-1224. 10.1534/genetics.118.300985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbonne P., Maddox P. S. and Labbe J.-C. (2015). daf-18/PTEN locally antagonizes insulin signalling to couple germline stem cell proliferation to oocyte needs in C. elegans. Development 142, 4230-4241. 10.1242/dev.130252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazdernik N. and Schedl T. (2013). Introduction to germ cell development in Caenorhabditis elegans. Adv. Exp. Med. Biol. 757, 1-16. 10.1007/978-1-4614-4015-4_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper A. S. R. S.-R., Killian D. J. and Hubbard E. J. A. (2003). Genetic analysis of Caenorhabditis elegans glp-1 mutants suggests receptor interaction or competition. Genetics 163, 115-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett C. L., Dietrich N., Chen J., Xiong C. and Kornfeld K. (2013). Mated progeny production is a biomarker of aging in Caenorhabditis elegans. G3 (Bethesda) 3, 2219-2232. 10.1534/g3.113.008664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preibisch S., Saalfeld S. and Tomancak P. (2009). Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25, 1463-1465. 10.1093/bioinformatics/btp184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z. and Hubbard E. J. A. (2015). Non-autonomous DAF-16/FOXO activity antagonizes age-related loss of C. elegans germline stem/progenitor cells. Nat. Commun. 6, 7107 10.1038/ncomms8107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband W. (2016). ImageJ. http://imagej.nih.gov/ij/.
- R Core Team (2013). R: A Language and Environment for Statistical Computing. http://www.r-project.org/.
- Roy D., Michaelson D., Hochman T., Santella A., Bao Z., Goldberg J. D. and Hubbard E. J. A. (2016). Cell cycle features of C. elegans germline stem/progenitor cells vary temporally and spatially. Dev. Biol. 409, 261-271. 10.1016/j.ydbio.2015.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Studio Team (2015). RStudio: Integrated Development Environment for R. www.rstudio.com/.
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676-682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel H. S. and Kimble J. (2015). Cell-cycle quiescence maintains Caenorhabditis elegans germline stem cells independent of GLP-1/Notch. eLife 4, e10832 10.7554/eLife.10832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C. and Murphy C. T. (2014). Mating induces shrinking and death in Caenorhabditis mothers. Science 343, 536-540. 10.1126/science.1242958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H., Haupt K. A., Kershner A. M., Kroll-Conner P., Wickens M. and Kimble J. (2017). SYGL-1 and LST-1 link niche signaling to PUF RNA repression for stem cell maintenance in Caenorhabditis elegans. PLoS Genet. 13, e1007121 10.1371/journal.pgen.1007121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout P. E. and Fleiss J. L. (1979). Intraclass correlations: uses in assessing rater reliability. Psychol. Bull. 86, 420-428. 10.1037/0033-2909.86.2.420 [DOI] [PubMed] [Google Scholar]
- Spike C. A., Huelgas-Morales G., Tsukamoto T. and Greenstein D. (2018). Multiple mechanisms inactivate the LIN-41 RNA-binding protein to ensure a robust oocyte-to-embryo transition in Caenorhabditis elegans. Genetics 210, 1011-1037. 10.1534/genetics.118.301421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T. (2006). Maintenance of C. elegans. WormBook 1-11. 10.1895/wormbook.1.101.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel S. and Kipreos E. T. (2012). C. elegans cell cycle analysis. Methods Cell Biol. 107, 265-294. 10.1016/B978-0-12-394620-1.00009-6 [DOI] [PubMed] [Google Scholar]
- Ward S. and Carrel J. S. (1979). Fertilization and sperm competition in the nematode Caenorhabditis elegans. Dev. Biol. 73, 304-321. 10.1016/0012-1606(79)90069-1 [DOI] [PubMed] [Google Scholar]
- Wickham H. (2009). ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag. [Google Scholar]
- Williams G. C. (1957). Plieotropy, natural selection, and the evolution of senescence. Evolution 11, 398-411. 10.1111/j.1558-5646.1957.tb02911.x [DOI] [Google Scholar]
- Wolke U., Jezuit E. and Priess J. R. (2007). Actin-depenedent cytoplasmic streaming in C. elegans oogenesis. Development 134, 2227-2236. 10.1242/dev.004952 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.