ABSTRACT
The sensory nervous system of C. elegans comprises cells with varied molecular and functional characteristics, and is, therefore, a powerful model for understanding mechanisms that generate neuronal diversity. We report here that VAB-3, a C. elegans homolog of the homeodomain-containing protein Pax6, has opposing functions in regulating expression of a specific chemosensory fate. A homeodomain-only short isoform of VAB-3 is expressed in BAG chemosensory neurons, where it promotes gene expression and cell function. In other cells, a long isoform of VAB-3, comprising a Paired homology domain and a homeodomain, represses expression of ETS-5, a transcription factor required for expression of BAG fate. Repression of ets-5 requires the Eyes Absent homolog EYA-1 and the Six-class homeodomain protein CEH-32. We determined sequences that mediate high-affinity binding of ETS-5, VAB-3 and CEH-32. The ets-5 locus is enriched for ETS-5-binding sites but lacks sequences that bind VAB-3 and CEH-32, suggesting that these factors do not directly repress ets-5 expression. We propose that a promoter-selection system together with lineage-specific expression of accessory factors allows VAB-3/Pax6 to either promote or repress expression of specific cell fates in a context-dependent manner.
This article has an associated ‘The people behind the papers’ interview.
KEY WORDS: C. elegans, Chemosensory, Nervous system, VAB-3
Highlighted Article: Variants of VAB-3, the C. elegans homolog of Pax6 and Eyeless, have opposing functions in controlling expression of a specific neuronal fate that might also be recapitulated in the vertebrate nervous system.
INTRODUCTION
During nervous system development, an extraordinary diversity of neuron types is generated. The availability of neurons with different physiological properties and specified connectivity permits the generation of neural circuits that support complex behaviors. How different types of neurons arise during development remains a major issue in neuroscience. The roundworm C. elegans is an excellent model for investigating this. During development of the C. elegans hermaphrodite, 302 neurons are generated (Sulston et al., 1983). These neurons differ with respect to neurotransmitter identity, morphology and connectivity (White et al., 1986; Hobert et al., 2016). Altogether, the compact nervous system of C. elegans contains more than 100 discernible neuron types defined by structural and molecular criteria. Sensory neurons of C. elegans recapitulate this diversity in neuronal form and function on a smaller scale. They have the capacity to detect a wide range of environmental stimuli, e.g. chemical cues, heat, mechanical forces and light. Accordingly, each type of sensory neuron expresses genes that encode the molecules of sensory transduction and signaling required for its cognate modality. In addition, sensory neurons of C. elegans trigger innate behavioral programs that require connectivity between sensory neurons and specific motor circuits. Genetic analysis of sensory neuron development in C. elegans can determine developmental mechanisms that endow sensory neurons with their specific characteristics, and it is furthermore possible to determine the role of these mechanisms in neural circuit function through behavioral or physiological analysis of mutants.
C. elegans BAG neurons were orphan sensory neurons until only recently, when they were found to mediate chemosensation of the respiratory gases oxygen (O2) and carbon dioxide (CO2) (Hallem and Sternberg, 2008; Zimmer et al., 2009). Distinct cyclic GMP-based signaling pathways mediate detection of these gases. A soluble heme-containing guanylyl cyclase comprising GCY-31 and GCY-33 subunits is inhibited by oxygen and confers hypoxia sensitivity to BAG neurons. By contrast, CO2-sensing is mediated by the receptor-type guanylyl cyclase GCY-9, which is likely a receptor for CO2. The cyclic GMP signals generated by CO2 and hypoxia excite BAG neurons via heteromeric TAX-2/TAX-4 channels, which are homologs of cyclic nucleotide-gated (CNG) ion channels found in many types of vertebrate and invertebrate sensory neurons. Under normal cultivation conditions, activation of BAG neurons by hypoxia or CO2 triggers an acute avoidance response and negative chemotaxis, indicating that environments with high CO2 or low oxygen are aversive to C. elegans. BAG-mediated avoidance behavior requires that BAG neurons release the neurotransmitter glutamate and neuropeptides (Lee et al., 2017; Guillermin et al., 2017; Hallem and Sternberg, 2008). These neurochemical signals act on interneurons of the RIA, RIG, AIY and AIZ types, some of which receive direct synaptic inputs from BAGs and all of which are required for CO2 chemotaxis (Guillermin et al., 2017).
During development, therefore, BAG neurons are endowed with two chemosensory modalities, a compound neurotransmitter identity and connectivity to specific interneuron circuitry. Many of the molecules required for these characteristics are highly specific to BAGs, e.g. the guanylyl cyclases GCY-31, GCY-33 and GCY-9, and the FLP-17 neuropeptides (Zimmer et al., 2009; Hallem et al., 2011b; Brandt et al., 2012; Kim and Li, 2004; Guillermin et al., 2011). Screens for mutants with defective expression of specific markers of BAG neuron fate have identified genes required for normal BAG development and function (Gramstrup Petersen et al., 2013; Rojo Romanos et al., 2015; Brandt et al., 2012; Guillermin et al., 2011). These studies identified the ETS-family transcription factor ETS-5 as a critical regulator of gene expression in BAG neurons (Brandt et al., 2012; Guillermin et al., 2011). ETS-5 is required for expression of all tested BAG-specific genes. ETS-5 also promotes expression in BAG neurons of the more broadly expressed CNG genes tax-2 and tax-4, and it is required for expression of a glutamatergic neurotransmitter identity by BAGs (Serrano-Saiz et al., 2013). Furthermore, ETS-5 promotes transcription from the ets-5 locus, indicating that it positively regulates its own expression (Guillermin et al., 2011). On the basis of these properties, ETS-5 has been labeled a ‘terminal selector’, i.e. a factor whose expression at the end of a developmental program confers a stable cell-type identity (Deneris and Hobert, 2014).
Because ETS-5 is a critical determinant of BAG neuron fate, the question of how BAG neurons are specified during development might be reformulated as a question about the regulation of ETS-5 expression, i.e. what mechanisms pattern expression of ETS-5 during development and thereby restrict its function to lineages that generate BAG neurons? Here, we report the discovery of such a mechanism, which invokes the parallel and opposing functions of different isoforms of the C. elegans Pax6 homolog VAB-3. Pax6 is an evolutionarily ancient molecule with conserved functions in many animal phyla. We suggest that its dual roles in promoting and repressing specific neuronal fates in the C. elegans nervous system might also be recapitulated in the vertebrate nervous system.
RESULTS
The vab-3 locus encodes activities that promote and repress a BAG neuron fate
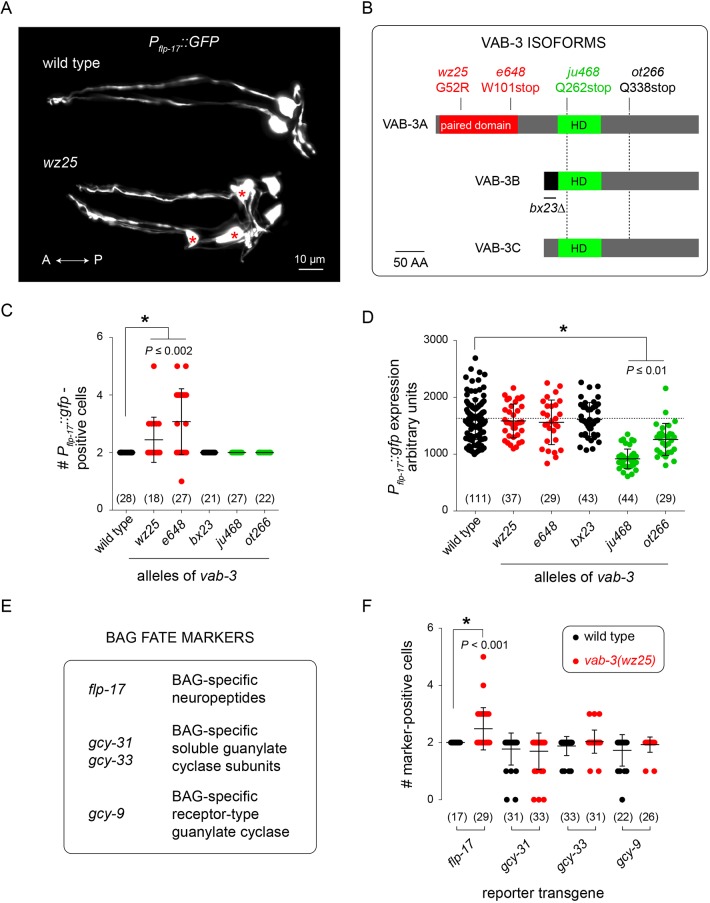
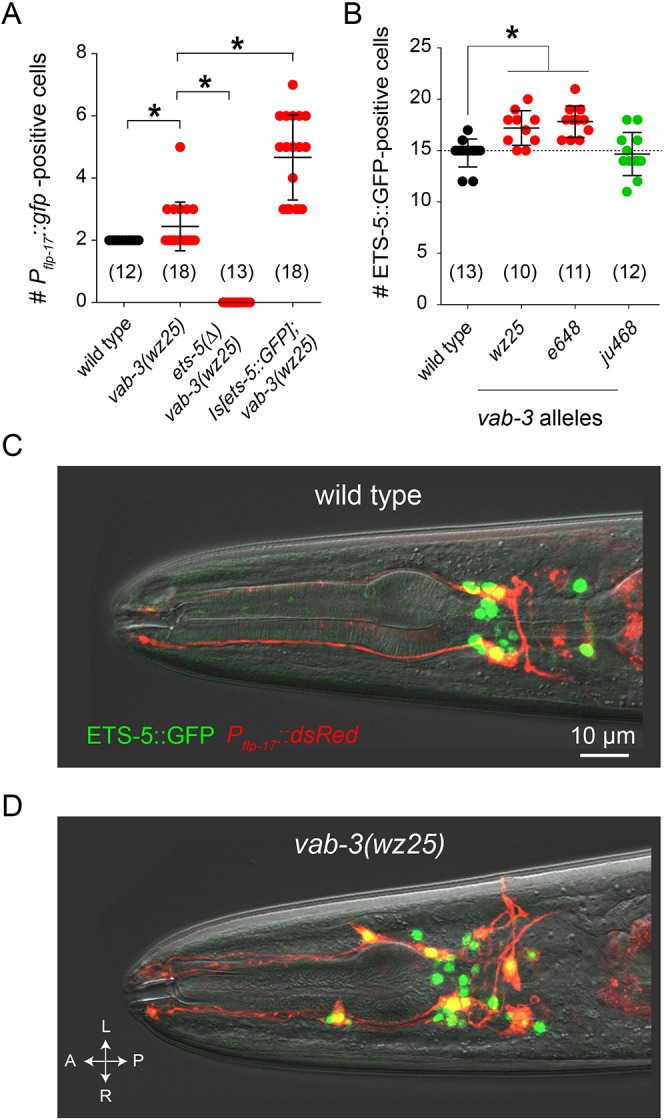
To identify genes required for the generation of chemosensory neurons that detect respiratory gases, we performed mutagenesis screens and isolated mutants with abnormal expression of the BAG-neuron-specific marker transgene Pflp-17::GFP. This screen yielded six mutants that failed to express Pflp-17::GFP. Four mutations were alleles of ets-5, which is an ETS-family transcription factor, and two were alleles of egl-13, which encodes a SOX-domain-containing protein (Fig. S1). ets-5 and egl-13 have established roles in promoting expression of a BAG neuron fate (Brandt et al., 2012; Gramstrup Petersen et al., 2013; Guillermin et al., 2011). We also isolated a mutation – wz25 – that caused Pflp-17::GFP expression in more than two cells. Many – but not all – wz25 mutants displayed GFP expression in three or more cells in the head (Fig. 1A).
Fig. 1.
Mutations affecting different VAB-3 isoforms have opposing effects on expression of a chemosensory neuronal fate. (A) Fluorescence micrographs of the heads of a wild-type (top) and wz25 mutant (bottom) hermaphrodite expressing a Pflp-17::GFP transcriptional reporter transgene. Extra Pflp–17::GFP-expressing cells are marked with red asterisks. (B) Schematic of vab-3 gene products showing mutations that affect different VAB-3 isoforms. (C) Number of Pflp-17::GFP-expressing cells in strains with various mutant alleles of vab-3. Mutants with alleles affecting the long isoform have extra Pflp-17::GFP-positive cells. (D) Expression levels of Pflp-17::GFP in BAG cells of strains with mutant alleles of vab-3. Mutants with alleles affecting the short isoform have reduced reporter expression levels. (E) BAG-specific markers and their gene products. (F) Effect of wz25, a mutation affecting the long isoform of vab-3, on the number of BAG-specific reporter-positive cells. Pflp-17::GFP is ectopically expressed in this mutant but expression of other markers is not significantly different between wz25 mutants and the wild type. In C,D,F, the numbers of animals assayed are indicated in brackets; data are mean±s.d. with individual data points indicated.
By mapping wz25 with respect to sequence variants in the polymorphic C. elegans strain CB4856 and, in parallel, sequencing the genome of wz25 mutants, we found that wz25 is an allele of vab-3. VAB-3 is a homolog of the homeodomain-containing transcription factor Pax6/Eyeless, which functions in sensory organ and nervous system development in animals from diverse phyla (Georgala et al., 2011; Nakayama et al., 2015; Shaham et al., 2012). Like its insect and vertebrate homologs, VAB-3 comprises a homeodomain and a Paired homology domain (Fig. 1B). The vab-3 locus also encodes other VAB-3 isoforms that lack a Paired homology domain and whose transcripts are generated from an alternate promoter. The wz25 mutation recovered from our screen is predicted to cause a glycine-to-arginine substitution in the Paired homology domain and, therefore, only to affect the long isoform of VAB-3 (Fig. 1B). The glycine residue affected by wz25 is strikingly conserved among insect and mammalian Paired homology domains, and the homologous residue in mammalian Pax6 defines the end of a helix that interacts with the major groove of B-form DNA (Fig. S2) (Xu et al., 1999). It is therefore likely that the wz25 mutation disrupts interactions between the VAB-3 Paired homology domain and DNA.
The e648 mutation, which also disrupts the long isoform of VAB-3, caused ectopic expression of Pflp-17::GFP like that caused by wz25 (Fig. 1C). By contrast, ju468 and ot266 mutations, which spare the Paired homology domain but disrupt the homeodomain of both long and short isoforms, did not cause ectopic expression of the BAG fate marker (Fig. 1C). However, ju468 and ot266 reduced expression of Pflp-17::GFP in the BAG neurons themselves (Fig. 1D), suggesting they maintain BAG cell fate in BAG neurons. The bx23 mutation, which eliminates expression of a distinct homeodomain-only isoform of VAB-3 required for development of the male tail (Zhang and Emmons, 1995) neither caused ectopic expression of a BAG fate marker nor affected marker expression in BAG neurons (Fig. 1C,D). We examined other reporters of a BAG-neuron fate in wz25 mutants. Reporters for the guanylyl cyclase genes gcy-9 and gcy-31 were expressed normally in wz25 mutants, i.e. their expression was restricted to the two BAG neurons proper, and although we occasionally observed a supernumerary gcy-33-expressing cell, the difference between wz25 mutants and the wild type did not reach statistical significance (Fig. 1E,F). From these data, we concluded that the wz25 mutation results in the generation of cells that ectopically express a partial BAG neuron fate, suggesting that the VAB-3 Paired homology domain and homeodomain have separable functions, with the Paired homology domain mediating repression of a BAG neuron fate in non-BAG cells.
Paired domain-containing and homeodomain-only VAB-3 isoforms are differentially expressed
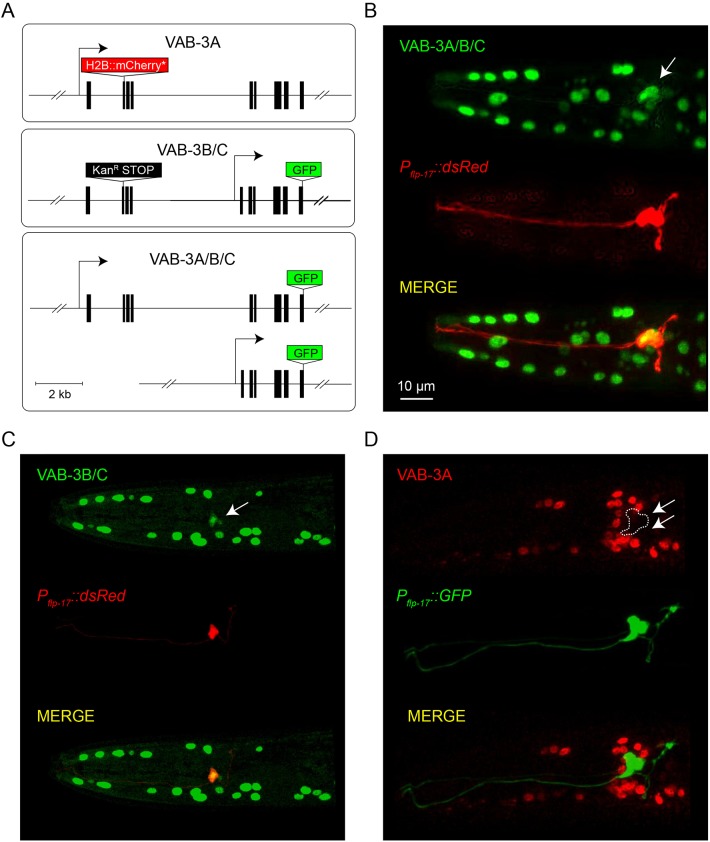
Expression of vab-3 in BAG neurons has been previously noted (Chisholm and Horvitz, 1995; Zhang and Emmons, 1995), which raised the question of why a gene that mediates repression of BAG fate would be expressed in those very same neurons? To address this, we examined the expression of specific VAB-3 isoforms. We generated a fosmid-based reporter that fuses GFP-coding sequences to the last exon of vab-3, which is included in all known vab-3 transcripts (Fig. 2A). This reporter, therefore, should be expressed in cells that express either long VAB-3 isoforms (Paired homology domain plus homeodomain) or short isoforms (homeodomain only). We generated a fosmid reporter that carries a cassette encoding nuclear-localized GFP in an exon that is only included in long vab-3 transcripts (Fig. 2A). We also modified the pan-vab-3 reporter with a kanamycin resistance cassette inducing an early stop in the long isoform in order to determine the expression pattern of the short isoform (Fig. 2A).
Fig. 2.
Long and short VAB-3 isoforms are differentially expressed. (A) Schematics of reporters for expression of long (VAB-3A), short (VAB-3B/C) and all (VAB-3A/B/C) isoforms of vab-3. (B) An adult hermaphrodite expressing a VAB-3A/B/C fosmid transcriptional reporter. This reporter is expressed in BAG neurons. A Pflp-17::dsRed transcriptional reporter marks BAG neurons. The white arrow indicates the location of the BAG neuron soma. (C) An adult hermaphrodite expressing a VAB-3B/C fosmid transcriptional reporter and Pflp-17::dsRed. Arrow indicates BAG neurons. (D) An adult hermaphrodite expressing a VAB-3A fosmid transcriptional reporter and Pflp-17::GFP. Arrows indicate BAG neurons.
Both the pan-VAB-3 reporter transgene and the reporter for short homeodomain-only VAB-3 isoforms were expressed in BAG neurons (Fig. 2B,C). By contrast, the reporter for long VAB-3 isoforms that include the Paired homology domain was not expressed in BAG neurons (Fig. 2D). We confirmed expression of the homeodomain-only isoform in BAGs using a reporter generated by Emmons and colleagues, who fused GFP to DNA sequences carrying the promoter for short vab-3 transcripts and vab-3 coding sequences sufficient to confer nuclear localization (Zhang and Emmons, 1995) (Fig. S3). Because the transgenes that specifically report expression of long and short VAB-3 isoforms are essentially transcriptional reporters, these data indicate that different promoters used for transcription of VAB-3 isoforms are active in different cell types and that the promoter required for expression of long repressive isoforms of VAB-3 is not active in BAG neurons.
The homeodomain of VAB-3 is required for BAG neuron chemosensory function
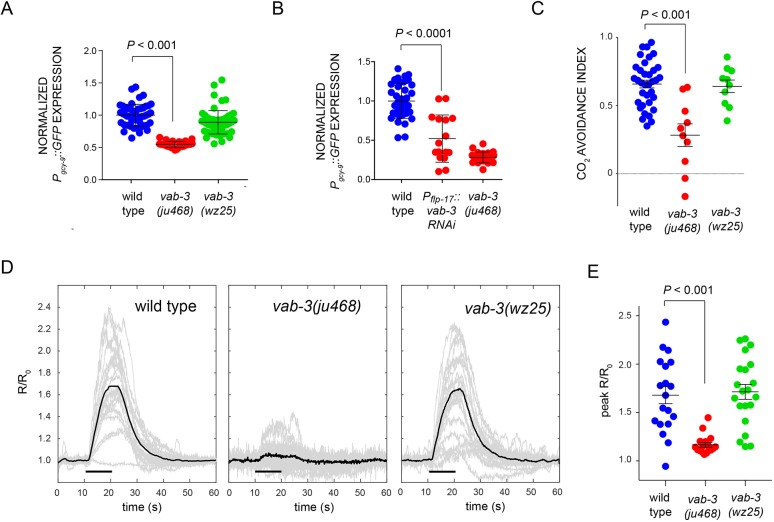
Because it is so prominently expressed in BAG neurons, we hypothesized that mutations affecting the VAB-3 homeodomain-only isoform might compromise BAG neuron chemosensory function. In support of this hypothesis, we observed that expression of the receptor-type guanylyl cyclase gcy-9, which is required for chemosensory function of BAG neurons (Hallem et al., 2011b), was significantly reduced in vab-3(ju468) mutants (Fig. 3A). We also observed that gcy-9 expression is significantly decreased in the context of BAG-specific RNAi knockdown of vab-3, which indicates that this role for VAB-3 is cell-autonomous (Fig. 3B). We also found that BAG-specific expression of vab-3c, which encodes a homeodomain-only variant of VAB-3, could partially rescue the reduction in gcy-9 levels seen in vab-3(ju468) mutants (Fig. S4A). These data indicate that a short, homeodomain-only VAB-3 variant promotes expression of BAG fate in a cell-autonomous manner.
Fig. 3.
A homeodomain-only VAB-3 isoform is required for function of chemosensory BAG neurons. (A) Levels of Pgcy-9::GFP expression in BAG neurons of vab-3 mutants in arbitrary fluorescence units (AFUs) normalized to the wild-type average. ju468, an allele affecting the short isoform, causes drastically decreased levels of the putative BAG CO2 receptor. (B) Levels of Pgcy-9::GFP expression in BAG neurons of vab-3 mutants in AFUs normalized to the wild-type average. BAG-specific knockdown of vab-3 causes drastically decreased levels of the putative BAG CO2 receptor. (C) CO2 avoidance indices of the wild type, and vab-3(ju468) and vab-3(wz25) mutants. Data are the mean avoidance indices±s.e.m. (D) Responses of wild-type, ju468 and wz25 BAG neurons to a 10 s pulse of 10% CO2 measured using the ratiometric calcium indicator cameleon YC3.60. Individual responses are plotted in light gray and the average response is plotted in black. A horizontal black bar indicates the time of stimulus presentation. (E) Peak responses of wild-type BAG neurons and BAG neurons of ju468 and wz25 mutants.
ets-5(tm1734) mutants express many BAG-neuron markers at low levels (Guillermin et al., 2011), which prompted us to investigate whether vab-3 is required for residual expression of these markers in an ets-5 mutant. We measured expression of tax-4 and gcy-33 reporter transgenes in the wild type, and vab-3(ju468), ets-5 and ets-5 vab-3(ju468) mutants. As reported by others (Guillermin et al., 2011), expression of these reporters was reduced, but not eliminated, by ets-5 mutations (Fig. S4B,C). We found that vab-3(ju468) decreased tax-4 expression to the same extent as ets-5 mutation. However, ju468 caused an increase in gcy-33 expression. ets-5 vab-3(ju468) double mutants were indistinguishable from ets-5 single mutants and we still observed residual expression of tax-4 and gcy-33 reporters (Fig. S4B,C). These data reveal that vab-3 and ets-5 have separable functions in regulating gene expression in BAGs. These data also indicate that other gene-regulatory mechanisms function in parallel to ETS-5 and VAB-3-dependent mechanisms to promote expression of a BAG fate.
To confirm the physiological relevance of these gene expression changes, we assayed BAG neuron function using a behavior assay in which animals navigate in a chamber that contains a discontinuous CO2 gradient. The wild type migrate to the low-CO2 sector of the chamber, whereas animals with defective BAG function migrate equally between the two sectors of the chamber. This behavior is measured as an avoidance index that ranges from +1 (all animals in the no-CO2 sector) through 0 (no preference) to −1 (all animals in the CO2 sector). vab-3(wz25) mutants were grossly normal for CO2 avoidance, but ju468 mutants were severely defective (Fig. 3C).
Next, we assayed BAG neuron physiology to determine whether VAB-3 is required for their function as chemosensors. In response to CO2 stimuli, BAGs display large calcium responses that result from activation of the GCY-9 receptor-type cyclase and subsequent activation of cyclic nucleotide-gated ion channels (Hallem et al., 2011b; Smith et al., 2013). We used the ratiometric calcium sensor cameleon YC3.60 (Nagai et al., 2004) to measure these calcium responses. wz25 mutant BAGs responded strongly to 10% CO2 stimuli with an average peak YC3.60 ratio change of 71% over baseline, which was comparable with changes we measured in wild-type BAG neurons (Fig. 3D-G). The kinetics of activation and inactivation of BAG neurons in wz25 mutants were also comparable with those we measured in wild-type BAG neurons (Fig. S5). By contrast, the ju468 BAG neurons were defective in responding to CO2 stimuli with a 17% average peak ratio change (Fig. 3E,G). Together, these data indicate that the long VAB-3 isoform comprising a Paired domain and a homeodomain is dispensable for BAG neuron function, whereas a short homeodomain-only isoform is required for gene expression in and function of BAG neurons.
Ectopic expression of BAG neuron fate in vab-3 mutants is caused by dysregulated expression of the terminal selector ETS-5
What is the mechanism by which mutation of long VAB-3 isoforms causes ectopic expression of a marker of BAG neuron fate? The ETS-family transcription factor ETS-5 is required for expression of many BAG neuron fate markers and for BAG chemosensory function (Brandt et al., 2012; Guillermin et al., 2011; Serrano-Saiz et al., 2013). ETS-5 also promotes its own expression (Guillermin et al., 2011), suggesting that it functions as a terminal selector in BAG neurons, i.e. that its expression is self-sustaining and supports expression of a battery of genes that confer a specific identity upon a terminally differentiated cell (Deneris and Hobert, 2014). We tested whether ectopic expression of a BAG neuron fate in vab-3 Paired-domain mutants requires ETS-5. We found that, indeed, ETS-5 is required for the ectopic expression of Pflp-17::GFP in non-BAG cells; in ets-5 vab-3(wz25) double mutants, we observed no expression of the reporter in BAG neurons or in any other cells (Fig. 4A). Furthermore, we found that overexpression of ETS-5, which has no effect on reporter expression in the wild type (Brandt et al., 2012), enhanced the ectopic expression of Pflp-17::GFP in vab-3(wz25) mutants (Fig. 4A).
Fig. 4.
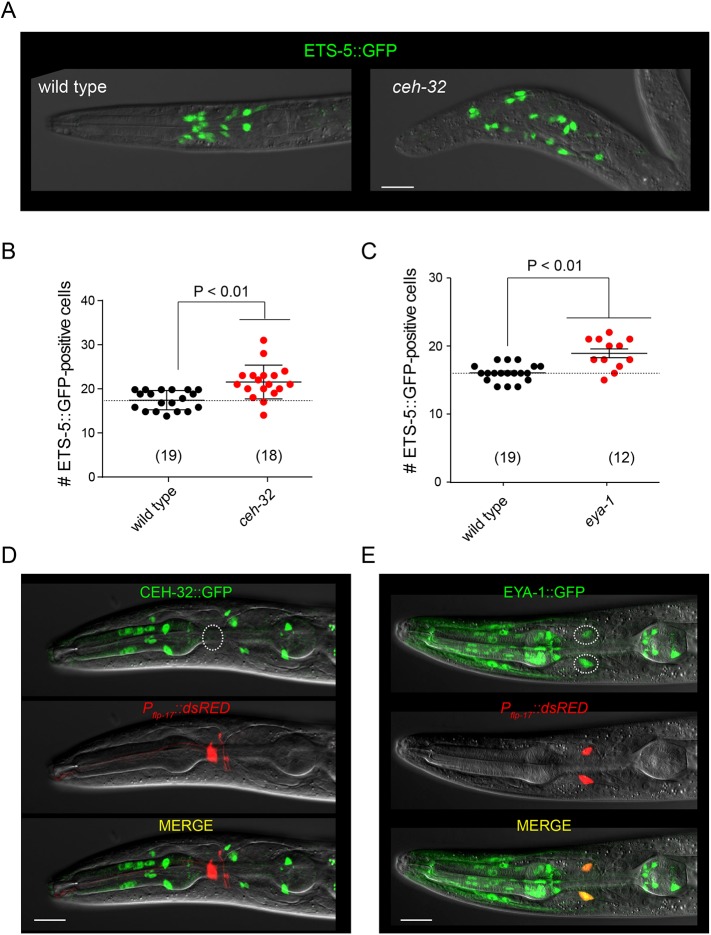
VAB-3 regulates expression of the terminal selector ETS-5. (A) Effects of ets-5 deletion and overexpression on Pflp-17::GFP expression in vab-3(wz25) mutants. (B) Mutations affecting the long isoform of vab-3 cause ectopic expression of ETS-5. Data are mean±s.d. *P<0.01. The number of animals assayed is indicated in brackets. (C) Fluorescence micrograph of a wild-type adult hermaphrodite expressing an integrated ETS-5::GFP translational reporter and a Pflp-17::dsRed transcriptional reporter. (D) Fluorescence micrograph of a wz25 mutant worm expressing ETS-5::GFP and Pflp-17::dsRed showing their co-expression in supernumerary Pflp-17::dsRed-expressing cells.
We next tested whether mutation of vab-3 affected expression of ETS-5 itself. In the wild type, an ETS-5::GFP fusion under the control of 3.5 kb of regulatory sequences from the ets-5 locus is expressed in 15 neurons (Fig. 4B). vab-3(wz25) mutants had, on average, 17 ETS-5::GFP-expressing cells. The e648 mutation caused a similar increase in the number of ETS-5::GFP-expressing cells. However, mutations that spared the Paired homology domain and did not cause ectopic expression of a BAG neuron fate (ju468) did not increase the number of ETS-5::GFP-expressing cells. To determine whether ectopic expression of ETS-5::GFP correlates with ectopic expression of a BAG fate marker, we introduced a Pflp-17::dsRed reporter into animals expressing ETS-5::GFP. Non-BAG cells that expressed Pflp-17::dsRed also expressed ETS-5::GFP (Fig. 4D). These data, together with the observed epistasis between the ets-5 mutant phenotype and that of vab-3, indicate that ectopic expression of a BAG neuron fate in vab-3 mutants is caused by dysregulated expression of ETS-5.
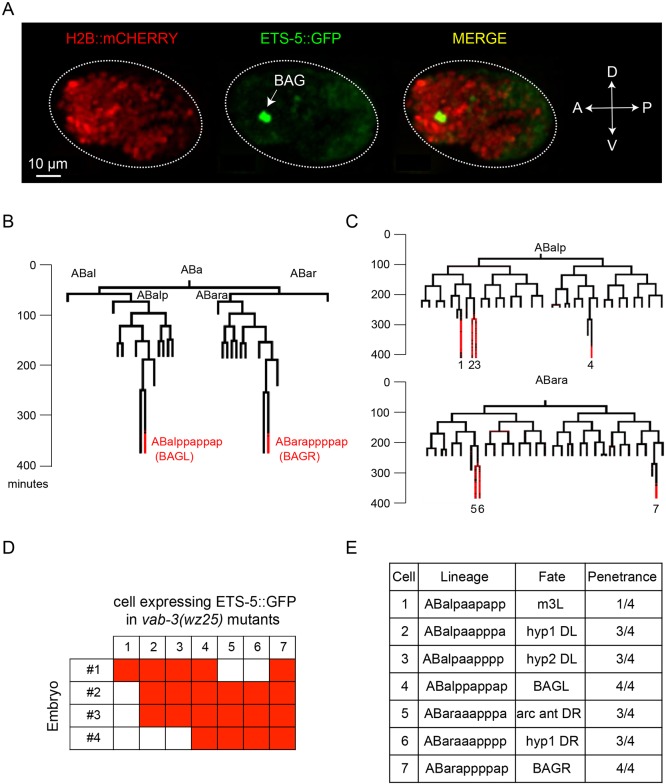
Does mutation of vab-3 alter neuronal lineages and cause the generation of extra BAG-like neurons? Or, are neuronal fates altered in vab-3 mutants such that non-BAG cells inappropriately express characteristics of BAG neurons? To distinguish these possibilities, we used machine-vision-assisted analysis of cell lineages in the C. elegans embryo to determine when and where ets-5 was expressed during development of the wild type and of vab-3(wz25) mutants. We generated transgenic animals that express in all cells a fusion of histone H2B with mCherry to monitor nuclear divisions and migrations, and also express an ETS-5::GFP fusion protein under the control of regulatory sequences from the ets-5 locus. In wild-type embryos, the first cells to express ETS-5::GFP are the embryonic BAG neurons (Fig. 5A), and these are the only cells that display strong expression of ETS-5::GFP during the first 470 min of embryonic development (Fig. 5B).
Fig. 5.
Mutation of the VAB-3 Paired homology domain causes a stereotyped transformation of cell fates. (A) Fluorescence micrograph of a comma-stage embryo expressing a H2B:mCherry and ETS-5::GFP reporter. GFP expression is limited to the two BAG cells at this developmental stage. (B) Lineage analysis of a wild-type worm up to 470 min of development. Developmental time is shown in minutes. Red lines represent the times when cells express ETS-5::GFP. (C) Lineage analysis of two vab-3(wz25) mutants, showing additional ETS-5::GFP-expressing cells. (D) Table of cells that expressed ETS-5::GFP in four lineaged vab-3(wz25) mutants. (E) Lineage, fate and frequency of cells aberrantly expressing ETS-5::GFP in vab-3(wz25) mutants.
When we analyzed the pattern of cell divisions and ETS-5::GFP expression in vab-3(wz25) mutants, we did not detect gross changes in the embryonic cell lineage, but we did observe inappropriate expression of ETS-5::GFP in non-BAG cells (Fig. 5C). In three out of four vab-3(wz25) embryos that were lineage traced, we observed expression in hypodermal cells ABalpaapppaa (hyp1DL), ABaraaapppp (hyp1DR), ABalpaapppp (hyp2DL) and ABaraaapppa (anterior arcade cell DR). In one wz25 embryo, we observed inappropriate expression of ETS-5::GFP in ABalpaapapp (pharyngeal muscle m3L). The hypodermal and arcade cells are generated in homologous lineages that descend from the anterior sister of a blast cell – ABalpp or ABarap – from which the BAG neurons descend. The mutation of the Paired homology domain of long VAB-3 isoforms, therefore, causes a reproducible fate-transformation in these lineages such that ets-5 expression is de-repressed and the BAG neuron fate is partially expressed.
VAB-3 functions together with an Eyes absent homolog and the Six-class homeodomain protein CEH-32 to repress expression of ETS-5
Studies of the Drosophila Pax6-like transcription factor Eyeless have defined a conserved pathway required for its function in eye development. Drosophila Eyeless promotes expression of a Six-class homeodomain factor Sine oculis, which regulates transcription of target genes that specify or define cell fates (Halder et al., 1998). Another factor required for function of the Eyeless pathway is Eyes absent, which has sequence similarity to protein phosphatases and is required for Sine oculis function (Jemc and Rebay, 2007). The C. elegans genome encodes four Six-class homeodomain proteins, including ceh-32, and a single homolog of Eyes absent, eya-1 (Dozier et al., 2001; Furuya et al., 2005). We examined expression of ETS-5 in mutants for these homologs of canonical Pax6/Eyeless partners to determine whether this conserved pathway mediates repression of ETS-5 during nervous system development. ceh-32 mutants (Fig. 6A,B) had on average four more ETS-5-expressing cells in the head than did the paired wild-type controls (21.6±0.9 cells in ceh-32, 17.6±0.5 in the wild type, mean±s.e.m.). eya-1 mutants (Fig. 6C) displayed a similar phenotype and had, on average, three more ETS-5-expressing cells in the head than did the paired wild-type controls (18.9±0.6 cells in eya-1, 16.1±0.3 in the wild type). These data indicate that the Eyes absent homolog EYA-1 and the six-class homeodomain protein CEH-32 also regulate expression of ETS-5 and suggest that a conserved Pax6/Eyeless pathway patterns expression of this terminal selector during development.
Fig. 6.
vab-3 functions together with the Eyes absent homolog eya-1 and the Six-class homeodomain gene ceh-32 to repress expression of ets-5. (A) Overlays of differential interference contrast and fluorescence micrographs of a wild-type and ceh-32 L1 hermaphrodite expressing ETS-5::GFP. (B) Number of ETS-5::GFP-expressing cells in wild type and ceh-32 mutants. (C) Number of ETS-5::GFP-expressing cells in wild type and eya-1 mutants. Data are mean±s.d. The number of animals assayed is indicated in brackets. (D) Fluorescence micrograph of an adult hermaphrodite expressing Pceh-32::GFP and Pflp-17::dsRed. (E) Fluorescence micrograph of an adult hermaphrodite expressing EYA-1::GFP and Pflp-17::dsRed. The BAG neurons are outlined. Scale bars: 10 μm.
We examined the expression of ceh-32 and eya-1 to determine whether these factors, which are required for the VAB-3-dependent repression of ets-5, are expressed in BAG neurons. A Pceh-32::GFP reporter was not expressed in BAG neurons (Fig. 6D). By contrast, an EYA-1::GFP reporter was expressed in BAG neurons as well as many non-BAG cells (Fig. 6E). These data indicate that not only do BAG neurons not express the repressive isoform of VAB-3, they also do not express Sine oculis, a crucial component of the pathway in which this VAB-3 isoform functions.
The ets-5 locus lacks high-affinity binding sites for VAB-3 and CEH-32
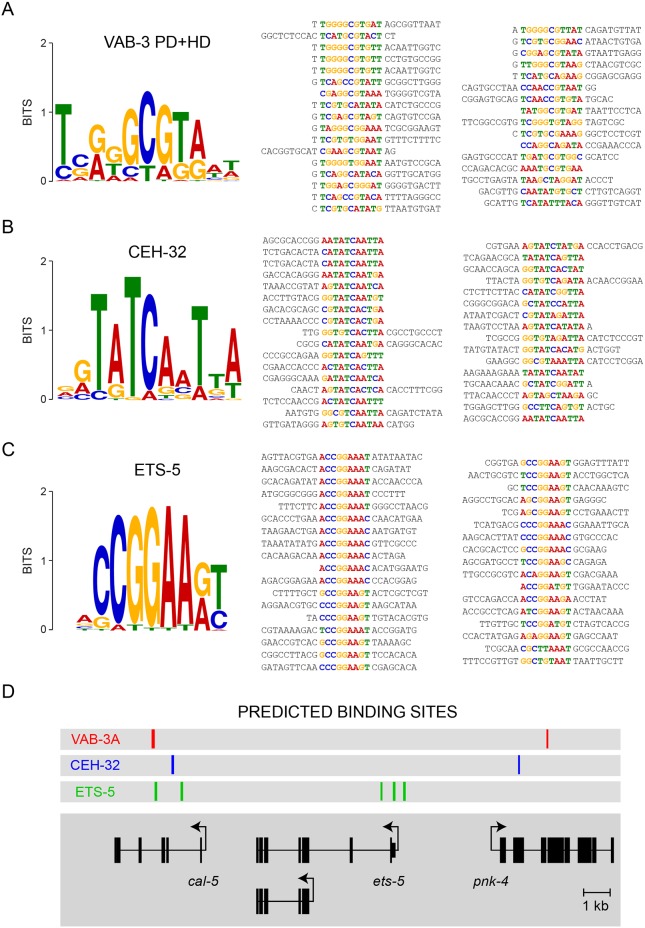
Do either VAB-3 or CEH-32 directly interact with sequences in the ets-5 locus to repress ets-5 expression? To address this question, we determined what sequences CEH-32 and the long isoform of VAB-3 bind using an optimized bacterial one-hybrid system. Because ETS-5 promotes its own expression, we expected that the ets-5 locus would include ETS-5 binding sites. We therefore also identified sequences that are preferentially bound by ETS-5. From a library of 2.0×108 potential transcription factor binding sites (Noyes et al., 2008), 33-35 sites were selected that bound to each transcription factor. Analysis of these sequences using the MEME suite for motif discovery (Bailey et al., 2015) generated consensus binding sequences for each of these transcription factors (Fig. 7A-C).
Fig. 7.
The ets-5 locus lacks high-affinity binding sites for VAB-3 and CEH-32. (A) MEME analysis of bacterial one-hybrid sequences yields a binding motif for the VAB-3 paired domain and homeodomains. (B) MEME analysis of bacterial one-hybrid sequences yields a binding motif for the entire CEH-32 protein. (C) MEME analysis of bacterial one-hybrid sequences yields a binding motif for the entire ETS-5 protein. Aligned sequences recovered from one-hybrid screens are shown on the right of each sequence logo. (D) FIMO analysis of the ets-5 locus reveals ETS-5 binding sites, yet no VAB-3- or CEH-32-binding sites, suggesting the indirect regulation of ets-5 expression.
ETS-5-binding sequences defined a hexanucleotide motif CCGGAA, which is identical to a sequence motif determined by analysis of a mammalian ETS-5 homolog, Pet1 (Wyler et al., 2016). A motif with the core sequence GCGTA was selected by VAB-3. This motif is also at the core of sequences bound by mammalian Pax6 that were determined by sequencing Pax6-associated chromatin (Sun et al., 2015). CEH-32-binding sequences defined a motif that matches the GTATCA consensus sequence previously found to define high-affinity binding sites for Six-class homeodomain proteins (Noyes et al., 2008). The similarities between the motifs determined by the bacterial one-hybrid system and the motifs known to support binding of mammalian homologs of ETS-5, VAB-3 and CEH-32 indicate that these motifs can accurately predict binding sites in the genome for these factors.
The presence of VAB-3 or CEH-32 binding sites in the ets-5 locus would support the hypothesis that these factors directly regulate transcription of ets-5. By contrast, the absence of VAB-3 or CEH-32 binding sites would strongly suggest that repression is mediated by other factors possibly downstream of VAB-3 and its partners. We used the FIMO module of the MEME suite for motif analysis (Bailey et al., 2015) to identify binding sites for VAB-3, CEH-32 and ETS-5 in the C. elegans genome, and inspected the ets-5 locus for the presence of such sites (Fig. 7D). As expected, the ets-5 locus contained binding sites for ETS-5, which is known to regulate its own expression (Guillermin et al., 2011). However, neither VAB-3- nor CEH-32-binding sites were detected in the ets-5 locus (Fig. 7D). This analysis supports a model wherein VAB-3 and its accessory factors indirectly regulate expression of ets-5 as opposed to directly repressing transcription of ets-5. However, it is also possible that long VAB-3 variants require co-factors to bind cryptic sequences in the ets-5 locus, something that would be missed by the bacterial one-hybrid system as implemented.
DISCUSSION
Pax6-like transcription factors are conserved among diverse animal phyla and, within a species, have many functions in brain and sense-organ development (Georgala et al., 2011; Shaham et al., 2012). How does one gene perform so many different functions during nervous system development? Our data suggest a mechanism that diversifies the functions of the C. elegans Pax6 homolog vab-3 such that it promotes a chemosensory neural fate in some lineages and represses that fate in others. Within a cell, the function of vab-3 is switched from activator of a chemosensory fate to repressor of that fate when two conditions are satisfied: (1) the transcriptional program of a cell selects a promoter in the vab-3 locus that generates a long vab-3 transcript that encodes a Paired homology domain together with a homeodomain; and (2) that cell expresses the Eyes absent homolog EYA-1 and the Six class homeodomain protein CEH-32. This regulation of VAB-3 function is part of a combinatorial logic that restricts expression of a BAG cell fate to only the two BAG cells proper. By contrast, a short isoform of VAB-3 is required for expression of genes required for BAG neuron function (Fig. 8). Therefore, vab-3 supports opposing functions because it encodes functionally distinct transcriptional regulators, each of which can cooperate with accessory factors that are differentially expressed during development. The permutation of transcription factor isoforms and accessory factors to create diversified regulators of transcription might be especially important for nervous system development, which requires a large number of distinct transcriptional programs to generate different neuron types and to instruct their connectivity. It is worth noting that a role for diversified functions of vab-3 gene products was previously suggested to contribute to glial development (Yoshimura et al., 2008); together with our study, these observations might suggest a more widespread role for permutations of VAB-3 variants and their partners in development.
Fig. 8.
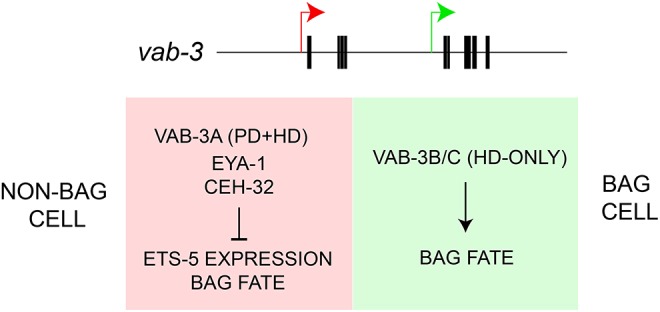
Model for the function of vab-3 in expression of chemosensory neuronal fate. Expression of the long isoform of vab-3 through promoter selection causes repression of chemosensory neuronal fate through collaboration with ceh-32 and eya-1, whereas selection of the short isoform promoter leads to activation of chemosensory neuronal fate.
How vab-3 patterns expression of a specific chemosensory fate illustrates the important role in neurodevelopment played by mechanisms that repress expression of programmed neural fates. Among studies of C. elegans neurodevelopment, there are many examples of mutations that cause ectopic expression of a neuronal fate by eliminating or reducing function of a repressor mechanism and thereby permitting the expression of another default fate. ASI chemosensory neurons require the Collier/Olf/EBP-like protein UNC-3 to repress expression of chemoreceptors normally restricted to non-ASI sensory neurons (Kim et al., 2005). The left and right ASE gustatory neurons adopt distinct fates in a stereotyped manner through the actions of an Nkx6-like transcriptional repressor COG-1 (Chang et al., 2003). Repression of alternate cell fates also patterns the motor system of C. elegans. Mutants lacking the homeodomain factor UNC-4 fail to differentiate VA and VB motoneurons (Miller et al., 1992; White et al., 1992), which share a cholinergic identity but have distinct connectivities within the motor system. Similarly, the cholinergic DA and DB motoneurons require the homeodomain protein VAB-7 to express distinct fates (Esmaeili et al., 2002). Within the same motor system, GABAergic DD and VD neurons acquire distinct characteristics through the actions of the nuclear hormone receptor UNC-55 (Zhou and Walthall, 1998). These studies reveal that, during development, many C. elegans neurons have the latent capacity to acquire alternate fates. The expression of alternate fates upon loss of a repressor mechanism has also been observed in genetic studies of insect and vertebrate nervous system development (e.g. Cayirlioglu et al., 2008), indicating that nervous system development in diverse animal phyla involves a dialog between mechanisms that promote and repress a specific neuronal fate.
Why is cell-fate specification in the nervous system of diverse species such a complicated pastiche of programs that promote and repress cell fates? It has been proposed that ancestral gene-regulatory programs are modified or co-opted by developmental programs throughout evolution and that mechanisms that repress gene expression are crucial agents of this evolutionary process. One study of cell-fate determination in the insect chemosensory system is noteworthy for how it illustrates a role for repression in generating the diversity of patterns of chemosensory fates seen in modern insect species. Cayirlioglu et al. describe a mutation that, like the vab-3 mutation we isolated, causes ectopic expression of genes that are normally expressed only by CO2-sensing neurons (Cayirlioglu et al., 2008). In Drosophila melanogaster, CO2 sensing is mediated by insect-specific odorant receptors that are restricted to antennal sensilla. Activation of these antennal sensilla triggers a powerful innate avoidance behavior (Suh et al., 2004). Loss of the microRNA mir-279 de-represses expression of CO2 chemoreceptors in neurons of the maxillary palp, which is the primary locus of CO2 chemosensation in insects that are attracted to CO2, e.g. the nectar-feeding moth Manduca sexta and Anopheles mosquitoes, which sense CO2 as a cue generated by hosts. Loss of mir-279 has been proposed to reveal an ancestral pattern of expression of CO2 chemosensitivity, and the phenotype of mir-279 mutants was suggested to resemble that of an evolutionary intermediate between an ancestor with maxillary palp expression of this fate and modern Drosophila species, which restrict this fate to sensory neurons in antennae (Cayirlioglu et al., 2008). Perhaps the phenotype of C. elegans mutants that lack a repressor isoform of VAB-3 also reflects an ancestral pattern of expression of the CO2-chemosensory modality. Because CO2 is a ubiquitous product of aerobic metabolism and a general regulator of cell physiology, it is an intriguing possibility that CO2 sensing in nematodes originally appeared in non-neural tissues and was subsequently co-opted by sensory neurons that control behavior.
Given that VAB-3 plays a crucial role in patterning expression of the terminal selector gene ets-5 in the developing nervous system, it is now important to understand the molecular mechanisms by which it regulates transcription at the ets-5 locus. Our studies suggest that neither VAB-3 nor its partner, the Six-class homeodomain protein CEH-32, directly binds to high-affinity sites in the ets-5 locus, which in turn suggests that regulation of ets-5 transcription by the VAB–3/CEH-32/EYA-1 pathway is indirect. One possibility is that VAB-3 and its partners regulate factors that directly regulate ets-5 transcription, e.g. promote the expression of repressors or inhibit the expression transcriptional activators. Another possibility is that VAB-3 and its partners establish genome-wide chromatin states that normally inhibit expression of terminal selector genes not associated with the normal fate of a cell. Recent studies demonstrate that the normal complement of terminal selectors in a cell generates a chromatin state that occludes the function of other terminal selectors (Patel and Hobert, 2017). As a consequence of promoting specific non-BAG fates, VAB-3, CEH-32 and EYA-1 might promote the acquisition of a repressed chromatin state at the ets-5 locus. Such a mechanism would not require specific high-affinity interactions between DNA-binding proteins and ets-5 regulatory sequences. The regulation of ets-5 expression is amenable to further genetic and molecular analysis. Our studies, therefore, lay a foundation for resolving these alternate possibilities and defining the mechanisms that regulate and pattern expression of terminal selector genes during normal development of the nervous system.
MATERIALS AND METHODS
Strains used in this study
Strains used in this study were: FQ402 ynIs64[Pflp-17::gfp], FQ462 ynIs64[Pflp-17::gfp]; ets-5(wz29), FQ649 ynIs64[Pflp-17::gfp]; ets-5(wz39), FQ453 ynIs64[Pflp-17::gfp]; ets-5(wz22), FQ452 ynIs64[Pflp-17::gfp]; ets-5(wz21), FQ466 ynIs64[Pflp-17::gfp];egl-13(wz33), FQ454 ynIs64[Pflp-17::gfp]; egl-13(wz23), FQ457 ynIs64[Pflp-17::gfp]; vab-3(wz25), FQ593 ynIs64[Pflp-17::gfp]; vab-3(e648), FQ540 ynIs64[Pflp-17::gfp]; vab-3(bx23), FQ1437 ynIs64[Pflp-17::gfp]; vab-3(ju468), FQ1533 ynIs64[Pflp-17::gfp];vab-3(ot266), FQ1094 vab-3(wz25); kyEx211[Pgcy–31::gcy-31::SL2::GFP], FQ1310 nIs242[Pgcy-33::GFP]; vab-3(wz25), FQ499 wzIs94[Pgcy-9::dsRed]; vab-3(wz25), FQ1092 wzEx302[Pflp-17:: dsRed+vab-3a/b/c::GFP], FQ553 nIs60[vab-3b/c::gfp]; wzEx36[Pflp-17::dsRed], FQ1869 ynIs64[Pflp-17::gfp]; wzEx500[vab-3a::mCherry::H2B], FQ2169 wzEx551[Pflp-17::dsRed+vab-3ΔA::GFP], FQ1546 wzIs113[Pgcy-9::gfp]; vab-3(ju468), FQ424 wzIs113[Pgcy-9::gfp], FQ2274 wzIs113[Pgcy-9 ::gfp]; wzEx580[Pflp-17::vab-3 RNAi], MT17370 lin-15A(n765); nIs242[Pgcy-33::gfp], FQ644 wzEx135[Pflp-17::YC3.60], FQ2278 nIs242 [Pgcy-33::gfp]; vab-3(ju468) ets-5(tm1734), FQ2268 nIs242[Pgcy-33::gfp]; ets-5(tm1734), FQ2144 nIs242[Pgcy-33::gfp]; vab-3(ju468), BC13862 dpy-5(e907); sEx13862[Ptax-4::gfp], FQ2279 ets-5(tm1734); sEx13862[Ptax-4::gfp], FQ2257 vab-3(ju468); sEx13862[Ptax-4::gfp], FQ1318 vab-3(wz25); wzEx135[Pflp–17::YC3.60], FQ1599 vab-3(ju468)/okIs57[Pmyo-2::gfp]; wzEx135[Pflp-17::YC3.60], CZ3391 vab-3(ju468), FQ502 ynIs64[Pflp-17::gfp]; ets-5(tm1734) vab-3(wz25), FQ517 ynIs64[Pflp-17::gfp]; wzIs80[ets-5::GFP]; vab-3(wz25), FQ237 wzIs80[ets-5::GFP], FQ501 wzIs80[ets-5::GFP]; vab-3(wz25), FQ1530 wzIs80[ets-5::GFP];vab-3(e648), FQ1527 wzIs80[ets-5::GFP]; vab-3(ju468), FQ341 wzIs80[ets-5::GFP]; wzEx36[Pflp-17::dsRed], FQ1095 wzIs80[ets-5::GFP]; vab-3(wz25); wzEx36[Pflp-17::dsRed], FQ580 wzIs80[ets-5::GFP]; stIs10266[Phis-72::his-24::mCherry]; itIs37[Ppie-1:: mCherry::H2B], FQ764 wzIs80[ets-5::GFP]; stIs10266[Phis-72::his-24:: mCherry]; itIs37[Ppie-1::mCherry::H2B];vab-3(wz25), FQ1335 ceh-32(ok343)/nT1; wzIs80[ets-5::GFP], FQ1498 eya-1(ok654); wzIs80[ets-5::GFP], FQ1591 wzEx441[Pceh-32::gfp+Pflp-17::dsRed] and FQ1538 tjEx25[eya-1::GFP]; wzEx36[Pflp-17::dsRed].
Mutagenesis screen for genes that function in BAG neuron development
Mutagenesis was performed as described previously (Brenner, 1974). F2 progeny of EMS-mutagenized hermaphrodites carrying the ynIs64[Pflp-17::gfp] were screened for abnormal GFP expression. Approximately 50,000 animals were screened non-clonally. We also screened progeny of 5000 singled F1 progeny. In total, ∼35,000 mutagenized haploid genomes were screened. Alleles of ets-5 were initially identified as X-linked mutations that caused a strong defect in ynIs64[Pflp-17::gfp] expression. These mutations were then tested for their ability to complement the GFP expression defect of ynIs64[Pflp-17::GFP]; ets-5(tm1734) mutants. The ets-5 locus was sequenced by conventional Sanger sequencing to identify mutations in the ets-5 locus. Alleles of egl-13 were initially identified as mutants with strong defects in ynIs64[Pflp-17::gfp] expression and severe egg-laying defects because of vulval abnormalities. Whole-genome sequencing revealed that these mutants had mutations in egl-13, which is known to promote expression of a BAG neuron fate (Gramstrup Petersen et al., 2013).
Mapping and cloning of vab-3(wz25)
High resolution SNP-mapping of wz25 was performed by crossing wz25 mutants to the polymorphic strain CB4856 and identifying crossovers using restriction fragment polymorphisms (Davis et al., 2005). This experiment placed wz25 in a 0.4 Mb interval on LG X. Whole-genome sequencing of the wz25 mutant revealed that wz25 contains a G-to-A substitution predicted to change Gly to Arg in isoform A of vab-3 and did not reveal other mutations in annotated protein-coding sequences.
Quantification of expression of transgene markers
For cell counts and measurements of transgene expression, young adult animals were mounted on 2% agarose pads made in M9 medium and immobilized with 30 mM sodium azide. Samples were imaged with a Zeiss AxioImager M2 upright microscope equipped with an Andor Clara EMCCD camera and an X-cite epifluorescence light source (Excelitas). A 20×0.8 NA air objective was used. For ynIs64[Pflp-17::GFP], 10 ms exposure and 12% lamp intensity were used to acquire images. For wzIs113[Pgcy-9::gfp], 200 ms exposure and 12% X-cite lamp intensity were used to acquire images. For nIs242[Pgcy-33::gfp], 25 ms exposure and 100% X-cite lamp intensity were used to acquire images. For sEx13862[Ptax-4::gfp], 25 ms exposure and 12% X-cite lamp intensity were used to acquire images. Ten to 20 animals were analyzed per genotype. To determine expression levels of fluorescent reporters, measurements of the mean pixel intensity in a circle 50 pixels wide and centered on the BAG cell body were made.
Confocal microscopy
Young adults were mounted on a 2% agarose pad made in M9 medium and anesthetized with 30 mM sodium azide. Z stacks were acquired using a Zeiss LSM700 laser-scanning confocal microscope, and maximum projection images were created using ImageJ. For display purposes, some micrographs were cropped and mounted on a rectangular background colored to match the background of the micrograph.
CO2-avoidance assays
Avoidance assays were performed essentially as previously described (Martínez-Velázquez and Ringstad, 2018). A total of 20-30 adult hermaphrodites were placed on an unseeded 10 cm NGM plate. A custom-made chamber (Fig. S6) was pressed into the NGM media, trapping the animals in the chamber. Gas mixes were introduced at 1.5 ml min−1 using a syringe pump (New Era). After 30 min, animals were scored as being either on the side with the CO2 inlet or on the side with the air inlet.
In vivo calcium imaging
Calcium imaging was performed essentially as previously described (Hallem et al., 2011a). Young adults were immobilized with cyanoacrylate veterinary glue (Surgi-Lock; Meridian Animal Health) on a cover glass coated with a 2% agarose pad made with 10 mM HEPES (pH 7.4). The cover glass was affixed to a custom-made air chamber. The specimen was illuminated with 435 nm excitation light and imaged using a 20× Nikon long-working distance objective (0.8 numerical aperture). The emission image was passed through a DV2 image splitter (Photometrics), and the CFP and YFP emission images were projected onto two halves of a cooled CCD camera (Andor). Images were acquired at 10 Hz, with exposure times between 10 and 50 ms. Gas perfusion was controlled using three-way valves (Numatics) driven by a custom-made valve controller unit. Custom certified gas mixes used for imaging were obtained from Airgas. Excitation light, image acquisition and hardware control were performed by the Live Acquisition software package (FEI/Till Photonics). Post-acquisition analysis of ratio plots was performed using Matlab (MathWorks) scripts that subtracted linear baseline drift from traces and then applied a five-frame boxcar filter to the time-series.
Generation of fosmid-based reporters
Primers were designed to add homology arms to GFP-coding sequences and insert them into the fosmid WRM069dG06 into the predicted 3′ end of the vab-3 locus. Fosmid recombineering was carried out as described previously (Tursun et al., 2009). For the short isoform reporter fosmid, a kan resistance cassette was amplified with overhangs homologous to the second exon of vab-3a. Recombination was induced by heat shock. The recombineered fosmid was linearized by digestion with NotI and co-injected at 10 ng/μl together with 15 ng/µl pJB134 (Pflp-17::dsRed) and 150 ng/μl EcoRV-digested N2 genomic DNA.
Embryonic imaging, lineage tracing and determination of cell identities
Gravid adult hermaphrodites with one row of embryos in the uterus were dissected in M9 buffer and two- to four-cell embryos were mounted. Multiple (up to six) embryos were transferred to a ∼2 μl drop of M9 buffer with 100-200 20 μm diameter polystyrene beads (Polysciences) on to a coverslip. A smaller coverslip was then laid on top and sealed with melted Vaseline along the perimeter to prevent evaporation (the embryos were slightly compressed but not crushed because of the presence of the polystyrene beads). Embryos were imaged using 491 and 561 nm lasers, with only the 561 nm laser for the first 200 min. For two-color imaging, both red and green channels were simultaneously acquired using a pair of aligned EM-CCD cameras (C9100-13) on a Zeiss AxioObserver Z1 inverted microscope frame with Yokogawa CSU-X1 spinning disk. Imaging was performed using MetaMorph software (Molecular Devices). The objective used for all imaging was a 40× oil immersion objective. Multiple stage positions were imaged on the same run at 75 to 90 s interval with 30 slices in total with 1 μm between slices. The image-analysis pipeline was similar to that described by Du et al. (2014), where StarryNite (Santella et al., 2014) was used for image processing, nuclear segmentation and tracking, and AceTree (Katzman et al., 2018) was used to manually correct any tracking errors. Entire lineages were manually curated to the 200-cell stage, and cells of interest were traced through the final division.
Statistical analyses
Statistical tests were carried out using GraphPad Prism. P values from comparisons of GFP expression levels between BAG neurons from different mutants were made using a Mann–Whitney test and corrected for multiple comparisons. P values from comparisons of the numbers of cells expressing fate markers were made using Fisher's exact test.
Bacterial one-hybrid screens
Bacterial one-hybrid screens were conducted as previously described (Noyes et al., 2008). Sequences of bait proteins are shown in Fig. S7. In short, 1 µg of bait plasmid was transformed with 50 ng of binding-site library into the selection strain. Cells were recovered first in SOC (super optimal broth with catabolite repression) for 1 h, and then in complete NM medium (minimal glucose medium lacking histidine) for another 1 h before being titrated by 10-fold serial dilutions on rich media plates supplemented with antibiotics to determine the total number of transformants. The following day, ∼5×107 cells were placed on NM (minimal glucose medium lacking histidine) media plates without histidine or uracil containing either 2, 5 or 10 mM 3 amino-1,2,4-triazole (3AT). Cells were grown under selection for 36 to 48 h at 37°C. Colony PCR was performed on selected colonies to determine prey sequences, which were analyzed using MEME motif discovery tool (meme.sdsc.edu/meme/intro.html) and compiled into sequence logos using the WebLogo tool (weblogo.berkeley.edu/logo.cgi).
Supplementary Material
Acknowledgements
Tugba Colak-Champollion isolated some ets-5 mutants described in this study, and Elver Ho generated a fosmid-based vab-3a reporter. Gira Bhabha helped generate a homology model of the VAB-3 Paired domain. Dr Asako Sugimoto (Tohoku University) generously shared eya-1::gfp transgenic worms. Other strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH Office of Research Infrastructure Programs (P40OD010440).
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: J.P.B., M.R., N.R.; Methodology: Z.B.; Software: Z.B.; Validation: J.P.B., M.R.; Formal analysis: J.P.B., M.R., Z.D., D.I., K.B., A.C., M.N., Z.B., N.R.; Investigation: J.P.B., M.R., Z.D., D.I., K.B., A.C., Z.B.; Data curation: J.P.B., M.R., Z.D., D.I., K.B., A.C., M.N., Z.B., N.R.; Writing - original draft: J.P.B., M.R., N.R.; Writing - review & editing: J.P.B., M.R., N.R.; Supervision: M.N., Z.B., N.R.; Project administration: N.R.; Funding acquisition: Z.B., N.R.
Funding
This work was supported by a National Institute of General Medical Sciences grant (R35 GM122573) and a Hirschl Weill-Caulier Foundation career development award (both to N.R.); by National Institutes of Health grants R01GM097576 and R24OD016474, and a National Institutes of Health center grant P30CA008748 to the Memorial Sloan Kettering Cancer Center (awarded to Z.B.). M.R. received support from National Institutes of Health training grants T32GM007308 and T32HD007520, and National Institutes of Health grant F30HD094483; D.I. received support from National Institutes of Health training grant T32GM066704. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.168153.supplemental
References
- Bailey T. L., Johnson J., Grant C. E. and Noble W. S. (2015). The MEME suite. Nucleic Acids Res. 43, W39-W49. 10.1093/nar/gkv416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J. P., Aziz-Zaman S., Juozaityte V., Martínez-Velázquez L. A., Petersen J. G., Pocock R. and Ringstad N. (2012). A single gene target of an ETS-family transcription factor determines neuronal CO2-chemosensitivity. PLoS ONE 7, e34014 10.1371/journal.pone.0034014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayirlioglu P., Kadow I. G., Zhan X., Okamura K., Suh G. S. B., Gunning D., Lai E. C. and Zipursky S. L. (2008). Hybrid neurons in a microRNA mutant are putative evolutionary intermediates in insect CO2 sensory systems. Science 319, 1256-1260. 10.1126/science.1149483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Johnston R. J. Jr and Hobert O (2003). A transcriptional regulatory cascade that controls left/right asymmetry in chemosensory neurons of C. elegans. Genes Dev. 17, 2123-2137. 10.1101/gad.1117903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm A. D. and Horvitz H. R. (1995). Patterning of the Caenorhabditis elegans head region by the Pax-6 family member vab-3. Nature 377, 52-55. 10.1038/377052a0 [DOI] [PubMed] [Google Scholar]
- Davis M. W., Hammarlund M., Harrach T., Hullett P., Olsen S. and Jorgensen E. M. (2005). Rapid single nucleotide polymorphism mapping in C. elegans. BMC Genomics 6, 118 10.1186/1471-2164-6-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneris E. S. and Hobert O. (2014). Maintenance of postmitotic neuronal cell identity. Nat. Neurosci. 17, 899-907. 10.1038/nn.3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier C., Kagoshima H., Niklaus G., Cassata G. and Bürglin T. (2001). The Caenorhabditis elegans six/sine oculis class homeobox gene ceh-32 is required for head morphogenesis. Dev. Biol. 236, 289-303. 10.1006/dbio.2001.0325 [DOI] [PubMed] [Google Scholar]
- Du Z., Santella A., He F., TionGSON M. and Bao Z. (2014). De novo inference of systems-level mechanistic models of development from live-imaging-based phenotype analysis. Cell 156, 359-372. 10.1016/j.cell.2013.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili B., Ross J. M., Neades C., Miller D. M. III and Ahringer J (2002). The C. elegans even-skipped homologue, vab-7, specifies DB motoneurone identity and axon trajectory. Development 129, 853-862. [DOI] [PubMed] [Google Scholar]
- Furuya M., Qadota H., Chisholm A. D. and Sugimoto A. (2005). The C. elegans eyes absent ortholog EYA-1 is required for tissue differentiation and plays partially redundant roles with PAX-6. Dev. Biol. 286, 452-463. 10.1016/j.ydbio.2005.08.011 [DOI] [PubMed] [Google Scholar]
- Georgala P. A., Carr C. B. and Price D. J. (2011). The role of Pax6 in forebrain development. Dev. Neurobiol. 71, 690-709. 10.1002/dneu.20895 [DOI] [PubMed] [Google Scholar]
- Gramstrup Petersen J., Rojo Romanos T., Juozaityte V., Redo Riveiro A., Hums I., Traunmüller L., Zimmer M. and Pocock R. (2013). EGL-13/SoxD specifies distinct O2 and CO2 sensory neuron fates in Caenorhabditis elegans. PLoS Genet. 9, e1003511 10.1371/journal.pgen.1003511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillermin M. L., Castelletto M. L. and Hallem E. A. (2011). Differentiation of carbon dioxide-sensing neurons in Caenorhabditis elegans requires the ETS-5 transcription factor. Genetics 189, 1327-1339. 10.1534/genetics.111.133835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillermin M. L., Carrillo M. A. and Hallem E. A. (2017). A single set of interneurons drives opposite behaviors in C. elegans. Curr. Biol. 27, 2630-2639.e6. 10.1016/j.cub.2017.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G., Callaerts P., Flister S., Walldorf U., Kloter U. and Gehring W. J. (1998). Eyeless initiates the expression of both sine oculis and eyes absent during Drosophila compound eye development. Development 125, 2181-2191. [DOI] [PubMed] [Google Scholar]
- Hallem E. A. and Sternberg P. W. (2008). Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 105, 8038-8043. 10.1073/pnas.0707469105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem E. A., Dillman A. R., Hong A. V., Zhang Y., Yano J. M., Demarco S. F. and Sternberg P. W. (2011a). A sensory code for host seeking in parasitic nematodes. Curr. Biol. 21, 377-383. 10.1016/j.cub.2011.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem E. A., Spencer W. C., Mcwhirter R. D., Zeller G., Henz S. R., Rätsch G., Miller D. M., Horvitz H. R., Sternberg P. W. and Ringstad N. (2011b). Receptor-type guanylate cyclase is required for carbon dioxide sensation by Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 108, 254-259. 10.1073/pnas.1017354108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O., Glenwinkel L. and White J. (2016). Revisiting neuronal cell type classification in Caenorhabditis elegans. Curr. Biol. 26, R1197-R1203. 10.1016/j.cub.2016.10.027 [DOI] [PubMed] [Google Scholar]
- Jemc J. and Rebay I. (2007). The eyes absent family of phosphotyrosine phosphatases: properties and roles in developmental regulation of transcription. Annu. Rev. Biochem. 76, 513-538. 10.1146/annurev.biochem.76.052705.164916 [DOI] [PubMed] [Google Scholar]
- Katzman B., Tang D., Santella A. and Bao Z. (2018). AceTree: a major update and case study in the long term maintenance of open-source scientific software. BMC Bioinformatics 19, 121 10.1186/s12859-018-2127-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. and Li C. (2004). Expression and regulation of an FMRFamide-related neuropeptide gene family in Caenorhabditis elegans. J. Comp. Neurol. 475, 540-550. 10.1002/cne.20189 [DOI] [PubMed] [Google Scholar]
- Kim K., Colosimo M. E., Yeung H. and Sengupta P. (2005). The UNC-3 Olf/EBF protein represses alternate neuronal programs to specify chemosensory neuron identity. Dev. Biol. 286, 136-148. 10.1016/j.ydbio.2005.07.024 [DOI] [PubMed] [Google Scholar]
- Lee J. S., Shih P.-Y., Schaedel O. N., Quintero-Cadena P., Rogers A. K. and Sternberg P. W. (2017). FMRFamide-like peptides expand the behavioral repertoire of a densely connected nervous system. Proc. Natl. Acad. Sci. USA 114, E10726-E10735. 10.1073/pnas.1710374114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Velázquez L. A. and Ringstad N. (2018). Antagonistic regulation of trafficking to Caenorhabditis elegans sensory cilia by a Retinal Degeneration 3 homolog and retromer. Proc. Natl. Acad. Sci. USA 115, E438-e447. 10.1073/pnas.1712302115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. M., Shen M. M., Shamu C. E., Burglin T. R., Ruvkun G., Dubois M. L., Ghee M. and Wilson L. (1992). C. elegans unc-4 gene encodes a homeodomain protein that determines the pattern of synaptic input to specific motor neurons. Nature 355, 841-845. 10.1038/355841a0 [DOI] [PubMed] [Google Scholar]
- Nagai T., Yamada S., Tominaga T., Ichikawa M. and Miyawaki A. (2004). Expanded dynamic range of fluorescent indicators for Ca(2+) by circularly permuted yellow fluorescent proteins. Proc. Natl. Acad. Sci. USA 101, 10554-10559. 10.1073/pnas.0400417101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Fisher M., Nakajima K., Odeleye A. O., Zimmerman K. B., Fish M. B., Yaoita Y., Chojnowski J. L., Lauderdale J. D., Netland P. A. et al. (2015). Xenopus pax6 mutants affect eye development and other organ systems, and have phenotypic similarities to human aniridia patients. Dev. Biol. 408, 328-344. 10.1016/j.ydbio.2015.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes M. B., Christensen R. G., Wakabayashi A., Stormo G. D., Brodsky M. H. and Wolfe S. A. (2008). Analysis of homeodomain specificities allows the family-wide prediction of preferred recognition sites. Cell 133, 1277-1289. 10.1016/j.cell.2008.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T. and Hobert O. (2017). Coordinated control of terminal differentiation and restriction of cellular plasticity. eLife 6, 249 10.7554/eLife.24100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo Romanos T., Petersen J. G., Riveiro A. R. and Pocock R. (2015). A novel role for the zinc-finger transcription factor EGL-46 in the differentiation of gas-sensing neurons in Caenorhabditis elegans. Genetics 199, 157-163. 10.1534/genetics.114.172049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santella A., Du Z. and Bao Z. (2014). A semi-local neighborhood-based framework for probabilistic cell lineage tracing. BMC Bioinformatics 15, 217 10.1186/1471-2105-15-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Saiz E., Poole R. J., Felton T., Zhang F., De La Cruz E. D. and Hobert O (2013). Modular control of glutamatergic neuronal identity in C. elegans by distinct homeodomain proteins. Cell 155, 659-673. 10.1016/j.cell.2013.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham O., Menuchin Y., Farhy C. and Ashery-Padan R. (2012). Pax6: a multi-level regulator of ocular development. Prog. Retin. Eye Res. 31, 351-376. 10.1016/j.preteyeres.2012.04.002 [DOI] [PubMed] [Google Scholar]
- Smith E. S., Martinez-Velazquez L. and Ringstad N. (2013). A chemoreceptor that detects molecular carbon dioxide. J. Biol. Chem. 288, 37071-37081. 10.1074/jbc.M113.517367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh G. S., Wong A. M., Hergarden A. C., Wang J. W., Simon A. F., Benzer S., Axel R. and Anderson D. J. (2004). A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature 431, 854-859. 10.1038/nature02980 [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G. and Thomson J. N. (1983). The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64-119. 10.1016/0012-1606(83)90201-4 [DOI] [PubMed] [Google Scholar]
- Sun J., Rockowitz S., Xie Q., Ashery-Padan R., Zheng D. and Cvekl A. (2015). Identification of in vivo DNA-binding mechanisms of Pax6 and reconstruction of Pax6-dependent gene regulatory networks during forebrain and lens development. Nucleic Acids Res. 43, 6827-6846. 10.1093/nar/gkv589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tursun B., Cochella L., Carrera I. and Hobert O. (2009). A toolkit and robust pipeline for the generation of fosmid-based reporter genes in C. elegans. PLoS ONE 4, e4625 10.1371/journal.pone.0004625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G., Southgate E., Thomson J. N. and Brenner S. (1986). The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 314, 1-340. 10.1098/rstb.1986.0056 [DOI] [PubMed] [Google Scholar]
- White J. G., Southgate E. and Thomson J. N. (1992). Mutations in the Caenorhabditis elegans unc-4 gene alter the synaptic input to ventral cord motor neurons. Nature 355, 838-841. 10.1038/355838a0 [DOI] [PubMed] [Google Scholar]
- Wyler S. C., Spencer W. C., Green N. H., Rood B. D., Crawford L., Craige C., Gresch P., Mcmahon D. G., Beck S. G. and Deneris E. (2016). Pet-1 switches transcriptional targets postnatally to regulate maturation of serotonin neuron excitability. J. Neurosci. 36, 1758-1774. 10.1523/JNEUROSCI.3798-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. E., Rould M. A., Xu W., Epstein J. A., Maas R. L. and Pabo C. O. (1999). Crystal structure of the human Pax6 paired domain-DNA complex reveals specific roles for the linker region and carboxy-terminal subdomain in DNA binding. Genes Dev. 13, 1263-1275. 10.1101/gad.13.10.1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S., Murray J., Lu Y., Waterston R. and Shaham S. (2008). mls-2 and vab-3 control glia development, hlh-17/Olig expression and glia-dependent neurite extension in C. elegans. Development 135, 2263-2275. 10.1242/dev.019547 [DOI] [PubMed] [Google Scholar]
- Zhang Y. and Emmons S. W. (1995). Specification of sense-organ identity by a Caenorhabditis elegans Pax-6 homologue. Nature 377, 55-59. 10.1038/377055a0 [DOI] [PubMed] [Google Scholar]
- Zhou H. M. and Walthall W. W. (1998). UNC-55, an orphan nuclear hormone receptor, orchestrates synaptic specificity among two classes of motor neurons in Caenorhabditis elegans. J. Neurosci. 18, 10438-10444. 10.1523/JNEUROSCI.18-24-10438.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer M., Gray J. M., Pokala N., Chang A. J., Karow D. S., Marletta M. A., Hudson M. L., Morton D. B., Chronis N. and Bargmann C. I. (2009). Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron 61, 865-879. 10.1016/j.neuron.2009.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.