ABSTRACT
Vascular pruning is crucial for normal development, but its underlying mechanisms are poorly understood. Here, we report that retinal vascular pruning is controlled by the oxygen-sensing mechanism in local astrocytes. Oxygen sensing is mediated by prolyl hydroxylase domain proteins (PHDs), which use O2 as a substrate to hydroxylate specific prolyl residues on hypoxia inducible factor (HIF)-α proteins, labeling them for polyubiquitylation and proteasomal degradation. In neonatal mice, astrocytic PHD2 deficiency led to elevated HIF-2α protein levels, expanded retinal astrocyte population and defective vascular pruning. Although astrocytic VEGF-A was also increased, anti-VEGF failed to rescue vascular pruning. However, stimulation of retinal astrocytic growth by intravitreal delivery of PDGF-A was sufficient to block retinal vascular pruning in wild-type mice. We propose that in normal development, oxygen from nascent retinal vasculature triggers PHD2-dependent HIF-2α degradation in nearby astrocytic precursors, thus limiting their further growth by driving them to differentiate into non-proliferative mature astrocytes. The physiological limit of retinal capillary density may be set by astrocytes available to support their survival, with excess capillaries destined for regression.
This article has an associated ‘The people behind the papers’ interview.
KEY WORDS: Vascular pruning, Angiogenesis, Retinal astrocytes, Hypoxia, PHD2
Highlighted Article: Targeted disruption of astrocytic PHD2 led to HIF-2α accumulation and prolonged expansion of the retinal astrocyte population and the resulting supra-physiological astrocyte abundance prevented retinal vascular pruning in neonatal mice.
INTRODUCTION
Retinal vascular development is spatially and temporally coordinated with the development of the astrocytic network (Dorrell et al., 2002; Duan et al., 2017; Gariano et al., 1996; Ling et al., 1989). In rodents, retinal astrocytic development begins as early as the late-gestation stages, with astrocytic progenitor cells (APCs) migrating out of the rim of the optic nerve head (ONH) and onto the inner retinal surface (Ling et al., 1989). APCs are strongly Pax2+ in their nuclei but negative for glial fibrillary acidic protein (GFAP) (Chan-Ling et al., 2009; Duan et al., 2017). At birth, APCs have spread out to approximately halfway between the ONH and retinal periphery (Duan et al., 2017; Huxlin et al., 1992; Ling et al., 1989), but mostly remain in the progenitor stage. Once retinal angiogenesis begins in newborn mice, APCs undergo partial differentiation to become immature astrocytes (IACs) ahead of the vascular front, and full differentiation to become mature astrocytes (mASCs) within the vascularized area. IACs remain strongly Pax2+as in APCs, but distinguish themselves from APCs by gaining weak GFAP expression. By contrast, mASCs express lower levels of Pax2, but are strongly GFAP+ (Duan et al., 2017). APCs and IACs are astrocytic precursors at different stages of differentiation towards full maturity. Astrocytic precursors are proliferative, but mASCs are generally quiescent. Furthermore, both APCs and IACs are highly migratory, with IACs approaching the retinal periphery by postnatal day (P) 3 in mice (Dorrell et al., 2002; Duan et al., 2017).
In rodents, retinal angiogenesis begins at birth, with nascent blood vessels sprouting out of the rim of the ONH (Fruttiger, 2002). In the subsequent week, the vascular front continues to advance in radial directions towards the retinal periphery, led by vascular tip cells (Fruttiger, 2002; Gerhardt et al., 2003; Scott et al., 2010). Whereas retinal angiogenesis is first induced by APCs after birth, subsequent vascular growth towards the retinal periphery depends on IACs. As they form, new blood vessels provide oxygen to nearby IACs, driving them to differentiate into mASCs (Duan et al., 2017). In addition to oxygen, endothelial cell (EC)-derived factors, such as leukemia inhibitory factor and the apelin/APJ system, also promote retinal astrocytic differentiation (Fukushima et al., 2009; Sakimoto et al., 2012).
Oxygen is a substrate of prolyl hydroxylase domain (PHD) proteins (also referred to as EGLNs), a subfamily of oxoglutarate and Fe2+-dependent dioxygenases that hydroxylate specific prolyl residues in hypoxia inducible factor (HIF)-α (Bruick and McKnight, 2001; Epstein et al., 2001; Ivan et al., 2002). Once hydroxylated, HIF-α proteins undergo pVHL (VHL)- and E3 ligase-dependent polyubiquitylation, followed by degradation in proteasomes (Epstein et al., 2001; Huang et al., 2002; Maxwell et al., 1999). Under normal physiological conditions, HIF-α protein levels are tightly controlled by tissue oxygen levels. Rising oxygen levels due to vascular development may activate PHD2 (EGLN1), triggering HIF-α hydroxylation and degradation. In neonatal mouse retinas, expression of HIF-2α (EPAS1) in astrocytic precursors (APCs and IACs) is crucial for the maintenance of their proliferative capacity (Duan et al., 2014), whereas diminished HIF-2α levels may cause APCs and IACs to differentiate into proliferation-incompetent mASCs (Duan et al., 2017, 2014).
The development of the primary retinal vasculature proceeds in two phases: (1) formation of nascent vascular plexus by sprouting angiogenesis; and (2) remodeling of the honeycomb-patterned nascent vascular plexus into a mature vascular tree displaying hierarchical organization of larger trunks and smaller branches (Fruttiger, 2007). Although angiogenesis has been studied extensively (Bentley et al., 2014; Costa et al., 2016; Hellström et al., 2007; Jakobsson et al., 2010; Pitulescu et al., 2017; Sawamiphak et al., 2010; Tamagnone and Mazzone, 2011; Tammela et al., 2011), much less is known about developmental remodeling. Nonetheless, it is well recognized that developmental remodeling involves multiple regulated processes, including recruitment of mural cells, regulation of vascular calibers, and pruning (regression) of excess microvessels (Arreola et al., 2018; Cheng et al., 2012; Chu et al., 2016; Ehling et al., 2013; Ishida et al., 2003; Korn et al., 2014; Phng et al., 2009; Scott et al., 2010; Simonavicius et al., 2012; Watson et al., 2016; Zaitoun et al., 2015).
In this study, we demonstrate that oxygen sensing in retinal astrocytes controls endothelial growth and apoptosis, morphologically manifested as regulation of vascular pruning and capillary caliber. Blocking oxygen sensing in astrocytes by targeted disruption of the Phd2 locus caused HIF-2α accumulation, lingering of astrocytes in immature states, expansion of the retinal astrocyte population, and defective vascular pruning. These findings indicate that oxygen targets retinal astrocytes instead of endothelial cells to initiate developmental vascular regression.
RESULTS
Disruption of Phd2 in retinal astrocytes
We disrupted the Phd2 gene in astrocytes to cripple their oxygen-sensing mechanisms. Although several different GFAPCre mouse lines are available to delete floxed genes in astrocytes, only one of them (Jackson Laboratory stock number 004600, originating from the Messing Lab, University of Wisconsin, WI, USA) has demonstrated Cre activity in the precursors of retinal astrocytes (Duan et al., 2014; Zhuo et al., 2001). Thus, the Jackson Laboratory 004600 GFAPCre line was chosen for this project to achieve Phd2 disruption early in retinal astrocyte development.
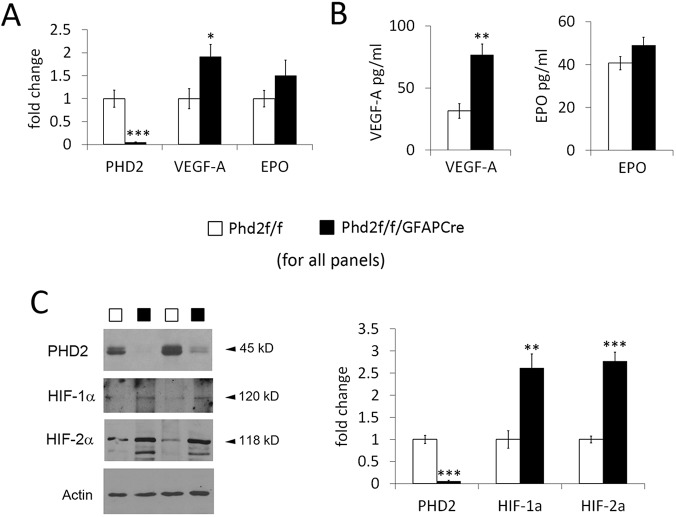
To assess Phd2 targeting efficiency, we quantified PHD2 mRNA and protein levels in neonatal retinal astrocytes cultured according to an established protocol, with minor modifications (Fig. S1) (Scheef et al., 2005). According to both quantitative RT-PCR (qPCR) and western blotting, astrocytic PHD2 level in Phd2f/f/GFAPCre mice was reduced to 6% or less relative to floxed controls (Fig. 1). qPCR and ELISA also revealed that VEGF-A expression was increased in PHD2-deficient retinal astrocytes (Fig. 1A,B), but EPO expression was not significantly affected. In addition, PDGF-A expression was undetectable in both floxed and PHD2-deficient astrocytes. Western blotting demonstrated that HIF-1α and HIF-2α were increased by 1.6- and 1.8-fold, respectively, in Phd2f/f/GFAPCre mice compared with controls (Fig. 1C). However, HIF-1α bands remained relatively weak even after a 1.6-fold upregulation owing to a very low basal level. Interestingly, astrocytic HIF-2α but not HIF-1α was previously found to be crucial for retinal astrocytic and vascular development in neonatal mice (Duan et al., 2014).
Fig. 1.
PHD2 deficiency promotes HIF-α accumulation and VEGF-A expression in retinal astrocytes. (A) Quantitative RT-PCR (qPCR) analysis for PHD2, VEGF-A and EPO. Total RNA used for qPCR was isolated from primary retinal astrocytes cultured from neonatal mice (P3). All data points were normalized against β-actin values. (B) ELISA for VEGF-A and EPO. Conditioned media from retinal astrocyte cultures were collected and assayed with VEGF and EPO ELISA kits. (C) Western blotting of nuclear extracts isolated from cultured retinal astrocytes. For quantification, PHD2, HIF-1α and HIF-2α signals were normalized to that of α-actin. n=5 mice/group. *P<0.05; **P<0.01; ***P<0.001.
Astrocytic PHD2 deficiency causes retinal astrocytes to linger in less mature states
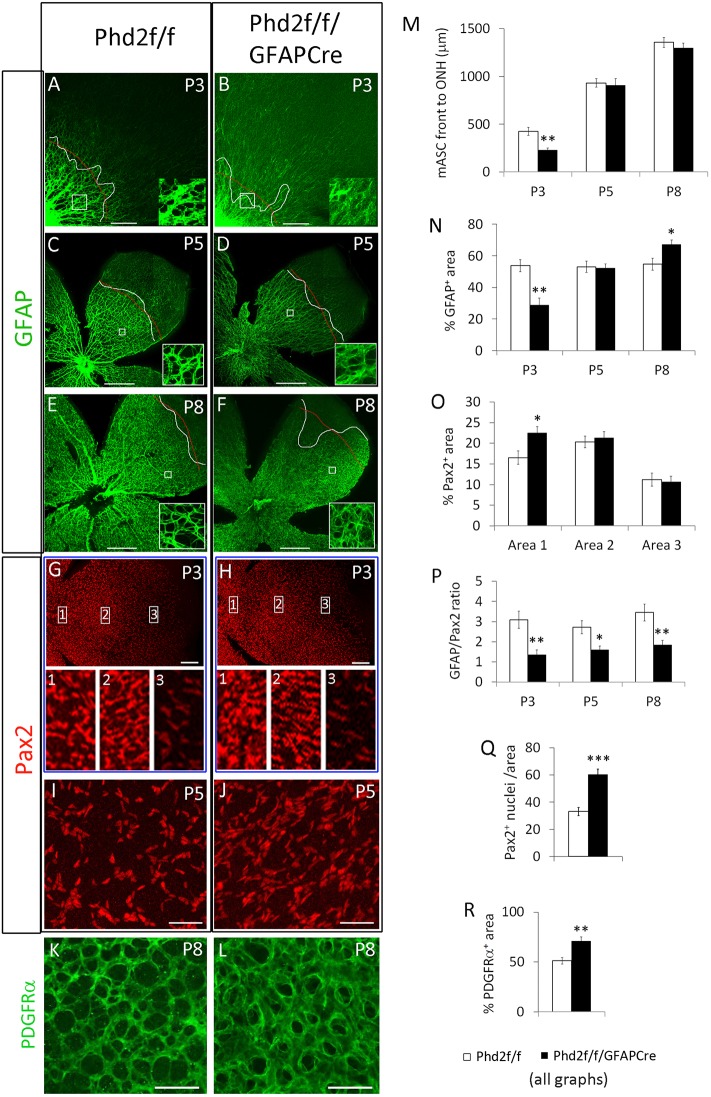
Retinal astrocytic differentiation was examined by whole-mount anti-GFAP and anti-Pax2 immunofluorescence (IF) staining, followed by laser confocal imaging of flat-mount retinas (Fig. 2A-J). In normal retinal development, a boundary is typically discernable between the strongly GFAP+ region on the central side and weakly GFAP+ region on the peripheral side. This boundary represents the front edge of the mature astrocyte cell population, and is referred to as the mASC front (Duan et al., 2017). At P3, the mASC front was less advanced towards the retinal periphery in Phd2f/f/GFAPCre mice than in floxed controls (Fig. 2A,B,M). At P5 and P8, however, the mASC front was at similar positions in floxed and Phd2f/f/GFAPCre mice (Fig. 2C-F,M).
Fig. 2.
Astrocytic PHD2 deficiency suppresses astrocyte maturation in Phd2f/f/GFAPCre mice. (A-F) Flat-mount confocal images of anti-GFAP-stained mouse retinas at P3, P5 and P8. Curved white lines indicate mASC front. Thin red lines indicate approximate average positions of the mASC front. Insets show magnifications of the boxed areas. (G-J) Anti-Pax2-stained retinas. Insets 1-3 show magnifications of the boxed areas in G and H. Images in I and J are located midway between the ONH and retinal periphery. (K,L) P8 retinas stained with anti-PDGFRα. Images are from midway between ONH and retinal periphery. (M-R) Quantification of astrocyte development. (M) Distance from ONH to the mASC front. (N) % GFAP+ area, defined as percentage of an area (white boxes in A-F) occupied by GFAP+ pixels. (O) % Pax2+ area in areas 1-3, as indicated in G and H. (P) GFAP+/Pax2+ ratios. Quantification was carried out in areas similar to those indicated by white boxes in A-F. (Q) Number of Pax2+ nuclei per 0.02 mm2 area in P5 retinas. Quantification was done approximately midway between ONH and retinal periphery. (R) % PDGFRα+ area in K and L. n=6 mice/group. *P<0.05; **P<0.01. Scale bars: 200 µm (A,B); 500 µm (C-F); 200 µm (G,H); 50 µm (I-L).
Behind the mASC front, strongly GFAP+ structures organized into hierarchical patterns in floxed mice but appeared as a random collection of branches in Phd2f/f/GFAPCre mice. The edges of GFAP+ structures were typically fuzzy in Phd2f/f/GFAPCre mice, appearing as though they were out of focus (Fig. 2A-F, insets). In addition, large GFAP+ structures in Phd2f/f/GFAPCre mice often appeared dimmer than in their floxed controls. These features partially resembled those in the peripheral side of the mASC front in floxed mice, implying that the retinal astrocytes might be less mature in Phd2f/f/GFAPCre mice.
As a first step towards analyzing retinal astrocyte differentiation in Phd2f/f/GFAPCre mice, we quantified the GFAP+ signals as a percentage of GFAP+ pixels per area (% GFAP+ area) (Fig. 2N). Areas selected for quantification were approximately halfway between the ONH and the mASC front in Phd2f/f mice, or at a comparable distance to the ONH in Phd2f/f/GFAPCre mice (Fig. 2A,C,E). As summarized in Fig. 2N, Phd2f/f/GFAPCre mice had lower % GFAP+ area at P3, but caught up with Phd2f/f mice by P5, and eventually surpassed Phd2f/f mice by P8. Of note, % GFAP+ area values should be interpreted with caution, because they are influenced not only by the differentiation status of astrocytes but also their population density. For example, a very crowded population of weakly GFAP+ immature astrocytes could potentially lead to high % GFAP+ area values because of more numerous cells in the region. Thus, proper assessment of retinal astrocyte differentiation would require normalization of GFAP+ signals to astrocyte abundance.
Pax2 is an appropriate marker for normalization because it is expressed in all retinal astrocytes, with expression gradually declining as differentiation progresses to more mature states. However, the Pax2 expression pattern itself is also informative of astrocyte distribution in different retinal regions. Therefore, we first characterized the retinal distribution of Pax2+ staining signals in floxed and Phd2f/f/GFAPCre mice. At P3, Pax2 expression patterns appeared grossly similar between floxed and Phd2f/f/GFAPCre mice (Fig. 2G,H). However, closer inspection indicated that in Phd2f/f/GFAPCre mice, Pax2+ cells were more densely packed near the ONH (Fig. 2G,H). This observation was confirmed by quantification of % Pax2+ area (Fig. 2O).
Next, we quantified retinal astrocyte differentiation by normalizing % GFAP+ area to % Pax2+ area in double-stained retinas. A higher GFAP/Pax2 ratio indicates more advanced astrocyte differentiation, whereas a reduced ratio suggests less mature status (Duan et al., 2017). Phd2f/f/GFAPCre mice had reduced GFAP/Pax2 ratios at all three stages examined (P3, P5 and P8) (Fig. 2P). Thus, retinal astrocytes in Phd2f/f/GFAPCre mice were at less mature states than in floxed mice. Also, although Phd2f/f/GFAPCre mice displayed modestly elevated % GFAP+ area values at P8, this feature by itself did not indicate more advanced astrocyte differentiation. Instead, because the GFAP/Pax2 ratio was lower, we can conclude that retinal astrocytes were also less mature in P8 Phd2f/f/GFAPCre mice. The range of GFAP/Pax2 ratio in Phd2f/f/GFAPCre is between 1 and 2. According to the criteria defined in a previous study (Duan et al., 2017), these values indicate intermediate differentiation status. Overall, these data demonstrate that maturation of retinal astrocytes is hindered in Phd2f/f/GFAPCre mice.
As immature astrocytes are proliferative whereas mature astrocytes are quiescent (Nahirnyj et al., 2013), the less mature status of retinal astrocytes in Phd2f/f/GFAPCre mice might lead to an expanded astrocyte population. In support of this notion, the number of Pax2+ nuclei was indeed increased in PHD2-deficient mice (Fig. 2Q). Retinal astrocyte abundance may be also quantified as % PDGFRα+ area in anti-PDGFRα-stained retinas. Because PDGFRα is expressed in immature as well as mature astrocytes (Fruttiger, 2002; Mudhar et al., 1993), % PDGFRα+ area values are inclusive of astrocytes at different stages of differentiation. As shown in Fig. 2K,L,R, % PDGFRα+ area values were higher in Phd2f/f/GFAPCre mice, demonstrating that retinal astrocyte abundance was increased.
Astrocytic PHD2 deficiency leads to defective APC migration in a small percentage of mice
The phenotypes presented above were observed in 91% of Phd2f/f/GFAPCre mice, and are referred to as Phenotype A. The remaining 9% of Phd2f/f/GFAPCre mice displayed Phenotype B, in which Pax2+ cells failed to migrate towards the retinal periphery (Fig. S2). Also, astrocyte differentiation was mostly limited to the immediate vicinity of ONH, whereas the majority of Pax2+ cells beyond this region remained GFAP− as late as P5, indicating that they remained at the APC stage (Fig. S2F, area 1). In normal development, APCs are abundantly present only in newborn mice, but quickly become IACs or mASCs by P1, depending on their relative location with the developing vasculature (Duan et al., 2017). Although failed APC migration was unique to Phenotype B, persistence of APCs was probably a more severe form of differentiation defects seen in Phenotype A.
Because mice used for these studies were in a mixed strain background, we wondered whether backcrossing to C57BL/6 would result in improved consistency. In mice with 97% C57BL/6 background, Phenotype B was not observed. Perplexingly, Phenotype B also disappeared in mice with 95% CD1 background. Although these outcomes did not allow us to identify the exact strain background responsible for Phenotype B, they nevertheless indicated that the existence of two different phenotypes was related to a peculiar mix of strain background. Given that Phenotype A was relatively insensitive to strain background (i.e. the penetrance was at least 91% in outbred background), we mostly focused on Phenotype A, while also performing some preliminary characterization of Phenotype B.
Astrocytic PHD2 deficiency leads to vascular overcrowding
In Phd2f/f/GFAPCre mice, a major vascular defect at P3 and P5 was the overcrowding of endothelial cells near the ONH, with the central retina being uniformly IB4+ (Fig. S3A-D). Further away from the ONH, individual blood vessels were discernable but failed to organize into a hierarchical pattern. Retinal blood vessels mostly had similar sizes instead of remodeling into large vascular trunks and smaller branches. Vascular density was apparently higher than in floxed controls, except in the area just behind the vascular front. Consistent with these qualitative observations, % IB4+ area in the central retina was significantly higher in Phd2f/f/GFAPCre mice (Fig. S3G). However, the narrow area immediately behind the vascular front appeared similar to floxed mice.
In mice displaying Phenotype B, ECs clumped together near the ONH but failed to migrate towards the retinal periphery (Fig. S3E,F). Because EC migration during retinal vascular development is normally dependent on immature astrocytes (IACs) ahead of the vascular front, failed EC migration in Phenotype B appeared to be a secondary defect to the lack of IACs.
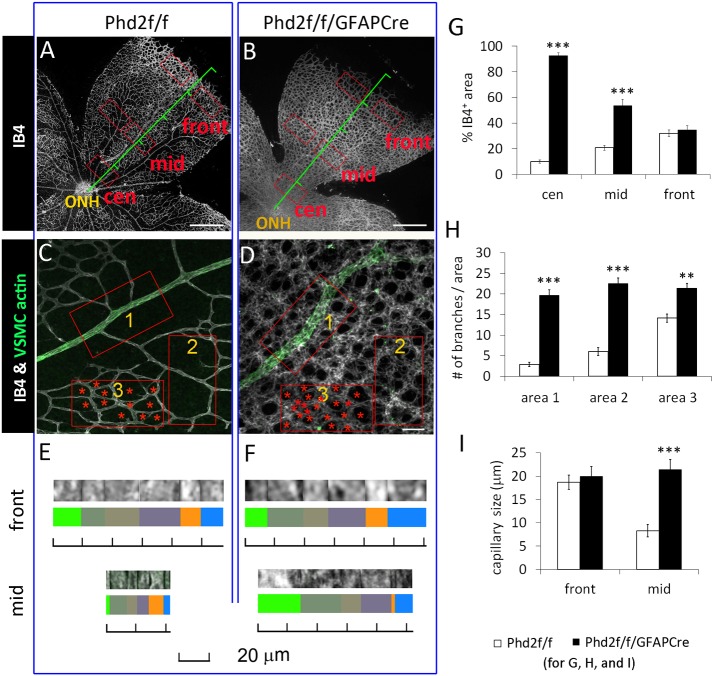
For more detailed analysis of the vascular defects, we focused on Phenotype A. At P8, the retinal vasculature had undergone extensive developmental remodeling in floxed mice but not in Phd2f/f/GFAPCre mice (Fig. 3A,B). Similar to earlier stages (P3 and P5), Phd2f/f/GFAPCre mice had higher % IB4+ area values in most retinal areas, except just behind the vascular front (Fig. 3A,B,G). In addition to % IB4+ area, we also quantified the number of vascular branches in mid-retinal areas (i.e. halfway between the ONH and retinal periphery). Within this general area, three different sub-areas were quantified separately. At all three locations, Phd2f/f/GFAPCre mice had more numerous vascular branches than did floxed mice, but this was most pronounced near arterioles (Fig. 3C,D,H).
Fig. 3.
Increased retinal vascular density and capillary diameters in Phd2f/f/GFAPCre mice. (A,B) Laser confocal images of IB4-stained, flat-mount P8 retinas. Red rectangles labeled ‘cen’, ‘mid’ and ‘front’ represent central, mid-retinal, and vascular front areas, respectively. (C,D) Mid-retinal images from retinas double stained with IB4 and anti-actin α-smooth muscle-FITC. Rectangles 1, 2 and 3 indicate different positions relative to arterioles. Red asterisks in area 3 mark non-vascular tissues. (E,F) Determination of capillary sizes. For each measurement, six random capillary segments were aligned as shown, and the total distance from the left to right was divided by six to yield average of the set. (G) % IB4+ area at locations marked by red rectangles in A and B; where two rectangles are marked side by side, the average of each set of rectangles was used as one data point. (H) Vascular density (number of vascular branches per 0.02 mm2) in the areas1-3, as indicated in C and D. (I) Capillary sizes at two different retinal locations, near vascular front and mid-retinal area. n=7 mice/group. **P<0.01; ***P<0.001. Scale bars: 500 µm (A,B); 50 µm (C,D).
Microvascular diameter was measured at two retinal locations: near the vascular front and in the mid-retinal area. To minimize the inherent inaccuracy of measuring small capillary sizes, we first measured the sum of diameters for each set of six capillaries as shown in Fig. 3E,F. All six capillaries were from the same specified retinal area, and the average of each set from the same mouse was used as one data point for statistical analysis. The results indicated that in mid-retinal areas, capillaries in Phd2f/f/GFAPCre mice were larger than those in the floxed group (Fig. 3I). Just behind the vascular front, the two groups had similarly large capillaries, which was not surprising because in normal mice the capillaries near the vascular front are immature and relatively large.
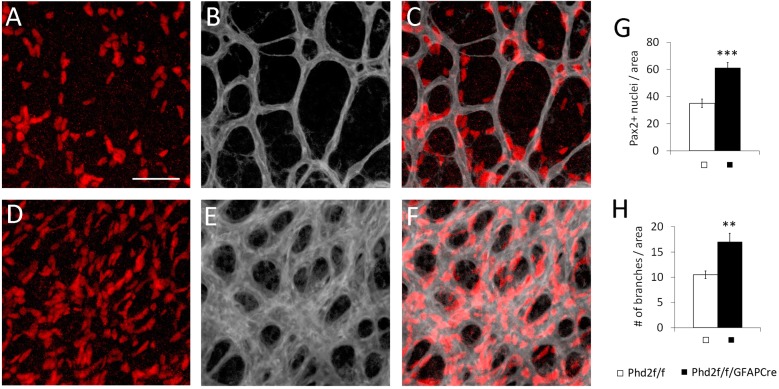
Because astrocyte abundance and vascular density were both increased in Phd2f/f/GFAPCre mice (Phenotype A), we wondered whether the two phenotypes were related to each other. Therefore, we directly compared astrocyte abundance and capillary density in P5 retinas double stained with IB4 and anti-Pax2. In Phd2f/f/GFAPCre mice, higher capillary density matched the increased number of Pax2+ cells at the same retinal location (Fig. 4). Because Phd2 disruption was achieved with astrocyte-specific Cre, we propose that astrocytic phenotypes caused the vascular phenotypes.
Fig. 4.
Simultaneously increased retinal astrocyte abundance and capillary density in Phd2f/f/GFAPCre mice. (A-F) Whole-mount P5 retinas were double stained with IB4-Alexa Fluor 647 (gray) and rabbit anti-Pax2/donkey anti-rabbit IgG-Cy3 (red). Stained retinas were flat-mounted, and confocal images were taken midway between the ONH and vascular front. (G) Astrocyte abundance, measured as number of Pax2+ nuclei per 0.02 mm2 area. (H) Number of vascular branches per 0.02 mm2 area. n=5 mice/group. **P<0.01; ***P<0.001. Scale bar: 50 µm.
Astrocytic PHD2 deficiency promotes EC proliferation but suppresses tip cell filopodia formation
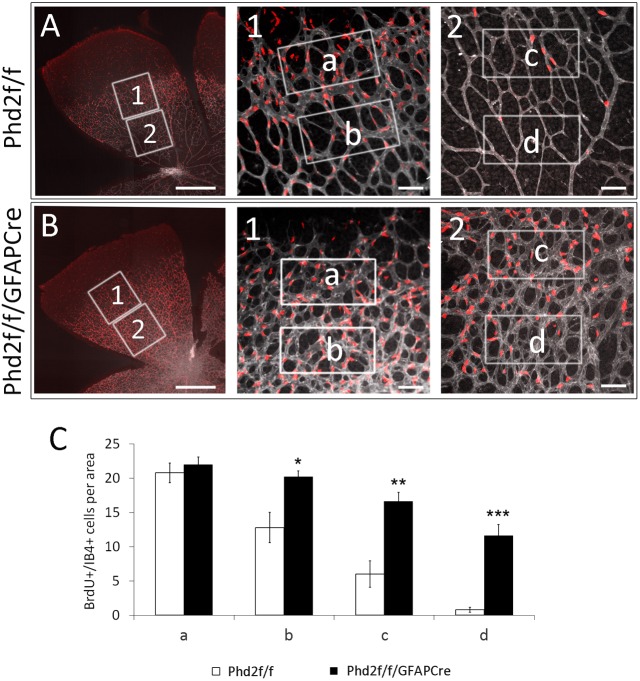
We examined EC proliferation by measuring 5′-bromo-2′-deoxyuridine (BrdU) incorporation (Fig. 5). Quantification of IB4+/BrdU+ cells was carried out at four retinal locations, indicated by rectangles a, b, c and d (Fig. 5A,B). Just behind the vascular front (a), IB4+/BrdU+ cells were abundant in both Phd2f/f and Phd2f/f/GFAPCre mice. Further behind (b, c and d), IB4+/BrdU+ cells were significantly more abundant in Phd2f/f/GFAPCre mice (Fig. 5A-C). These observations indicate that astrocytic PHD2 deficiency led to persistent EC proliferation in areas where they were normally quiescent, thus contributing to increased capillary caliber.
Fig. 5.
Astrocytic PHD2 deficiency sustains EC proliferation. (A,B) Neonatal mice were injected with BrdU at P6, and retinas were dissected an hour later. Retinas were analyzed by IB4-Alexa 647 (gray) and anti-BrdU (red) double staining. Numbered boxes are expanded and shown to the right of the main images. Proliferative ECs were identified as IB4+/BrdU+ cells. White rectangles are at different distances from the vascular front, with ‘a’ near the vascular front and ‘d’ being furthest behind. (C) Number of IB4+/BrdU+ cells per 0.02 mm2 in rectangular areas a-d. n=5 mice/group. *P<0.05; **P<0.01; ***P<0.001. Scale bars: 500 µm (A,B, main panels); 50 µm (A,B, panels 1 and 2).
To monitor sprouting angiogenesis directly, we quantified the number of vascular tip cells (Fig. 6). Phd2f/f/GFAPCre mice had normal numbers of tip cells (Fig. 6A,B,E), but there were fewer filopodia per tip cell (Fig. 6C-E). Nonetheless, microvascular density immediately behind the tip cells was similar in Phd2f/f and Phd2f/f/GFAPCre mice. Because pruning and other remodeling events in this nascent vascular area is negligible, vascular abundance just behind the tip cells mostly reflect the activity of angiogenesis. Thus, our data suggest that sprouting angiogenesis was essentially normal in Phd2f/f/GFAPCre mice displaying Phenotype A.
Fig. 6.
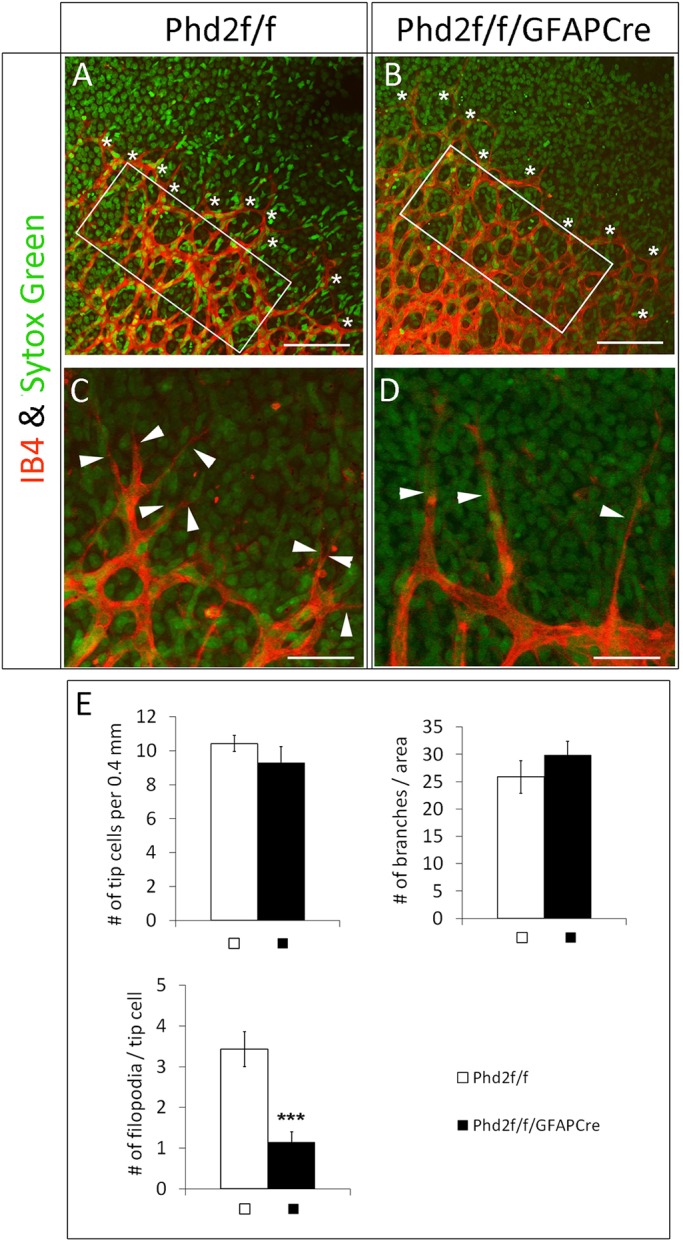
Astrocytic PHD2 deficiency hinders tip cell filopodia development. (A,B) Vascular front from P5 retinas double stained with IB4-Alexa 594 and SYTOX Green, with tip cells indicated by white asterisks. (C,D) High-magnification images showing filopodia (white arrowheads). (E) Number of vascular branches per 0.02 mm2 in white rectangles in A and B, number of tip cells per 0.4 mm length of vascular front, and filopodia per tip cell. n=7 mice/group. ***P<0.001. Scale bars: 100 µm (A,B); 40 µm (C,D).
Astrocytic PHD2 deficiency suppresses EC apoptosis and vascular regression
EC apoptosis was examined by double staining of whole-mount retinas with IB4-Alexa 594 and anti-caspase 3 (directed to the active fragment, Cas3) (Fig. 7). To validate Cas3 whole-mount IF staining, we tested the antibody in positive controls generated by overnight exposure of P7 mice to 75% O2. This pilot test demonstrated the presence of numerous IB4+/Cas3+ in the retinas (Fig. 7A,D,G,J). The effect of astrocytic PHD2 deficiency on EC apoptosis was assessed in P6 mice maintained under normoxia at all times. In the Phd2f/f group, seven IB4+/Cas3+ apoptotic ECs were detected per 1.0 mm2 retinal area, but such cells were virtually undetectable in the Phd2f/f/GFAPCre group (<1 per mm2) (Fig. 7B,C,E,F,H,I,K-M). These findings indicate that astrocytic PHD2 deficiency blocked retinal EC apoptosis.
Fig. 7.
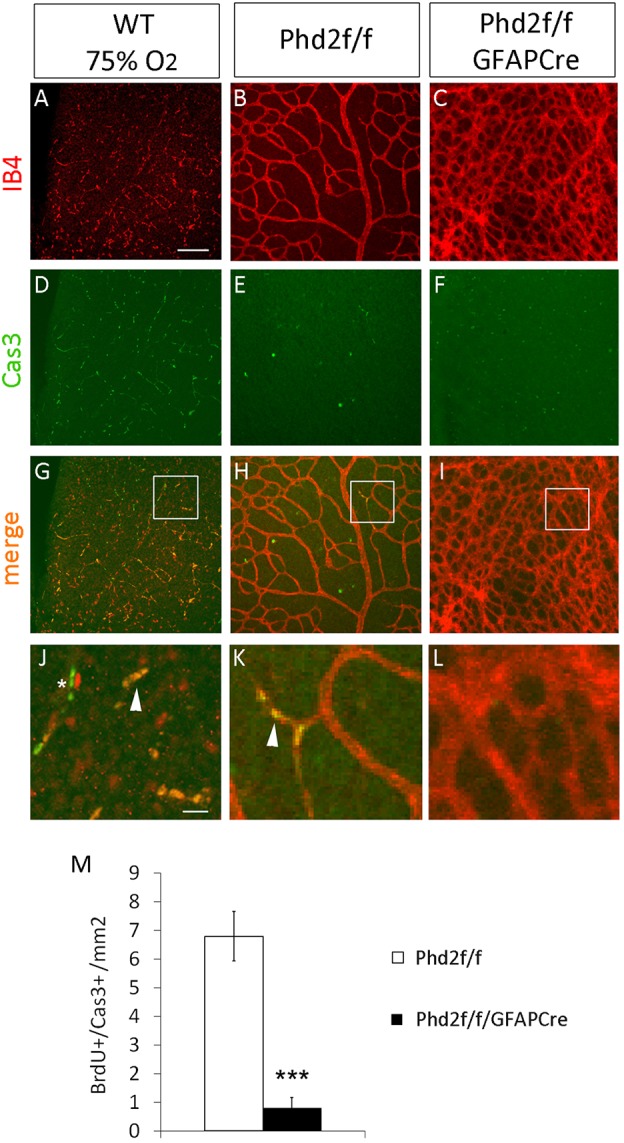
Astrocytic PHD2 deficiency blocks EC apoptosis. (A-L) IB4 staining (A-C), anti-caspase 3 (Cas3) staining (D-F) and merged images (G-I), with magnifications of the boxed areas shown below (J-L), of A and D, B and E, and C and F, respectively. Arrowheads in J and K indicate apoptotic ECs (IB4+/Cas3+). Asterisk in J indicates Cas3+ cells. The numerous IB4+/Cas3+ cells in G and J validate the staining protocol. Retinas from Phd2f/f and Phd2f/f/GFAPCre mice were dissected at P6, without prior exposure to hyperoxia. (M) Number of apoptotic ECs per 1 mm2 retinal area. n=5 mice/group. ***P<0.001. Scale bars: 100 µm (A-I); 20 µm (J-L).
In an alternative method of monitoring microvascular regression, we visualized remnant microvascular fragments by double staining with IB4-Alexa 594 and anti-collagen IV (Fig. 8A-F). Capillary regression transiently leaves behind remnants of their original vascular components, such as collagen in the basement membrane, fragments of EC cells (IB4+), or a mixture of both. The exact content and relative proportions of these remnant structures may depend on which component degrades faster in a particular microvascular environment. To simplify the analysis, we grouped regressing microvessels into three categories: (1) collagen IV+/IB4−; (2) collagen IV+/IB4+; and (3) collagen IV−/IB4+. Examples for each are illustrated in Fig. 8G,H. In both floxed and PHD2-deficient mice, regressing microvessels were infrequent near the vascular front (Fig. 8C,D). Further behind, all three categories were abundantly present in floxed mice, but rare in Phd2f/f/GFAPCre mice (Fig. 8E,F,I). Taken together, our data demonstrate that astrocytic PHD2 deficiency blocked developmental vascular regression.
Fig. 8.
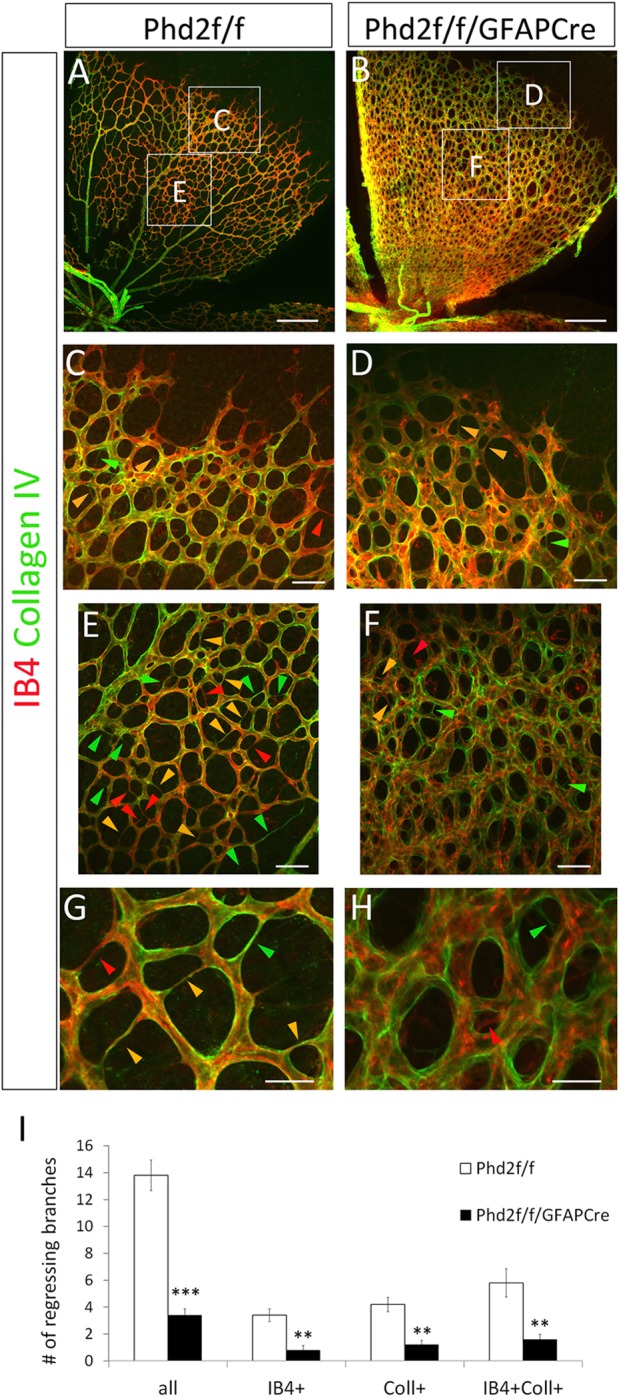
Astrocytic PHD2 deficiency blocks developmental vascular regression in neonatal retinas. (A,B) Flat-mount confocal images of P6 retinas double stained with IB4 and anti-collagen IV. Lettered rectangles are shown at higher magnifications in C-F. Regressing microvessels are seen as very thin threads lacking cell bodies, and fall into three categories: collagen IV+/IB4− (green arrowheads); collagen IV+/IB4+ (yellow arrowheads); and collagen IV−/IB4+ (red arrowheads). (G,H) High-magnification images showing details of different types of regressing microvessels. (I) Number of regressing vessels per 0.1 mm2. n=5 mice/group. **P<0.01; ***P<0.001. Scale bars: 250 µm (A,B); 50 µm (C-F); 25 µm (G,H).
Increased retinal astrocyte population density, but not VEGF-A or T-cell abnormality, blocks vascular pruning
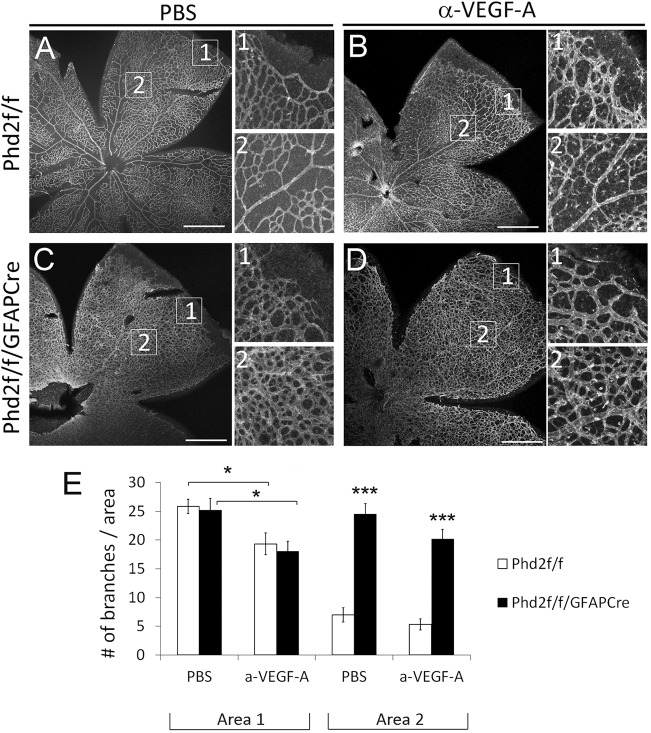
In exploring the potential mechanisms underlying blocked retinal vascular pruning in Phd2f/f/GFAPCre mice, we first considered whether increased VEGF-A expression might be responsible for the blockade of capillary regression. To examine this possibility, we chose to perform intravitreal injection of anti-VEGF. Because overdosage of anti-VEGF might block sprouting angiogenesis altogether, which would preclude the analysis of vascular pruning, pilot experiments were performed to optimize anti-VEGF dosage. Subsequently, we injected P4 Phd2f/f/GFAPCre mice with 1.8 µg of anti-VEGF per eye, or PBS in the contralateral eye. When examined at P8, retinas from anti-VEGF-injected eyes displayed partially inhibited sprouting angiogenesis, confirming that VEGF-A was effectively neutralized (Fig. 9). However, vascular pruning remained blocked. These data indicate that although astrocytic VEGF-A expression was elevated in Phd2f/f/GFAPCre mice, it may not be responsible for blocked vascular pruning.
Fig. 9.
Anti-VEGF does not rescue vascular pruning in Phd2f/f/GFAPCre mice. (A-D) IB4-stained P8 retinas from Phd2f/f and Phd2f/f/GFAPCre mice pre-treated with intravitreal injection of anti-VEGF or PBS at P4. Images numbered 1 or 2 are expanded from corresponding boxed areas in the main panels. (E) Quantification of vascular branches per 0.02 mm2 area. Vascular density in area 1 mostly reflects sprouting angiogenesis, because vascular pruning is negligible near the vascular front. At 1.8 µg per eye, anti-VEGF (a-VEGF-A) moderately suppressed angiogenesis. Values in area 2 reflect the sum of the initial angiogenesis and subsequent pruning. n=7 mice/group. *P<0.05; ***P<0.001. Scale bars: 500 µm.
Next, we investigated whether failed vascular pruning in Phd2f/f/GFAPCre mice was related to any T-cell abnormality. This possibility was considered because of an earlier report suggesting that apoptotic signals from T cells were essential for EC apoptosis and retinal vascular pruning during development (Ishida et al., 2003). Because there is no known connection between oxygen sensing and T-cell function, we first sought to confirm that T cells were indeed necessary for retinal vascular pruning. Therefore, we examined retinal vascular development in SCID (severe combined immunodeficiency) mice. Unexpectedly, retinal vascular patterning was normal in neonatal SCID mice (Fig. S4). Therefore, at least in our hands, T cells did not appear to be important for retinal vascular pruning. Given this outcome, no further studies were carried out on T cells.
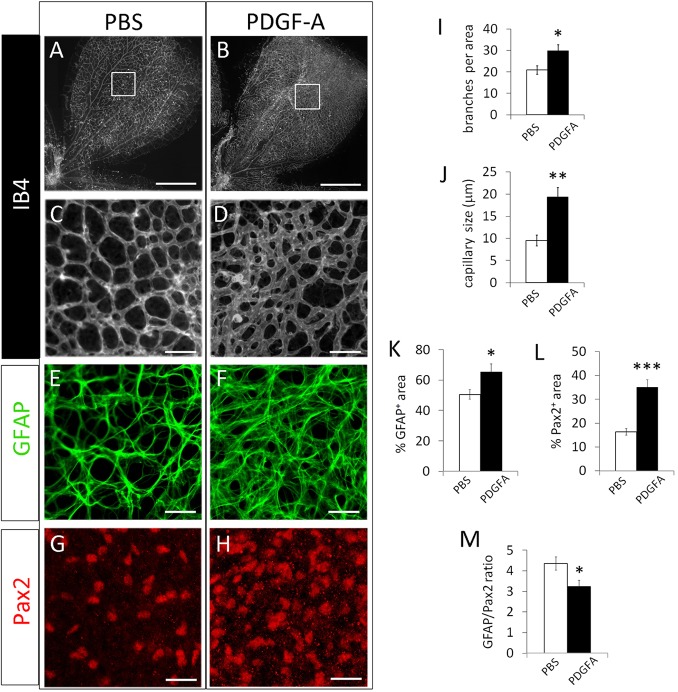
Finally, we turned our attention to the intriguing possibility of excessive astrocytic growth as a cause of failed vascular pruning. If astrocyte overgrowth was the underlying mechanism, then it should be possible to block vascular pruning in wild-type mice by stimulating sustained astrocyte growth without directly manipulating the oxygen-sensing system. Because PDGF-A is known to promote the proliferation and migration of immature astrocytes (Nakamura-Ishizu et al., 2012), we performed intravitreal injection of PDGF-A in Phd2f/f mice. Injection was performed at P4, and retinas were examined at P8. For confocal imaging analysis, we focused on the peripheral half of the retina because that was where vascular development occurred after injection (Fig. 10).
Fig. 10.
Intravitreal injection of PDGF-A promotes astrocytic growth and blocks developmental vascular pruning. (A-D) Phd2f/f mice were subjected to intravitreal injection of PDGF-A or PBS at P4. Retinas were dissected at P8, and stained with IB4. Boxed areas in A and B are expanded in C and D. (E-H) Anti-GFAP and anti-Pax2 double-stained retinal images from PDGF-A- or PBS-injected mice. Images were from similar retinal locations as the boxed areas in A and B. (I) Number of vascular branches per 0.02 mm2. (J) Capillary size, determined by the same method described for Fig. 2. (K) % GFAP+ area. (L) % Pax2+ area. (M) Ratio between % GFAP+ area and % Pax2+ area. n=5 mice/group. *P<0.05; **P<0.01; ***P<0.001. Scale bars: 500 µm (A,B); 50 µm (C,D); 25 µm (E-H).
PDGF-A effectively blocked retinal vascular pruning in Phd2f/f mice (Fig. 10A-D,I). Microvascular caliber was also larger in PDGF-A-injected mice (Fig. 10C,D,J). Given that PDGF-A is not known to directly promote retinal EC growth or survival, we suspected that its effects on the retinal vasculature might be due to increased astrocyte growth. Indeed, retinas from PDGF-A-injected eyes exhibited increased astrocyte abundance (Fig. 10E-H,K,L). More importantly, the GFAP/Pax2 ratio was reduced, suggesting that PDGF-A maintained the astrocytes in less mature states thus permitting their continued growth (Fig. 10M).
DISCUSSION
Astrocytes, oxygen, and vascular development
The earliest hint that retinal astrocytes had a role in vascular development came from the observation that the avascular retinas in possums were also free of astrocytes (Stone and Dreher, 1987). Subsequent studies indicated that hypoxia in retinal astrocytes may contribute to their proangiogenic functions. For example, Nr2e1 (nuclear receptor subfamily 2 group E member 1, a homolog of the Drosophila tailless gene Tll) is much more strongly expressed in immature retinal astrocytes in the hypoxic avascular region than in the mature astrocytes in vascularized retinal tissues. Knockout of Nr2e1/Tlx almost completely blocked retinal angiogenesis, implying that its proangiogenic role could be also hypoxia-related, in addition to its role in regulating fibronectin deposition (Miyawaki et al., 2004; Uemura et al., 2006). A more direct relationship between hypoxia in retinal astrocytes and angiogenesis was demonstrated in mice lacking astrocytic HIF-2α, where the astrocytic network was poorly developed and retinal angiogenesis significantly suppressed (Duan et al., 2014).
Oxygen is known to suppress retinal vascular development or cause existing microvessels to regress. For example, exposure of newborn mice to 75% oxygen completely blocked retinal vascular development (West et al., 2005). In the mouse model of oxygen-induced retinopathy, exposure of neonatal mice to hyperoxia between P7 and P12 leads to massive obliteration of microvessels in the central retina, but the relevance of this disease model to developmental vascular pruning is uncertain (Connor et al., 2009; Smith et al., 1994). A potential role of oxygen in developmental vascular pruning is implied by the presence of avascular zones near arterioles, but its mechanism of action is unclear. Phd2f/f/GFAPCre mice generated in the present study provided a valuable tool for investigating developmental vascular pruning, and allowed us to identify retinal astrocytes as the major targets of oxygen to regulate vascular pruning.
Mechanisms of retinal vascular pruning
Because hypoxia upregulates VEGF-A expression, one might speculate that astrocytic PHD2 deficiency, which molecularly mimics hypoxia, blocks retinal vascular pruning by upregulating VEGF-A expression in retinal astrocytes. Although VEGF-A was indeed upregulated, anti-VEGF failed to rescue vascular pruning. Furthermore, it has been shown previously that astrocyte-specific VEGF-A disruption led to very minimal changes in retinal vascular patterning (Scott et al., 2010). Therefore, VEGF-A regulation does not appear to be directly involved in vascular pruning.
Our data suggest that the oxygen-sensing mechanism controls vascular pruning by regulating astrocyte abundance. Oxygen from the nascent vascular plexus may activate PHD2 in nearby astrocytes, leading to the downregulation of astrocytic HIF-2α protein levels. Note that astrocytes in newly vascularized areas are relatively immature when they are first exposed to blood vessels, but HIF-2α downregulation in response to rising oxygenation levels may cause them to quickly differentiate into mature astrocytes. This view is consistent with our earlier finding that astrocytic HIF-2α deficiency greatly accelerated retinal astrocyte differentiation towards the mature state (Duan et al., 2014). Data presented in this paper complement our previous finding in astrocyte-specific HIF-2α-deficient mice and demonstrate that astrocytic PHD2 deficiency hinders retinal astrocyte maturation, likely through HIF-2α upregulation. Because astrocytes are more proliferative in less mature states, retinal astrocyte growth likely continued for longer time periods in Phd2f/f/GFAPCre mice, thus leading to significantly expanded astrocyte populations.
Because blood vessels in the primary retinal vasculature are generally supported by adjacent astrocytes, we propose that the physiological limit of vascular density is set by local astrocyte abundance. In other words, only those blood vessels with adequate astrocytic support may survive. Capillaries in excess of available astrocytes are destined to regress (Fig. 11). We confirmed the pivotal role of retinal astrocyte abundance in determining capillary density by an independent approach. Intravitreal delivery of PDGF-A, which stimulates astrocyte growth through its receptor signaling but is not known to modulate the oxygen-sensing mechanism, not only increased astrocyte abundance but also blocked vascular pruning.
Fig. 11.
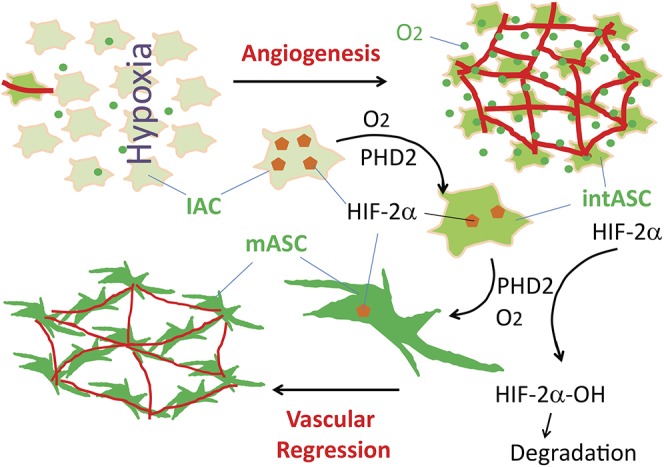
Regulation of developmental vascular pruning (regression) by the astrocytic oxygen-sensing mechanism. Peripheral to the vascularized area, retinal tissues are low in oxygen (fewer green dots), and astrocytes are immature (IACs). Induction of retinal angiogenesis (red lines) by immature astrocytes leads to progressively increasing oxygenation (more green dots). In parallel, astrocytic HIF-2α (brown pentagons, shown only in larger-sized drawings) levels gradually decline as a result of oxygen- and PHD2-catalyzed prolyl hydroxylation and subsequent polyubiquitylation and proteasomal degradation. Declining HIF-2α drives astrocyte differentiation, presumably through intermediately differentiated stages (intASC) before finally becoming fully differentiated mature astrocytes (mASCs). Because mASCs are incapable of further proliferation, the expansion of the astrocytic population comes to a halt once differentiation is complete. With astrocyte abundance being limited by oxygenation levels, many retinal capillaries may not find astrocytic support, and therefore undergo regression.
Dependence of APC migration on the oxygen-sensing mechanism
Besides regulating astrocyte differentiation and vascular pruning, astrocytic PHD2 also seems to participate in sensing the oxygen gradient. During normal development of the primary retinal vasculature, an oxygen gradient may exist across the retina, with the vascularized region being well oxygenated and the more peripheral avascular region being hypoxic (Gerhardt et al., 2003). As PHD2 uses O2 as a substrate, it follows that its hydroxylase activity in astrocytes may be dictated by local oxygen concentration, decreasing in the direction of the ONH towards the retinal periphery. In Phd2f/f/GFAPCre mice, astrocytes and their precursors are desensitized to oxygen owing to PHD2 deficiency. Therefore, the accumulation of APCs near the ONH in Phenotype B mice suggests that their migration depends on PHD2-mediated oxygen sensing. It is unclear why the migration defect is present only in a low percentage of Phd2f/f/GFAPCre mice, but future studies should examine whether double or triple knockout of different PHD isoforms might increase the penetrance of this phenotype.
Conclusions
In summary, developmental vascular pruning may be viewed as a mutual feedback mechanism between the nascent microvascular plexus and the associated astrocytes. Rising oxygen levels accompanying retinal angiogenesis may limit the expansion of the astrocytic population by driving maturation differentiation, thus forcing excess microvessels without astrocytic support to undergo regression. Given that astrocyte abundance is controlled by vascularization via astrocytic oxygen-sensing mechanism, it can be said that vascular pruning is ultimately auto-regulated by the vascularization process itself.
MATERIALS AND METHODS
Mice
All animals were handled according to procedures approved by the Institutional Animal Care and Use Committee (IACUC) at the UConn Health in compliance with Animal Welfare Assurance. Floxed Phd2 mice were originally generated in our lab, using C57BL/6/129 S6 hybrid embryonic stem cells targeted at the Phd2 locus (Takeda et al., 2006). GFAPCre transgenic mice were purchased from the Jackson laboratory (stock number 004600), and were in FVB strain background upon arrival. Phd2f/f/GFAPCre mice used were in mixed background of CD1/C57BL/6/129 S6 /FVB, at estimated relative proportion of 0.13:0.56:0.06:0.25. SCID mice were purchased from the Jackson Laboratory (stock number 001303).
Genotyping
Ear punch tissues from weaned mice were used for DNA extraction and PCR. For neonatal mice (P8 or younger), toe clipping was performed to identify individual mice and provide tissues for genotyping. PCR primers are summarized in Table S1.
Primary cultures of neonatal retinal astrocytes
Retinas were isolated from neonatal mice at P3, and minced with fine dissection scissors. The retinal pieces were incubated in digestion medium for 45 min at 37°C, with gentle rocking and intermittent pipetting up and down to further break the pieces. Digestion medium was serum-free DMEM (pre-equilibrated in 5% CO2) supplemented with Collagenase Type I (1 mg/ml, Life Technologies) and DNase I (100 µg/ml, Life Technologies). After incubation, cell suspensions were mixed with equal volume of pre-warmed (37°C) 0.05% trypsin (Life Technologies), and incubated for another 2 min. Trypsin digestion was terminated by the addition of fetal bovine serum (FBS) to 10%. Cells were centrifuged at 1000 g for 5 min, re-suspended in DMEM containing 10% FBS, and filtered through 40 µm cell strainers (Thermo Fisher). Cells that passed through the filters were incubated with anti-CD31 (PECAM1)-coated magnetic micro-beads (Miltenyi Biotech) to deplete endothelial cells (1 h at 4°C with gentle rocking). After removing magnetic beads using a Dynal magnetic bar, cells were collected from the suspension by centrifugation at 1000 g for 5 min, and re-suspended in DMEM containing 10% FBS, 10 ng/ml PDGF-A (R&D Systems, now part of Thermo Fisher), 20 ng/ml γ-interferon (ProSpec), 2 mM L-glutamine, 2 mM sodium pyruvate, 1% non-essential amino acids, 100 mg/ml streptomycin and 100 U/ml penicillin (all from Life Technologies). Re-suspended cells were plated out on feeder cells (mitomycin-treated mouse embryonic fibroblasts). Cells were incubated in a hypoxia cell culture incubator (5% O2, 5% CO2, 37°C). Astrocytes remained viable for several weeks whereas other cell types gradually died out. The astrocyte identity of the surviving cells was validated by anti-GFAP and anti-Pax2 double immunofluorescence staining. The absence of ECs was confirmed by lack of CD31+ cells.
Quantitative RT-PCR and ELISA
Total RNA was purified from cultured retinal astrocytes using TriZol (Invitrogen, now Life Technologies) according to the manufacturer's instructions. Quantitative real-time RT-PCR (qPCR) analyses were performed as described previously (Duan et al., 2011), using primers listed in Table S2. β-Actin was used as the base for normalization.
For ELISA, conditioned media were collected from retinal astrocyte cultures, and used for VEGF-A and EPO ELISA kits, respectively (R&D Systems, now part of Thermo Fisher; catalog numbers MMV00 for VEGF kit, and MEP00B for EPO kit).
Western blotting
Nuclear protein extracts were prepared from primary retinal astrocyte cultures for western blotting. Cells were homogenized in ice-cold Buffer A, consisting of 10 mM HEPES-KOH (pH 7.9), 10 mM KCl, 1.5 mM MgCl2, 0.5 mM dithiothreitol, 0.1% NP-40, 1× complete protease inhibitor cocktail (Roche), 0.2 mM PMSF (phenylmethylsulfonyl fluoride) and 0.2 mM deferoxamine (Sigma-Aldrich). Nuclei were pelleted by centrifugation at 4500 g for 5 min, and re-suspended in ice-cold Buffer C, which contains 20 mM HEPES-KOH (pH 7.9), 1.5 mM MgCl2, 420 mM NaCl, 0.5 mM dithiothreitol, 0.2 mM deferoxamine, 1× protease inhibitor cocktail, 0.2 mM PMSF and 25% glycerol. Antibodies are described in Table S3.
Whole-mount staining of neonatal retinas and laser confocal microscopy
IF staining of neonatal retinas was performed using antibodies validated in previous publications (Duan et al., 2017, 2014). Neonatal mice (P8 or younger) were euthanized by decapitation, and whole eyes were isolated by enucleation. Eyes were fixed immediately in 4% paraformaldehyde (PFA) for 45 min at room temperature, and then retinas were isolated. Each retina received four radial incisions, leaving four petals attached to one another at the optic nerve head. To visualize blood vessels, retinas were stained as whole mounts with IB4-Alexa Fluor 594 or IB4-Alexa Fluor 647 in retina staining buffer (RSB), which is PBS containing 1 mM CaCl2, 1 mM MgCl2, 1% Triton X-100 and 1% bovine serum albumin. Staining was carried out overnight at 4°C, with gentle rocking. Retinas were washed three times in RSB before being flat-mounted to slides. Whole-mount IF staining was carried out similarly to IB4 staining. Vascular smooth muscle cells (VSMCs) were stained by anti-actin α-smooth muscle-FITC under the same conditions as IB4 staining. Detailed information for antibodies and IB4 are provided in Table S3.
Stained retinas were flat-mounted in mounting media (50% glycerol in PBS, 1 mM CaCl2 and 1 mM MgCl2). Laser confocal microscopy was carried out with a Zeiss LSM 880, and images were processed with ZEN software (Zeiss). Analysis of z-stack confocal images was carried out with Imaris software (Bitplane).
Identification of proliferative and apoptotic ECs and vascular tip cells
To label proliferating cells, neonatal mice (P6) were injected with BrdU intraperitoneally at 120 mg/kg (Roche Applied Science). Mice were euthanized an hour later, and whole eyes were isolated and fixed in 4% PFA. Fixed eyes were stored in 70% ethanol overnight. Retinas were isolated as described in the preceding section. Isolated retinas were incubated sequentially in PBS containing 1% Triton X-100 for 30 min at room temperature, then in 2 mM HCl for 1 h at 37°C, and finally in RSB for 2 h. BrdU incorporation into vascular structures was detected by whole-mount double staining with biotin-conjugated rat anti-BrdU and Streptavidin-DyLight-549 (Table S3). Stained retinas were flat-mounted for confocal microscopy.
Apoptotic ECs were identified as IB4+/Cas3+ cells in retinas double stained with IB4-Alexa Fluor-594 and anti-caspase 3 directed to the active fragment (Cell Signaling). Secondary antibody for anti-caspase 3 was goat anti-rabbit IgG-Alexa Fluor-488. As a positive control, neonatal mice (P7) were exposed to 75% oxygen for 16 h to cause extensive EC apoptosis. Oxygen-exposed neonates were euthanized, and eyes and retinas were dissected and fixed as described in earlier sections.
Tip cells were identified essentially according to Gerhardt et al. (2003) Briefly, whole-mount retinas (P5) were double stained with SYTOX green and IB4-Alexa Fluor-594, and 6 µm z-stack images were taken. Tip cells were identified as IB4+ positive cells at the leading edge of the vasculature containing SYTOX positive nuclei, with filopodia extending further into avascular areas.
Intravitreal injection of PDGF-A and anti-VEGF
Intravitreal injection was performed with a custom tip 33 gauge needle (Hamilton) attached to a 10 µl gastight syringe (Hamilton). PDGF-A injection was performed at P4 in anesthetized mice. Each mouse was injected with 1 µl of PDGF-A (100 ng/µl in PBS, R&D Systems-Thermo Fisher) in one eye, and 1 µl of PBS in the contralateral eye. Injected mice were euthanized at P8, and retinas were dissected, fixed and stained as described in preceding sections.
Anti-VEGF was injected at 1 µl per eye (1.8 µg/µl, Cedarlane), with the contralateral eye injected with PBS as control.
Quantification of vascular content
Vascular content was quantified as number of vascular branches per area (0.02 mm2) or percentage of an area occupied by IB4+ pixels (% IB4+ area), as indicated where appropriate. Because vascular density may vary substantially at different retinal locations, all quantifications refer to specified retinal positions. To minimize sampling errors, at least two samples were quantified per specified location in each mouse, and the average of the two or more equivalent areas in each mouse was used as one data point.
Where it was impractical to directly count vascular branches because of overcrowding, the number of non-vascular spaces surrounded by blood vessels was enumerated. Mathematically, the two sets of the numbers are identical as long as all vascular branches meet at their ends to form enclosed loops, which is mostly true in the retina. At the perimeters of each specified rectangular area, every two non-vascular spaces crossing the line were counted as one. All quantifications were assisted by NIH ImageJ software.
Quantification of astrocyte development
Astrocytes were quantified in specified areas as % GFAP+ area (percentage of an area occupied by GFAP+ pixels), % Pax2+ area, and the number of Pax2+ nuclei per 0.02 mm2 area. Astrocyte differentiation was quantified as the ratio between % GFAP+ area and % Pax2+ area. Astrocyte quantification was assisted by NIH ImageJ software.
Statistical analysis
Data were evaluated by two-tailed Student's t-tests using Excel, and are presented as mean±s.e.m. All n values refer to number of mice. P<0.05 was considered significant.
Supplementary Material
Acknowledgements
We thank the Imaging Core and Molecular Core at the UConn Heath Center for technical support.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: G.-H.F.; Methodology: L.-J.D., G.-H.F.; Validation: L.-J.D.; Formal analysis: G.-H.F.; Investigation: L.-J.D.; Data curation: L.-J.D., G.-H.F.; Writing - original draft: G.-H.F.; Writing - review & editing: G.-H.F.; Supervision: G.-H.F.; Project administration: L.-J.D., G.-H.F.; Funding acquisition: G.-H.F.
Funding
This study was supported by the National Institutes of Health (NIH) (5R01EY019721). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.175117.supplemental
References
- Arreola A., Payne L. B., Julian M. H., de Cubas A. A., Daniels A. B., Taylor S., Zhao H., Darden J., Bautch V. L., Rathmell W. K. et al. (2018). Von Hippel-Lindau mutations disrupt vascular patterning and maturation via Notch. JCI Insight 3, 92193 10.1172/jci.insight.92193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley K., Franco C. A., Philippides A., Blanco R., Dierkes M., Gebala V., Stanchi F., Jones M., Aspalter I. M., Cagna G. et al. (2014). The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat. Cell Biol. 16, 309-321. 10.1038/ncb2926 [DOI] [PubMed] [Google Scholar]
- Bruick R. K. and McKnight S. L. (2001). A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294, 1337-1340. 10.1126/science.1066373 [DOI] [PubMed] [Google Scholar]
- Chan-Ling T., Chu Y., Baxter L., Weible Ii M. and Hughes S. (2009). In vivo characterization of astrocyte precursor cells (APCs) and astrocytes in developing rat retinae: differentiation, proliferation, and apoptosis. Glia 57, 39-53. 10.1002/glia.20733 [DOI] [PubMed] [Google Scholar]
- Cheng C., Haasdijk R., Tempel D., van de Kamp E. H. M., Herpers R., Bos F., Den Dekker W. K., Blonden L. A. J., de Jong R., Bürgisser P. E. et al. (2012). Endothelial cell-specific FGD5 involvement in vascular pruning defines neovessel fate in mice. Circulation 125, 3142-3159. 10.1161/CIRCULATIONAHA.111.064030 [DOI] [PubMed] [Google Scholar]
- Chu M., Li T., Shen B., Cao X., Zhong H., Zhang L., Zhou F., Ma W., Jiang H., Xie P. et al. (2016). Angiopoietin receptor Tie2 is required for vein specification and maintenance via regulating COUP-TFII. eLife 5, e21032 10.7554/eLife.21032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor K. M., Krah N. M., Dennison R. J., Aderman C. M., Chen J., Guerin K. I., Sapieha P., Stahl A., Willett K. L. and Smith L. E. H. (2009). Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 4, 1565-1573. 10.1038/nprot.2009.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa G., Harrington K. I., Lovegrove H. E., Page D. J., Chakravartula S., Bentley K. and Herbert S. P. (2016). Asymmetric division coordinates collective cell migration in angiogenesis. Nat. Cell Biol. 18, 1292-1301. 10.1038/ncb3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell M. I., Aguilar E. and Friedlander M. (2002). Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest. Ophthalmol. Vis. Sci. 43, 3500-3510. [PubMed] [Google Scholar]
- Duan L.-J., Takeda K. and Fong G.-H. (2011). Prolyl hydroxylase domain protein 2 (PHD2) mediates oxygen-induced retinopathy in neonatal mice. Am. J. Pathol. 178, 1881-1890. 10.1016/j.ajpath.2010.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L.-J., Takeda K. and Fong G.-H. (2014). Hypoxia inducible factor-2alpha regulates the development of retinal astrocytic network by maintaining adequate supply of astrocyte progenitors. PLoS ONE 9, e84736 10.1371/journal.pone.0084736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L.-J., Pan S. J., Sato T. N. and Fong G.-H. (2017). Retinal angiogenesis regulates astrocytic differentiation in neonatal mouse retinas by oxygen dependent mechanisms. Sci. Rep. 7, 17608 10.1038/s41598-017-17962-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehling M., Adams S., Benedito R. and Adams R. H. (2013). Notch controls retinal blood vessel maturation and quiescence. Development 140, 3051-3061. 10.1242/dev.093351 [DOI] [PubMed] [Google Scholar]
- Epstein A. C. R., Gleadle J. M., McNeill L. A., Hewitson K. S., O'Rourke J., Mole D. R., Mukherji M., Metzen E., Wilson M. I., Dhanda A. et al. (2001). C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43-54. 10.1016/S0092-8674(01)00507-4 [DOI] [PubMed] [Google Scholar]
- Fruttiger M. (2002). Development of the mouse retinal vasculature: angiogenesis versus vasculogenesis. Invest. Ophthalmol. Vis. Sci. 43, 522-527. [PubMed] [Google Scholar]
- Fruttiger M. (2007). Development of the retinal vasculature. Angiogenesis 10, 77-88. 10.1007/s10456-007-9065-1 [DOI] [PubMed] [Google Scholar]
- Fukushima M., Setoguchi T., Komiya S., Tanihara H. and Taga T. (2009). Retinal astrocyte differentiation mediated by leukemia inhibitory factor in cooperation with bone morphogenetic protein 2. Int. J. Dev. Neurosci. 27, 685-690. 10.1016/j.ijdevneu.2009.07.006 [DOI] [PubMed] [Google Scholar]
- Gariano R. F., Sage E. H., Kaplan H. J. and Hendrickson A. E. (1996). Development of astrocytes and their relation to blood vessels in fetal monkey retina. Invest. Ophthalmol. Vis. Sci. 37, 2367-2375. [PubMed] [Google Scholar]
- Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A., Jeltsch M., Mitchell C., Alitalo K., Shima D. et al. (2003). VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163-1177. 10.1083/jcb.200302047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström M., Phng L.-K., Hofmann J. J., Wallgard E., Coultas L., Lindblom P., Alva J., Nilsson A.-K., Karlsson L., Gaiano N. et al. (2007). Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445, 776-780. 10.1038/nature05571 [DOI] [PubMed] [Google Scholar]
- Huang L. E., Pete E. A., Schau M., Milligan J. and Gu J. (2002). Leu-574 of HIF-1alpha is essential for the von Hippel-Lindau (VHL)-mediated degradation pathway. J. Biol. Chem. 277, 41750-41755. 10.1074/jbc.M207280200 [DOI] [PubMed] [Google Scholar]
- Huxlin K. R., Sefton A. J. and Furby J. H. (1992). The origin and development of retinal astrocytes in the mouse. J. Neurocytol. 21, 530-544. 10.1007/BF01186955 [DOI] [PubMed] [Google Scholar]
- Ishida S., Yamashiro K., Usui T., Kaji Y., Ogura Y., Hida T., Honda Y., Oguchi Y. and Adamis A. P. (2003). Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat. Med. 9, 781-788. 10.1038/nm877 [DOI] [PubMed] [Google Scholar]
- Ivan M., Haberberger T., Gervasi D. C., Michelson K. S., Gunzler V., Kondo K., Yang H., Sorokina I., Conaway R. C., Conaway J. W. et al. (2002). Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc. Natl. Acad. Sci. USA 99, 13459-13464. 10.1073/pnas.192342099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson L., Franco C. A., Bentley K., Collins R. T., Ponsioen B., Aspalter I. M., Rosewell I., Busse M., Thurston G., Medvinsky A. et al. (2010). Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 12, 943-953. 10.1038/ncb2103 [DOI] [PubMed] [Google Scholar]
- Korn C., Scholz B., Hu J., Srivastava K., Wojtarowicz J., Arnsperger T., Adams R. H., Boutros M., Augustin H. G. and Augustin I. (2014). Endothelial cell-derived non-canonical Wnt ligands control vascular pruning in angiogenesis. Development 141, 1757-1766. 10.1242/dev.104422 [DOI] [PubMed] [Google Scholar]
- Ling T. L., Mitrofanis J. and Stone J. (1989). Origin of retinal astrocytes in the rat: evidence of migration from the optic nerve. J. Comp. Neurol. 286, 345-352. 10.1002/cne.902860305 [DOI] [PubMed] [Google Scholar]
- Maxwell P. H., Wiesener M. S., Chang G.-W., Clifford S. C., Vaux E. C., Cockman M. E., Wykoff C. C., Pugh C. W., Maher E. R. and Ratcliffe P. J. (1999). The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271-275. 10.1038/20459 [DOI] [PubMed] [Google Scholar]
- Miyawaki T., Uemura A., Dezawa M., Yu R. T., Ide C., Nishikawa S., Honda Y., Tanabe Y. and Tanabe T. (2004). Tlx, an orphan nuclear receptor, regulates cell numbers and astrocyte development in the developing retina. J. Neurosci. 24, 8124-8134. 10.1523/JNEUROSCI.2235-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudhar H. S., Pollock R. A., Wang C., Stiles C. D. and Richardson W. D. (1993). PDGF and its receptors in the developing rodent retina and optic nerve. Development 118, 539-552. [DOI] [PubMed] [Google Scholar]
- Nahirnyj A., Livne-Bar I., Guo X. and Sivak J. M. (2013). ROS detoxification and proinflammatory cytokines are linked by p38 MAPK signaling in a model of mature astrocyte activation. PLoS ONE 8, e83049 10.1371/journal.pone.0083049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Ishizu A., Kurihara T., Okuno Y., Ozawa Y., Kishi K., Goda N., Tsubota K., Okano H., Suda T. and Kubota Y. (2012). The formation of an angiogenic astrocyte template is regulated by the neuroretina in a HIF-1-dependent manner. Dev. Biol. 363, 106-114. 10.1016/j.ydbio.2011.12.027 [DOI] [PubMed] [Google Scholar]
- Phng L.-K., Potente M., Leslie J. D., Babbage J., Nyqvist D., Lobov I., Ondr J. K., Rao S., Lang R. A., Thurston G. et al. (2009). Nrarp coordinates endothelial Notch and Wnt signaling to control vessel density in angiogenesis. Dev. Cell 16, 70-82. 10.1016/j.devcel.2008.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitulescu M. E., Schmidt I., Giaimo B. D., Antoine T., Berkenfeld F., Ferrante F., Park H., Ehling M., Biljes D., Rocha S. F. et al. (2017). Dll4 and Notch signalling couples sprouting angiogenesis and artery formation. Nat. Cell Biol. 19, 915-927. 10.1038/ncb3555 [DOI] [PubMed] [Google Scholar]
- Sakimoto S., Kidoya H., Naito H., Kamei M., Sakaguchi H., Goda N., Fukamizu A., Nishida K. and Takakura N. (2012). A role for endothelial cells in promoting the maturation of astrocytes through the apelin/APJ system in mice. Development 139, 1327-1335. 10.1242/dev.072330 [DOI] [PubMed] [Google Scholar]
- Sawamiphak S., Seidel S., Essmann C. L., Wilkinson G. A., Pitulescu M. E., Acker T. and Acker-Palmer A. (2010). Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature 465, 487-491. 10.1038/nature08995 [DOI] [PubMed] [Google Scholar]
- Scheef E., Wang S., Sorenson C. M. and Sheibani N. (2005). Isolation and characterization of murine retinal astrocytes. Mol. Vis. 11, 613-624. [PubMed] [Google Scholar]
- Scott A., Powner M. B., Gandhi P., Clarkin C., Gutmann D. H., Johnson R. S., Ferrara N. and Fruttiger M. (2010). Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature. PLoS ONE 5, e11863 10.1371/journal.pone.0011863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonavicius N., Ashenden M., van Weverwijk A., Lax S., Huso D. L., Buckley C. D., Huijbers I. J., Yarwood H. and Isacke C. M. (2012). Pericytes promote selective vessel regression to regulate vascular patterning. Blood 120, 1516-1527. 10.1182/blood-2011-01-332338 [DOI] [PubMed] [Google Scholar]
- Smith L. E., Wesolowski E., McLellan A., Kostyk S. K., D'Amato R., Sullivan R. and D'Amore P. A. (1994). Oxygen-induced retinopathy in the mouse. Invest. Ophthalmol. Vis. Sci. 35, 101-111. [PubMed] [Google Scholar]
- Stone J. and Dreher Z. (1987). Relationship between astrocytes, ganglion cells and vasculature of the retina. J. Comp. Neurol. 255, 35-49. 10.1002/cne.902550104 [DOI] [PubMed] [Google Scholar]
- Takeda K., Ho V. C., Takeda H., Duan L.-J., Nagy A. and Fong G.-H. (2006). Placental but not heart defects are associated with elevated hypoxia-inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol. Cell. Biol. 26, 8336-8346. 10.1128/MCB.00425-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnone L. and Mazzone M. (2011). Semaphorin signals on the road of endothelial tip cells. Dev. Cell 21, 189-190. 10.1016/j.devcel.2011.07.017 [DOI] [PubMed] [Google Scholar]
- Tammela T., Zarkada G., Nurmi H., Jakobsson L., Heinolainen K., Tvorogov D., Zheng W., Franco C. A., Murtomäki A., Aranda E. et al. (2011). VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat. Cell Biol. 13, 1202-1213. 10.1038/ncb2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura A., Kusuhara S., Wiegand S. J., Yu R. T. and Nishikawa S. (2006). Tlx acts as a proangiogenic switch by regulating extracellular assembly of fibronectin matrices in retinal astrocytes. J. Clin. Invest. 116, 369-377. 10.1172/JCI25964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson E. C., Koenig M. N., Grant Z. L., Whitehead L., Trounson E., Dewson G. and Coultas L. (2016). Apoptosis regulates endothelial cell number and capillary vessel diameter but not vessel regression during retinal angiogenesis. Development 143, 2973-2982. 10.1242/dev.137513 [DOI] [PubMed] [Google Scholar]
- West H., Richardson W. D. and Fruttiger M. (2005). Stabilization of the retinal vascular network by reciprocal feedback between blood vessels and astrocytes. Development 132, 1855-1862. 10.1242/dev.01732 [DOI] [PubMed] [Google Scholar]
- Zaitoun I. S., Johnson R. P., Jamali N., Almomani R., Wang S., Sheibani N. and Sorenson C. M. (2015). Endothelium expression of Bcl-2 is essential for normal and pathological ocular vascularization. PLoS ONE 10, e0139994 10.1371/journal.pone.0139994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo L., Theis M., Alvarez-Maya I., Brenner M., Willecke K. and Messing A. (2001). hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis 31, 85-94. 10.1002/gene.10008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.