ABSTRACT
Human mesenchymal stem cells (hMSCs), during in vitro expansion, gradually lose their distinct spindle morphology, self-renewal ability, multi-lineage differentiation potential and enter replicative senescence. This loss of cellular function is a major roadblock for clinical applications which demand cells in large numbers. Here, we demonstrate a novel role of substrate stiffness in the maintenance of hMSCs over long-term expansion. When serially passaged for 45 days from passage 3 to passage 18 on polyacrylamide gel of Young's modulus E=5 kPa, hMSCs maintained their proliferation rate and showed nine times higher population doubling in comparison to their counterparts cultured on plastic Petri-plates. They did not express markers of senescence, maintained their morphology and other mechanical properties such as cell stiffness and cellular traction, and were significantly superior in adipogenic differentiation potential. These results were demonstrated in hMSCs from two different sources, umbilical cord and bone marrow. In summary, our result shows that a soft gel is a suitable substrate to maintain the stemness of mesenchymal stem cells. As preparation of polyacrylamide gel is a well-established, and well-standardized protocol, we propose that this novel system of cell expansion will be useful in therapeutic and research applications of hMSCs.
KEY WORDS: Cell mechanics, Mesenchymal stem cells, Senescence, Stemness, Substrate rigidity
Summary: hMSCs retain their stemness by delaying senescence when expanded in vitro on soft polyacrilamide gel.
INTRODUCTION
Human mesenchymal stem cells (hMSCs), due to their multi-lineage differentiation potential, immuno-suppressive capability, and immuno-modulatory effects have been used with varying degree of success to treat cardiovascular, musculoskeletal, immune-related and hemopoietic diseases (Pittenger et al., 1999; Ranganath et al., 2012; Tuan, 2013; Wang et al., 2016). Though MSCs are available from multiple adult tissues (Caplan and Bruder, 2001), critically low availability of MSCs in the isolated sample is a major roadblock for clinical trials. For example, in bone marrow aspirates, only 0.001–0.01% of the nucleated cells are MSCs, while a dose of roughly about 100 million cells are required to treat a person 70 kg in weight (Ren et al., 2012). As a result, a long-term in vitro expansion is essential to reach a significant number of cells for autologous treatment.
However, on in vitro expansion, MSCs lose their proliferative ability and multilineage differentiation potential (Banfi et al., 2000; Wagner et al., 2008). Like any other primary somatic cells, after a certain number of cell divisions, they enter a senescence state, which is morphologically characterized by enhanced spreading area and shape irregularity (Bonab et al., 2006; Wagner et al., 2010b). More importantly, they lose their multilineage potential, migration and homing ability (De Becker and Van Riet, 2016; Honczarenko et al., 2006), making them unsuitable for clinical use (Kassem, 2006; Ullah et al., 2015). Though multiple approaches have been tried to maintain MSC stemness over prolonged expansion (Saei Arezoumand et al., 2017), finding an easy-to-use culture system to achieve the same is still an unmet need. In this context, it might be noted that the NIH on their website has listed six points that need to be addressed to realize the potential of stem cell-based therapies. The first one in that list is “Stem cells must be reproducibly made to proliferate extensively and generate sufficient quantities of cells for making tissue” (Stem Cell Basics IV. | stemcells.nih.gov, 2017, https://stemcells.nih.gov/info/basics/1.htm). A culture system that can fulfill this need may help to progress regenerative medicine significantly.
Controlling the physical microenvironment of the cell culture system might offer a solution in this context. In the past 15 years, it has been shown that mechanical cues such as stiffness of cell culture substrate, shear stress, mechanical strain, cell morphology, substrate topology, etc., influence a wide array of cell behavior and cell fate including survival, proliferation and differentiation (Anderson et al., 2016; Engler et al., 2006; Gilbert et al., 2010; Lutolf et al., 2009; Murphy et al., 2014; Winer et al., 2009; Yeung et al., 2005). It has also been shown that such mechanical cues may play an important role in maintaining MSCs stemness. For example, MSCs cultured on micro-contact printed islands as spheroids and on nano-patterns were shown to retain multipotency and proliferative capacity (Cesarz and Tamama, 2016; Lee et al., 2015; McMurray et al., 2011; Zhang and Kilian, 2013). However, both micro-contact printing and spheroid culture restrict the proliferation of MSCs leading to limited or no expansion in cell number. Moreover, creating micro-patterns or nano-patterns for a large area is a daunting task and demands huge infrastructure and cost.
In this work, we have shown that hMSCs maintain their stemness over long passages when cultured on an optimally soft polyacrylamide (PAA) gel. The soft substrate also preserves cellular morphology. Staining for β-gal and BrdU respectively showed that in these cells onset of senescence is delayed and proliferative potential is maintained. Staining for other senescence-related changes such as loss of Lamin B and gain of Lamin A confirmed this observation. Not only the proliferative potential but the cells cultured on gel could differentiate into the adipo lineage, as shown by the expression of PPAR-gamma and accumulation of oil droplets, while cells cultured on tissue culture plastic (TCP) lose their adipogenic differentiation potential. Finally, we have shown that surface markers, used to characterize MSCs, remain unaltered in the cells cultured on soft substrate ensuring the maintenance of cellular identity.
RESULTS AND DISCUSSION
Loss of cell morphology and induction of senescence during long-term in vitro expansion
To study the effect of substrate stiffness on maintenance of stemness, we cultured umbilical cord-derived hMSCs (UC-hMSCs) on polyacrylamide gel and on TCP, both coated with collagen I, from passage 3 (P3) to passage 13 (P13) (Fig. 1). These cells were well characterized (SI appendix, Fig. S1) and applicable bio-safety and ethical guidelines were followed. For better understanding of the long-term effect of passaging on cellular behavior, we grouped our results as ‘early passage’ (EP), ‘mid passage’ (MP), and ‘late passage’ (LP), which were defined as passage number (P≤6), (P=7–10), and (P>10), respectively. This classification, though arbitrary, was done based on the prevalent practice that MSCs are generally used till maximum P6 for research and clinical applications (Binato et al., 2013; Bonab et al., 2006).
Fig. 1.
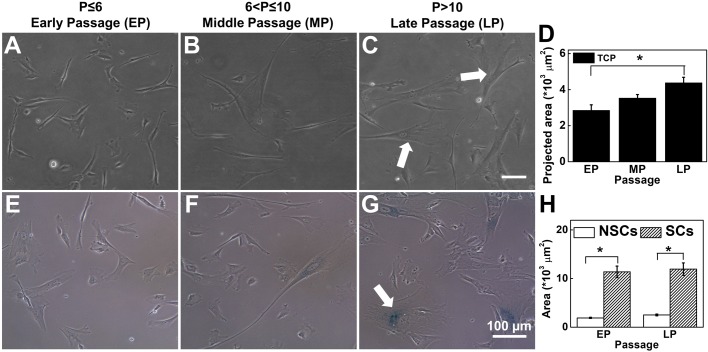
hMSCs lose their morphology and enter into replicative senescence on long-term passaging on TCP. Representative micrographs from (A) EP (passage number ≤6) (B) MP (passage number >6 and ≤10) and (C) LP (passage number >10) show that during in vitro expansion, MSCs lose their spindle morphology and become large and flat. White arrows show large cells with irregular shapes. (D) Over passage, average cell-spread area increases significantly from ∼3000–4500 µm2. (N=3, n=at least 150). (E–G) β-gal staining (a senescence marker) significantly increased in MP and LP cells compared to EP cells. White arrow indicates β-gal positive cells. (H) Spreading area of senescent cells and non-senescent cells: senescent cells have more spreading area than non-senescent cells, irrespective of passage (N=3, n>100). Results are expressed as mean±s.e.m., *P<0.05. Scale bars: 100 µm.
To measure spread area, cells were imaged after 24 h of cell seeding and average cell area was computed considering at least 150 randomly selected cells for each passage. We found that when cultured on TCP, UC-hMSCs with increasing passage lose their spindle morphology (Fig. 1A–C) and go into replicative senescence (Fig. 1E–G). The majority of the cells became flat and took irregular shapes with the passage as shown by the white arrows in Fig. 1C. The change in the projected area is quantified in Fig. 1D. Also, more debris and more granularity in the cytoplasm were observed for later passage cells (data not shown). To check the onset of senescence, we trypsinized the cells from their respective substrates and re-plated them on glass coverslips. After 24 h, we stained the cells with SA-β-gal, a well-established method to capture the senescent cells. We observed that while for EP cells only very few cells (<5%) were β-gal positive (Fig. 1E), it increases gradually to finally reach at about 20% for LP cells (Fig. 1G). Further, to check if the increase in the cell area and increased senescence are correlated, we estimated the average spread area of β-gal positive and β-gal negative cells for EP as well as for LP. We found that irrespective of passage number, senescent cells are always significantly larger compared to the non-senescent cells (Fig. 1H). Also, the average area of senescent and non-senescent cells remains almost unaltered over passage implying a possibility of close association of cell spreading with the onset of senescence. This observation led us to the hypothesis that maintaining cell size using soft substrates made of polyacrylamide gel may delay or stop senescence resulting in more efficient in vitro expansion of MSCs.
Selection of substrate for further study based on cell morphology and proliferation
It is known from the literature that cells spread less and take a rounded morphology on soft substrates (Jalali et al., 2015; Tee et al., 2011). To find the suitable substrate that restricts cell spreading, we cultured LP cells on PAA gels of a stiffness range varying from 0.5–20 kPa and TCP (∼GPa) (SI Appendix, Fig. S2A–G). We found that on soft gels (E≤5 kPa), LP cells had a cell-spread area smaller than or equivalent to EP cells cultured on TCP (Fig. 1D and SI Appendix, Fig. S2G). This observation implied that the cell-spread area can be kept restricted over passages if cultured on soft gels (E≤5 kPa). However the question remains, how soft can we go? To answer this question, we need to consider another parameter, i.e. the effect of substrate stiffness on cell proliferation. It is known that soft gel induces reversible cell cycle arrest or quiescence in hMSCs (Rumman et al., 2018; Winer et al., 2009). As a result, the very soft gel cannot be used for cell number expansion. To find the optimum range of stiffness, we cultured cells on substrates of different stiffness. After 48 h of culture on these gels, which is sufficient to induce quiescence (Rumman et al., 2018), we gave a 4 h pulse of BrdU that tags the replicating DNA. We found that while cells on 1 or 2 kPa gel showed critically less replicating DNA, cells on gels of 5 kPa and higher stiffness had more than 30% of dividing cells which is equivalent to that on TCP (SI Appendix, Fig. S2H–L).
Putting these two observations together, we selected 5 kPa gel for all our ongoing studies to compare the effect of substrate stiffness on long-term in vitro culture.
Soft substrate maintains cellular morphology and self-renewal ability of UChMSCs
When continuously cultured on 5 kPa gel for 45 days, it was observed that UChMSCs maintain their cellular morphology and proliferative potential better than the cells cultured on TCP. For P3 to P18, the cells were trypsinized in every 72 h and re-seeded on the respective substrates at 1000 cells/cm2 seeding density. Fig. 2A–D show that while cells lose their morphology both in terms of size and shape when cultured on TCP for multiple passages, they maintain the same morphology if cultured on the gel. The average area of cells on 5 kPa gel was maintained with the passage at about 1500 µm2, but the same was increased from 3000 µm2 to 4500 µm2 on TCP (Fig. 2E). On the gel we observed more spindle-shaped cells and fewer irregular protrusions, as can be seen in Fig. 2A–D and F. The same observations were made when the cells from EP. MP, and LP from both gel and TCP were trypsinized and seeded on to the plastic (Fig. S3). To have a blind test, we showed the cells under the microscope to multiple independent observers who were well versed with hMSCs morphology. They could not differentiate between EP and LP cells from the gel. However, the difference between EP and LP on TCP was obvious. Other than spread area and protrusions, cells expanded on the gel for LP also showed significantly lower traction (Fig. 2G) than the cells on TCP when tested on 5 kPa gel for traction force microscopy (TFM).
Fig. 2.
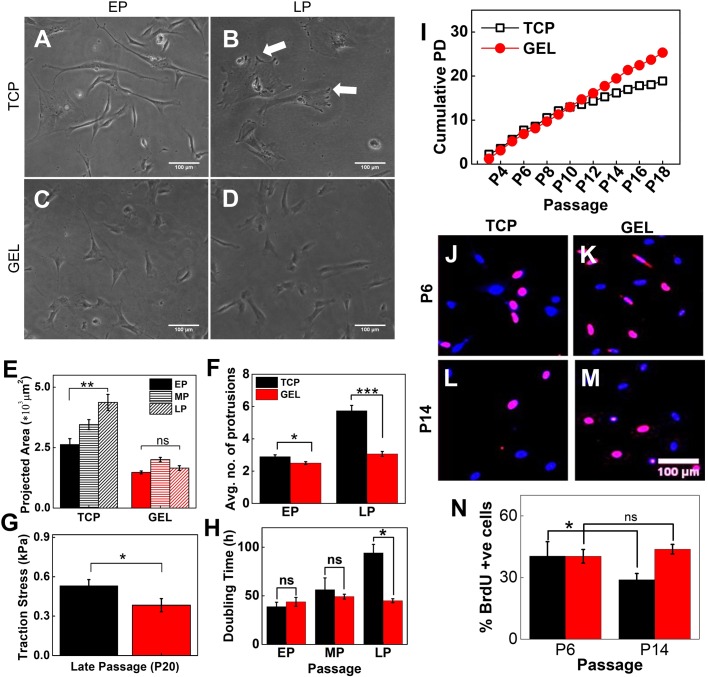
Soft substrate maintains cellular morphology, rate of expansion and proliferation during serial passage. Representative phase contrast images of (A) EP (passage number ≤6) and (B) LP (passage number >10) UC-hMSCs on collagen-coated TCP and (C) EP and (D) LP on soft gel. (E) Cell spread area increases with passage on TCP; however, spreading area of cells on gel showed no significant difference across passages. (N=3, n=150). (F) The number of protrusions, as shown by white arrows in B, are significantly increased in LP cells cultured on TCP (N=3, n=150). (G) LP UChMSCs on gel showed significantly lower traction than that on TCP. The experiment was performed using two biological samples (N=3, n=20). (H) DT of UC-hMSCs cultured on gel and TCP over the passage. While DT increases with passage when cultured on TCP, it remains unaffected when expanded on 5 kPa gel. The difference in DT for EP and MP are negligible while for LP the difference is significant (N=3, n=1). (I) CPD of UC-hMSCs cultured on gel and TCP over the passage. CPD increases linearly for UChMSCs on the gel but approaches a plateau for cells cultured on TCP (N=1, n=11). (J) Immunofluorescence images of nuclei co-stained with DAPI (blue) and BrdU (red) capturing a relative percentage of cycling cells. (K) Percentage BrdU positive cell for P14 (LP) from gels are significantly higher than the cells from TCP, though for EP (P6) cells there was no significant difference between gel and TCP. This data reconfirms that cells maintain their proliferative potential if cultured on the soft gel. Results are expressed as mean±s.e.m. *P<0.05, **P<0.001, ***P<0.0001. Scale bars: 100 µm.
To check if along with maintenance of morphology, self-renewal efficiency is also maintained, we assessed the effect of a soft substrate on cellular expansion of UC-hMSCs in the long-term passage. Using microscopic images, as described in the Materials and Methods section, we counted cell number twice; first, after 4 h of seeding and second, just before harvesting. From the number of cells seeded and harvested we calculated population doubling (PD), doubling time (DT) and cumulative population doubling (CPD) as described in the Materials and Methods section. Fig. 2H compares DT values for EP, MP, and LP cells. We found that the DT for cells on TCP increased slightly from 40–50 h for EP to MP and then jumped significantly to more than 80 h for LP cells. However, the DT value remains almost constant for all three groups of cells (∼40 h) when cultured on 5 kPa PAA gels.
This observation is also reflected in the CPD (Fig. 2I). We observed that CPD on TCP increased almost linearly till P9 and then gradually slowed down. For gel, CPD continues to increase linearly till the end of the experiment. By P18, we observed a difference of 9 in CPD signifying that 29 or 512 times more UChMSCs can be obtained from gel than from TCP. This difference signifies that while one single cell will give us 4 million cells after 18 passages if cultured on TCP, the same cell will give 2000 million or 2 billion cells if cultured on the soft gel.
Further, to confirm that this reduction in CPD on TCP compared to that on the gel is indeed due to the maintenance of proliferation and not a cell culture artifact, we performed BrdU assay to estimate the fraction of cells in S phase after harvesting them from their respective substrate and then reseeding on the glass. We found that there was no significant difference in the percentage of S phase cells between TCP and gels for EP population. However, for LP cells, the same was significantly higher when cells were harvested from the gels than their counterparts harvested from TCP (Fig. 2J–N). This data conclusively proves that 5 kPa PAA gel maintains self-renewal ability of UC-hMSCs over long-term culture leading to a significant increase in cell number. To rule out the possibility of higher cell adhesion on gel compared to TCP resulting into higher CPD, we checked for plating efficiency on both the substrates and found no significant difference (SI Appendix, Fig. S4).
All the results mentioned here were done with technical triplicates. To check the reproducibility of the data, we used two independent biological replicates and MSCs derived from bone marrow. We found similar observations in all these cases, as shown in the SI Appendix and Figs S5–S9.
Soft substrate delays senescence
To verify if this maintained rate of expansion on soft gels is a result of reduced senescence as we proposed earlier, we stained the cells with SA-β-gal, a standard technique to estimate senescence. We found that on TCP, the fraction of senescent cells increases with the passage, reaching >20% of the population for late passages (Fig. 3A–C,G). However, when cultured on the gel, although there was an increase in senescent population from EP to MP, the percentage of this population did not increase further and remained <10%, which is significantly less compared to the same for cells cultured on TCP (Fig. 3D–G). To reconfirm, we also checked for expression of vimentin, which is known to overexpress in senescence fibroblast (Frescas et al., 2017; Nishio et al., 2001). Immunofluorescence analysis revealed a fourfold increase in vimentin expression in cells cultured on TCP compared to the same on gel (SI Appendix, Fig. S10).
Fig. 3.
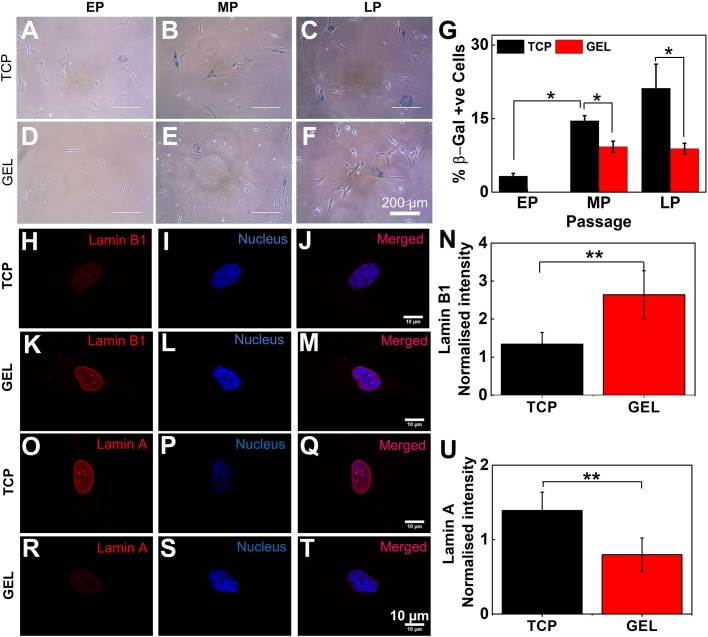
Soft substrate delays senescence. (A–G) Cells stained for β-gal (blue), a well-established senescence marker, show that while senescence gradually increases for TCP over the passage, on the gel after an initial increase it remains constant (EP: passage number ≤6, MP: passage number >6 and ≤10, LP: passage number >10). The same is quantified in G (N=3, n=400). Results are expressed as mean±s.e.m. *P<0.05. Scale bars: 200 µm. (H–N) Comparison of expression of total nuclear Lamin B1 (red) between LP UC-MSCs grown on TCP and gel. The decrease of Lamin B1 expression on TCP (H–J) compared to UC-hMSCs on the gel (K–M). Quantification of intensity shows that Lamin B1 is maintained in late passage UC-hMSCs on the gel (N) (N=2, n=22). Further, comparison of Lamin A expression for late passage UC-hMSCs on plastic (TCP) (O–Q) and on the gel (R–T) reveals the fact that Lamin A expressed more on TCP. The same is quantified in figure (U) (N=2, n=20). Results are expressed as mean±s.d. **P<0.001. Scale bars: (H–T) 10 µm.
We also checked for Lamin A and Lamin B1, two nuclear envelope-associated proteins, expression of which is known to vary differentially in senescence. Loss of Lamin B1 and accumulation of Lamin A are known to increase in senescent cells and are used as novel biomarkers for senescence (Bellotti et al., 2016; Freund et al., 2012; Wang et al., 2017). To investigate the expression of Lamin A and Lamin B, we trypsinized cells from gel and TCP and plated them on glass coverslips. After 24 h of cell seeding, we stained the cells with respective antibody and quantified Lamin A and B1 expression for the whole nucleus. We indeed found that expression of Lamin B goes down (Fig. 3H–N) and Lamin A goes up (Fig. 3O–U) for cells from TCP compared to the cells harvested from gels. Both of these observations, along with SA-β-gal staining and vimentin expression, show that culturing UC-hMSCs on soft gel delays senescence.
Long-term culture on gel did not alter surface marker expression but helps the stem cells to maintain differentiation potential
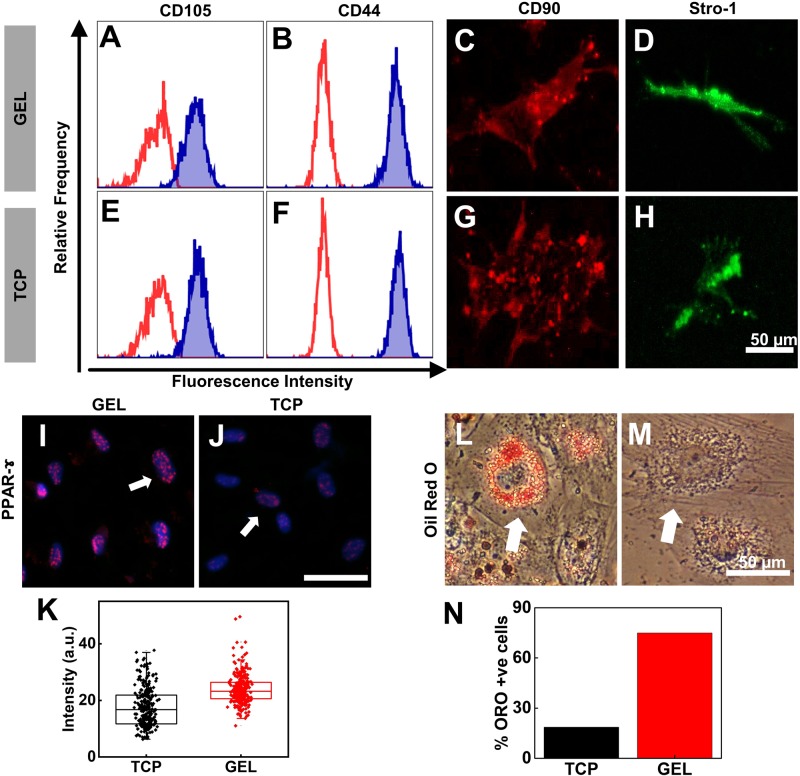
Finally, to confirm the identity of our cells after long-term culture, we investigated the effect of substrate on the expression of surface pluripotency markers. Surface marker analysis of late passage (P22) UChMSCs using flow cytometry and immune-cytometry demonstrated that cells cultured either on gel or on TCP express characteristic positive surface markers, CD105 (Fig. 4A,E), CD44 (Fig. 4B,F), CD90 (Fig. 4C,G) and Stro-1 (Fig. 4D,H). This is in accordance with the previous studies which showed that the MSC surface marker expression remains same with increasing passage (Kundrotas et al., 2016). However, the difference appears in their differentiation potential. While LP cells on TCP lose their adipogenic potential, LP cells on gel did not (Fig. 4I–N). We harvested the cells from their respective substrates and cultured them on TCP in adipogenic induction media for 7 days. Then the cells were fixed and stained for the early adipo transcription factor, PPAR-γ. It is evident from the images that LP cells from gel expressed a much higher level of PPAR-γ than the LP cells from TCP in similar condition (Fig. 4I–K). The same difference was observed after 14 days of adipo induction in terms of accumulation of oil droplets that were detected with Oil Red O dye (Fig. 4L,M). Approximately 75% of LP cells on gel cells differentiated into adipocytes as determined by ORO in comparison to only 18% of LP cells on TCP cells staining (Fig. 4N).
Fig. 4.
Long-term culture on gel did not alter surface marker expression but helps stem cells maintain differentiation potential. Flow cytometry analysis of the expression of surface pluripotency markers of UC-hMSCs at the LP (P22) from the gel (A–D) and TCP (E–H) was determined. The expression of surface markers, CD105, CD44, CD90, and Stro-1 was not altered by the long-term culture of UChMSCs on the gel. The red curve in A, B, E and F is auto-fluorescence of the cells and the blue filled histogram is the fluorescence signal of the stained marker. (I–N) Adipogenic differentiation: LP hMSCs (P14) from gel and TCP were cultured in adipogenic induction media. (I–K) The early adipogenic marker PPAR-γ expression was checked after 7 days of adipo-induction using immunostaining of UC-hMSCs from gel (I) and TCP (J) (magenta puncta in the blue nucleus, shown by white arrow). The UC-hMSCs serially passaged on gel showed higher expression of PPAR-γ compared to the cells cultured on TCP, as quantified in K (N=2, n=273, ***P<0.001). (L–N) After 14 days of adipogenic induction, the lipid droplets accumulation was significantly higher in cells from the gel (L) than from TCP (M). Lipid droplets were identified by staining with Oil Red O. The percentage of Oil Red O-positive cells was higher on the gel substrate compared to TCP, as quantified in N (N=2, n=100). White arrow shows cells with and without oil droplet accumulation. Scale bars: 50 µm.
In this work, we have demonstrated that a soft PAA gel substrate of 5 kPa elastic modulus can maintain the stemness of UChMSCs in vitro by delaying the senescence process. Like any other primary cells, hMSCs undergo only a certain number of divisions before entering into replicative senescence. Replicative senescence is typically marked by large and flat morphology, and significantly slowed down proliferation rate (Banfi et al., 2000; Wagner et al., 2008). Multi-lineage differentiation potential also goes down with population expansion (Bonab et al., 2006; Wagner et al., 2010b). This decreased proliferation and loss of multi-lineage potential poses a serious challenge in using in vitro expanded hMSCs for clinical use. In this work we have shown that culturing UChMSCs on soft substrate maintains self-renewal ability, multi-lineage potential and surface markers much beyond the same when cultured on TCP.
As previous studies have shown that the span of culture before the onset of a senescence program can vary significantly depending on cellular origin, we first cultured our UChMSCs on TCP up to 13 passages and confirmed the onset of senescence with enlarged and flat morphology, and β-gal staining (Fig. 1) (Bonab et al., 2006; Vidal et al., 2012). We also showed that average area of non-senescent cells remains same over passages. Recently, Neurohr et al. showed that when cells grow too large, DNA required for proper cellular functions becomes limiting, leading to senescence (Neurohr et al., 2019). This observation opens up a possibility that restricting cell spreading may help in delaying senescence. Such hypothesis is supported by work from Killian's group showing that MSCs cultured on micro-patterned substrates to restrict cell spread area helps in maintaining the expressions of stemness markers STRO-1 and Endoglin (Zhang and Kilian, 2013; Lee et al., 2015). Although these results point towards the fact that stemness can be maintained by culturing cells on micro-patterned island thus restricting spreading, such approach is not very useful for MSC expansion as cells on micro-island stop proliferation and do not increase in number.
Earlier studies also showed that stemness in hMSCs could be maintained better by reducing actomyosin contractility or cellular traction using pharmacological inhibitor of ROCK and myosin (Zhang and Kilian, 2013). In a different study, it was shown that while mESCs (mouse embryonic stem cells) grown on TCP need LIF to stop spontaneous differentiation, a very soft substrate (600 Pa) can maintain them in their undifferentiated state without LIF (Chowdhury et al., 2010). All these results indicated that cell area/contractility plays an important role in the loss of stemness and both can be kept low if cultured on a soft substrate (McBeath et al., 2004; Tee et al., 2011). However, none of these works checked the effect of substrate stiffness in long-term culture. We demonstrated here that a long-term culture on soft substrate may inherently reduce the cellular traction (Fig. 2G) and thus can maintain stemness. To the best of our knowledge, this is the first work to demonstrate the effect of substrate stiffness on cellular traction and maintenance of stemness in long-term culture (for 20 passages i.e. ∼60 days) for any cell type.
Consistent with the previous report, we also found that though UC-hMSCs lose their self-renewal ability when cultured on TCP, they maintain the molecular signatures related to stemness (Fig. 4A–H) (McGrail et al., 2013). The cells, irrespective of cultured on gel or TCP were positive for CD105, CD44, CD90, and Stro-1. However, cells cultured on TCP lost their adipogenic differentiation ability whereas the same was maintained for the cells cultured on the gel (Fig. 4I–N). Loss of adipogenic potential over long term passages and dominance of osteogenic differentiation has been reported by many earlier researchers (Neuhuber et al., 2008; Wall et al., 2007). It was shown that flat cells that appear spontaneously over long-term culture, lose their adipogenic potential (McGrail et al., 2013). This observation is not unexpected if we look from the cell mechanics angle. It was established by Engler et al. in their seminal paper in 2006 that stiff substrate (34 kPa) induces osteogenic lineage in hMSCs (Engler et al., 2006) even in absence of chemical inducer. Similarly, it was also shown that cells that were made to spread more or to take the shape that induces high contractility, also were prone to osteogenic lineage commitment (Kilian et al., 2010; McBeath et al., 2004). So, it is expected that if for multiple passages, cells are continuously exposed to a substrate as rigid as TCP which increases cellular spreading and contractility, adipogenic potential would get diminished. However, a soft culture substrate, in contrast, should maintain the multi-lineage potential, as demonstrated by our result (Fig. 4).
Other than UChMSCs, we also used bone marrow-derived hMSCs and found a similar result proving that this effect might not be source specific. We have also found that the substrate stiffness for optimal growth of skin-derived keratinocytes is not the same as for MSCs (data not shown). How cell type and optimum substrate stiffness are inter-linked is open for future investigation.
One of the interesting observations in this work is that soft substrate delays senescence. It is known that acquiring replicative senescence over in vitro expansion may not be an obvious purposeful program but a result of the external environmental condition. For example, it has been shown that increased oxygen concentration may induce senescence faster. On the contrary, the hypoxic condition is known to maintain stemness for hMSCs (Basciano et al., 2011). However, the effect of substrate stiffness on senescence has not been studied before. We have demonstrated using four known markers of senescence – namely expression of β-gal, loss of Lamin B1, the gain of Lamin A and vimentin (Bellotti et al., 2016; Bonab et al., 2006; Freund et al., 2012) – that an optimally soft substrate may delay the onset of senescence significantly.
In summary, our data show that instead of using TCP, culturing cells on the soft substrate will help to solve the problem of limited availability of MSCs by increasing the number of available cells after extended expansion. This work offers a possibility to design a cell-specific culture substrate in the future. This work also demonstrates for the first time that replicative senescence in hMSCs can be delayed using substrates of physiological stiffness.
MATERIALS AND METHODS
Substrate preparation
Gels of polyacrylamide (PAA) of various stiffness were prepared by mixing 40% polyacrylamide and 2% bis-acrylamide solution, as described previously (Pelham and Wang, 1997). Substrate preparation protocols and modulus values were adopted from previously published work (Tse and Engler, 2010). Briefly, the gel solution for desired stiffness was mixed with APS (ammonium persulfate) 1:100 and TEMED (1:1000) and placed between a hydrophobic glass (octadecyltrichlorosilane treated; Sigma-Aldrich, 104817) and the transparency sheet 3-APTMS (Alfa Aesar, A17714) treated. Once polymerized, the hydrophobic plate was carefully removed. The gel was conjugated with sulfo-SANPAH and incubated with rat tail type I collagen (25 µg/ml) (Invitrogen, A1048301) at 4°C for overnight, as described (Venugopal et al., 2018). The tissue culture plates (TCP) (control) were also coated with type 1 collagen (25 µg/ml). The thickness of the gel was controlled by using the defined volume of the gel solution throughout the experiments.
Cell culture
Bone marrow-derived human MSCs were purchased from Lonza (Cat #PT-2501, Lot #482966) (authenticated and tested for contamination by the supplier), and fresh umbilical cord-derived MSCs were obtained from healthy individuals after due ethical clearance and bio-safety approval. For serial passage experiments, P4 cells were seeded on large area gels and on TCP (both collagen-I coated as mentioned above) with same seeding density (1000 cells/cm2) in MSCs qualified medium α-(MEM) (Invitrogen, A1049001). Low-glucose DMEM (HiMedia, AL006) supplemented with 16% MSC certified fetal bovine serum (FBS) (Invitrogen, 12662029), 1% Glutamax (Invitrogen, 35050061), and 1% pen-strep (Invitrogen, 15140122) in humidified incubator with 37°C and 5% CO2. After 72 h of culture, cells were trypsinized from PAA gels and TCP using TrypLE™ Enzyme Express (Invitrogen, 12604013) and were reseeded on fresh substrates respectively and cultured for next passage, this process was repeated until the TCP growth halted.
Cell count using image analysis
Images of the gels and TCP were acquired using a Magnus microscope at 10× magnification after 4 and 72 h of seeding to determine accurate cell number for calculating PD as described (Cristofalo et al., 1998). For PD counting, 20 random images per sample were captured (covering ∼3% of the total area of the gel), the average number of cells per frame was obtained and then divided by the total area of the frame to obtain seeding density (cells/cm2). The seeding density was then multiplied by the total area of the substrate (gel 20 cm2; TCP 25 cm2) to get the total number of cells seeded (4 h) and harvested (72 h) from a particular experimental condition (PAA gels and TCP) for respective passage. This was done in every passage, which was then used to calculate the CPD for each experimental condition as explained in the Eqns. 1–3 (Cristofalo et al., 1998):
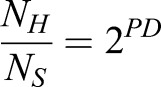 |
(1) |
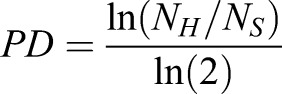 |
(2) |
where NH is the number of harvested cells, NS is the number of cells seeded,
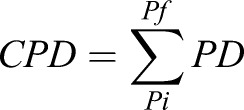 |
(3) |
where Pi is the initial passage number and Pf is the final passage number.
Quantification of cell morphology
Cell images were captured at different passages at 48 h post seeding using EVOS-FL auto inverted microscope (Life Technologies) at 10× magnification. Cell spreading area was determined using ImageJ (National Institutes of Health) software by manually tracing around the perimeter of an individual cell. For each sample minimum, 150 random cells were analyzed. The number of protrusions of cells was quantified from phase contrast images manually using ImageJ.
Plating efficiency
To compare the cell adhesion efficiency, P9 bone marrow MSCs were seeded on the 5 kPa gel and collagen-coated coverslip. After 15 min of seeding, 15 random images were captured and analyzed to get the number of cells seeded. The plate was kept in the incubator for 1 h and 30 min, then media was aspirated and fresh media was added in each well. Again, 15 random images were captured and analyzed to determine the number of cells attached. From the number of cells seeded and the number of cells attached, we calculated the plating efficiency as:
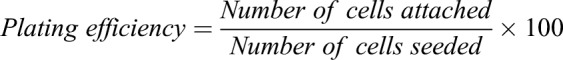 |
(4) |
100% plating efficiency shows all cells attached.
BrdU assay
To check the percentage of S-phase cells in the cell cycle, cells from EP, MP and LP were trypsinized from gels and TCP and were seeded on collagen-coated glass coverslips as described above. After 48 h of seeding, BrdU reagent (Invitrogen, 000103) was added in 1:100 (v/v) ratio in media and incubated for 4 h at 37°C in a humidified incubator with 5% CO2. Thereafter cells were fixed (4% paraformaldehyde), permeabilized (0.5% Triton-X), denatured (2 M HCl), blocked (1.5% bovine serum albumin), and incubated with anti-BrdU antibody (Invitrogen, B35128, 1:100) and counterstained with AlexaFluor 568 (Invitrogen, A11061, 1:400). Immunofluorescence images were captured using EVOS-FL auto and BrdU positive and negative cells were counted manually using ImageJ.
Senescence assays
Senescence-associated β-galactosidase (SA-β-gal) was used to detect MSCs senescence using SA-β-gal staining kit (Abcam, AB65351) according to the manufacturer’s instructions. Briefly, cells from EP, MP, and LP were seeded in a six-well plate and incubated in growth media for 48 h. Afterwards, cells were fixed, stained with β-gal solution and incubated at 37°C without CO2. Ten–15 random images were captured for each condition for analysis β-gal positive cells were counted manually.
Differentiation assays
EP and LP cells from gel and TCP were seeded in a 12-well culture plate in growth medium for 72 h and then incubated with differentiation media for adipogenic (Invitrogen, A1006501) and osteogenic (Invitrogen, A1006601) differentiation as per the manufacturer's instructions. MSCs cultured in growth media were used as a negative control. Post 14 days and 21 days incubation for adipo and osteo differentiation, respectively, adipocytes were assessed with Oil Red O (Sigma-Aldrich, O0625) solution and osteoblasts were assessed with Alizarin Red solution (Sigma-Aldrich, 3422613022311). Images were captured for qualitative and quantitative analysis using EVOS FL Auto.
Immunofluorescence staining
For nuclear Lamin A (Abcam, ab8980, 1:400), Lamin B1 (Abcam, ab16048, 1:400), and early adipogenic differentiation marker staining, EP and LP cells from gel and TCP were cultured on collagen-I-coated glass coverslips for 24 h. Cells were then fixed with 4% paraformaldehyde in PBS for 15 min at room temperature (RT) and blocked (3% bovine serum albumin in PBS) for 30 min and washed with cytoskeletal buffer, as described previously (Venugopal et al., 2018). Cells were incubated with respective primary antibodies for 4 h at 4°C, and then incubated with corresponding secondary antibodies for 1 h at RT. Primary and secondary antibodies were used in the following combinations: anti-PPAR-γ (Abcam, ab59256, 1:300) counterstained with AlexaFluor 488 (Invitrogen, A11034, 1:500), anti-Lamin A (Abcam, ab8980, 1:400) counterstained with AlexaFluor 568 (Abcam, ab175473, 1:400), anti-Lamin B1 (Abcam, ab16048, 1:400) counterstained with AlexaFluor 568 (Abcam, ab175470, 1:400), anti-Vimentin (Sigma-Aldrich, V5255, 1:300) counterstained with AlexaFluor 488 (Invitrogen, A11059, 1:500). Cell nuclei were stained with Hoechst 33342 (Invitrogen, H3570) (1:10,000) in PBS for 5 min at RT and mounted. Images were captured for qualitative and quantitative analysis using EVOS fluorescence microscope (Invitrogen).
Traction force microscopy (TFM)
Gels of 5 kPa were fabricated with embedded fluorescent beads to conduct TFM. Briefly, to make a single layer of the fluorescent bead (Fluka, 1 µm rhodamine), beads (1:50) were added to the pre-polymer solution (25 µl) and solidified over the normal gel of 5 kPa. The gel was then functionalized as described above. Cells were seeded on the gels, after 24 h of cell seeding images of stressed (before lysing) and unstressed gel (after lysing with 1% Triton-X) were captured by the EVOS FL Auto (Invitrogen). An average of 20 cells were analyzed per condition. A MATLAB algorithm was used to determine the cell-generated displacement field and traction forces as previously described (Butler et al., 2002). The TFM data was analyzed using MATLAB R2018a (IIT Bombay License).
Statistical analysis
Data is presented as means±standard error of the mean (s.e.m.). For statistical analysis, we used unpaired Student’s t-test and values of P<0.05 were considered statistically significant, if not otherwise stated. Data was plotted using Origin software (IIT Bombay License).
Supplementary Material
Acknowledgements
We thank Dr James P. Butler (Harvard Medical School, Department of Medicine, Boston, USA) for his TFM codes used for the traction force analysis. We thank IRCC, IIT Bombay for providing the fellowship to P.M. and confocal microscopy facility.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: A.M.; Methodology: S.K.K., P.M., A.M.; Formal analysis: S.K.K., P.M., A.K., R.J.; Investigation: S.K.K., P.M., A.K., V.K., R.J.; Resources: S.D., J.B.; Data curation: S.K.K., P.M., A.K., A.M.; Writing - original draft: S.K.K., P.M., A.M.; Writing - review & editing: S.K.K., P.M., A.K., J.B., A.M.; Visualization: A.M.; Supervision: A.M.; Project administration: A.M.; Funding acquisition: A.M.
Funding
This work was supported by Welcome Trust-DBT India Alliance [Project #IA/E/11/1/500419] and Indian Institute of Technology Bombay Seed Grant [14IRCCSG002].
Data availability
Raw data underlying the study is available and can be seen upon request to the corresponding author.
Supplementary information
Supplementary information available online at http://bio.biologists.org/lookup/doi/10.1242/bio.039453.supplemental
References
- Anderson H. J., Sahoo J. K., Ulijn R. V. and Dalby M. J. (2016). Mesenchymal stem cell fate: applying biomaterials for control of stem cell behavior. Front. Bioeng. Biotechnol. 4, 38 10.3389/fbioe.2016.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfi A., Muraglia A., Dozin B., Mastrogiacomo M., Cancedda R. and Quarto R. (2000). Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: implications for their use in cell therapy. Exp. Hematol. 28, 707-715. 10.1016/S0301-472X(00)00160-0 [DOI] [PubMed] [Google Scholar]
- Basciano L., Nemos C., Foliguet B., de Isla N., de Carvalho M., Tran N. and Dalloul A. (2011). Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status. BMC Cell Biol. 12, 12 10.1186/1471-2121-12-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellotti C., Capanni C., Lattanzi G., Donati D., Lucarelli E. and Duchi S. (2016). Detection of mesenchymal stem cells senescence by prelamin A accumulation at the nuclear level. Springerplus 5, 1427 10.1186/s40064-016-3091-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binato R., de Souza Fernandez T., Lazzarotto-Silva C., Du Rocher B., Mencalha A., Pizzatti L., Bouzas L. F. and Abdelhay E. (2013). Stability of human mesenchymal stem cells during in vitro culture: considerations for cell therapy. Cell Prolif. 46, 10-22. 10.1111/cpr.12002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonab M. M., Alimoghaddam K., Talebian F., Ghaffari S. H., Ghavamzadeh A. and Nikbin B. (2006). Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 7, 14 10.1186/1471-2121-7-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J. P., Tolić-Nørrelykke I. M., Fabry B. and Fredberg J. J. (2002). Traction fields, moments, and strain energy that cells exert on their surroundings. Am. J. Physiol. Cell Physiol. 282, C595-C605. 10.1152/ajpcell.00270.2001 [DOI] [PubMed] [Google Scholar]
- Caplan A. I. and Bruder S. P. (2001). Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol. Med. 7, 259-264. 10.1016/S1471-4914(01)02016-0 [DOI] [PubMed] [Google Scholar]
- Cesarz Z. and Tamama K. (2016). Spheroid culture of mesenchymal stem cells. Stem Cells Int. 2016, 9176357 10.1155/2016/9176357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury F., Li Y., Poh Y.-C., Yokohama-Tamaki T., Wang N. and Tanaka T. S. (2010). Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS ONE 5, e15655 10.1371/journal.pone.0015655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofalo V. J., Allen R. G., Pignolo R. J., Martin B. G. and Beck J. C. (1998). Relationship between donor age and the replicative lifespan of human cells in culture: a reevaluation. Proc. Natl. Acad. Sci. USA 95, 10614-10619. 10.1073/pnas.95.18.10614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Becker A. and Van Riet I. (2016). Homing and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy? World J. Stem Cells 8, 73-87. 10.4252/wjsc.v8.i3.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A. J., Sen S., Sweeney H. L. and Discher D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell 126, 677-689. 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- Frescas D., Roux C. M., Aygun-Sunar S., Gleiberman A. S., Krasnov P., Kurnasov O. V., Strom E., Virtuoso L. P., Wrobel M., Osterman A. L. et al. (2017). Senescent cells expose and secrete an oxidized form of membrane-bound vimentin as revealed by a natural polyreactive antibody. Proc. Natl. Acad. Sci. USA 114, E1668-E1677. 10.1073/pnas.1614661114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A., Laberge R.-M., Demaria M. and Campisi J. (2012). Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell 23, 2066-2075. 10.1091/mbc.e11-10-0884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P. M., Havenstrite K. L., Magnusson K. E. G., Sacco A., Leonardi N. A., Kraft P., Nguyen N. K., Thrun S., Lutolf M. P. and Blau H. M. (2010). Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078-1081. 10.1126/science.1191035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honczarenko M., Le Y., Swierkowski M., Ghiran I., Glodek A. M. and Silberstein L. E. (2006). Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 24, 1030-1041. 10.1634/stemcells.2005-0319 [DOI] [PubMed] [Google Scholar]
- Jalali S., Tafazzoli-Shadpour M., Haghighipour N., Omidvar R. and Safshekan F. (2015). Regulation of endothelial cell adherence and elastic modulus by substrate stiffness. Cell Commun. Adhes. 22, 79-89. 10.1080/15419061.2016.1265949 [DOI] [PubMed] [Google Scholar]
- Kassem M. (2006). Stem cells: potential therapy for age-related diseases. Ann. N. Y. Acad. Sci. 1067, 436-442. 10.1196/annals.1354.062 [DOI] [PubMed] [Google Scholar]
- Kilian K. A., Bugarija B., Lahn B. T. and Mrksich M. (2010). Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 107, 4872-4877. 10.1073/pnas.0903269107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundrotas G., Gasperskaja E., Slapsyte G., Gudleviciene Z., Krasko J., Stumbryte A. and Liudkeviciene R. (2016). Identity, proliferation capacity, genomic stability and novel senescence markers of mesenchymal stem cells isolated from low volume of human bone marrow. Oncotarget 7, 10788-10802. 10.18632/oncotarget.7456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Abdeen A. A., Kim A. S. and Kilian K. A. (2015). Influence of biophysical parameters on maintaining the mesenchymal stem cell phenotype. ACS Biomater. Sci. Eng. 1, 218-226. 10.1021/ab500003s [DOI] [PubMed] [Google Scholar]
- Lutolf M. P., Gilbert P. M. and Blau H. M. (2009). Designing materials to direct stem-cell fate. Nature 462, 433-441. 10.1038/nature08602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeath R., Pirone D. M., Nelson C. M., Bhadriraju K. and Chen C. S. (2004). Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483-495. 10.1016/S1534-5807(04)00075-9 [DOI] [PubMed] [Google Scholar]
- McGrail D. J., McAndrews K. M. and Dawson M. R. (2013). Biomechanical analysis predicts decreased human mesenchymal stem cell function before molecular differences. Exp. Cell Res. 319, 684-696. 10.1016/j.yexcr.2012.11.017 [DOI] [PubMed] [Google Scholar]
- McMurray R. J., Gadegaard N., Tsimbouri P. M., Burgess K. V., McNamara L. E., Tare R., Murawski K., Kingham E., Oreffo R. O. C. and Dalby M. J. (2011). Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat. Mater. 10, 637-644. 10.1038/nmat3058 [DOI] [PubMed] [Google Scholar]
- Murphy W. L., McDevitt T. C. and Engler A. J. (2014). Materials as stem cell regulators. Nat. Mater. 13, 547-557. 10.1038/nmat3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhuber B., Swanger S. A., Howard L., Mackay A. and Fischer I. (2008). Effects of plating density and culture time on bone marrow stromal cell characteristics. Exp. Hematol. 36, 1176-1185. 10.1016/j.exphem.2008.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurohr G. E., Terry R. L., Lengefeld J., Bonney M., Brittingham G. P., Moretto F., Miettinen T. P., Vaites L. P., Soares L. M., Paulo J. A. et al. (2019). Excessive cell growth causes cytoplasm dilution and contributes to senescence. Cell 176, 1083-1097. 10.1016/j.cell.2019.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio K., Inoue A., Qiao S., Kondo H. and Mimura A. (2001). Senescence and cytoskeleton: overproduction of vimentin induces senescent-like morphology in human fibroblasts. Histochem. Cell Biol. 116, 321-327. 10.1007/s004180100325 [DOI] [PubMed] [Google Scholar]
- Pelham R. J. and Wang Y.-L. (1997). Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 94, 13661-13665. 10.1073/pnas.94.25.13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D., Moorman M. A., Simonetti D. W., Craig S. and Marshak D. R. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284, 143-147. 10.1126/science.284.5411.143 [DOI] [PubMed] [Google Scholar]
- Ranganath S. H., Levy O., Inamdar M. S. and Karp J. M. (2012). Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 10, 244-258. 10.1016/j.stem.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G., Chen X., Dong F., Li W., Ren X., Zhang Y. and Shi Y. (2012). Concise review: mesenchymal stem cells and translational medicine: emerging issues. Stem Cells Transl. Med. 1, 51-58. 10.5966/sctm.2011-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumman M., Majumder A., Harkness L., Venugopal B., Vinay M. B., Pillai M. S., Kassem M. and Dhawan J. (2018). Induction of quiescence (G0) in bone marrow stromal stem cells enhances their stem cell characteristics. Stem Cell Res. 30, 69-80. 10.1016/j.scr.2018.05.010 [DOI] [PubMed] [Google Scholar]
- Saei Arezoumand K., Alizadeh E., Pilehvar-Soltanahmadi Y., Esmaeillou M. and Zarghami N. (2017). An overview on different strategies for the stemness maintenance of MSCs. Artif. Cells Nanomed. Biotechnol. 45, 1255-1271. 10.1080/21691401.2016.1246452 [DOI] [PubMed] [Google Scholar]
- Stem Cell Basics IV. | stemcells.nih.gov (2017).
- Tee S.-Y., Fu J., Chen C. S. and Janmey P. A. (2011). Cell shape and substrate rigidity both regulate cell stiffness. Biophys. J. 100, L25-L27. 10.1016/j.bpj.2010.12.3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse J. R. and Engler A. J. (2010). Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell Biol. 47, 10.16.1-10.16.16. 10.1002/0471143030.cb1016s47 [DOI] [PubMed] [Google Scholar]
- Tuan R. S. (2013). The coming of age of musculoskeletal tissue engineering. Nat. Rev. Rheumatol. 9, 74-76. 10.1038/nrrheum.2012.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah I., Subbarao R. B. and Rho G. J. (2015). Human mesenchymal stem cells - current trends and future prospective. Biosci. Rep. 35, 1-18. 10.1042/BSR20150025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal B., Mogha P., Dhawan J. and Majumder A. (2018). Cell density overrides the effect of substrate stiffness on human mesenchymal stem cells’ morphology and proliferation. Biomater. Sci. 6, 1109-1119. 10.1039/C7BM00853H [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M. A., Walker N. J., Napoli E. and Borjesson D. L. (2012). Evaluation of senescence in mesenchymal stem cells isolated from equine bone marrow, adipose tissue, and umbilical cord tissue. Stem Cells Dev. 21, 273-283. 10.1089/scd.2010.0589 [DOI] [PubMed] [Google Scholar]
- Wagner W., Horn P., Castoldi M., Diehlmann A., Bork S., Saffrich R., Benes V., Blake J., Pfister S., Eckstein V. et al. (2008). Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS ONE 3, e2213 10.1371/journal.pone.0002213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W., Ho A. D. and Zenke M. (2010b). Different facets of aging in human mesenchymal stem cells. Tissue Eng. Part B Rev. 16, 445-453. 10.1089/ten.teb.2009.0825 [DOI] [PubMed] [Google Scholar]
- Wall M. E., Bernacki S. H. and Loboa E. G. (2007). Effects of serial passaging on the adipogenic and osteogenic differentiation potential of adipose-derived human mesenchymal stem cells. Tissue Eng. 13, 1291-1298. 10.1089/ten.2006.0275 [DOI] [PubMed] [Google Scholar]
- Wang L.-T., Ting C.-H., Yen M.-L., Liu K.-J., Sytwu H.-K., Wu K. K. and Yen B. L. (2016). Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J. Biomed. Sci. 23, 76 10.1186/s12929-016-0289-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. S., Ong P. F., Chojnowsky A., Clavel C. and Dressen O. (2017). Loss of lamin B1 is a biomarker to quantify cellular senescence in photoaged skin. Sci. Rep. 7, 15678 10.1038/s41598-017-15901-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer J. P., Janmey P. A., McCormick M. E. and Funaki M. (2009). Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng. Part A 15, 147-154. 10.1089/ten.tea.2007.0388 [DOI] [PubMed] [Google Scholar]
- Yeung T., Georges P. C., Flanagan L. A., Marg B., Ortiz M., Funaki M., Zahir N., Ming W., Weaver V. and Janmey P. A. (2005). Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskelet. 60, 24-34. 10.1002/cm.20041 [DOI] [PubMed] [Google Scholar]
- Zhang D. and Kilian K. A. (2013). The effect of mesenchymal stem cell shape on the maintenance of multipotency. Biomaterials 34, 3962-3969. 10.1016/j.biomaterials.2013.02.029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.