Abstract
Objective
To test whether the assessment of growth in very low birth weight infants during the hospital stay using z-score differences (Zdiff) is confounded by gestational age (GA), birth weight percentiles (BW%ile), and length of the observation period (LOP). We hypothesize that Zdiff calculated from growth charts based on birth weight data introduces a systematic statistical error leading to falsely classified growth as restricted in infants growing similarly to the 50th percentile.
Methods
This observational study included 6,926 VLBW infants from the German Neonatal Network (2009 to 2015). Inclusion criterion was discharge between 37 and 41 weeks postmenstrual age. For each infant, Zdiff, weight gain velocity, and reference growth rate (50th percentile Fenton) from birth to discharge were calculated. To account for gestational age dependent growth rates, assessment of growth was standardized calculating the weight gain ratio (WGR) = weight gain velocity/reference growth rate. The primary outcome is the variation of the Zdiff-to-WGR relationship.
Results
Zdiff and WGR showed a weak agreement with a Zdiff of -0.74 (-1.03, -0.37) at the reference growth rate of the 50th percentile (WGR = 1). A significant proportion (n = 1,585; 23%) of infants with negative Zdiff had weight gain velocity above the 50th percentile’s growth rate. Zdiff to WGR relation was significantly affected by the interaction of GA x BW%ile x LOP.
Conclusion
This study supports the hypothesis that Zdiff, which are calculated using birth weights, are confounded by skewed reference data and can lead to misinterpretation of growth rates. New concepts like individualized growth trajectories may have the potential to overcome this limitation.
Introduction
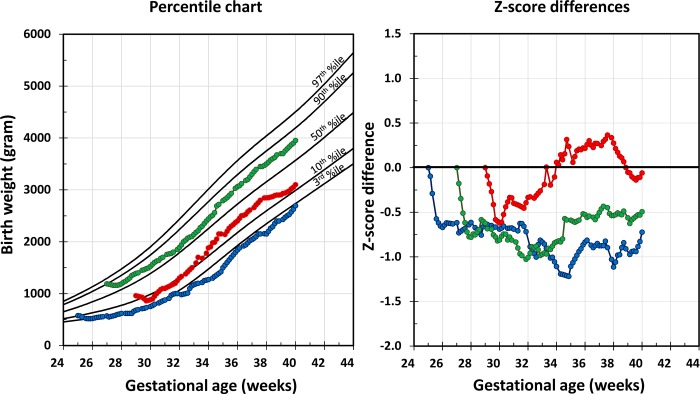
Changes in percentiles or z-scores during defined observation periods, such as from birth to discharge, are commonly used to assess growth of preterm infants. Tools presently applied in clinical routine for monitoring and guiding growth include growth charts and z-score plots (Fig 1).[1–3]
Fig 1.
Common tools for the assessment of growth: percentile charts (left panel) and z-score plots (right panel), modified from Fenton et al. 2013 and Griffin et al. 2014; colored lines and dots represent growth trajectories of three example infants.
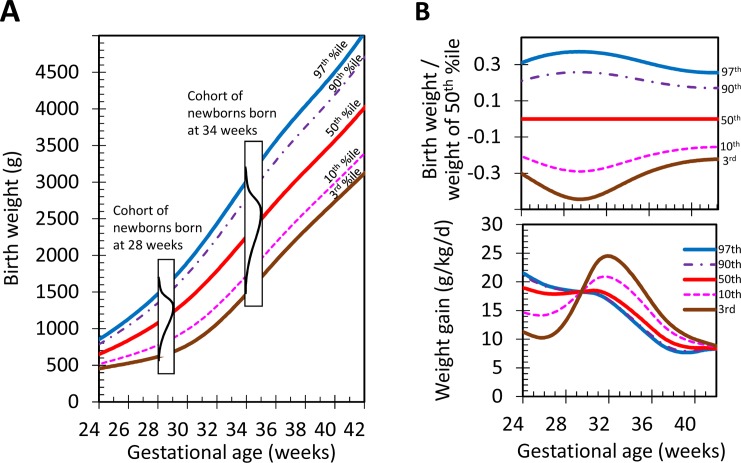
Cross-sectional data from pregnancies with known birth weights and gestational ages serve as the basis for percentile growth charts. A percentile is defined as the birth weight value below which a given percentage of observations in a selected cohort can be found. For example, the 10th percentile for a gestational age of 28 weeks is the birth weight value below which 10% of the neonates in the cohort fall at 28 weeks. Percentiles of birth weights for each gestational week are calculated using datasets of infants born at given weeks (e.g. in the 27th, 28th or 34th week) (Fig 2).[4]
Fig 2.
2A: Plotting of growth charts and calculation of z-scores, distribution (black line) of birth weights for a cohort of newborns with a gestational age of 28 and 34 weeks; 2B: Characteristics of growth charts: skewness of percentile distributions (top) and weight gain velocity for major percentiles (bottom).
Once birth weight percentiles have been built from the different cohorts, a growth chart is subsequently created by connecting these percentile values across gestational ages (e.g. from 24 to 42 weeks). However, linking distributions of percentile values between the weeks of gestation results in trajectories that are not representative of physiological growth.[5] Nonetheless, these trajectories are used in clinical practice as physiological growth curves in order to monitor growth and adjust feeding.
Z-scores are another commonly used parameter to compare individual weights with the cohort.[2, 6] A z-score (or a standard deviation score) is the number of standard deviations an individual weight is above or below the mean birth weight value. The z-score for a particular gestational age and sex is defined as:
For instance, a z-score of -1 depicts an individual birth weight that is one standard deviation below the mean birth weight of the cohort. Z-score differences (Zdiff) are used to analyze changes in growth for a particular observation period. Zdiff is defined as:
Z-scores and standard deviations have been defined for normally distributed data. If the distribution of birth weight data at different gestational ages is skewed, the accuracy of the z-score during particular observation periods, and thus its utility in monitoring growth, are impacted. This becomes even more significant if the skewness changes during the observation period.
Unfortunately, varying degrees of skewness across gestational ages is significantly present in most birth weight charts used for growth analyses nowadays. For the preterm period, the datasets inherently include a substantially high number of pregnancies and preterm births that are affected by pathologies, thus leading to the preterm termination of the pregnancy. This severely impacts the accuracy of the fetal growth data, resulting in a skewed distribution of birth weight percentiles mostly towards lower percentiles. As can be seen in Fig 2, Panel B (top), the relative distance between percentiles and standard deviations (standard deviation/mean) varies over the range of gestational ages. For example, the relative distance between the 3rd percentile (brown line) and the 50th percentile (red line) ((50th percentile - 3rd percentile) / 50th percentile) increases up to 29 weeks, followed by a decrease until term age.[1, 3, 6] Despite this unexplained variation, target growth trajectories of preterm infants are estimated by using these percentile lines in routine neonatal care.
Fig 2, Panel B (bottom) depicts a set of resulting weight gain velocities (g/kg/d) when growth trajectories follow major percentiles (3rd, 10th, 50th, 90th and 97th) on standard growth charts.[1] Considerable fluctuation in weight gain velocity can be seen, especially when following the 3rd and the 10th percentile curves. From a physiological perspective however, it is not evident why an infant should undergo such a weight gain velocity fluctuation during the last trimester of gestation. Moreover, the cause for the inverse order of the fluctuation seen for the 3rd and 10th percentile compared to the curves of the 50th or the 90th percentiles is unclear. These findings illustrate that the variation of skewness of percentiles across gestational ages might impact the accuracy of the Zdiff calculation.
We hypothesize that applying the current Zdiff approach will introduce a systematic error and may indicate growth restriction in preterm infants that are in fact growing at median rates of reference charts. Therefore, the objective of the current study is to investigate whether the assessment of growth of preterm infants by Zdiff is affected by the parameters gestational age, birth weight percentile, or by the length of the observation period. Mathematical models will be used to test the impact of the aforementioned three parameters on the Zdiff.
Methods
This observational study was performed using a data set of very low birth weight (VLBW, birth weight < 1,500 g) infants from the German Neonatal Network (2009 to 2015). All VLBW infants that were discharged between 37 to 41 weeks were included in this study. The study was approved by the Hamilton Integrated Research Ethics Board (HiREB) (2016-1696-C). The data were anonymized, and ethics committee waived the requirement for informed consent for this retrospective study.
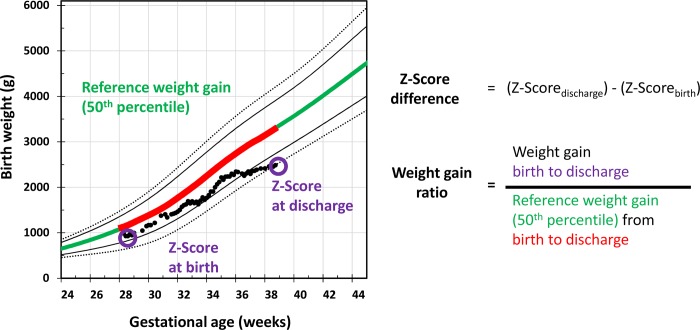
For each infant, the z-scores for weight at birth and weight at discharge were obtained using the LMS parameter from the Fenton chart to calculate the Zdiff (Fig 3).[1]
Fig 3.
Method for the analysis of z-score differences in relation to weight gain velocity; the left panel shows how the reference data for weight gain velocity and z-score calculation were selected; the right panel shows the corresponding equations for z-score difference and weight gain ratio calculation.
The z-scores were calculated using the equation:[7]
In order to account for gestational age dependent growth rates, assessment of growth was standardized by calculating the individual weight gain ratio (WGR). Adopted from recent approaches to growth analysis, the median weight data of intrauterine growth charts were used to standardize the WGR.[8] The WGR was defined as a ratio of the individual weight gain velocity from birth (t1) to discharge (t2) divided by weight gain velocity from reference data (50th percentile) for the same gestational age period (Fig 3).
The individual weight gain velocity as well as the reference weight gain velocity were calculated using the following equation:[9]
WGR is described using the following equation:
The reference data for the 50th percentile used were obtained from the Fenton growth chart 2013.[1]
The primary outcome is the degree of variation of the Zdiff-to-WGR relationship (homogeneity).
The effects of gestational age, birth weight percentile, and length of the observation period on the Zdiff were analyzed using regression models employing the r statistic function lm.
Model 1 tested the overall relationship between Zdiff and WGR using a linear regression analysis.
Model 2 analyzed the effects of the three parameters (gestational age (GA), birth weight percentile (BW%ile), and length of the observation period (LOP)) on the correlation with Zdiff.
The third model tested whether WGR, GA, BW%ile, and LOP affects the relationship between the Zdiff and the WGR. This model tested whether the interaction of the parameters had an amplified effect, compared to when the parameters were considered independently (Model 2). It was hypothesized that Zdiff are non-linearly related to WGR, GA, BW%ile, and LOP. Model 3 was defined as follows:
The difference between the models was analyzed using ANCOVA. The level of significance is p<0.05. The analysis was assisted by R software package for statistical analysis, R Foundation for Statistical Computing, version 3.5.0 (2018-04-23), Vienna, Austria.
Results
This study was comprised of 6,926 (male n = 3,461; 50%) VLBW infants. The patient characteristics are outlined in Table 1.
Table 1. Patient characteristics.
| All | Gestational age group | ||||
|---|---|---|---|---|---|
| ≤27 weeks | 28–31 weeks | ≥32 weeks | |||
| Birth (t1) | N | 6926 | 2539 | 3293 | 1094 |
| GA (weeks) | 29.0 ± 2.8 | 26.1 ± 1.2 | 29.8 ± 1.1 | 33.3 ± 1.1 | |
| Weight (g) | 1070 ± 280 | 820 ± 200 | 1170 ± 220 | 1340 ± 150 | |
| SGA n (%) | 1594 (23.0) | 297 (11.7) | 497 (15.1) | 800 (73.1) | |
| LGA n (%) | 168 (2.4) | 140 (5.5) | 28 (0.9) | 0 (0) | |
| z-score birth (t1) | -0.5 ± 1.0 | -0.1 ± 0.9 | -0.5 ± 0.7 | -1.7 ± 0.6 | |
| Length (cm) | 36.6 ± 3.6 | 33.6 ± 2.8 | 37.9 ± 2.8 | 39.8 ± 2.4 | |
| HC (cm) | 25.9 ± 2.5 | 23.6 ± 1.9 | 26.8 ± 1.7 | 28.5 ± 1.3 | |
| Discharge (t2) | PMA (weeks) | 38.7 ± 1.3 | 39.2 ± 1.4 | 38.5 ± 1.2 | 38.4 ± 1.1 |
| Weight (g) | 2620 ± 450 | 2780 ± 480 | 2630 ± 410 | 2250 ± 290 | |
| z-score difference (t2-t1) | -0.9 ± 0.8 | -1.2 ± 1.0 | -0.8 ± 0.7 | -0.5 ± 0.5 | |
| Weight gain velocity (t2-t1) (g/kg/d) | 13.8 ± 2.4 | 13.6 ± 2.0 | 13.7 ± 2.4 | 14.7 ± 2.8 | |
| Weight gain ratio | 1.0 ± 0.2 | 0.9 ± 0.1 | 0.9 ± 0.2 | 1.2 ± 0.3 | |
| Length (cm) | 46.2 ± 2.7 | 46.6 ± 2.9 | 46.4 ± 2.6 | 44.7 ± 2.3 | |
| HC (cm) | 33.0 ± 1.7 | 33.2 ± 1.8 | 33.2 ± 1.6 | 32.2 ± 1.4 | |
| Outcome | Sepsis | 1884 (27.2) | 678 (26.7) | 883 (26.8) | 323 (29.5) |
| IVH grade 3–4 | 371 (5.4) | 148 (5.8) | 161 (4.9) | 62 (5.7) | |
| ROP grade 3–5 | 330 (4.8) | 111 (4.4) | 172 (5.2) | 47 (4.3) | |
| NEC stage 2–3 | 143 (2.1) | 91 (3.6) | 42 (1.3) | 10 (0.9) | |
| severe BPD | 278 (4) | 104 (4.1) | 140 (4.3) | 34 (3.1) | |
GA-gestational age, PMA-postmenstrual age, HC-head circumference, t1 –birth, t2 –discharge
When using the linear regression model (Model 1), the following relationship between Zdiff and WGR was obtained:
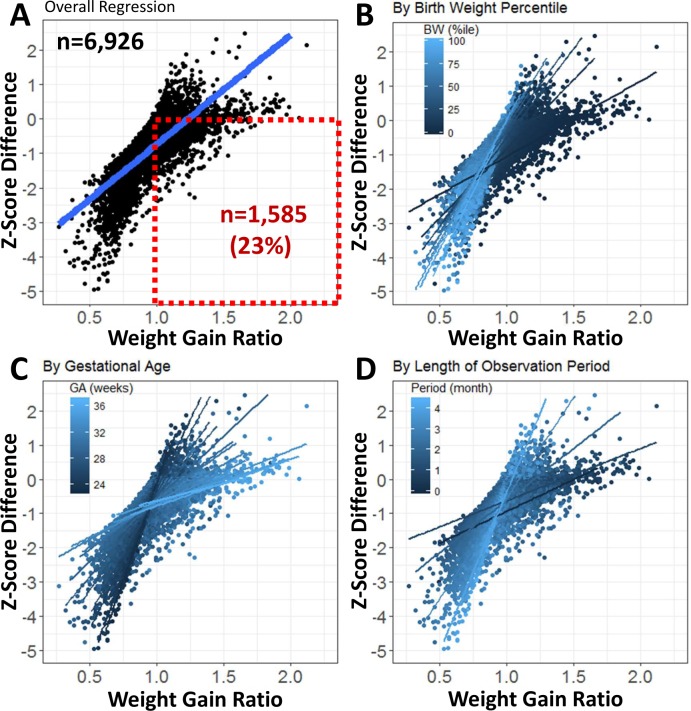
This analysis revealed that at a WGR = 1, which should correspond to a growth rate following the 50th percentile, the Zdiff was -0.74 at the regression line. The first and third quartiles of Zdiff were -1.03 and -0.37, respectively. As can be seen in Fig 4, Panel A, there is a significant proportion of infants n = 1,585 (23%) which show a WGR > 1, indicating growth faster than the 50th percentile, when Zdiff is calculated, the result is negative. In contrast, the number of infants with WGR ≤ 1 and positive Zdiff is 18 (0.3%).
Fig 4. Longitudinal assessment of growth: Zdiff versus WGR relationship.
Scatter plots show individual data and the regression line calculated for the complete data set (panel A), as well as a set of regression lines stratified by birth weight (panel B), gestational age (panel C) and length of observation period (panel D), colours represents stratification for four different parameters as indicated in legends. Dotted red box (panel A) shows proportion of infants which show a WGR>1 and negative z-score.
This subset of infants is highlighted by the dotted red box in Fig 4, Panel A. These infants are growing at the median rate, but would be classified as growth restricted because of this negative Zdiff. Moreover, there was also a high inter-individual variation in the Zdiff of infants with the same WGR. The Zdiff differed by up to 4 z-scores for the same WGR (Fig 4).
The shape of the residuals plot of the linear model (Model 1) demonstrated a non-random distribution with a residuals standard error of 0.56 Zdiff (S1 Fig) indicating that additional factors affect the Zdiff and that the Zdiff is not linearly related to the WGR.
Fig 4 shows the effect of gestational age (GA), birth weight percentile (BW%ile), and length of the observation period (LOP) on the Zdiff-to-WGR relationship. The slope of the regression lines for the infants with lower GA, BW%ile, and shorter LOP is steeper, which translates into larger changes of Zdiff with changes in WGR.
Model 2 revealed that gestational age, birth weight percentile, and length of the observation period have significant effects on the Zdiff (R2 = 0.60; p<0.0001). The comparison of Model 1 and Model 2 by ANCOVA showed a significant improvement of Zdiff prediction by Model 2 (F value = 236; p<0.0001).
Model 3 demonstrated that the interaction between the parameters (WGR, gestational age, birth weight percentile and length of the observation period) significantly affected the Zdiff (R2 = 0.95; p<0.0001).
The ANCOVA analysis revealed an increase in the explanatory power of Model 3 over 2 with an F value of 4,308 and a small p-value (p<0.0001). This indicates that Model 3 predicted Zdiff closer to the actual values in comparison to Model 2. The variation of the residuals also decreased when Model 3 was applied compared to Model 2. Thus, the interaction of the variables WGR, GA, BW%ile and length of the observation period has a stronger effect on the Zdiff, compared to when these parameters are considered independently.
In summary, these mathematical findings imply that the relation between Zdiff and WGR is dependent on the variables gestational age, birth weight percentile, and length of the observation period. The analysis showed no sex-specific effects.
Discussion
In this study, we found that z-score differences (Zdiff) and weight gain velocity from birth to discharge do not correlate well in a significant portion of in VLBW infants. This finding supports the hypothesis that the approach of using Zdiff to assess growth in preterm infants is confounded by skewed reference data and thus, does not provide an accurate reflection of growth. Gestational age, birth weight percentile, and the length of the observation period have significant effects on the Zdiff. The influence of these factors is supported by statistically significant mathematical findings. Thus, an unadjusted Zdiff cannot be accurately translated into growth rates or growth trajectories.
One explanation for the study’s findings could be that there is a significant variation of the standard deviation of birth weight data from early preterm to term age (Fig 2), leading to the paradoxical phenomenon that infants with the same growth rate have different z-scores. Between 28 and 30 weeks, the relative standard deviation (standard deviation divided by the mean) is twice the relative standard deviation at term gestational age.[3, 10] This widening affects the Zdiff calculation. For instance, assuming a mean weight of 1,000 g and a standard deviation of 200 g (~20%) at 28 weeks, an infant with a weight of 0.8 of the mean weight (800 g) would have a z-score of -1 (800 g– 1,000 g / 200 g). If this infant were to grow with the growth rate of the 50th percentile, the infant would reach 0.8 of the mean weight by term age (2,720 g). The standard deviation at term age would be about half (~10% of the mean weight; 340 g). The z-score of this infant at term age would then be -2 (2,720 g– 3,400 g / 340 g) and the Zdiff would thus be -1. Another infant born at the mean weight and also growing with the growth rate of the 50th percentile would have a z-score of 0 (1,000 g– 1,000 g / 200 g) at 28 weeks and a z-score of 0 at term age (3,400 g—3,400 g / 340 g), resulting in a Zdiff of 0 (S1 Table). Thus, even though both these infants are growing at the same rate, the z-score and Zdiff are different, illustrating that reliance solely on Zdiff for growth prediction and feeding adjustments is problematic.
In order for an infant to maintain an unchanged z-score, the infant would need to grow according to the fluctuating pattern of percentiles or standard deviations across the gestational weeks as depicted in Fig 2, Panel B (bottom). The variation is significant.[11] When expressed in percentiles, at 24 weeks of gestation, the ratio between the 3rd and the 50th percentile is 0.66. At 29, 34 and 40 weeks however, it is 0.57, 0.64 and 0.72, respectively. Refer to Fig 2, Panel B (top). There is, however, no physiological explanation justifying an infant following a fluctuating growth trajectory such as the ones observed for the 3rd or the 10th percentiles. This observation supports the claim that the Zdiff calculation and resulting Zdiff are dependent on the gestational age, and also on when the observation period for the given infant is complete.
The physiological condition of postnatal adaptation is not reflected by the z-score approach. Postnatal adaptation includes weight loss during the first few days of life, mostly due to an irreversible, one-time loss of extracellular water volume.[12–14] As can be seen in Fig 2, Panel A, absolute distances (in grams) between the birth weight percentiles for gestational age are similar between major percentiles (see distances between the 90th and 97th percentile, or between the 3rd and 10th percentile).[13, 14] During the period of postnatal adaptation, relative weight loss expressed as a percentage of birth weight has been found to be similar across infants of the same gestational age. Therefore, infants born at the same gestational age but in higher birth weight percentiles will cross more percentiles during the period of postnatal adaptation as compared to infants born at lower birth weight percentiles. An issue arises because in the z-score calculation, a fixed value for the standard deviation is applied to infants of different birth weights, experiencing a similar relative weight loss (e.g., 10%). For instance, an infant at the 97th percentile with a birth weight of 1,430 g would undergo the 10% physiological weight loss, resulting in a weight of 1,290 g (74th percentile). This infant has thus crossed 23 percentiles. Another infant at the 10th percentile with a birth weight of 700 g undergoing the same 10% weight loss would result in a weight of 630 g (3rd percentile). This infant has thus crossed only seven percentiles. The Zdiff would consequently be higher from birth to day 5 of life in infants with higher birth weight percentiles. Thus, this finding supports the hypothesis that birth weight percentile impacts Zdiff.
In summary, the relation between Zdiff and weight gain velocity is weak when Zdiff is calculated within the postmenstrual age range from preterm (e.g. 24 weeks) to term age. This was shown in the current study using a large cohort of very low birth weight infants which stayed an average of 5 to 13 weeks in the NICU. We have identified that this finding is determined by the characteristics of the reference data used for z-score calculations. The reference data are cross-sectional birth weight data, which were developed to assess the nutritional status at birth. Employing these birth weight data for the analysis of postnatal growth diverges from the intended use of the data and introduces systematic errors in growth analysis.
The findings suggest that a novel approach to assess growth and rates of growth is necessary. A promising approach would be to apply the concept of individualized growth trajectories as recently proposed.[12, 15, 16] An individualized growth trajectory combines physiological considerations and data established for the different periods of growth after preterm birth. These periods include: 1) intrauterine growth until birth, 2) the physiological postnatal weight loss mainly due to contraction of extracellular water spaces and adjustment to the new postnatal trajectory, 3) stable growth and 4) transition to healthy term infant equivalent trajectories at post-term age. Individual growth trajectories would allow clinicians to calculate the absolute deviation, in grams, from the predicted weight trajectory at any given point in time. This would indicate to the clinician how well an individual infant is growing and allow for prompt adjustment of nutrition to achieve optimal growth.[12, 15, 16]
In conclusion, the calculation of z-scores, percentiles and Zdiff is dependent on the reference data. When birth weight data are used to assess longitudinal growth, the interpretation should be done with caution. Individualized growth trajectories are a promising alternative. Nonetheless, their use in clinical practice requires validation.
Supporting information
(DOCX)
(DOCX)
Data Availability
The study was approved by the Hamilton Integrated Research Ethics Board (HiREB) at McMaster University. To comply with the privacy legislation and data security, the access to the data is restricted by Hamilton Integrated Research Ethics Board. Data access can be requested from German Neonatal Network office, Luebeck, Germany (contact via email: Maria.Klingenberg@uksh.de).
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC pediatrics. 2013;13:59 10.1186/1471-2431-13-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffin IJ. Perinatal Growth and Nutrition: CRC Press; 2014. [Google Scholar]
- 3.Voigt M, Rochow N, Schneider KT, Hagenah HP, Scholz R, Hesse V, et al. [New percentile values for the anthropometric dimensions of singleton neonates: analysis of perinatal survey data of 2007–2011 from all 16 states of Germany]. Z Geburtshilfe Neonatol. 2014;218(5):210–7. 10.1055/s-0034-1385857 . [DOI] [PubMed] [Google Scholar]
- 4.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002;(246):1–190. . [PubMed] [Google Scholar]
- 5.Lampl M, Thompson AL. Growth chart curves do not describe individual growth biology. Am J Hum Biol. 2007;19(5):643–53. 10.1002/ajhb.20707 . [DOI] [PubMed] [Google Scholar]
- 6.Kramer MS, Platt RW, Wen SW, Joseph KS, Allen A, Abrahamowicz M, et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108(2):E35 . [DOI] [PubMed] [Google Scholar]
- 7.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11(10):1305–19. Epub 1992/07/01. . [DOI] [PubMed] [Google Scholar]
- 8.Gliozzi AS, Guiot C, Delsanto PP, Iordache DA. A novel approach to the analysis of human growth. Theor Biol Med Model. 2012;9:17 Epub 2012/05/19. 10.1186/1742-4682-9-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel AL, Engstrom JL, Meier PP, Jegier BJ, Kimura RE. Calculating postnatal growth velocity in very low birth weight (VLBW) premature infants. J Perinatol. 2009;29(9):618–22. 10.1038/jp.2009.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voigt M, Rochow N, Straube S, Briese V, Olbertz D, Jorch G. Birth weight percentile charts based on daily measurements for very preterm male and female infants at the age of 154–223 days. J Perinat Med. 2010;38(3):289–95. 10.1515/JPM.2010.031 . [DOI] [PubMed] [Google Scholar]
- 11.Fenton TR, Sauve RS. Using the LMS method to calculate z-scores for the Fenton preterm infant growth chart. Eur J Clin Nutr. 2007;61(12):1380–5. 10.1038/sj.ejcn.1602667 . [DOI] [PubMed] [Google Scholar]
- 12.Rochow N, Raja P, Liu K, Fenton T, Landau-Crangle E, Gottler S, et al. Physiological adjustment to postnatal growth trajectories in healthy preterm infants. Pediatr Res. 2016. 10.1038/pr.2016.15 . [DOI] [PubMed] [Google Scholar]
- 13.Bauer K, Versmold H. Postnatal weight loss in preterm neonates less than 1,500 g is due to isotonic dehydration of the extracellular volume. Acta Paediatr Scand Suppl. 1989;360:37–42. . [DOI] [PubMed] [Google Scholar]
- 14.Shaffer SG, Bradt SK, Meade VM, Hall RT. Extracellular fluid volume changes in very low birth weight infants during first 2 postnatal months. J Pediatr. 1987;111(1):124–8. . [DOI] [PubMed] [Google Scholar]
- 15.Landau-Crangle E, Rochow N, Fenton TR, Liu K, Ali A, So HY, et al. Individualized postnatal growth trajectories for preterm infants JPEN J Parenter Enteral Nutr. 2018. [DOI] [PubMed] [Google Scholar]
- 16.Rochow N, Landau-Crangle E, Thommandram A, Fusch C. Individualized Postnatal Growth Trajectory for Preterm Infants—Online Calculator 2016. [2016-10-22]. Available from: http://www.growthcalculator.org/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
The study was approved by the Hamilton Integrated Research Ethics Board (HiREB) at McMaster University. To comply with the privacy legislation and data security, the access to the data is restricted by Hamilton Integrated Research Ethics Board. Data access can be requested from German Neonatal Network office, Luebeck, Germany (contact via email: Maria.Klingenberg@uksh.de).