Abstract
Background
Insulin resistance and related metabolic disturbances are major risk factors for the higher T2D risk and associated morbidity and mortality amongst South Asians. The contribution of physical activity to the increased prevalence of insulin resistance and related disturbances amongst South Asians is unknown.
Methods
We recruited 902 South Asian and European men and women, aged 35–85 years from the ongoing LOLIPOP study. Clinical characterisation comprised standardised questionnaire and measurement of height, weight, waist and hip circumference and blood pressure. Fasting bloods were taken for assessment of glucose, insulin, lipids and HbA1c. Physical activity was quantified using a validated accelerometer, Actigraph GT3X+, worn for 7 days. Univariate and multivariate approaches were used to investigate the relationship between ethnicity, physical activity, insulin resistance and related metabolic disturbances.
Results
Total physical activity was ~31% (P = 0.01) lower amongst South Asians compared to Europeans (Mean MET.minutes [SD]: 1505.2 [52] vs. 2050.9 [86.6], P<0.001). After adjusting for age and sex, total physical activity had a negative association with HOMA-IR (B [SE]: -0.18 [0.08], P = 0.04) and fasting glucose levels (B[SE]: -0.11 [0.04], P = 0.02). There was no association between physical activity and other glycemic and lipid parameters. Total physical activity per week contributed towards the differences in insulin resistance and associated metabolic disturbances between South Asians and Europeans.
Conclusion
Lower levels of physical activity may contribute to the increased insulin resistance in South Asians compared to Europeans. Our results suggest that lifestyle modification through increased physical activity may help to improve glucose metabolism and reduce the burden of excess T2D and related complications amongst South Asians.
Background
People of South Asian ancestry (originating from India, Pakistan, Sri lanka and Bangladesh) are at high risk of type 2 diabetes (T2D). According to the IDF report 2015, worldwide there were an estimated 415 million South Asians with T2D in 2015, and this number is expected to increase to 642 million by 2040. Population studies from the non-migrant South Asians show that T2D affects ~15% of urban South Asians, compared to ~5% of their rural counterparts[1]. In the UK, prevalence of T2D amongst South Asians is reported to be up to three times higher than in the native European population (22% vs. 7%)[2].
The increased risk of T2D amongst South Asians is closely associated with the presence of insulin resistance and related metabolic disturbances (raised fasting insulin and glucose, central adiposity, high triglycerides and low high density lipid [HDL] cholesterol)[3]. Population studies report a higher prevalence of insulin resistance and related metabolic disturbances amongst South Asians compared to Europeans[2, 4]. This higher risk of insulin resistance and metabolic disturbances amongst UK South Asians is not explained by differences in common genetic variants[5], adiposity[6] or dietary macronutrient intake[7].
Observational and prospective studies show that increased physical activity is associated with improvement in insulin sensitivity and reduction in insulin resistance and related metabolic disturbances[8–12]. A reduction of ~7% (P<0.05) in fasting insulin levels has been reported for a 30-minute increase in moderate-intensity physical activity[13]. In addition, physical activity is reported to be associated with improvements in glucose metabolism, reduction in triglyceride concentration and an increase in high-density lipoprotein (HDL)[14, 15], even in the absence of weight loss[16, 17].
Cross-sectional studies in the UK report that South Asians have lower levels of physical activity than Europeans[18–20], raising the possibility that low levels of physical activity may contribute to the increased risk of T2D in South Asians. However, published studies have almost all used physical activity questionnaires, an approach known to be of limited accuracy and confounded by reporting bias[21]. Moreover, none of the questionnaires used for measuring physical activity levels have been validated for use amongst South Asians. Inaccurate quantification of physical activity levels amongst South Asians is a significant limitation to understanding the potential contribution of physical activity levels towards the higher risk of insulin resistance, associated metabolic disturbances and T2D in South Asians compared to Europeans.
Accelerometers are electronic sensors for objective measurement of physical activity, validated for use in free-living settings[22]. Compared to direct calorimetry and doubly-labelled water, accelerometers provide substantially greater accuracy for quantification of energy expenditure during physical activity than subjective methods (diaries, self-reports, questionnaires). In this study, we quantified physical activity by using accelerometer amongst South Asian and European men and women, to compare physical activity levels between the two populations, and to determine the contribution of physical activity to the higher prevalence of insulin resistance and related metabolic disturbances in South Asians.
Methods
Participants
We recruited 902 South Asian and European men and women, aged 35–85 years, from Dec 2013 till Oct 2015 to the study. All participants were recruited from the ongoing London Life Sciences Prospective Population (LOLIPOP) study amongst South Asians and Europeans. South Asians were defined as having all 4 grandparents born on the Indian subcontinent (India, Pakistan, Sri lanka and Bangladesh); Europeans were of self-reported white ancestry and born in the UK. People with musculoskeletal disorders which impaired habitual physical activity were excluded from the study. This study was approved by the London-Fulham Research Ethics Committee (ref 07/H0712/150), and all participants gave an informed written consent.
Clinical characterisation
Participants attended a dedicated research clinic between 0900h and 1200h following an overnight fast. An interview-administered questionnaire was used to collect data on medical history, family history, current medications, personal and social history. Weight, height, waist circumference, hip circumference and blood pressure were measured by standardized protocols. Weight was measured to the nearest 0.1kg using digital scales mounted on a hard and flat surface. Height, waist and hip circumference were measured to the nearest 0.1cm. Height was measured using a stadiometer, mounted on a hard and flat surface. Waist circumference was defined as the point midway between the iliac crest and lowest rib. Hip circumference was defined as the maximum circumference over the greater trochanters and buttocks. Blood pressure was measured using an OMRON 705CP blood pressure monitor. Three measurements were taken and an average of these was documented.
Fasting venous blood samples were collected for glucose, insulin, lipid profile, and HbA1c. Blood samples were analysed in the Clinical Chemistry laboratories at Ealing Hospital NHS Trust, London, UK using the commercially available assays (S1 Table). Insulin resistance was assessed using the homeostatic model assessment (HOMA- IR), where HOMA-IR = [Glucose (mmol/L) x Insulin (mU/L)] / 22.5. HOMA-IR is a highly validated measure of insulin resistance which correlates closely with the gold standard euglycaemic-clamp method (r = 0.8, P<0.05)[23] for measurement of insulin resistance.
Assessment of physical activity
Physical activity was measured objectively using Actigraph GT3X+, a tri-axial accelerometer that has been validated for assessment of free-living physical activity in Europeans, through comparison with gold standard doubly-labelled water and calorimetry. All participants were asked to wear the accelerometer for 7 consecutive days and nights (while sleeping), and only remove it during water based activities (swimming or bathing). The accelerometer was worn on the waist, using an elasticated belt. Participants were contacted on alternate days to ensure they were wearing the device. The accelerometers were returned at the end of the 7-day study period.
Analysis of physical activity data
Accelerometer data were analysed using the manufacturer software (Actilife version 6.10.4). Non-wear time was identified and removed from analysis using the Troiano et al algorithm[24]. The algorithm identified periods of consecutive 60 minutes with zero acceleration recorded allowing a spike tolerance of 2 minutes, indicating that the device had not been worn during that hour. Participants who had worn the accelerometer for less than 7 days and for less than 10 hours per day (averaged over the 7 days) were excluded from final analysis.
Raw acceleration measured by the Actigraph GT3X+ was converted into counts, based on the vector magnitude (i.e. the sum of acceleration in all three axes). Counts are directly proportional to the frequency and intensity of the raw acceleration and hence the physical activity performed. Counts were used to classify data collected from the accelerometer into level of energy expenditure. The cut points defining different physical activity intensities were: (i) Light: 0–2690 counts per minute (CPM), (ii) Moderate: 2691–6166 CPM, (iii) Vigorous: 6167–9642 CPM, (iv) Very Vigorous: 9643 - ∞ CPM. Time (minutes per week) spent in different physical activity intensity categories was based on the above-mentioned cut points.
The Freedson VM3 Combination (2011) algorithm was used to calculate kilocalories consumed during physical activity over the 7-day period. This option combines the Freedson VM3 (2011) formula[25] with the Williams Work-Energy (1998) equation[26]. Freedson VM3 (2011) equation uses vector magnitude to estimate energy expenditure, which is only valid if the epoch counts exceed 2453 per minute. For counts less than 2453 per minute, Williams Work-Energy (1998) equation is used.
Metabolic Equivalents (METs) per minute were calculated from vertical axis counts using the Freedson adult (1998) equation[27]. Mets were then summed over the recording period to calculate MET.minutes in total physical activity over the 7-day period. MET.minutes were also calculated for light (METs<3), moderate (3–6 METS) and vigorous (METS>6) physical activity performed over the 7-day period.
Statistical analysis
Statistical analyses were performed using SPSS version 22. Continuous data was expressed as mean ± standard deviation (SD), and categorical data as percentage (%). Distribution of the study variables was assessed using histograms. Univariate tests of the differences were carried out between South Asians and Europeans using the independent samples t-test and chi squared test for continuous and categorical variables respectively. Pearson’s correlation co-efficient was used for initial univariate assessment of the relationships between continuous variables. Finally, multivariate linear regression was used to investigate the relationships between ethnicity, physical activity, insulin resistance and related metabolic disturbances, taking account of potential confounding effects. All participants with type 2 diabetes were excluded from primary analysis.
Results
Characteristics of participants
We studied 902 South Asian and European men and women. Out of these, 219 participants did not wear accelerometer for at least 10 hours per day for 7 days and were excluded from analysis. This left 683 participants with complete data (183 Europeans and 500 South Asians), who were used for all analyses.
Clinical characteristics of the participants are shown in Table 1. South Asians were on average younger than Europeans (Mean [SD]: 53 [9] vs. 59 [10], P<0.001). Body mass index was similar (P = 0.3), but waist hip ratio higher in South Asians compared to Europeans (Mean [SD]: 0.95 [0.08] vs. 0.92 [0.08], P<0.001). South Asians had higher HOMA-IR, fasting glucose, HbA1c, and fasting insulin compared to Europeans (all P<0.001). Triglyceride levels were higher while HDL cholesterol was lower in South Asians than in Europeans (P<0.001). The prevalence of T2D was ~3 times higher in South Asians compared to Europeans (17.7% vs. 5.9%, P<0.001). Cigarette smoking and alcohol intake was lower amongst South Asians compared to Europeans (P<0.001).
Table 1. Clinical characteristics of South Asian and European participants.
Results are presented as mean (SD) and percentages (%) for continuous and categorical variables respectively. P-values are determined from the independent student t-test (continuous variables) and Fisher’s exact test (categorical variables).
| Europeans | South Asians | ||
|---|---|---|---|
| Mean (SD) | Mean (SD) | p | |
| N | 183 | 500 | |
| Age (years) | 59.3 (10.1) | 56.4 (9.0) | <0.001 |
| Male (%) | 42.7 | 53.1 | 0.02 |
| Type 2 Diabetes (%) | 5.9 | 17.7 | <0.001 |
| Hypertension (%) | 25.9 | 35.7 | 0.01 |
| Coronary heart disease (%) | 6.5 | 8.3 | 0.4 |
| Height (cm) | 167.9 (8.8) | 164.0 (9.4) | <0.001 |
| Weight (kg) | 78.7 (15.3) | 74.1 (14.5) | <0.001 |
| Body mass index (kg/m2) | 27.8 (4.9) | 27.4 (4.6) | 0.3 |
| Waist circumference (cm) | 96.3 (12.6) | 96.9 (11.7) | 0.5 |
| Hip circumference (cm) | 104.3 (9.2) | 101.9 (8.4) | 0.001 |
| Waist-hip ratio | 0.92 (0.08) | 0.95 (0.08) | <0.001 |
| Systolic blood pressure (mmHg) | 131.3 (17.6) | 132.6 (17.4) | 0.4 |
| Diastolic blood pressure (mmHg) | 78.0 (9.9) | 79.2 (10.1) | 0.2 |
| Cholesterol (mmol/l) | 5.20 (0.97) | 4.95 (1.01) | 0.002 |
| Triglycerides (mmol/l) | 1.18 (0.55) | 1.46 (0.86) | <0.001 |
| HDL cholesterol (mmol/l) | 1.69 (0.45) | 1.44 (0.42) | <0.001 |
| LDL cholesterol (mmol/l) | 2.98 (0.91) | 2.85 (0.90) | 0.07 |
| Glucose (mmol/l) | 5.28 (1.08) | 5.87 (1.87) | <0.001 |
| HbA1c (%) | 5.70 (0.66) | 6.21 (1.16) | <0.001 |
| Insulin (mmol/l) | 9.79 (6.16) | 13.59 (9.39) | <0.001 |
| HOMA-IR (mmol/l) | 2.38 (1.71) | 3.71 (3.10) | <0.001 |
Physical activity levels in South Asians and Europeans
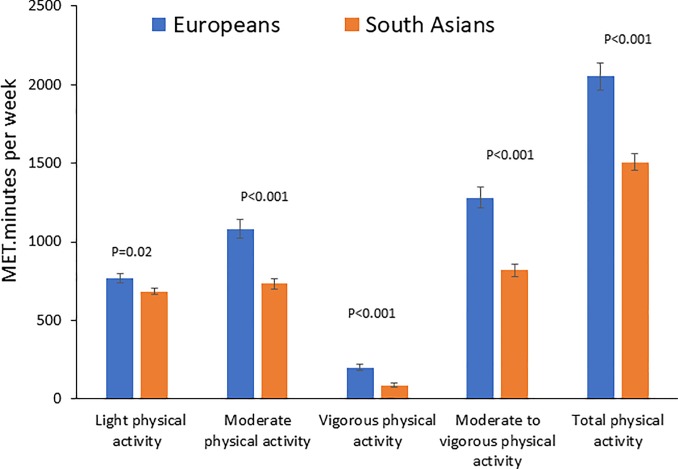
Physical activity levels amongst South Asians and Europeans are summarised in Fig 1. Total physical activity levels expressed as MET.minutes per week were ~31% lower amongst South Asians than in Europeans (Mean MET.minutes [SD]: 1505.2 [52] vs. 2050.9 [86.6], P<0.001). In addition, compared to Europeans, South Asians showed lower levels of physical activity in all pre-specified intensity domains (light, moderate, vigorous and moderate to vigorous physical activity). Differences in age and sex did not contribute to the observed differences in physical activity between the two populations.
Fig 1. Physical activity levels (MET.minutes per week) amongst South Asians and Europeans.
Error bars represent standard error of the mean. P-values are for the difference in physical activity between South Asians and Europeans in regression analysis with adjustment for age and sex.
Relationship of physical activity with insulin resistance
The univariate relationships between physical activity and insulin resistance, measured by HOMA-IR, showed a negative correlation between total physical activity levels per week and HOMA-IR (r = -0.1, P = 0.02, S2 Table). We used multiple regression analysis to evaluate the relationship between MET.minutes in total physical activity per week and HOMA-IR, after adjusting for differences in age, sex and ethnicity. Results revealed a negative association between total physical activity and HOMA-IR (B [SE]: -0.18 [0.08], P = 0.04, Table 2). Adjusting for adiposity (waist hip ratio) did not alter the association between physical activity and HOMA-IR (B [SE]: -0.20 [0.07], P = 0.01, S3 Table)
Table 2. Relationship of total physical activity with glucose, HbA1c, Insulin and HOMA-IR in regression analysis with adjustment for age, sex and ethnic group.
Results are presented as the change (effect [SE]) in metabolic parameter per 1000 MET.minutes of total physical activity per week.
| Total MET.minutes per week Effect (SE) |
p | |
|---|---|---|
| Glucose (mmol/l) | -0.11 (0.04) | 0.02 |
| HbA1c (%) | -0.05 (0.03) | 0.1 |
| Insulin (IU/l) | -0.42 (0.28) | 0.1 |
| HOMA-IR (mmol/l) | -0.18 (0.08) | 0.04 |
Relationship of physical activity with glycaemic and lipid parameters
We investigated the relationship of MET.minutes in physical activity with biochemical measures of glucose and lipid metabolism. MET.minutes per week in total physical activity were inversely correlated with fasting glucose levels (r = -0.1, P<0.05) and HbA1c (r = -0.1, P<0.05). On the other hand, there was no significant correlation found between total physical activity and fasting insulin levels, HDL cholesterol or triglycerides (S2 Table). After adjusting for differences in age, sex and ethnicity, increased total physical activity per week was associated with lower fasting glucose levels (B[SE]: -0.11 [0.04], P = 0.02, Table 2). The association between fasting glucose levels and physical activity per week remained significant after additional adjustment for waist hip ratio (B[SE]: -0.11 [0.04], P = 0.01, S3 Table). Multiple regression analysis showed no evidence for a relationship of total physical activity with HbA1c (B[SE]: -0.05 (0.03), P = 0.1) or fasting insulin levels (B[SE]: -0.42 (0.28), P = 0.1, Table 2).
Sensitivity analysis
We carried out sensitivity analyses to evaluate the robustness of our findings. Analysis of physical activity levels amongst South Asians and Europeans showed similar results using alternate metrics of physical activity as a sensitivity analysis (total counts, kilocalories and time spent in total physical activity, S4 Table). The relationships of physical activity with HOMA-IR, glycaemic parameters and lipid parameters were not materially altered using alternate classification schemes for physical activity, log-transformation of positively skewed variables or by inclusion of people with T2D (S5, S6 and S7 Tables).
Contribution of physical activity to metabolic disturbances in South Asians compared to Europeans
We used multiple regression to quantify the contribution of physical activity to metabolic disturbances amongst South Asians compared to Europeans (Table 3). Results are expressed as difference in respective metabolic parameters amongst South Asians and Europeans, after adjustment for potential predictors. Results showed that glucose concentrations were 0.17mmol/L (SE:0.08, P = 0.03) higher amongst South Asians than Europeans. After adjusting for total physical activity, the difference in fasting glucose between South Asians and Europeans was reduced to up to 0.13mmol/L (SE:0.08) and was no longer statistically significant. In contrast, physical activity, although associated with a decrease in HOMA-IR, did not account for the differences in HOMA-IR and other glycaemic and lipid parameters between South Asians and Europeans (Table 3).
Table 3. Contribution of physical activity towards the excess levels of metabolic disturbances amongst South Asians compared to Europeans.
Beta coefficient from multiple linear regression represents the difference in metabolic parameters between South Asians (SA) and Europeans (EW) before and after adjustment for total physical activity (MET.minutes) per week.
| Metabolic parameters | Unadjusted for physical activity | p | Adjusted for total MET.minutes/week | p |
|---|---|---|---|---|
| Glucose | 0.17 (0.08) | 0.03 | 0.13 (0.08) | 0.1 |
| HbA1c | 0.29 (0.05) | <0.001 | 0.27 (0.05) | <0.001 |
| Insulin | 3.2 (0.8) | <0.001 | 2.9 (0.8) | <0.001 |
| HOMA-IR | 0.9 (0.2) | <0.001 | 0.8 (0.2) | <0.001 |
| Cholesterol | -0.2 (0.09) | 0.03 | -0.2 (0.09) | 0.04 |
| Triglycerides | 0.2 (0.07) | 0.002 | 0.2 (0.07) | 0.006 |
| HDL | -0.2 (0.04) | <0.001 | -0.2 (0.04) | <0.001 |
| LDL | -0.1 (0.08) | 0.1 | -0.1 (0.08) | 0.2 |
Discussion
Our results show that total physical activity levels are ~31% lower amongst South Asians than Europeans in the UK. Total physical activity is independently and inversely associated with glucose and insulin resistance but not with other metabolic parameters. Low levels of physical activity do not account for the higher levels of insulin resistance amongst South Asians. However, lower physical activity levels amongst South Asians may contribute to their elevated glucose concentrations compared to Europeans. Our results suggest that lifestyle modification through increased physical activity may help to improve glucose metabolism and thus reduce the excess burden of T2D amongst South Asians.
The ~3 fold higher prevalence of type 2 diabetes in South Asians compared to Europeans in our study, is consistent with the existing literature. The Southall and Brent Revisited Study (SABRE) study, 20 year follow-up study of participants in the Southall study, reported that the prevalence of T2D was 22% amongst South Asians compared to 7% amongst Europeans[2]. Holman et al[28] compared the prevalence of T2D between South Asians and Europeans living in England in 2010. Results from this study estimated that the prevalence of T2D was ~2 times higher in South Asians compared to Europeans (14.0% vs. 6.9% respectively). A meta-analysis of international epidemiological studies and health examination surveys, by Danaei et al[29], showed similar results. Our findings thus reaffirm the high burden of type 2 diabetes in the South Asian population.
Though previous studies have suggested that levels of leisure time and total physical activity are lower amongst South Asians than Europeans, they have exclusively used semi-quantitative methods that are not validated amongst South Asians[18, 30, 31]. A recent study reported low levels of physical activity amongst South Asians compared to Europeans but was carried out in a population sample with low response rate (<5%) and exclusively male, and therefore unrepresentative of the UK South Asian population[32]. In the present study, we used a validated tri- axial accelerometer[33] to quantify physical activity levels amongst a representative population sample of South Asian and European men and women recruited from general practitioner lists (response rates averaged 62% in South Asians and 61% in Europeans). We show that total physical activity levels are ~31% lower amongst South Asians than Europeans, as measured by energy expenditure during free living activity. Lower levels of physical activity amongst South Asians were most evident in the moderate and vigorous physical activity domains, but also present in light physical activity. Low levels of physical activity amongst South Asians were independent of and not explained by differences in age, sex or adiposity, compared to Europeans. Our results extend current knowledge by providing quantitative assessment of physical activity in a representative sample of South Asian men and women. The reasons underlying low levels of physical activity amongst South Asians are not known, but may include a lack of understanding regarding the health benefits associated with regular physical activity, as well as cultural expectations and barriers to participation in recreational activities, especially among South Asian women[34].
We did not find an association of physical activity with HbA1c, triglycerides or HDL cholesterol concentrations. However, our results do show an association of lower levels of physical activity with higher insulin resistance and fasting glucose concentrations. In regression analysis, the relationship of physical activity with insulin resistance and glucose was not accounted for by differences in age, sex or adiposity, suggesting that physical activity has an independent effect on insulin resistance and fasting glucose concentrations. Our results are consistent with published data which show that increased physical activity is associated with a reduction in insulin resistance and glucose concentrations in European populations[35–38]. Findings of the current study are in line with experimental studies which show that physical activity stimulates translocation of GLUT4 glucose transporters, the rate-limiting step in glucose metabolism, in skeletal muscle[39].
Our results show that insulin resistance, as measured by HOMA-IR, and related insulin and lipid disturbances are more common amongst South Asians than Europeans. Lower physical activity alone did not explain the presence of these metabolic abnormalities amongst South Asians. However, we found that insulin resistance and glucose concentrations were reduced significantly after adjusting for total physical activity. Our findings suggest that higher concentrations of glucose amongst South Asians are in part accounted for by their lower levels of physical activity compared to Europeans. Abnormal glucose metabolism underlies T2D and impaired glucose tolerance, both major risk factors for cardiovascular disease. Prospective studies show that elevated glucose despite being in the normal range is a risk factor for cardiovascular disease[2, 40]. Low physical activity amongst South Asians may therefore have a role in the increased risk of cardiovascular disease in this population, through an effect on glucose metabolism.
This is the first study to quantify physical activity levels using an objective, validated tool in a representative sample of South Asian and European men and women. However, this study does have some limitations. Our study is cross-sectional rather than prospective and so may be susceptible to confounding effects (“reverse causation”). It is possible that our sample of participants may not be fully representative of the spectrum of South Asian and European populations. This is unlikely though as participants were recruited from general practitioner lists and showed the well-established patterns of insulin resistance and related metabolic abnormalities amongst South Asians. Modest sample size is a potential explanation for the failure to find the previously reported associations of physical activity with fasting insulin levels and related lipid abnormalities though this relationship is not consistently identified in other studies[41, 42]. We also used HOMA-IR, a surrogate measure of insulin resistance, which is closely correlated with the gold standard Hyperinsulinaemic euglycaemic clamp but which remains a less accurate measure of insulin resistance. Although physical activity has been measured objectively in the present study, ‘cardio respiratory fitness’, which has been indicated as a strong predictor of metabolic health, was not determined[43]. Furthermore, the detrimental effect of smoking on cardiorespiratory fitness and physical activity is well established[44]. Future research should consider including this important risk factor in the analysis too. Finally, although accelerometers are an objective measure of physical activity and their use has been validated against the gold standard under close supervision, their accuracy in free living individuals is lower, and our estimates of physical activity may be imprecise. In addition, the “regression dilution”[45] bias might have underestimated the strength of relationship between physical activity and metabolic parameters. To avoid the regression dilution bias, prospective studies should be conducted where physical activity levels are measured on more than one occasion.
In conclusion, we show that physical activity levels are lower amongst South Asians and may contribute to their increased fasting glucose concentrations and insulin resistance, compared with Europeans. Our results suggest the potential for increased physical activity to improve glucose metabolism and insulin resistance amongst South Asians, and help reduce their excess risk of T2D and cardiovascular disease.
Supporting information
(DOCX)
(DOCX)
Results are presented as the change (effect [SE]) in glucose levels for a 1000 MET.minutes per week increase in physical activity after adjustment for age, sex, ethnicity and waist hip ratio.
(DOCX)
Similar results were obtained using alternate metrics of physical activity as a sensitivity analysis (total counts, kilocalories and time total physical activity per week).
(DOCX)
(DOCX)
Outcome variables, with a positively skewed distribution, have been log-transformed.
(DOCX)
T2D cases have also been included in the analysis. Results are presented as the change (effect [SE]) in glucose or HbA1c for physical activity per week: *per 100000 counts/week, **per 100 minutes/week, ***per 1000 kilocalories or MET.minutes/week.
(DOCX)
Data Availability
Data underlying the results contain potentially identifying and sensitive patient information, hence there are ethical and legal restrictions on sharing the data. This restriction has been implemented by the London-Fulham Research Ethics Committee (ref 07/H0712/150). Data requests may be sent to Ninha Silva (ninha.silva@imperial.ac.uk), School of public health, Faculty of Medicine, Imperial college London, UK.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Jayawardena R, Ranasinghe P, Byrne NM, Soares MJ, Katulanda P, and Hills AP, Prevalence and trends of the diabetes epidemic in South Asia: a systematic review and meta-analysis. BMC Public Health, 2012. 12: p. 380 10.1186/1471-2458-12-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tillin T, Forouhi NG, McKeigue PM, Chaturvedi N, and Group SS, Southall And Brent REvisited: Cohort profile of SABRE, a UK population-based comparison of cardiovascular disease and diabetes in people of European, Indian Asian and African Caribbean origins. Int J Epidemiol, 2012. 41(1): p. 33–42. 10.1093/ije/dyq175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakker LE, Sleddering MA, Schoones JW, Meinders AE, and Jazet IM, Pathogenesis of type 2 diabetes in South Asians. Eur J Endocrinol, 2013. 169(5): p. R99–R114. 10.1530/EJE-13-0307 [DOI] [PubMed] [Google Scholar]
- 4.Mather HM and Keen H, The Southall Diabetes Survey: prevalence of known diabetes in Asians and Europeans. Br Med J (Clin Res Ed), 1985. 291(6502): p. 1081–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kooner JS, Saleheen D, Sim X, Sehmi J, Zhang W, Frossard P, et al. , Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat Genet, 2011. 43(10): p. 984–9. 10.1038/ng.921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sevak L, McKeigue PM, and Marmot MG, Relationship of hyperinsulinemia to dietary intake in south Asian and European men. Am J Clin Nutr, 1994. 59(5): p. 1069–74. 10.1093/ajcn/59.5.1069 [DOI] [PubMed] [Google Scholar]
- 7.Misra A, Sharma R, Pandey RM, and Khanna N, Adverse profile of dietary nutrients, anthropometry and lipids in urban slum dwellers of northern India. Eur J Clin Nutr, 2001. 55(9): p. 727–34. 10.1038/sj.ejcn.1601214 [DOI] [PubMed] [Google Scholar]
- 8.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. , Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care, 2010. 33(12): p. 2692–6. 10.2337/dc10-1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer-Davis EJ, D'Agostino R Jr., Karter AJ, Haffner SM, Rewers MJ, Saad M, et al. , Intensity and amount of physical activity in relation to insulin sensitivity: the Insulin Resistance Atherosclerosis Study. JAMA, 1998. 279(9): p. 669–74. [DOI] [PubMed] [Google Scholar]
- 10.Esteghamati A, Khalilzadeh O, Rashidi A, Meysamie A, Haghazali M, Asgari F, et al. , Association between physical activity and insulin resistance in Iranian adults: National Surveillance of Risk Factors of Non-Communicable Diseases (SuRFNCD-2007). Prev Med, 2009. 49(5): p. 402–6. 10.1016/j.ypmed.2009.09.005 [DOI] [PubMed] [Google Scholar]
- 11.Assah FK, Brage S, Ekelund U, and Wareham NJ, The association of intensity and overall level of physical activity energy expenditure with a marker of insulin resistance. Diabetologia, 2008. 51(8): p. 1399–407. 10.1007/s00125-008-1033-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizzo NS, Ruiz JR, Oja L, Veidebaum T, and Sjostrom M, Associations between physical activity, body fat, and insulin resistance (homeostasis model assessment) in adolescents: the European Youth Heart Study. Am J Clin Nutr, 2008. 87(3): p. 586–92. 10.1093/ajcn/87.3.586 [DOI] [PubMed] [Google Scholar]
- 13.Irwin ML, Mayer-Davis EJ, Addy CL, Pate RR, Durstine JL, Stolarczyk LM, et al. , Moderate-intensity physical activity and fasting insulin levels in women: the Cross-Cultural Activity Participation Study. Diabetes Care, 2000. 23(4): p. 449–54. [DOI] [PubMed] [Google Scholar]
- 14.Monda KL, Ballantyne CM, and North KE, Longitudinal impact of physical activity on lipid profiles in middle-aged adults: the Atherosclerosis Risk in Communities Study. J Lipid Res, 2009. 50(8): p. 1685–91. 10.1194/jlr.P900029-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwin ML, Ainsworth BE, Mayer-Davis EJ, Addy CL, Pate RR, and Durstine JL, Physical activity and the metabolic syndrome in a tri-ethnic sample of women. Obes Res, 2002. 10(10): p. 1030–7. 10.1038/oby.2002.140 [DOI] [PubMed] [Google Scholar]
- 16.Ekelund U, Franks PW, Sharp S, Brage S, and Wareham NJ, Increase in physical activity energy expenditure is associated with reduced metabolic risk independent of change in fatness and fitness. Diabetes Care, 2007. 30(8): p. 2101–6. 10.2337/dc07-0719 [DOI] [PubMed] [Google Scholar]
- 17.van der Heijden GJ, Toffolo G, Manesso E, Sauer PJ, and Sunehag AL, Aerobic exercise increases peripheral and hepatic insulin sensitivity in sedentary adolescents. J Clin Endocrinol Metab, 2009. 94(11): p. 4292–9. 10.1210/jc.2009-1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams ED, Stamatakis E, Chandola T, and Hamer M, Physical activity behaviour and coronary heart disease mortality among South Asian people in the UK: an observational longitudinal study. Heart, 2011. 97(8): p. 655–9. 10.1136/hrt.2010.201012 [DOI] [PubMed] [Google Scholar]
- 19.Hayes L, White M, Unwin N, Bhopal R, Fischbacher C, Harland J, et al. , Patterns of physical activity and relationship with risk markers for cardiovascular disease and diabetes in Indian, Pakistani, Bangladeshi and European adults in a UK population. J Public Health Med, 2002. 24(3): p. 170–8. [DOI] [PubMed] [Google Scholar]
- 20.Pomerleau J, McKeigue PM, and Chaturvedi N, Factors associated with obesity in South Asian, Afro-Caribbean and European women. Int J Obes Relat Metab Disord, 1999. 23(1): p. 25–33. [DOI] [PubMed] [Google Scholar]
- 21.Neilson HK, Robson PJ, Friedenreich CM, and Csizmadi I, Estimating activity energy expenditure: how valid are physical activity questionnaires? Am J Clin Nutr, 2008. 87(2): p. 279–91. 10.1093/ajcn/87.2.279 [DOI] [PubMed] [Google Scholar]
- 22.Rothney MP, Schaefer EV, Neumann MM, Choi L, and Chen KY, Validity of physical activity intensity predictions by ActiGraph, Actical, and RT3 accelerometers. Obesity (Silver Spring), 2008. 16(8): p. 1946–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okita K, Iwahashi H, Kozawa J, Okauchi Y, Funahashi T, Imagawa A, et al. , Homeostasis model assessment of insulin resistance for evaluating insulin sensitivity in patients with type 2 diabetes on insulin therapy. Endocr J, 2013. 60(3): p. 283–90. [DOI] [PubMed] [Google Scholar]
- 24.Troiano RP, Large-scale applications of accelerometers: new frontiers and new questions. Med Sci Sports Exerc, 2007. 39(9): p. 1501 10.1097/mss.0b013e318150d42e [DOI] [PubMed] [Google Scholar]
- 25.Sasaki JE, John D, and Freedson PS, Validation and comparison of ActiGraph activity monitors. J Sci Med Sport, 2011. 14(5): p. 411–6. 10.1016/j.jsams.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 26.Kido Y, Burks DJ, Withers D, Bruning JC, Kahn CR, White MF, et al. , Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J Clin Invest, 2000. 105(2): p. 199–205. 10.1172/JCI7917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freedson PS, Melanson E, and Sirard J, Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc, 1998. 30(5): p. 777–81. [DOI] [PubMed] [Google Scholar]
- 28.Holman N, Forouhi NG, Goyder E, and Wild SH, The Association of Public Health Observatories (APHO) Diabetes Prevalence Model: estimates of total diabetes prevalence for England, 2010–2030. Diabet Med, 2011. 28(5): p. 575–82. 10.1111/j.1464-5491.2010.03216.x [DOI] [PubMed] [Google Scholar]
- 29.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. , National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet, 2011. 378(9785): p. 31–40. 10.1016/S0140-6736(11)60679-X [DOI] [PubMed] [Google Scholar]
- 30.Fischbacher CM, Hunt S, and Alexander L, How physically active are South Asians in the United Kingdom? A literature review. J Public Health (Oxf), 2004. 26(3): p. 250–8. [DOI] [PubMed] [Google Scholar]
- 31.Daniel M and Wilbur J, Physical activity among South Asian Indian immigrants: an integrative review. Public Health Nurs, 2011. 28(5): p. 389–401. 10.1111/j.1525-1446.2010.00932.x [DOI] [PubMed] [Google Scholar]
- 32.Ghouri N, Purves D, McConnachie A, Wilson J, Gill JM, and Sattar N, Lower cardiorespiratory fitness contributes to increased insulin resistance and fasting glycaemia in middle-aged South Asian compared with European men living in the UK. Diabetologia, 2013. 56(10): p. 2238–49. 10.1007/s00125-013-2969-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Afaq S, Tan S-T, Afzal U, Loh M, Dimarco A, Kooner J, et al. , 117 Validation of Accelerometers for Measurement of Physical Activity Energy Expenditure in South Asians and Europeans. Heart, 2014. 100(Suppl 3): p. A66–A67. [Google Scholar]
- 34.Jepson R, Harris FM, Bowes A, Robertson R, Avan G, and Sheikh A, Physical activity in South Asians: an in-depth qualitative study to explore motivations and facilitators. PLoS One, 2012. 7(10): p. e45333 10.1371/journal.pone.0045333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen ND, Dunstan DW, Robinson C, Vulikh E, Zimmet PZ, and Shaw JE, Improved endothelial function following a 14-month resistance exercise training program in adults with type 2 diabetes. Diabetes Res Clin Pract, 2008. 79(3): p. 405–11. 10.1016/j.diabres.2007.09.020 [DOI] [PubMed] [Google Scholar]
- 36.Thomas DE, Elliott EJ, and Naughton GA, Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev, 2006(3): p. CD002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sigal RJ, Kenny GP, Boule NG, Wells GA, Prud'homme D, Fortier M, et al. , Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med, 2007. 147(6): p. 357–69. [DOI] [PubMed] [Google Scholar]
- 38.Sigal RJ, Kenny GP, Wasserman DH, and Castaneda-Sceppa C, Physical activity/exercise and type 2 diabetes. Diabetes Care, 2004. 27(10): p. 2518–39. [DOI] [PubMed] [Google Scholar]
- 39.Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, and Kraus WE, Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol (1985), 2004. 96(1): p. 101–6. [DOI] [PubMed] [Google Scholar]
- 40.Tillin T, Hughes AD, Godsland IF, Whincup P, Forouhi NG, Welsh P, et al. , Insulin resistance and truncal obesity as important determinants of the greater incidence of diabetes in Indian Asians and African Caribbeans compared with Europeans: the Southall And Brent REvisited (SABRE) cohort. Diabetes Care, 2013. 36(2): p. 383–93. 10.2337/dc12-0544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ball GD, Shaibi GQ, Cruz ML, Watkins MP, Weigensberg MJ, and Goran MI, Insulin sensitivity, cardiorespiratory fitness, and physical activity in overweight Hispanic youth. Obes Res, 2004. 12(1): p. 77–85. 10.1038/oby.2004.11 [DOI] [PubMed] [Google Scholar]
- 42.Bunt JC, Salbe AD, Harper IT, Hanson RL, and Tataranni PA, Weight, adiposity, and physical activity as determinants of an insulin sensitivity index in pima Indian children. Diabetes Care, 2003. 26(9): p. 2524–30. [DOI] [PubMed] [Google Scholar]
- 43.Solomon TP, Malin SK, Karstoft K, Knudsen SH, Haus JM, Laye MJ, et al. , Association between cardiorespiratory fitness and the determinants of glycemic control across the entire glucose tolerance continuum. Diabetes Care, 2015. 38(5): p. 921–9. 10.2337/dc14-2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikolakaros G, Vahlberg T, Auranen K, Sillanmaki L, Venetoklis T, and Sourander A, Obesity, Underweight, and Smoking Are Associated with Worse Cardiorespiratory Fitness in Finnish Healthy Young Men: A Population-Based Study. Front Public Health, 2017. 5: p. 206 10.3389/fpubh.2017.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tripepi G, Jager KJ, Dekker FW, Wanner C, and Zoccali C, Bias in clinical research. Kidney Int, 2008. 73(2): p. 148–53. 10.1038/sj.ki.5002648 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Results are presented as the change (effect [SE]) in glucose levels for a 1000 MET.minutes per week increase in physical activity after adjustment for age, sex, ethnicity and waist hip ratio.
(DOCX)
Similar results were obtained using alternate metrics of physical activity as a sensitivity analysis (total counts, kilocalories and time total physical activity per week).
(DOCX)
(DOCX)
Outcome variables, with a positively skewed distribution, have been log-transformed.
(DOCX)
T2D cases have also been included in the analysis. Results are presented as the change (effect [SE]) in glucose or HbA1c for physical activity per week: *per 100000 counts/week, **per 100 minutes/week, ***per 1000 kilocalories or MET.minutes/week.
(DOCX)
Data Availability Statement
Data underlying the results contain potentially identifying and sensitive patient information, hence there are ethical and legal restrictions on sharing the data. This restriction has been implemented by the London-Fulham Research Ethics Committee (ref 07/H0712/150). Data requests may be sent to Ninha Silva (ninha.silva@imperial.ac.uk), School of public health, Faculty of Medicine, Imperial college London, UK.