Abstract
Canine parvovirus first emerged in domestic dogs (Canis familiaris), most likely as a variant of the feline panleucopaenia virus. Relatively recently, canine parvovirus-2a and canine parvovirus-2b infections have been identified in both symptomatic and asymptomatic domestic cats, while canine parvovirus infections have also been demonstrated in wild felids. This report documents the first known case of canine parvovirus-2b detected in unvaccinated serval (Leptailurus serval) from South Africa. The serval presented with clinical signs of vomiting, anorexia and diarrhoea that responded to symptomatic treatment. Two weeks later, severe leucopaenia, thrombocytopenia and death occurred. Typical enteric histological lesions of parvovirus infection were not observed on histopathological examination of the small intestine; however, histological lesions consistent with septicaemia were present. Canine parvovirus was detected in formalin-fixed paraffin-embedded small intestine using polymerase chain reaction. Phylogenetic analysis of the sequence of the canine parvovirus viral capsid protein gene showed similarities between the sample from the serval and canine parvovirus-2b isolates from domestic dogs in Argentina and South Africa. A case of canine parvovirus-2b in a domestic dog from South Africa in 2012 that fell within the same clade as the serval sample appears distantly related because of the long branch length. The significance of these findings is explored. More extensive surveys of canine parvovirus in domestic and wild felids and canids are needed to understand the epidemiology of canine parvovirus in non-domestic felids in South Africa.
Introduction
Parvoviruses (family Parvoviridae) and their associated diseases affect various carnivores including felids and canids (Siegl et al. 1985). Feline panleucopaenia virus (FPLV) infection was one of the first viral diseases identified in domestic cats (Felis catus), during the 1930s and 1940s, and also infects non-domestic felids (Parrish 1990). At roughly the same time (~1947), parvoviral gastroenteritis was identified in farmed mink (Mustela vison), and within 15 years, it was observed on mink ranches throughout Canada, the United States, Europe and Scandinavia (Parrish 1990; Pearson & Gorham 1987); it is currently known as mink enteritis virus (MEV) (Barker & Parrish 2001).
During the late 1970s, canine parvovirus (CPV) first emerged in domestic dogs (Canis familiaris), most likely as a variant of FPLV or a closely related parvovirus. Although the exact mechanism of emergence is unclear, amplification of parvovirus DNA sequences intermediate between FPLV and CPV from red foxes (Vulpes vulpes) suggests that wildlife may have played a role in the adaptation of the virus in a new host (Parrish 1995; Truyen et al. 1996, 1998). The original strain (CPV type 2) spread worldwide rapidly, shortly thereafter being replaced by type 2a and a few years later by type 2b. These two variants, however, differ very little from the original strain (Parrish 1995; Truyen et al. 1996). Strain CPV type 2c was diagnosed much later (~2000), from domestic dogs in Italy, and has been proven to induce disease in cats as well (Buonavoglia et al. 2001; Nakamura et al. 2001).
Host range seems to be complex, as even within affected families only certain genera or species have been reported to be susceptible (Barker & Parrish 2001). The natural hosts of CPV (dogs), FPLV (cats) and MEV (mink) have been identified; however, the natural host ranges of feline subgroup parvoviruses are poorly defined (Barker & Parrish 2001). Wild felids that have been reported to be susceptible to FPLV include cheetah (Acinonyx jubatus), Siberian tiger (Panthera tigris altaica), African wildcat (Felis lybica), puma (Puma concolor), ocelot (Leopardus pardalis) and spotted cat (Leopardus tigrinus) (Filoni et al. 2006; Hoelzer & Parrish 2010; Steinel et al. 2000). In addition, FPLV has been reported in many non-felid species, for example raccoon (Procyon lotor), mink (Neovison vison) and arctic fox (Vulpes lagopus) (Hoelzer & Parrish 2010; Steinel et al. 2001; Van Vuuren et al. 2000).
Domestic cats have been shown to be both symptomatic and asymptomatic carriers of CPV-2a, 2b and 2c (Buonavoglia et al. 2001; Clegg et al. 2012; Nakamura et al. 2001), while infection has also been demonstrated in wild felids such as cheetah and Siberian tiger (Steinel et al. 2000). Similarly, both domestic dogs and wild canids are known to be susceptible to CPV, while antibodies against CPV have been found in coyote (Canis latrans), grey wolf (Canis lupus), maned wolf (Chrysocyon brachyurus), crab-eating fox (Cerdocyon thous), bush dog (Speothos venaticus), dingo (Canis lupus dingo), raccoon dog (Nyctereutes procyonoides) and African wild dog (Lycaon pictus) (Alexander et al. 1993; Barker & Parrish 2001; Van Heerden et al. 1995).
Genetic material from FPLV has been detected in a serval (Leptailurus serval) with histological lesions typical for parvoviral disease and in another with salmonellosis (Lane et al. 2016). This case explores the significance of the detection of CPV-2b in a serval from South Africa with bacterial septicaemia.
Materials and methods
Case presentation
A captive adult male serval that presented with inappetence, anorexia, vomiting and diarrhoea was hospitalised at a veterinary practice in Nelspruit, Mpumalanga. The animal reacted well to symptomatic treatment and regained its appetite. Fifteen days later, however, it presented with epistaxis, mild icterus, anaemia (haematocrit = 28), severe leucopaenia and thrombocytopenia (subjectively evident on a blood smear). Treatment was initiated but the animal died the following day. No evidence of warfarin or other anticoagulant exposure was reported. No further clinical tests or culture were performed, and no bone marrow was submitted for evaluation or further analysis.
A necropsy showed that the serval was in good condition with mild icterus, a slightly enlarged liver and mild fluid accumulation in the body cavities. The cause of death was suspected to be because of aspiration of blood from the epistaxis. Selected tissue samples preserved in 10% buffered formalin were processed routinely and stained with haematoxylin–eosin stain. The histopathological examination confirmed septicaemia (multifocal hepatic and pulmonary necrosis associated with fine bacterial bacilli, and fibrin deposition in the splenic red pulp sinuses). Hepatic macrophages contained bile pigment in the cytoplasm. The urinary bladder mucosa showed multifocal mucosal ulceration and haemorrhage with mild submucosal lymphoplasmacytic interstitial infiltration and fibroplasia. An unspecified lymph node was mildly active and cortical follicular structures contained central hyalinisation of the stroma and possible fibrin deposition. The small intestine did not reveal any enteric lesions including changes characteristic of parvovirus-induced necrosis.
The molecular diagnostics and phylogenetic analyses formed part of a larger project at the National Zoological Garden, South African National Biodiversity Institute (NZG, SANBI), which investigates the molecular identification and genetic diversity of FPLV in both wild and domestic felids (Lane et al. 2016). Here, we compared the serval sample (PV19) to other cases, which include PV0 to PV30 (various felids, Lane et al. 2016), PV31 (a domestic dog from Namibia) and PV35, PV36, PV38 and PV40 (domestic dogs from Pretoria) as summarised in Table 1. No domestic dog samples from Mpumalanga were available to include in the report.
TABLE 1.
Sample type, species, histopathological diagnosis and molecular analysis of parvoviral samples collected as part of the National Zoological Garden, South African National Biodiversity Institute wildlife disease database.
| Lab no. | Sample type | Species | Histopathological diagnosis† | Molecular analysis |
|---|---|---|---|---|
| PV10‡ | F/F | Serval | Suspected non-FPLV | FPLV Clade 1 |
| PV15‡ | FFPE | Serval | Suspected FPLV | FPLV Clade 2 |
| PV19 | FFPE | Serval | Suspected FPLV | CPV-2b |
| PV36 | FFPE | Domestic dog | CPV | CPV-2a |
| PV38 | FFPE | Domestic dog | CPV | CPV-2a |
| PV40 | FFPE | Domestic dog | CPV | CPV-2a |
| PV31 | FFPE | Domestic dog | CPV | CPV-2b |
| PV35 | FFPE | Domestic dog | CPV | CPV-2b |
| PV1‡ | F/F | African black footed cat | FPLV | FPLV Clade 1 |
| PV24‡ | FFPE | Caracal | Suspected FPLV | FPLV Clade 1 |
| PV0‡ | F/F | Cheetah | Suspected FPLV | FPLV Clade 1 |
| PV4‡ | FFPE | Cheetah | FPLV | FPLV Clade 1 |
| PV7‡ | FFPE | Cheetah | FPLV | FPLV Clade 1 |
| PV8‡ | FFPE | Cheetah | FPLV | FPLV Clade 1 |
| PV9‡ | FFPE | Cheetah | FPLV | FPLV Clade 1 |
| PV11‡ | F/F | Cheetah | FPLV | FPLV Clade 1 |
| PV12‡ | F/F | Cheetah | FPLV | FPLV Clade 1 |
| PV26‡ | F/F | Cheetah | FPLV | FPLV Clade 1 |
| PV27‡ | Rectal swab | Cheetah | FPLV | FPLV Clade 1 |
| PV28‡ | F/F | Cheetah | FPLV | FPLV Clade 1 |
| PV29‡ | F/F | Cheetah | FPLV | FPLV Clade 1 |
| PV14‡ | FFPE | Lion | FPLV | FPLV Clade 1 |
| PV23‡ | FFPE | Lion | FPLV | FPLV Clade 1 |
| PV21‡ | FFPE | Ocelot | Suspected FPLV | FPLV Clade 1 |
| PV30‡ | FFPE | Caracal | Suspected FPLV | PCR negative |
| PV3‡ | F/F | Cheetah | Suspected FPLV | PCR negative |
| PV5‡ | F/F | Cheetah | FPLV | PCR negative |
| PV13‡ | FFPE | Cheetah | Suspected FPLV | PCR negative |
| PV2‡ | F/F | Domestic cat | Suspected FPLV | PCR negative |
| PV20‡ | F/F | Leopard | Non-FPLV | PCR negative |
| PV6‡ | F/F | Lion | Suspected FPLV | PCR negative |
| PV17‡ | F/F | Lion | Suspected FPLV | PCR negative |
| PV22‡ | FFPE | Lion | FPLV | PCR negative |
| PV25‡ | F/F | Ocelot | Non-FPLV | PCR negative |
| PV18‡ | FFPE | Puma | Non-FPLV | PCR negative |
FFPE, formalin-fixed paraffin-embedded; F/F, fresh or frozen; FPLV, feline panleucopaenia virus; CPV, canine parvovirus; PCR, polymerase chain reaction.
, Histopathological diagnosis was made on the presence of diagnostic or strongly suggestive lesions for parvoviral infection.
, Samples from Lane et al. (2016).
Nucleic acids from a formalin-fixed, paraffin-embedded (FFPE) intestinal sample (PV19) were extracted using the Epicentre MasterPure™ Complete DNA & RNA Purification Kit (Whitehead Scientific®) following the manufacturer’s specifications for FFPE tissue, and deparaffinisation of FFPE samples was performed using xylene.
Molecular analysis of parvovirus from the isolate was achieved by amplification of a ~1400 bp region of the viral capsid protein (VP2) gene (Table 2) using DreamTaq™ Green PCR (polymerase chain reaction) master mix (ThermoFischer Scientific) and previously published primers (Horiuchi et al. 1996; Meers et al. 2007; Steinel et al. 2000; Wasieri et al. 2009). A standard polymerase chain reaction (PCR) setup was used consisting of 8.5 µL nuclease-free water (supplied with DreamTaq™), 12.5 µL DreamTaq master mix, 1 µL of each primer (Forward and Reverse; 10 pmol) and 2 µL DNA sample standardised to a concentration of 50 ng/µL. Cycling conditions for tissue isolates using primer sets 2 to 5 were as follows: initial denaturation (94 °C for 5 minutes), 30 cycles of denaturation (94 °C for 30 seconds), annealing (58 °C for 50 s) and extension (72 °C for 1 min). A final extension step (72 °C for 10 min) concluded the cycling. Cycling conditions for primer sets 1 and 6 were identical except annealing occurred at 54 °C. Conditions were adjusted for FFPE isolates by decreasing the annealing temperatures to 54 °C for primer sets 2 to 5, and 50 °C for primer sets 1 and 6. Polymerase chain reaction setup was performed in a DNA-free hood and a DNA negative control was included.
TABLE 2.
Details of sequences and amplicon size of primers used in this study for polymerase chain reaction and sequencing spanning the viral capsid protein 2 gene region.
| Primer set | Name | Sequence (5’–3’) | Amplicon size (base pair) |
|---|---|---|---|
| 1 | CPV_EF† | GCCGGTGCAGGACAAGTA | 421 |
| M2R‡ | AGTTGCCAATCTCCTGGATT | ||
| 2 | PV_VP2-1§ | GAAAACGGATGGGTGGAAATC | 405 |
| PV_VP2-2§ | AGCTGCTGGAGTAAATGGCATAGT | ||
| 3 | PV_VP2-3§ | TAAAGACTGTTTCAGAATCTGCTACTCA | 424 |
| PV_VP2-4§ | AGAAATGGTGGTAAGCCCAATG | ||
| 4 | PV_VP2-5§ | ATACTGGAACTAGTGGCACACC | 437 |
| PV_VP2-5ii¶ | ATTTAAAACACCTATTGCAGCAGGAC | ||
| 5 | PV_VP2-5iiR¶ | GTCCTGCTGCAATAGGTGTTTTAAAT | 440 |
| PV_VP2-8§ | GCATCAGGATCATATTCATTTGTTAA | ||
| 6 | Primer 22 F†† | TGTCAAAATAATTGTCCTGG | 564 |
| CPV_JS2R† | CAACCCACACCATAACAACA |
All positive amplicons were purified using an exonuclease I and alkaline phosphatase PCR purification protocol, sequenced using BigDye V3.1 (Applied Biosystems) chemistry and analysed on an ABI3500 genetic analyser. Sequences were aligned using the ClustalX function incorporated in MEGA6 (Tamura et al. 2011) and phylogenetically analysed using neighbour-joining (NJ) in MEGA6. The Tamura 3-parameter model with gamma distribution (T92+G; G = 0.12) was determined to be the best-fit model of sequence evolution under the Akaike Information Criterion (AIC) in jModeltest (Posada 2008). Phylogenetic analyses were performed on 61-taxon, trimmed to the shortest sequence (~1200 bp), VP2 gene data set including 11 FPLV positive isolates, two FPLV vaccine strains (Fel-o-vax IV®, Boehringer Ingelheim; Felocell®, Zoetis), six CPV positive case samples and 42 parvovirus reference strains (Table 3) from the National Centre for Biotechnology Information (NCBI) online database, GenBank (https://www.ncbi.nlm.nih.gov/). Identical field isolate sequences were selectively removed, to reduce the phylogenetic tree size, so that only representative isolates remained.
TABLE 3.
GenBank reference sequences including species, country of origin, year of collection and accession number.
| Species | Virus | Collection origin and year | Accession number |
|---|---|---|---|
| Cell line | CPV | Unknown; 1990 | M38245 |
| Domestic dog | CPV-2 | ITA; 2005 | FJ222824 |
| Vaccine | CPV-2 | CHN; 2008 | FJ432718 |
| Fox | CPV-2 | CHN; 2009 | GU392236 |
| Domestic dog | CPV-2 | RSA; 2010 | HQ602985 |
| Domestic dog | CPV-2 | RSA; 2010 | HQ602986 |
| Cell line | CPV-2a | CHN; 2007 | EU310373 |
| Domestic dog | CPV-2a | CHN; 2011 | JQ268283 |
| Domestic dog | CPV-2a | CHN; 2011 | JX660690 |
| Domestic dog | CPV-2a | URY; 2011 | KC196111 |
| Domestic dog | CPV-2a | CHN; 2009 | KF482472 |
| Domestic dog | CPV-2a | CHN; 2011 | KF785797 |
| Domestic dog | CPV-2a | CHN; 2014 | KT382542 |
| Domestic dog | CPV-2b | ARG; 2003 | JF414817 |
| Domestic dog | CPV-2c | DEU; 1997 | FJ005196 |
| Domestic dog | CPV-2c | BEL; 2008 | FJ005247 |
| Domestic dog | CPV-2c | GRC; 2008 | GQ865518 |
| Domestic dog | CPV-2c | URY; 2008 | KC196105 |
| Domestic dog | CPV-2c | ITA; 2010 | KF373598 |
| Domestic dog | CPV-2c | ITA; 2009 | KF385386 |
| Blue fox | BFPV | CHN; 2008 | GQ857595 |
| Domestic cat | FPLV | RSA; 1999 | AJ249556 |
| Cheetah | FPLV | RSA; 1999 | AJ249557 |
| Cell line | FPLV | RUS; 2004 | AY665655 |
| Vaccine Purevax | FPLV | Merial; 2008 | EU498680 |
| Vaccine Felocell | FPLV | Pfizer; 2008 | EU498681 |
| Domestic cat | FPLV | KOR; 2008 | HQ184200 |
| Raccoon | FPLV | US; 1978 | JN867596 |
| Domestic cat | FPLV | TWN; 2011 | JX048608 |
| Cougar | FPLV | US; 2010 | JX475253 |
| Domestic cat | FPLV | CHN; 2014 | KP280068 |
| Cell line | FPLV | US; 1988 | M24002 |
| Cell line | FPLV | Unknown; 1988 | M24004 |
| Cell line | FPLV | Unknown; 1990 | M38246 |
| Wild cat | FPLV | US; 1990 | U22187 |
| Cell line | MEV | RUS; 1998 | AF201477 |
| Cell line | MEV | CHN; 2007 | EF428258 |
| Mink | MEV | CHN; 2009 | GU272028 |
| Mink | MEV | CHN; 2010 | JX535284 |
| Mink | MEV | CHN; 2012 | KC713592 |
| Mink | MEV | CHN; 2011 | KP008112 |
| Mink | MEV | CHN; 2014 | KT899745 |
ARG, Argentina; US, United States; PRT, Portugal; URY, Uruguay; ITA, Italy; BEL, Belgium; CHN, China; GRC, Greece; DEU, Germany; TWN, Taiwan; RSA, South Africa; KOR, Korea; RUS, Russia; CPV, canine parvovirus; FPLV, feline panleucopaenia virus; BFPV, blue fox parvovirus.
Ethical considerations
Ethical approval was obtained from the NZG Research Ethics and Scientific Committee (NZG/RES/P/16/10).
Results
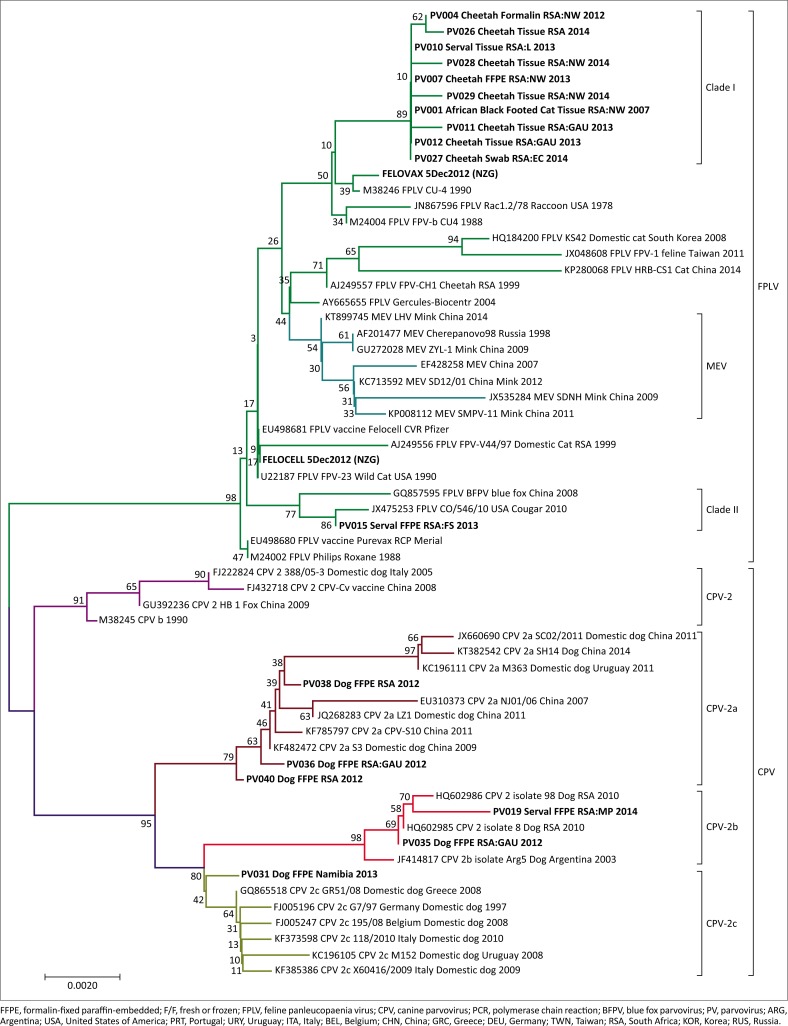
Results from the NJ analysis (1000 bootstrap replicates) illustrate a clear separation between FPLV and CPV isolates, as indicated in Figure 1. Additionally, the CPV clade consists of four distinct clusters representing CPV-2, -2a, -2b and -2c. The serval isolate from this study (PV19) grouped with the CPV-2b cluster with a domestic dog sample from the NZG, SANBI database (PV35), and with previously reported NCBI reference sequences from domestic dogs in Argentina (Gallo Calderón et al. 2012) and South Africa (Dogonyaro et al. 2013). Isolate PV19, however, appears to be distantly related to these isolates as indicated by the long branch length. The Namibian domestic dog isolate (PV31) from our database grouped with the CPV-2c cluster on a separate branch. Domestic dog isolates PV36, PV38 and PV40 grouped with the CPV-2a cluster also on separate branches. The majority of the South African felid samples grouped with the FPLV Clade I, including a sample from a serval with salmonellosis (PV10) (Lane et al. 2016). The South African Clade II contained one serval with histological lesions of FPLV (PV15) with a seemingly unique viral strain (Lane et al. 2016) that is more similar to a Felocell vaccine strain than to Clade I FPLV.
FIGURE 1.
Evolutionary relationships of detected parvoviral taxa using trimmed nucleotide sequences and the neighbour-joining method. The tree was generated using the Tamura 3-parameter model with Gamma distribution and 1000 bootstrap replicates. Cases from the NZG wildlife disease database and the study by Lane et al. (2016) are prefixed with laboratory numbers (PV), vaccine strains are prefixed with the vaccine name, and reference strains collected from GenBank are prefixed with the accession number. Rooted with CPV as the out-group.
Discussion
This study reports the first detection of CPV-2b in a captive serval from South Africa, while the study by Lane et al. (2016) previously detected FPLV in two serval isolates. Although CPV infection has previously been detected in wild felids, such as cheetah and Siberian tiger (Steinel et al. 2000), as well as domestic cats, the CPV-2b strain detected in the serval corresponds more closely with those detected in domestic dogs from South Africa (Dogonyaro et al. 2013) and Argentina (Gallo Calderón et al. 2012).
The significance of the presence of CPV and FPLV in tissues from servals with septicaemia is uncertain. Parvovirus is known to cause lymphoid necrosis and atrophy in lymphoid organs such as the thymus, gut-associated lymphoid tissue and lymph nodes as well as intestinal epithelial necrosis. The resultant immunosuppression and breach of intestinal integrity make affected animals susceptible to secondary systemic bacterial infection. Typically, domestic dogs and cats that die as a result of septicaemia secondary to parvoviral infection show histological lesions of intestinal epithelial necrosis. Detection of FPLV in a serval (PV15) with typical intestinal necrosis suggests that the disease, in FPLV at least, may show a similar course in servals. However, as domestic cats have been shown to be asymptomatic carriers of CPV-2a, -2b and -2c (Buonavoglia et al. 2001; Clegg et al. 2012; Nakamura et al. 2001), we cannot rule out the possibility that servals may be asymptomatic carriers of both FPLV and CPV-2b. Alternatively, servals may at times show mild transient intestinal infections with either virus that result in immune suppression, secondary bacterial enteritis and septicaemia. This could possibly explain the 2-week period between the initial gastrointestinal signs and the terminal septicaemia in this case (PV19). It is unclear what role the damaged bladder mucosa played in this case; it may have been the route of bacterial infection.
The presence of CPV-2a and CPV-2b in the samples collected from domestic dogs from South Africa 2 years before the serval case indicates the existence of these strains in the domestic dog population in the country, and this may also indicate a likely source of infection for the serval (PV19). Information on the contact or not between domestic dogs and this serval was not available. Further investigation of samples PV36, PV38 and PV40 may reveal whether or not these isolates represent unique variants of CPV-2a. The case of CPV-2c isolated from a domestic dog sample collected in Namibia is most likely an artefact of shortening the sequences to the shortest sequence in the data set, as this virus variant has not been reported in Africa or Australia (Decaro et al. 2005; Dogonyaro et al. 2013; Meers et al. 2007; Sykes 2013). Additionally, the results of the NCBI website based Basic Local Alignment Search Tool (BLAST) which compares an input sequence to the GenBank database of sequences, identified the full length sequence of this virus as CPV-2b.
Canine parvoviruses have spread widely across the globe since the 1970s because of contact with infected animals, inanimate fomites, flies or other reservoirs (Bagshaw et al. 2014; Barker & Parrish 2001). Over the last 15 years (2001–2016), however, very little information about the genetic diversity of parvoviruses and possible variants in South African domestic and non-domestic carnivores has been published. The epidemiology of these viruses is therefore largely unknown, including the transmission between domestic and non-domestic species and whether or not vaccination with live virus in either group affects the epidemiology. Natural recombination has been reported in porcine, mink and rodent parvoviruses (Shackelton & Hoelzer 2007). Similarly, recombination between FPLV and CPV-2, CPV-2a and CPV-2c, as well as vaccine and field strains has been reported but the exact mechanism is unknown (Mochizuki et al. 2008; Ohshima & Mochizuki 2009; Pérez et al. 2014). Therefore, recombination remains an important factor to consider when studying the evolution and genetic diversity of parvoviruses. Extensive surveys of parvoviral strains present in wild South African carnivores, as well as domestic dogs and cats, will help determine the strain diversity, geographic and host species distribution as well as possible sources of infection. How this recombination mechanism affects vaccine efficacy is largely unknown. As it has been previously shown that domestic cats, cheetah, tiger and serval (at present) are susceptible to both CPV and FPLV, the revision of currently accepted vaccination strategies, which primarily involves vaccinating felids against FPLV only, is required.
Acknowledgements
The authors wish to thank the staff of the NZG Biobank, and Pathology section of the Department of Paraclinical Studies, Faculty of Veterinary Science, University of Pretoria. They also thank the Ann van Dyk Cheetah Centre, IDEXX Laboratories, Dr Peter Caldwell of the Old Chapel Veterinary Clinic, Dr Katja Koeppel of the Johannesburg Zoo and Dr Lucia Lange of PathCare for submitting samples to the NZG Biobank that was used in this study.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors’ contributions
A.O. and H.B. were responsible for all the molecular work conducted and preparation of the manuscript. E.C.d.P. made the initial diagnosis and histopathological identification. E.P.M. confirmed the histopathological findings and facilitated collaboration. R.J. facilitated student supervision and edited the manuscript. D.L.D. and A.K. made sufficient resources and facilities available for the molecular and pathological work, and edited the manuscript. All authors contributed to the write-up and review of this article.
Funding information
This study was supported by the South African National Research Foundation (NRF) with a funding grant to H.B. (grant no 80457), as well as through NZG core grant allocations for the molecular work in this study.
Footnotes
How to cite this article: Oosthuizen, A., Brettschneider, H., Dalton, D.L., Du Plessis, E.C., Jansen, R., Kotze, A. et al., 2019, ‘Canine parvovirus detected from a serval (Leptailurus serval) in South Africa’, Journal of the South African Veterinary Association 90(0), a1671. https://doi.org/10.4102/jsava.v90i0.1671
References
- Alexander K.A., Conrad P.A., Gardner I.A., Parrish C., Appel M., Levy M.G. et al. , 1993, ‘Serologic survey for selected microbial pathogens in African wild dogs (Lycaon pictus) and sympatric domestic dogs (Canis familiaris) in Maasai Mara, Kenya’, Journal of Zoo and Wildlife Medicine 24, 140–144. [Google Scholar]
- Barker I.K. & Parrish C.R., 2001, ‘Parvovirus infections’, in Williams E.S. & Barker I.K. (eds.), Infectious disease of wild mammals, 3rd edn., pp. 131–146, Iowa State Press, Ames, IA. [Google Scholar]
- Bagshaw C., Isdell A.E., Thiruvaiyaru D.S., Brisbin I.L. & Sanchez S., 2014, ‘Molecular detection of canine parvovirus in flies (Diptera) at open and closed canine facilities in the eastern United States’, Preventive Veterinary Medicine 114(3–4), 276–284. 10.1016/j.prevetmed.2014.02.005 [DOI] [PMC free article] [PubMed]
- Buonavoglia C., Martella V., Pratelli A., Tempesta M., Cavalli A., Buonavoglia D. et al. , 2001, ‘Evidence for evolution of canine parvovirus type 2 in Italy’, Journal of General Virology 82, 3021–3025. 10.1099/0022-1317-82-12-3021 [DOI] [PubMed] [Google Scholar]
- Clegg S.R., Coyne K.P., Dawson S., Spibey N., Gaskell R.M. & Radford A.D., 2012, ‘Canine parvovirus in asymptomatic feline carriers’, Veterinary Microbiology 157, 78–85. 10.1016/j.vetmic.2011.12.024 [DOI] [PubMed] [Google Scholar]
- Decaro N., Desario C., Campolo M., Elia G., Martella V., Ricci D. et al. , 2005, ‘Clinical and virological findings in pups naturally infected by canine parvovirus type 2 Glu-426 mutant’, Journal of Veterinary Diagnostic Investigation 17, 133–138. 10.1177/104063870501700206 [DOI] [PubMed] [Google Scholar]
- Dogonyaro B.B., Bosman A., Sibeko K.P., Venter E.H. & Van Vuuren M., 2013, ‘Genetic analysis of the VP2-encoding gene of canine parvovirus strains from Africa’, Veterinary Microbiology 165(3–4), 460–465. 10.1016/j.vetmic.2013.04.022 [DOI] [PubMed] [Google Scholar]
- Filoni C., Catao-Dias L., Bay G., Luiz Durigon E., Pinto Jorge R.S., Lutz H. et al. , 2006, ‘First evidence of feline herpesvirus, calicivirus, parvovirus, and ehrlichia exposure in Brazilian free-ranging felids’, Journal of Wildlife Diseases 42, 470–477. 10.7589/0090-3558-42.2.470 [DOI] [PubMed] [Google Scholar]
- Gallo Calderón M., Wilda M., Boado L., Keller L., Malirat V., Iglesias M. et al. , 2012, ‘Study of canine parvovirus evolution: Comparative analysis of full-length VP2 gene sequences from Argentina and international field strains’, Virus Genes 44(1), 32–39. 10.1007/s11262-011-0659-8 [DOI] [PubMed] [Google Scholar]
- Hoelzer K. & Parrish C.R., 2010, ‘The emergence of parvoviruses of carnivores’, Veterinary Research 41, 39 10.1051/vetres/2010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzer K., Shackelton L.A., Parrish C.R. & Holmes E.C., 2008, ‘Phylogenetic analysis reveals the emergence, evolution and dispersal of carnivore parvoviruses’, Journal of General Virology 89, 2280–2289. 10.1099/vir.0.2008/002055-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi M., Yuri K., Soma T., Katae H., Nagasawa H. & Shinagawa M., 1996, ‘Differentiation of vaccine virus from field isolates of feline panleukopenia virus by polymerase chain reaction and restriction fragment length polymorphism analysis’, Veterinary Microbiology 53, 283–293. 10.1016/S0378-1135(96)01225-4 [DOI] [PubMed] [Google Scholar]
- Lane E.P, Brettschneider H., Caldwell P., Oosthuizen A., Dalton D.L., Du Plessis L. et al. , 2016, ‘Feline panleukopaenia virus in captive non-domestic felids in South Africa’, Onderstepoort Journal of Veterinary Research 83(1), a1099 10.4102/ojvr.v83i1.1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meers J., Kyaw-Tanner M., Bensink Z. & Zwijnenberg R., 2007, ‘Genetic analysis of canine parvovirus from dogs in Australia’, Australian Veterinary Journal 85, 392–396. 10.1111/j.1751-0813.2007.00206.x [DOI] [PubMed] [Google Scholar]
- Mochizuki M., Ohshima T., Une Y. & Yachi A., 2008, ‘Recombination between vaccine and field strains of canine parvovirus is revealed by isolation of virus in canine and feline cell cultures’, Journal of Veterinary Medical Science 70(12), 1305–1314. 10.1292/jvms.70.1305 [DOI] [PubMed] [Google Scholar]
- Nakamura K., Sakamoto M., Ikeda Y., Sato E., Kawakami K., Miyazawa T. et al. , 2001, ‘Pathogenic potential of canine parvovirus types 2a and 2c in domestic cats’, Clinical and Diagnostic Laboratory Immunology 8(3), 663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima T. & Mochizuki M., 2009, ‘Evidence for recombination between feline panleukopenia virus and canine parvovirus type 2’, Journal of Veterinary Medical Science 71(4), 403–408. 10.1292/jvms.71.403 [DOI] [PubMed] [Google Scholar]
- Parrish C.R., 1995, ‘Pathogenesis of feline panleukopenia virus and canine parvovirus’, Baillières Clinical Haematology 8, 57–71. 10.1016/S0950-3536(05)80232-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish C.R., 1990, ‘Emergence, natural history, and variation of canine, mink, and feline parvoviruses’, Advances in Virus Research 38, 403–450. 10.1016/S0065-3527(08)60867-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R.C. & Gorham J.R., 1987, ‘Mink virus enteritis’, in Appel M.J. (ed.), Virus infections of carnivores, pp. 248–254, Elsevier Science, Amsterdam. [Google Scholar]
- Pérez R., Calleros L., Marandino A., Sarute N., Iraola G., Grecco S. et al. , 2014, ‘Phylogenetic and genome-wide deep-sequencing analyses of canine parvovirus reveal co-infection with field variants and emergence of a recent recombinant strain’, PLoS One 9(11), e111779. 10.1371/journal.pone.0111779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D., 2008, ‘jModelTest: Phylogenetic model averaging’, Molecular Biology and Evolution 25, 1253–1256. 10.1093/molbev/msn083 [DOI] [PubMed] [Google Scholar]
- Shackelton L. & Hoelzer K., 2007, ‘Comparative analysis reveals frequent recombination in the parvoviruses’, Journal of General Virology 88, 3294–3301. 10.1099/vir.0.83255-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., Bates R.C., Berns K.I., Carter B.J., Kelly D.C., Kurstak E. et al. , 1985, ‘Characteristics and taxonomy of Parvoviridae’, Intervirology 23, 61–73. 10.1159/000149587 [DOI] [PubMed] [Google Scholar]
- Steinel A., Munson L., Van Vuuren M. & Truyen U., 2000, ‘Genetic characterization of feline parvovirus sequences from various carnivores’, Journal of General Virology 81, 345–350. 10.1099/0022-1317-81-2-345 [DOI] [PubMed] [Google Scholar]
- Steinel A., Parrish C.R., Bloom M.E. & Truyen U., 2001, ‘Parvovirus infections in wild carnivores’, Journal of Wildlife Diseases 37, 594–607. 10.7589/0090-3558-37.3.594 [DOI] [PubMed] [Google Scholar]
- Sykes J.E., 2013, ‘Chapter 14: Canine parvovirus infections and other viral enterides’, in Sykes J.E. (ed.), Canine and feline infectious diseases, pp. 141–151, Elsevier, St Louis, MO. ISBN: 978-1437707953. [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M. & Kumar S., 2011, ‘MEGA5: Molecular evolutionary genetics analysis using maximum likelihood evolutionary distance and maximum parsimony methods’, Molecular Biology and Evolution 28, 2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truyen U., Evermann J.F., Vieler E. & Parrish C.R., 1996, ‘Evolution of canine parvovirus involved loss and gain of feline host range’, Virology 215, 186–189. 10.1006/viro.1996.0021 [DOI] [PubMed] [Google Scholar]
- Truyen U., Muller T., Heindrich R., Tackmann K. & Carmichael L.E., 1998, ‘Survey on viral pathogens in wild red foxes (Vulpes vulpes) in Germany with emphasis on parvoviruses and analysis of a DNA sequence from a red fox parvovirus’, Epidemiology and Infection 121, 433–440. 10.1017/S0950268898001319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heerden J., Mills M.G.L., Van Vuuren M.J., Kelly P.J. & Dreyer M.J., 1995, ‘An investigation into the health status and diseases of wild dogs (Lycaon pictus) in the Kruger National Park’, Journal of the South African Veterinary Association 66, 18–27. [PubMed] [Google Scholar]
- Van Vuuren M., Steinel A., Goosen T., Lane E., Van der Lugt J., Pearson J. et al. , 2000, ‘Feline panleukopenia virus revisited: Molecular characteristics and pathological lesions associated with three recent isolates’, Journal of the South African Veterinary Association 71(3), 140–143. 10.4102/jsava.v71i3.702 [DOI] [PubMed] [Google Scholar]
- Wasieri J., Schmiedeknecht G., Forster C., Konig M. & Reinacher M., 2009, ‘Parvovirus infection in a Eurasian lynx (Lynx lynx) and in a European wildcat (Felis silvestris silvestris)’, Journal of Comparative Pathology 140, 203–207. 10.1016/j.jcpa.2008.11.003 [DOI] [PubMed] [Google Scholar]